Abstract
The anaplerotic odd-medium-chain triglyceride triheptanoin is used in clinical trials for the chronic dietary treatment of patients with long-chain fatty acid oxidation disorders. We previously showed (Kinman RP, Kasumov T, Jobbins KA, Thomas KR, Adams JE, Brunengraber LN, Kutz G, Brewer WU, Roe CR, Brunengraber H. Am J Physiol Endocrinol Metab 291: E860–E866, 2006) that the intravenous infusion of triheptanoin increases lipolysis traced by the turnover of glycerol. In this study, we tested whether lipolysis induced by triheptanoin infusion is accompanied by the potentially harmful release of long-chain fatty acids. Rats were infused with heptanoate ± glycerol or triheptanoin. Intravenous infusion of triheptanoin at 40% of caloric requirement markedly increased glycerol endogenous Ra but not oleate endogenous Ra. Thus, the activation of lipolysis was balanced by fatty acid reesterification in the same cells. The liver acyl-CoA profile showed the accumulation of intermediates of heptanoate β-oxidation and C5-ketogenesis and a decrease in free CoA but no evidence of metabolic perturbation of liver metabolism such as propionyl overload. Our data suggest that triheptanoin, administered either intravenously or intraduodenally, could be used for intensive care and nutritional support of metabolically decompensated long-chain fatty acid oxidation disorders.
inherited fatty acid oxidation disorders (FOD) can affect the carnitine transporter, the “carnitine cycle” [carnitine palmitoyltransferase (CPT) I, translocase, CPT II], or the mitochondrial β-oxidation spiral (for a review, see Ref. 17). Although the phenotype of long-chain FOD is variable, patients often suffer from muscle weakness, hypotonia, cardiac arrhythmia, and cardiomyopathy. Acute episodes, triggered by an infection or trauma, often involve hypoketotic hypoglycemia, massive rhabdomyolysis with release of creatine kinase in plasma, shock, and death (22).
Since the early 1980s, the chronic dietary treatment of long-chain FOD involved 1) a moderately high-carbohydrate diet with cornstarch at bed time, 2) decreasing long-chain fats to about 20% of the calories, including essential fatty acids, and 3) providing medium-chain triglycerides (1) since the corresponding C8 and C10 fatty acids enter mitochondria as carboxylates, which, after activation, require only those β-oxidation enzymes with medium- and short-chain specificity (10). The treatment with medium-chain triglycerides is restricted to long-chain FOD and is contraindicated for medium- and short-chain FOD (17).
In 2002, we proposed to replace the even-medium-chain triglycerides with odd-chain triheptanoin (19). Heptanoate, like octanoate, enters mitochondria without passing through the CPT system (10). Unlike octanoate, which is β-oxidized to acetyl-CoA, heptanoate is oxidized to acetyl-CoA and propionyl-CoA (Fig. 1). The latter is anaplerotic for the citric acid cycle. We reasoned that, during episodes of metabolic decompensation, such as long-chain FOD, the release of large molecules from cells, e.g., creatine kinase, is probably accompanied by the release of small molecules, including citric acid cycle intermediates. The latter carry acetyl groups as they are oxidized to CO2. This would explain the muscle weakness often encountered in long-chain FOD patients. Chronic anaplerotic therapy with triheptanoin improved the clinical status and quality of life of a number of patients (18, 20).
Fig. 1.
Interrelations between C4-ketogenesis from even-chain fatty acids, C5-ketogenesis from odd-chain fatty acids, and anaplerosis in the liver. The numbers in italics refer to the following enzymes: 3-ketoacyl-CoA thiolase (1), hydroxymethylglutaryl (HMG)-CoA synthase (2), HMG-CoA lyase (3), and β-hydroxybutyrate (BHB) dehydrogenase (4). This figure also shows the link between propionyl-CoA and the citric acid cycle (CAC) via anaplerosis (modified from Ref. 3). BHP, β-hydroxypentanoate; AcAc, acetoacetate; BKP, β-ketopentanoate; HEG, 3-hydroxy-3-ethylglutaryl.
In another study (8), we explored the metabolism of triheptanoin administered to rats as an intravenous vs. an intraduodenal emulsion. In the liver, the propionyl moiety of heptanoate β-oxidation has two main fates (Fig. 1), 1) anaplerotic gluconeogenesis and 2) formation of C5-ketone bodies (3) β-hydroxypentanoate (BHP) and β-ketopentanoate (BKP). C5-ketogenesis proceeds via the hydroxymethylglutaryl-CoA (HMG-CoA) cycle that forms the C4-ketone bodies β-hydroxybutyrate (BHB) and acetoacetate (AcAc). However, the intermediates of C5-ketogenesis are BKP-CoA and 3-hydroxy-3-ethylglutaryl-CoA (HEG-CoA). In peripheral tissues, C5-ketone bodies are rapidly metabolized (9) via 3-oxoacid-CoA transferase and converted to acetyl-CoA and propionyl-CoA. Thus, both heptanoate and C5-ketone bodies are anaplerotic in peripheral tissues.
When triheptanoin was infused intravenously, we measured high concentration ratios [heptanoate]/[C5-ketone bodies] in arterial blood (8). In contrast, when triheptanoin was infused intraduodenally, the [heptanoate]/[C5-ketone bodies] ratio was low. This results from the obligatory passage through the liver of all heptanoate derived from the hydrolysis of enteral triheptanoin. This results in the conversion of a large fraction of heptanoate derived from triheptanoin to C5-ketone bodies. In addition, we observed an apparent increase in lipolysis, which was measured from the dilution of tracer [13C3]glycerol during intravenous infusion of triheptanoin. This was not the case when triheptanoin was infused enterally. Activation of lipolysis would not be desirable during the treatment of decompensated long-chain FOD patients. The goal of the present study was to fully characterize the lipolytic process during the intravenous administration of triheptanoin. We explored the possibility that long-chain fatty acids, released in adipocytes by hormone-sensitive lipase during intravenous infusion of triheptanoin, could be reesterified in the same adipocytes. Thus, activation of lipolysis by intravenous triheptanoin would not lead to a flooding of muscle and heart cells with long-chain fatty acids. Our data show that this is indeed the case.
METHODS
Materials.
[13C6]glucose, [6,6-2H2]glucose, [1,1,2,3,3-2H5]glycerol, [2-13C]glycerol, [13C3]glycerol, [2H13]heptanoic acid, [13C18]oleate, 2H2O, NaO2H, sodium borodeuteride (NaB2H4), and general chemicals were purchased from Isotec/Sigma-Aldrich (St. Louis, MO). The derivatizing reagent bis(trimethylsilyl)trifluoroacetamide plus 10% trimethylchlorosilane was purchased from Regis (Morton Grove, IL). An internal standard of R,S-β-hydroxy-[2H5]pentanoate was prepared by incubating ethyl BKP in 2H2O plus NaO2H overnight followed by reducing β-keto-[2H4]pentanoate with sodium borodeuteride (9). A 10% sterile emulsion of triheptanoin was kindly donated by Sasol (Witten, Germany). As per Sasol's quality control data, the triheptanoin contained 97.5% triester, with the remaining 2.5% being monoester and diester. Before use, the [13C18]oleate was complexed with dialyzed fatty acid-free bovine serum albumin (Serologicals, Norcross, GA).
Animal experiments.
The animal experiments were approved by the Institutional Animal Care and Utilization Committee of the School of Medicine of Case Western Reserve University.
Preparation of rats.
Male Sprague-Dawley rats were fed ad libitum with Harlan Teklad rat chow. After an overnight fast to initiate a catabolic state, rats (250–310 g) were anesthetized with 2–2.5% isoflurane in pure oxygen. Catheters were inserted in a jugular vein and in a carotid artery. Some rats were also fitted with a catheter in the duodenum for infusion. Following successful placement of catheters, saline (58.3 μl/min) was infused into the jugular vein for a 20-min equilibration period. A basal blood sample (150 μl) was obtained from the carotid artery before the start of each protocol.
Protocols.
Six groups of six to seven rats (Table 1) were used. Group 1 was the control group. Groups 2 to 6 received Na-heptanoate or triheptanoin in amounts that provided 40% of the caloric requirement from the heptanoate moiety. These rates, calculated on the basis of the weight of each rat, and the caloric content of heptanoic acid (7.7 kcal/g) were on average 42.3 ± 0.09 μmol heptanoate equivalents·min−1·kg−1. The substrates and isotopic tracers infused, as well as the routes of administration, are indicated in Table 1.
Table 1.
Rates of administration of substrates and isotopic tracers
| Group | Substrate(s) Infused and Route of Administration | Rate of Heptanoate Equivalent Administration | Rate of Glycerol Equivalent Administration | Rate of Tracer [6,6-2H2]Glucose iv Infusion | Rate of Tracer [13C3]Glycerol iv Infusion | Rate of Tracer [13C18]Oleate iv Infusion |
|---|---|---|---|---|---|---|
| 1 (control) | Saline iv | 0 | 0 | 1.2 | 1.5 | 0.3 |
| 2 | Heptanoate-Na iv | 42.3 | 0 | 1.2 | 3.7 | 0.6 |
| 3 | Heptanoate-Na + glycerol iv | 42.3 | 28.2 | 1.2 | 3.7 | 0.6 |
| 4 | Triheptanoin iv | 42.3 | 28.2 | 1.2 | 3.7 | 0.6 |
| 5 | Triheptanoin + glucose + insulin iv | 42.3 | 28.2 | 1.2 | 3.7 | 0.6 |
| 6 | Triheptanoin id | 42.3 | 28.2 | 1.2 | 3.7 | 0.6 |
Substrates and tracers were infused intravenously (iv) or intraduodenally (id) at the indicated rates expressed in μmol•kg−1•min−1. Heptanoate was infused as the sodium salt (150 mM solution) or as a 10% triheptanoin emulsion. In groups 4, 5, and 6, the glycerol infused had 2 components: 1) the glycerol moiety of triheptanoin and 2) free glycerol used as an emulsifier. In group 3, free glycerol was infused at the same rate as the total glycerol infused in groups 4, 5, and 6. The [13C18]oleate tracer was complexed with dialyzed fatty acid-free bovine serum albumin. Also in group 5, glucose and insulin were infused iv at 145 μmol•kg−1•min−1 and 4 mU•kg−1•min−1, respectively. In group 6, [1,1,2,3,3-2H5]glycerol was infused id (3.7 μmol•kg−1•min−1) in addition to [13C3]glycerol iv.
Arterial blood samples (150 μl) were taken at 0 (basal), 20, 40, 60, 75, 90, 105, 120, and 125 min. After the last blood sampling the liver was rapidly removed, quick-frozen (24), and stored in liquid nitrogen.
Analytical procedures.
Arterial blood samples (150 μl) were centrifuged immediately to obtain plasma. For the assays of glucose and glycerol, 20 μl of plasma was pipetted into glass tubes containing 275 μl of an aqueous solution of internal standards: [13C6]glucose (50 nmol), [1,1,2,3,3-2H5]glycerol (5 nmol in groups 1, 2, 3, 4, and 5), or [2-13C]glycerol (0.16 nmol in group 6). For the assays of heptanoate, C4-ketone bodies, C5-ketone bodies, and oleate, another 40 μl of plasma was pipetted into glass tubes containing a 660-μl solution of internal standards: [2H13]heptanoate (20 nmol), R,S-β-hydroxy-[2H6]butyrate (40 nmol), R,S-β-hydroxy-[2H5]pentanoate (20 nmol), and heptadecanoic acid (16 nmol). After quick mixing, the solutions were treated with 100 μl of 1 M sodium borodeuteride in 0.1 M NaOH. The treatment with sodium borodeuteride converts unstable AcAc and BKP to the stable M1 BHB and M1 BHP, respectively, which can be distinguished by GC-MS from the unlabeled BHB and BHP (9). It also converts glucose to M1 sorbitol. Plasma samples were stored at −20°C until being assayed. Livers and adipose tissues were stored at −80°C until being assayed.
Assays of glucose and glycerol.
The borodeuteride-treated plasma samples were acidified with 100 μl of 12 N HCl (to destroy excess borodeuteride) and evaporated under nitrogen. The residue was reacted with 150 μl of acetic anhydride and 300 μl of pyridine, heated for 1 h at 100°C, and then left overnight at room temperature. The next day, samples were extracted three times with diethyl ether, and the combined extract was dried over Na2SO4 before evaporation. The residue was dissolved in 50 μl of ethyl acetate. Either 1 (glycerol assay) or 2 μl (sorbitol derived from glucose assay) was injected into an Agilent 6890 gas chromatograph linked to a 5973 MSD mass spectrometer. The chromatograph was equipped with a 30-m OV-225 capillary column (Quadrex). The carrier gas was helium, and the injection mode was either split (glycerol) or splitless (sorbitol derived from glucose). It was necessary to use the splitless mode for the sorbitol derivative because it does not extract well with diethyl ether. The GC injector temperature was set at either 190 (glycerol) or 235°C (glucose), and the transfer line was held at 240°C. For glycerol, the column temperature was increased from 80°C by 4°C/min to 190°C and then by 50°C/min from 190 to 220°C, where it was held for 10 min. For glucose, the column temperature was increased from 100°C by 20°C/min to 190°C, by 5°C/min from 190 to 220°C, and then by 20°C/min from 220 to 235°C, where it was held for 45 min. The mass spectrometer was operated under ammonia positive chemical ionization, with the source pressure adjusted to obtain the maximal signal. The retention times and ions monitored were as follows: glycerol [6.9 min; mass-to-charge ratio (m/z) 236, 237, 238, 239, and 241] and glucose converted to sorbitol (25.6 min; m/z 453, 454, 455, and 459).
Assay of heptanoate, C4-ketone bodies, C5-ketone bodies, and oleate.
Plasma samples were deproteinized with 3 ml of acetonitrile-methanol (vol/vol, 7:3). After centrifugation, the supernatant was transferred to new tubes and evaporated. The residue was reacted with 100 μl of trimethylsilyl reagent and heated for 1 h at 90°C. Two microliters were injected into the same gas chromatograph-mass spectrometer as described above. The chromatograph was equipped with a 60-m Varian CP 9017 VF-5 capillary column. The carrier gas was helium (10.3 ml/min), and the injection mode was splitless. The injector temperature was set at 290°C, and the transfer line was held at 290°C. The column temperature was increased from 100°C by 2°C/min to 135°C, by 10°C/min from 135 to 200°C, by 4°C/min from 200 to 300°C, where it was held for 5 min, and then by 50°C/min from 300 to 310°C, where it was held for 10 min. The mass spectrometer was operated under ammonia positive chemical ionization, with the source pressure adjusted to obtain the maximal signal. The retention times and ions monitored were as follows: heptanoate (13.8 min; m/z 220 and 233); BHB (13.2 min; m/z 249, 250, and 255); BHP (16.4 min; m/z 263, 264, and 268); oleate (38.3 min; m/z 372 and 390).
Assay of short-chain and medium-chain acyl-CoAs in liver tissue.
Frozen powdered liver samples (∼200 mg) were homogenized with a Polytron homogenizer in a 50-ml screw-cap tube containing 4 ml of methanol-H2O (vol/vol, 1:1), with 2% acetic acid and 1 nmol [2H5]propionyl-CoA as internal standard. The centrifuged extract was loaded onto a Supelco solid-phase extraction cartridge [2-(pyridyl)-ethyl functionalized silica gel] preconditioned with 3 ml of methanol and then 3 ml of buffer A (1:1 methanol-H2O with 2% acetic acid). The cartridge was then washed with 3 ml of buffer A to elute impurities, followed sequentially by 3 ml of buffer B (1:1 methanol-H2O with 50 mM ammonium formate), 3 ml of buffer C (3:1 methanol-H2O with 50 mM ammonium formate), and 3 ml of methanol to elute the acyl-CoAs. The eluent was evaporated under nitrogen.
After the residue in 100 μl of mobile phase A was dissolved (100 mM ammonium formate in 5% acetonitrile, pH 5.0), 15 μl of sample was injected on a Hypersil Gold C18 column (150 × 2.1 mm, 5-μm particle size; Thermo Electron) protected by a guard column (10 × 2.1 mm, 5 μm; Hypersil Gold) in a Dionex Ultimate 3000 liquid chromatograph. The flow rate was constant at 0.2 ml/min. For elution, 1) for the first 7 min, mobile phase A was 98%; 2) from 7 to 25 min, mobile phase B was 2–60% (5 mM ammonium formate in 95% acetonitrile); 3) from 25 to 26 min, mobile phase B was increased to 90%; 4) from 26 to 31 min, mobile phase B was 90%; and 5) for reequilibration, the mobile phase was brought back to 98% (A) over 1 min and held for 10 min.
The order of acyl-CoA elution (min) was malonyl-CoA (4.8), methylmalonyl-CoA (9.7), succinyl-CoA (12.4), HMG-CoA (13.1), acetyl-CoA (13.9), BHB-CoA, AcAc-CoA (14.2), HEG-CoA (14.7), BHP-CoA, BKP-CoA (15.4), propionyl-CoA (15.4), pentanoyl-CoA (18.5), and heptanoyl-CoA (21.7). In the absence of standards, HEG-CoA, BHP-CoA, and BKP-CoA were identified from 1) mother/daughter ion pairs, 2) comparison with the spectra of analogs (HMG-CoA, BHB-CoA, acetoacetyl-CoA), and 3) labeling in rat livers perfused with [5,6,7-13C3]heptanoate (not shown).
The liquid chromatograph was coupled to an API 4000 QTrap mass spectrometer (Applied Biosystems, Foster City, CA) operated under positive ionization mode with the following source settings: turbo ion spray source at 500°C under N2 nebulization at 60 psi, N2 heater gas at 60 psi, curtain gas at 30 psi, collision-activated dissociation gas pressure held at high, turbo ion spray voltage at 4,500 V, declustering potential at 90 V, entrance potential at 10 V, and collision cell exit potential at 50 V. The Analyst software (version 1.4.2; Applied Biology) was used for data registration.
Plasma insulin concentrations were assayed in samples of plasma taken at 125 min using the rat/mouse insulin ELISA kit (Linco Research). Liver glycogen contents were assayed by the standard acid-alcohol extraction, followed by glycogen acid hydrolysis and enzymatic assay of glucose.
Data analysis and calculations.
Total C4-ketone bodies were calculated as the sum of BHB and AcAc, whereas total C5-ketone bodies were the sum of BHP and BKP. The rates of appearance (Ra) of glucose, glycerol, and oleate were calculated according to the steady-state equation
| (1) |
where IEinfusate is the isotopic enrichment of the infused tracer, IEplasma is the isotopic enrichment of the tracee in plasma, and INF is the rate of the tracer infusion (μmol·kg−1·min−1). Whenever exogenous unlabeled glucose (group 5) or glycerol (groups 3–6) was infused, the corresponding total Ra were corrected for the amounts of exogenous substrate(s) infused:
| (2) |
where Ginf is the total exogenous glycerol or glucose infusion rate (μmol·kg−1·min−1). Note that the glycerol content of the triheptanoin emulsion (467 μmol/ml) has two almost equal components, the glycerol moiety of triheptanoin and the free glycerol, which keeps the emulsion stable.
In group 6, rats were infused intraduodenally with triheptanoin and M5 [2H5]glycerol. The other tracers, including M3 [13C3]glycerol, were infused intravenously. The two glycerol tracers were infused at the same rate (3.7 μmol·kg−1·min−1). The total glycerol Ra (endogenous + exogenous) was calculated from the arterial [13C3]glycerol enrichment (Eq. 1). We calculated the endogenous glycerol Ra according to the equation
| (3) |
where (M5/M3)arterial is the labeling ratio of glycerol in arterial plasma. This ratio represents the fraction of the intraduodenally infused glycerol (as triheptanoin + free glycerol) that escaped uptake by the liver. The identical rates of infusion of the M3 and M5 glycerol tracers are not included in Eq. 3 because they cancel out.
The rate of lipolysis was calculated as the endogenous Ra of glycerol, i.e., total Ra minus glycerol infused as such (group 3), or as a component of triheptanoin, plus as an emusifying agent in the triheptanoin emulsion (groups 4–6). Assuming that oleate accounts for one-fourth of plasma long-chain fatty acids, the percentage of the long-chain fatty acids released by lipolysis that was reesterified in the same cells was calculated as 100 [(3 × glycerol Ra) − (4 × oleate Ra)]/(3 × glycerol Ra).
Statistics.
Data are reported as means ± SE. We used one-way ANOVA with Tukey post hoc comparisons, using Graphpad Prism Software version 3.03 (Graphpad Software, La Jolla, CA), to identify significant differences between groups. The significance level was set at P < 0.05. Since plasma heptanoate and glycerol concentrations, as well as M18 oleate enrichment, reached steady state at 90 min, statistics were performed on 90- to 125-min data (105–125 min for plasma oleate concentration). For other plasma metabolites (glucose and C4- and C5-ketone bodies), statistics were performed on the 125-min data. All statistical comparisons between groups are presented in Table 2. Comparisons between a given group and the control group are also presented in the text.
Table 2.
Tukey test comparisons between profiles of plasma metabolite concentrations in 6 groups of experiments
|
P Value |
|||||||
|---|---|---|---|---|---|---|---|
| Groups Being Compared | Heptanoate | C5-Ketone Bodies | C4-Ketone Bodies | Glycerol | Glucose | Oleate Enrichment | Oleate |
| Group 1 vs. 2 | 0.001 | 0.001 | 0.001 | NS | 0.05 | 0.001 | 0.05 |
| Group 1 vs. 3 | 0.001 | 0.001 | 0.01 | 0.01 | 0.05 | 0.001 | NS |
| Group 1 vs. 4 | 0.001 | 0.001 | 0.01 | 0.001 | 0.001 | 0.001 | 0.01 |
| Group 1 vs. 5 | 0.001 | 0.05 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Group 1 vs. 6 | NS | 0.001 | 0.001 | NS | 0.001 | 0.001 | 0.01 |
| Group 2 vs. 3 | NS | NS | NS | 0.001 | NS | 0.01 | NS |
| Group 4 vs. 5 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Group 4 vs. 6 | 0.001 | NS | NS | 0.001 | NS | 0.001 | 0.001 |
NS, not significantly different. Since plasma heptanoate and glycerol concentrations as well as M18 oleate enrichment reached steady state at 90 min, statistics were performed on 90- to 125-min data (105–125 min for plasma oleate concentration). For other plasma metabolites (glucose, C4- and C5-ketone bodies), statistics were performed on the 125-min data. The compounds infused in the 6 groups are as follows: saline iv (group 1), heptanoate iv (group 2), heptanoate + glycerol iv (group 3), triheptanoin iv (group 4), triheptanoin + glucose + insulin iv (group 5), and triheptanoin id (group 6).
RESULTS
Figure 2 shows the profile of plasma heptanoate concentrations in the six groups of rats. Baseline heptanoate was undetectable in all groups and in the saline-infused control group. The infusion of Na-heptanoate at 40% of the caloric requirement resulted in stable heptanoate concentration of 0.34 ± 0.016 mM between 90 and 125 min (P < 0.001 compared with control; for other comparisons, see Table 2). Addition of glycerol to the Na-heptanoate infusion did not appreciably increase plasma heptanoate concentration. However, the infusion of triheptanoin markedly increased heptanoate concentration to 1.58 ± 0.11 mM compared with controls (P < 0.001). This was despite the fact that the infusions of triheptanoin and of Na-heptanoate plus glycerol supplied the same amounts of heptanoate and of glycerol equivalents. Addition of glucose plus insulin to the triheptanoin infusion further increased heptanoate concentration to 1.78 ± 0.11 mM compared with controls (P < 0.001). In contrast, as shown previously (8), the intraduodenal infusion of triheptanoin led to very low arterial plasma concentrations of heptanoate, ∼0.069 ± 0.021 mM (not significantly different from control).
Fig. 2.
Profile of heptanoate concentrations in rat plasma. In this and subsequent figures, data are presented as means ± SE (n = 6). iv, Intravenous; TH, triheptanoin; id, intraduodenal.
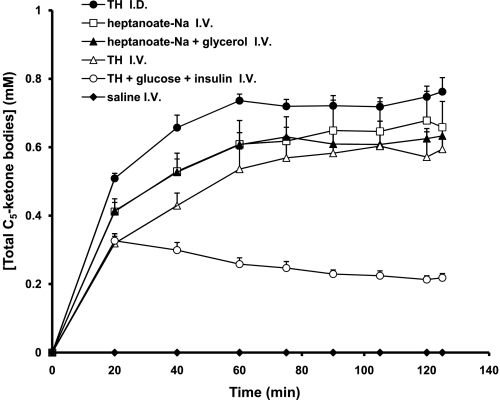
The basal concentration of C5-ketone bodies (Fig. 3) was undetectable in all groups and did not change during saline infusion. Similar concentrations of C5-ketone bodies were achieved at the end of infusions of either Na-heptanoate (0.66 ± 0.076 mM), Na-heptanoate plus glycerol (0.63 ± 0.036 mM), or triheptanoin (0.59 ± 0.070 mM). The accumulation of C5-ketone bodies was markedly blunted to 0.22 ± 0.013 mM (P < 0.05) when glucose plus insulin was added to the triheptanoin infusion. As shown previously, the intraduodenal infusion of triheptanoin led to the highest concentrations of C5-ketone bodies, 0.76 ± 0.041 mM (Fig. 3), and the lowest concentrations of heptanoate, except for the controls (Fig. 2).
Fig. 3.
Profile of total C5-ketone body concentrations in plasma.
The basal concentration of C4-ketone bodies (Fig. 4) was high in all groups (1.37 ± 0.10 to 2.08 ± 0.17 mM). This is the result of anesthesia that decreases the uptake of C4-ketone bodies by the brain (25). In the saline-infused rats, the concentration of C4-ketone bodies increased steadily to 2.48 ± 0.28 mM, presumably as a result of increased fasting duration. In the rats infused intravenously with Na-heptanoate, the concentration of C4-ketone bodies did not change. However, intravenous infusion of Na-heptanoate plus glycerol or triheptanoin slowly decreased the concentration of C4-ketone bodies. The same profile was observed during intraduodenal infusion of triheptanoin. The addition of glucose plus insulin to the intravenous infusion of triheptanoin lead to a 95% decrease in C4-ketone body concentration (P < 0.001 compared with controls) over 2 h.
Fig. 4.
Profile of total C4-ketone body concentrations in plasma.
In all groups, the [BHB]/[AcAc] and [BHP]/[BKP] ratios, measured in plasma, were stable from 60 to 125 min. The [BHB]/[AcAc] ratio was 1.49 ± 0.19 (SD; n = 216, all groups). The [BHP]/[BKP] ratio was 1.09 ± 0.18 (SD; n = 180, groups 2–6). Since both ratios are in equilibrium with the [NADH]/[NAD+] ratio in liver mitochondria via BHB dehydrogenase, we calculated the midpotential (in mV) of the BHP/BKP system using the equation E′0BHP/BKP = −297 − 13.5 log {[BHB]/[AcAc]/[BHP]/[BKP]}, where −297 mV is the midpotential of the BHB/AcAc couple (23). Using all of the ratios measured between 60 and 125 min, the calculated midpotential of the BHP/BKP couple is −298.8 ± 0.6 mV (SD; n = 180).
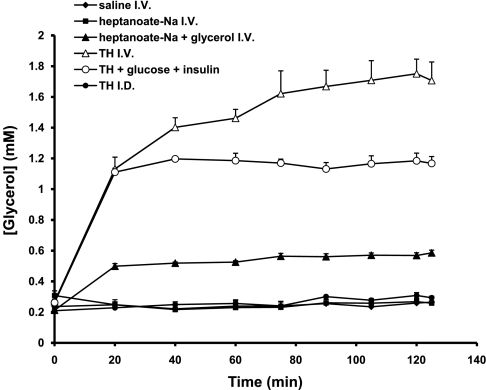
The plasma glycerol concentrations were 0.21 ± 0.011 mM in control (Fig. 5) and were not affected by the intravenous infusion of Na-heptanoate (0.26 ± 0.019 mM) or by the intraduodenal infusion of triheptanoin (0.29 ± 0.011 mM). Addition of glycerol to the intravenous Na-heptanoate infusion significantly increased glycerol concentration from 0.21 ± 0.011 to 0.57 ± 0.017 mM (P < 0.001). Intravenous infusions of triheptanoin led to very high glycerol concentrations (1.71 ± 0.11 mM, P < 0.001 compared with controls); this again is despite the fact that the infusions of triheptanoin and of Na-heptanoate plus glycerol supplied the same amounts of heptanoate and glycerol equivalents. Addition of glucose plus insulin to the triheptanoin infusion blunted about one-third of the glycerol concentration compared with the infusion of triheptanoin alone (P < 0.001). In rats infused with triheptanoin intraduodenally (group 6), M3 and M5 glycerol were infused intravenously and intraduodenally at equal rates (Table 1). The M5/M3 enrichment ratio in plasma glycerol was stable at 0.28 ± 0.018 (n = 6). Thus, ∼30% of the glycerol content of the intraduodenal infusion of triheptanoin escaped uptake by the liver.
Fig. 5.
Profile of glycerol concentrations in plasma.
The basal glucose concentration (6.6–7.4 mM; Fig. 6) was slightly above physiological, presumably because of the surgical stress. During saline infusion, glucose concentration decreased to almost normal values (5.75 ± 0.33 mM). Intravenous infusion of Na-heptanoate or Na-heptanoate plus glycerol significantly increased glucose concentration to 7.88 ± 0.16 (P < 0.05) or 7.98 ± 0.15 mM (P < 0.05) compared with controls, respectively. Infusion of triheptanoin, whether intravenous or intraduodenal, significantly increased glucose concentration to 9.12 ± 0.089 (P < 0.001) or 10.3 ± 0.29 mM (P < 0.001) compared with controls, respectively. Last, addition of glucose plus insulin to the intravenous triheptanoin infusion brought glucose concentration to 12.4 ± 0.26 mM (P < 0.001 compared with controls). The liver glycogen content (Table 3) was very low in the control group, 0.76 mg glucose equivalent/g, as expected for rats deprived of food for ∼18 h. In groups 2–6, which were infused for 2 h with gluconeogenic substrate(s) (and glucose + insulin in group 5), the liver glycogen content increased to a maximum of 6.9 mg glucose equivalents/g in group 5.
Fig. 6.
Profile of glucose concentrations in plasma.
Table 3.
Liver glycogen contents at 125 min and endogenous Ra of glucose, glycerol, and oleate
| Group | Compound(s) infused | Liver Glycogen | Glucose Ra | Glycerol Ra | Oleate Ra | %FA Reesterification |
|---|---|---|---|---|---|---|
| 1 (control) | Saline iv | 0.76 ± 0.12 | 29.9 ± 3.4 | 26.2 ± 3.1 | 15.3 ± 1.0 | 22 |
| 2 | Heptanoate iv | 1.08 ± 0.14 | 46.9 ± 1.4* | 35.2 ± 3.8 | 14.5 ± 0.8 | 45 |
| 3 | Heptanoate + glycerol iv | 3.65 ± 0.62* | 46.7 ± 1.4* | 34.8 ± 2.2 | 19.3 ± 1.2 | 26 |
| 4 | Triheptanoin iv | 4.59 ± 0.42* | 45.8 ± 2.2* | 282 ± 11* | 17.2 ± 0.7 | 92 |
| 5 | Triheptanoin + glucose + insulin iv | 6.93 ± 0.46*† | −15 ± 5.2*† | 145 ± 14*† | 8.3 ± 1.2*† | 92 |
| 6 | Triheptanoin id | 5.55 ± 0.27* | 32.3 ± 0.9† | 11.1 ± 0.9*† | 12.8 ± 1.5† | −53 |
Liver glycogen contents are expressed as mg glucose equivalents/g (means ± SE; n = 6). Ra, rate of appearance corrected for infusion of exogenous tracer or tracer + unlabeled substrate; FA, fatty acid. All rates are expressed in μmol•kg−1•min−1 (means ± SE; n = 6). In groups 2–6, heptanoate-Na or triheptanoin was infused iv or id at 42.3 μmol heptanoate equivalents•min−1•kg−1. In group 5, glucose was infused at 145 μmol•min−1•kg−1. Assuming that oleate accounts for ¼ of plasma long-chain fatty acids, the percentage of the long-chain fatty acids released by lipolysis that was reesterified in the same cells was calculated as 100 [(3 × glycerol Ra) − (4 × oleate Ra)]/(3 × glycerol Ra). The 2 values in italics are not considered precise values (see results).
P < 0.05 compared with control group;
P < 0.05, comparison between groups 4 and 5 or between groups 4 and 6.
The concentration of plasma oleate (Fig. 7B) remained stable from the start in all groups, except in the group infused intravenously with triheptanoin. However, in the latter group, the oleate concentration stabilized after 1 h. The M18 enrichment of plasma oleate remained stable in all groups (Fig. 7A). The concentrations of plasma palmitate and stearate remained stable in all groups (data not shown). Since steady-state concentration and enrichment of oleate were achieved in all groups during the last 30 min of the experiments, we calculated the turnover of oleate using the standard steady-state equation (Table 3). The endogenous Ra of oleate was not affected by the intravenous infusion of Na-heptanoate or of triheptanoin. The lack of increase in the endogenous Ra of oleate by intravenous triheptanoin contrasts with the 10.8-fold increase in glycerol endogenous Ra induced by the intravenous infusion of triheptanoin. When glucose plus insulin was added to the intravenous infusion of triheptanoin, the endogenous Ra of oleate was halved, whereas the endogenous Ra of glycerol was 5.5 times that of controls. Last, the intraduodenal infusion of triheptanoin did not change the endogenous Ra of oleate.
Fig. 7.
Profile of oleate M18 enrichment (A) and concentration (B) in plasma. MPE, molar %enrichment.
The plasma insulin concentration remained between 0.3 and 0.4 ng/ml in groups 1, 2, 3, 4, and 6 (data not shown). It rose to 4.4 ng/ml in group 5 (infused with triheptanoin + glucose + insulin; data not shown). We had expected some increase in insulin concentration in groups 4 and 6, where triheptanoin was infused alone intravenously or intraduodenally, respectively, and where plasma glucose concentration went ≤9–10 mM. The assays were repeated with the same results. We ascribe the absence of glucose-induced insulin release to isoflurane anesthesia (see discussion).
Table 4 shows the concentrations of medium- and short-chain acyl-CoAs, as well as free CoA, in the liver of the rats. In all rats infused with heptanoate or triheptanoin, the concentration of free CoA decreased as the concentrations of acyl-CoAs derived from heptanoate (heptanoyl-CoA, pentanoyl-CoA, and propionyl-CoA) increased. The sum of assayed concentrations of acyl-CoA plus free CoA increased by up to one-half in livers of rats infused with triheptanoin plus glucose plus insulin intravenously or triheptanoin intraduodenally. The concentrations of the intermediates of C4-ketogenesis (HMG-CoA, acetoacetyl-CoA) and C5-ketogenesis (HEG-CoA, BKP-CoA) remained fairly constant despite vastly different rates of C4- and C5-ketogenesis.
Table 4.
Liver acyl-CoA concentrations
| Group 1 (Saline iv) | Group 2 (Heptanoate iv) | Group 3 (Heptanoate + Glycerol iv) | Group 4 (Triheptanoin iv) | Group 5 (Triheptanoin + Glucose + Insulin iv) | Group 6 (Triheptanoin id) | |
|---|---|---|---|---|---|---|
| Free CoA | 14.7 ± 1.5 | 3.44 ± 1.08* | 2.04 ± 0.07* | 1.70 ± 0.10* | 3.05 ± 0.54* | 4.58 ± 1.47* |
| Acetyl-CoA | 44.3 ± 1.8 | 35.0 ± 1.5* | 36.9 ± 0.76* | 30.7 ± 2.2* | 36.8 ± 0.78*† | 33.2 ± 1.8* |
| Propionyl-CoA | 4.66 ± 0.34 | 4.59 ± 0.63 | 5.52 ± 0.42 | 4.52 ± 0.61 | 5.38 ± 0.53 | 9.20 ± 1.69*† |
| Malonyl-CoA | 1.78 ± 0.04 | 1.46 ± 0.09* | 1.57 ± 0.08* | 1.33 ± 0.07* | 4.56 ± 0.55*† | 1.45 ± 0.09* |
| Methylmalonyl-CoA | 2.31 ± 0.11 | 1.97 ± 0.14 | 2.48 ± 0.14 | 1.89 ± 0.15* | 2.90 ± 0.21*† | 2.41 ± 0.39 |
| Succinyl-CoA | 6.32 ± 0.84 | 4.75 ± 0.76 | 7.14 ± 0.60 | 4.15 ± 0.35* | 12.1 ± 1.1*† | 7.18 ± 0.70† |
| AcAc-CoA | 0.22 ± 0.02 | 0.20 ± 0.03 | 0.28 ± 0.01* | 0.30 ± 0.01* | 0.74 ± 0.02*† | 0.76 ± 0.02*† |
| BHP-CoA | 0.13 ± 0.02 | 0.65 ± 0.07* | 0.49 ± 0.03* | 0.54 ± 0.09* | 0.57 ± 0.05* | 1.18 ± 0.19*† |
| BKP-CoA | 0.08 ± 0.01 | 0.17 ± 0.05 | 0.30 ± 0.02* | 0.32 ± 0.01* | 0.72 ± 0.02*† | 0.74 ± 0.03*† |
| Pentanoyl-CoA | 0.76 ± 0.10 | 10.8 ± 0.41* | 11.8 ± 1.2* | 17.5 ± 1.3* | 15.0 ± 1.0* | 22.4 ± 2.9* |
| HMG-CoA | 15.3 ± 1.2 | 18.6 ± 1.1 | 16.0 ± 1.2 | 14.9 ± 0.70 | 17.0 ± 0.55† | 17.7 ± 0.53† |
| HEG-CoA | 0.81 ± 0.02 | 0.82 ± 0.04 | 0.83 ± 0.03 | 0.89 ± 0.03* | 0.55 ± 0.03*† | 0.96 ± 0.03* |
| Heptanoyl-CoA | 0.00 | 17.4 ± 1.0* | 15.8 ± 2.0* | 36.6 ± 2.7* | 35.2 ± 1.08* | 36.6 ± 2.5* |
| Total assayed CoA esters | 92.6 ± 2.2 | 106 ± 4.4* | 108 ± 3.8* | 116 ± 6.3* | 138 ± 4.2*† | 140 ± 6.2*† |
All concentrations are expressed in nmol/g (means ± SE; n = 6–8). AcAc, acetoacetate; BHP, β-hydroxypentanoate; BKP, β-ketopenanoate; HMG, hydroxymethylglutaryl; HEG, 3-hydroxy-3-ethylglutatyl.
P < 0.05 compared with control group 1;
P <0.05, comparison between groups 4 and 5 or between groups 4 and 6.
DISCUSSION
Considerations on triglyceride metabolism.
The metabolism of an odd-medium-chain triglyceride like triheptanoin differs from that of an even-medium-chain triglyceride like trioctanoin or tridecanoin (components of medium-chain triglyceride oil). First, in the liver, even- and odd-chain fatty acids are partially converted to C4- and C5-ketone bodies (3), respectively. Second, odd-chain fatty acids are gluconeogenic in liver and kidney. Third, odd-chain fatty acids are anaplerotic in liver and in all organs that can use odd-medium-chain fatty acids and C5-ketone bodies. The anaplerotic property of heptanoate and of C5-ketone bodies was the basis of the clinical trials that used triheptanoin for the chronic treatment of patients with long-chain FOD (19, 20) and for the treatment of one patient with a severe pyruvate carboxylase deficiency (12).
It is well known that the infusion of a triglyceride emulsion (even-long chain or mixed even-long + even-medium chain) induces the activation of plasma lipoprotein lipase, which hydrolyzes the infused triglyceride (13). In addition, human studies based on arterio-venous differences in fatty acid concentration across adipose tissue and the arm provided evidence that the infusion of a triglyceride emulsion (intralipid) results in an increase in long-chain fatty acid release from adipose tissue (5, 6, 21). In a previous study (8), we found that intravenous administration of a triheptanoin emulsion to normal rats induces an increase the endogenous Ra of glycerol using tracer [13C3]glycerol. The data were corrected for the exogenous glycerol infused in the triheptanoin emulsion. The increase in the endogenous Ra of glycerol reflected an activation of lipolysis, presumably in adipose tissue. Such an increase in adipose tissue lipolysis by triheptanoin, if accompanied by an increase in long-chain fatty acid release from adipose tissue, would be detrimental to decompensated long-chain FOD patients. However, triheptanoin decreases rhabdomyolysis in long-chain FOD patients, as reflected by a decrease in creatine kinase activity in plasma. These observations prompted the present study, in which five different modalities of heptanoate administration were used and in which the endogenous Ra of both glycerol and long-chain fatty acids were measured with tracer [13C3]glycerol and [13C18]oleate.
Profiles of metabolite concentrations and Ra.
The intravenous infusion of equimolar amounts of heptanoate (alone, mixed with glycerol, as triheptanoin, or as triheptanoin + glucose + insulin, groups 2–5; Fig. 1) resulted in different plasma concentrations of heptanoate (Fig. 2). Although adding free glycerol to the free heptanoate infusion did not affect heptanoate concentration, infusion of triheptanoin resulted in much higher heptanoate concentrations. Since the heptanoate concentrations reached plateaus during the 2nd hour of the experiments, the rates of heptanoate metabolism were identical in all rats in groups 2–5, albeit under very different plasma heptanoate concentrations. Therefore, some factors were inhibiting the metabolism of heptanoate derived from triheptanoin until the influence of such factors was balanced by higher heptanoate concentrations. In group 5 (infused with triheptanoin + glucose + insulin), the marked hyperglycemia (Fig. 6) and massive exogenous hyperinsulinemia favored glucose over heptanoate utilization. Similarly, in group 4, infused with triheptanoin alone, the 4-mM increase in glucose concentration compared with controls (Fig. 6) interfered with heptanoate utilization.
As shown in our previous study (8), the intraduodenal infusion of triheptanoin (group 6) resulted in very low arterial heptanoate concentrations (Fig. 2) because the whole heptanoate load passed first through the liver. This explains also why the concentration of C5-ketone bodies was the highest in group 6 (Fig. 3). In rats infused intravenously with heptanoate, heptanoate plus glycerol, or triheptanoin (groups 2–4), the profiles of C5-ketone body concentrations were similar (Fig. 3). Also, in the same groups, the profiles of C4-ketone body concentration were similar (Fig. 4). However, the accumulations of C4- and C5-ketone bodies were inhibited in rats infused with triheptanoin plus glucose plus insulin (Figs. 3 and 4, open circles). The rapid inhibition of C5-ketogenesis by supraphysiological concentrations of insulin in the presence of the highest heptanoate concentrations (Fig. 2) shows that insulin can interfere with mitochondrial C5-ketogenesis in a time frame shorter than that needed for modulation of gene expression. This is confirmed by the very strong inhibition of C4-ketogenesis in the same rats (Fig. 4).
The arterial concentration of glycerol (Fig. 5) was not affected by either the intravenous infusion of heptanoate or the intraduodenal infusion of triheptanoin. This confirms that the glycerol resulting from the enteral hydrolysis of triheptanoin and the free glycerol contained in the triheptanoin emulsion are taken up entirely by the liver in a single pass of portal vein blood. In contrast, the intravenous infusion of equimolar amounts of glycerol (mixed with heptanoate, as triheptanoin, or as triheptanoin + glucose + insulin, groups 2–5; Fig. 1) resulted in different plasma concentrations of glycerol (Fig. 5). This results from the fact that the intravenous administration of triheptanoin led to a major increase in the production of endogenous glycerol from lipolysis (groups 4 and 5; Table 3). In these groups, the load of lipolytic glycerol was equivalent to 10 and five times the load of glycerol infused as part of the triheptanoin emulsion (28.2 μmol·min−1·kg−1).
The intravenous administration of heptanoate did not significantly increase endogenous lipolysis (groups 1–3; Table 3). The intravenous infusion of triheptanoin (without or with glucose + insulin, groups 4 and 5) resulted in large increases in glycerol Ra, as shown by others with different triglycerides (5, 6, 21). However, the 10.8-fold increase in endogenous glycerol Ra we measured in the present study is much larger than the twofold increase we measured in our previous study (8). Actually, in group 5 of the present study (triheptanoin + glucose + insulin iv), the endogenous glycerol Ra was also much higher than any value in our previous study. The only difference between the two studies is the mode of anesthesia. In the previous study, the rats were anesthetized with pentobarbital sodium intraperitoneally followed by a continuous iv infusion. In the present study, the rats were anesthetized with isoflurane. In reviewing the metabolic effects of isoflurane anesthesia, we found reports showing that isoflurane induces hyperglycemia, inhibits glucose-induced insulin secretion, and stimulates lipolysis (4, 15, 26). These effects, ascribed to the stimulation of growth hormone by isoflurane (15), were not observed during anesthesia with pentobarbital sodium (26). The inhibition of insulin secretion by isoflurane probably explains why, in the present experiments, plasma insulin concentration increased only in group 5, when exogenous insulin was infused. In groups 2, 3, 4, and 6, insulin concentration did not increase despite hyperglycemia. This may be related to differences in the effect of triheptanoin on the Ra of glucose between our previous study (Ra increased by one-half with triheptanoin intraduodenally but was not significantly increased by iv infusion) and the present study (opposite findings).
In rats infused with triheptanoin, the 10.8-fold increase in glycerol endogenous Ra (Table 3) may be caused by an amplification by isoflurane of the stimulation of lipolysis induced by the infusion of triheptanoin. The stimulation of lipolysis was not accompanied by any significant increase in the endogenous Ra of plasma oleate traced with [13C18]oleate. The latter tracer was chosen instead of the more commonly used [13C]palmitate or [13C]stearate because the unavoidable contamination of glassware and reagents by ubiquitous palmitate and stearate makes the assay of the enrichment of palmitate and stearate in small samples of rat plasma very imprecise. Figure 7, A and B, shows that the enrichment and concentration of plasma oleate were stable at the end of the experiments. From the endogenous Ra of glycerol and oleate, we estimated the percentage of the lipolytic long-chain fatty acids that were reesterified in adipose tissue. The estimation assumes that oleate accounts for one-fourth of the turnover of plasma long-chain fatty acids in all groups (16). On the basis of this assumption, the percent of reesterification is 22% in the control rats and about 90% in rats infused intravenously with triheptanoin (± glucose + insulin, groups 4 and 5). We acknowledge that there is some uncertainty in the estimated percent reesterification; however, the comparison of the glycerol and oleate endogenous Ra in groups 4 and 5 supports our conclusion that, in rats infused intravenously with triheptanoin, most of the long-chain fatty acids derived from adipose tissue lipolysis are reesterified in the same cells.
In rats infused with triheptanoin intraduodenally (group 6), the above calculation yields an impossible −55% reesterification. Because the low-glycerol endogenous Ra in group 6 was calculated using two glycerol tracers, the calculation of fatty acid reesterification is admittedly imprecise.
The plasma glucose concentrations (Fig. 6) and the endogenous Ra of glucose (Table 3) must be interpreted taking into account 1) the concentrations of gluconeogenic precursors (heptanoate, glycerol; Figs. 2 and 5), 2) the concentrations of competing energy fuels (heptanoate, C5- and C4-ketone bodies; Figs. 2, 3, and 4), and 3) the metabolic loads corresponding to the supply of exogenous and endogenous gluconeogenic precursors (exogenous glycerol, propionyl moiety of exogenous heptanoate, endogenous glycerol). In Fig. 8, these metabolic loads are compared with the endogenous glucose Ra and to the increase in the concentration of liver glycogen in groups 2–6 compared with group 1. Note that all rates presented in Fig. 8 are expressed in (μmol glucose equivalents·min−1·kg−1. These comparisons are somewhat difficult because our measurements of the glucose endogenous Ra do not distinguish 1) the two components of the glucose Ra, glycogenolysis and gluconeogenesis, and 2) the contributions of gluconeogenic precursors, amino acids derived from proteolysis, glycerol, and propionyl-CoA derived from heptanoate. In all experiments, the enrichment of glucose was stable during the last 30 min. Thus Ra was equal to rates of disappearance.
Fig. 8.
Comparison between the glucose endogenous rates of appearance (Ra) (open bars), the rate of liver glycogen synthesis over control (cross-hatched bars), and the potential glucose productions from 1) endogenous glycerol Ra (vertically hatched bars), 2) exogenous glycerol (diagonally hatched bars), and 3) exogenous propionyl equivalents derived from heptanoate or triheptanoin (black bars). All rates are expressed in μmol glucose equivalents·min−1·kg−1. SE for endogenous glycerol and glucose Ra are shown in Table 3.
In rats infused intravenously with gluconeogenic heptanoate (alone, + glycerol, or as triheptanoin, groups 2–4), the endogenous Ra of glucose was about 1.5 times that of the control group (Table 3). It is not clear why the glucose endogenous Ra was the same in these three groups given that groups 3 and 4 (unlike group 2) were given exogenous glycerol in addition to the same load of propionyl precursor. Also, group 4 (triheptanoin intravenous) and group 5 (triheptanoin + glucose + insulin intravenous) had to dispose of a large load of gluconeogenic glycerol derived from lipolysis. Clearly, this large load of glycerol was not converted to free glucose. In groups 3–6, some of the exogenous plus endogenous glycerol ended up stored as liver glycogen (Fig. 8). However, the increase in liver glycogen content would account for only a small fraction of the metabolized glycerol. Also, the small increase in glycogen content has a component of gluconeogenesis from the propionyl moiety of heptanoate (groups 3–6). Last, a small fraction of the glucose infused in group 5 probably contributed to liver glycogen synthesis. Overall, the observed increases in liver glycogen contents represent only a small fraction of the potential glycogen precursors available. It is likely that there was some accumulation in muscle glycogen in groups 4 and 5, but we did not assay muscle glycogen content.
The α-glycerophosphate used for fatty acid reesterification in adipose tissue can be derived from glucose catabolism and possibly from glycerolneogenesis via phosphoenolpyruvate carboxykinase (2, 14). One can wonder whether the propionyl moiety of heptanoate could be glyceroneogenic in adipose tissue. However, the supply of propionyl-CoA equivalents during intravenous infusion of triheptanoin (42.3 μmol·min−1·kg−1) is much smaller than the amount of fatty acid released by lipolysis that needs to be reesterified. The latter is three times the glycerol Ra, i.e., 3 × 282 = 846 μmol·min−1·kg−1 (Table 3). Thus, when triheptanoin was infused, the reesterification of long-chain fatty acid derived from lipolysis could not be supported by glyceroneogenesis from the propionyl moiety of heptanoate. Thus, the supply of α-glycerophosphate for fatty acid reesterification must be derived from glycolysis stimulated by hyperglycemia (Fig. 6) and possibly by glyceroneogenesis from pyruvate derived from glycolysis (14).
Profiles of acyl-CoA concentrations in liver.
Administration of heptanoate or triheptanoin led to substantial changes in the concentrations of some acyl-CoA and of free CoA in the liver (Table 4). The sum of the concentrations of the assayed short- and medium-chain acyl-CoAs increased by up to 50% compared with saline-infused controls. This reflects a redistribution of CoA from long-chain to short- and medium-chain esters. Concern that the administration of some substrates or drugs could lead to “CoA sequestration, toxicity, or redistribution” has been expressed (11). In particular, the trapping of CoA into unusually large pools of some acyl-CoAs would decrease the availability of free CoA and impact on the rate of CoA-dependent reactions. In our experiments, the trapping of CoA in the intermediates of heptanoate β-oxidation (heptanoyl-CoA, pentanoyl-CoA, BKP-CoA, BHP-CoA, and propionyl-CoA) resulted in up to 10-fold and one-third decreases in the concentrations of free CoA and acetyl-CoA, respectively. However, the plateauing of the plasma heptanoate concentrations (Fig. 2), albeit at different levels, indicated that all of the infused heptanoate equivalents were being metabolized. Also, despite an up to one-third decrease in liver acetyl-CoA concentration, there was a large flow of acetyl groups into C4- and C5-ketogenesis, except when insulin was infused (Figs. 3 and 4). Ketogenesis removes from the liver acetyl groups that cannot be oxidized in the citric acid cycle, the rate of which is linked to the rate of ATP regeneration (7). Therefore, the trapping of free CoA by heptanoate metabolism did not impair the energy metabolism of the liver.
Despite very large differences in rates of C4- and C5-ketogenesis between rats infused with saline and rats infused with heptanoate or triheptanoin (Figs. 3 and 4), there were little variations in the concentrations of HMG-CoA and HEG-CoA. This reflects the high activities of HMG-CoA synthase and HMG-CoA lyase. Thus, under the conditions of the experiments, the rates of C4- and C5-ketogenesis depend on the supply of acetyl and propionyl groups to the HMG-CoA cycle.
Conclusions and clinical implications.
First, although the intravenous administration of triheptanoin leads to a stimulation of lipolysis, presumably in adipose tissue, there is no increase in fatty acid endogenous Ra because of intense reesterification of these fatty acids. Thus, the intravenous administration of triheptanoin should be considered for the acute treatment of decompensated long-chain FOD patients. Second, enteral treatment of these patients with triheptanoin (by nasogastric tube) remains an option because this treatment does not lead to increased lipolysis; it actually decreases basal lipolysis (Table 3). Third, intravenous infusion of triheptanoin does not need to be supplemented with glucose infusion because the gluconeogenic property of the infusion leads to moderate hyperglycemia. We realize that the normal rats used in the present investigation, although starved overnight, were not in a severely stressed condition comparable with that of decompensated long-chain FOD and other patients requiring intensive care support. In these patients, the massive basal lipolysis and the lipolysis induced by intravenous triheptanoin intravenous infusion may not be fully balanced by the reesterification processes demonstrated in our study. So these patients may benefit from the administration of insulin. This question should be investigated in animals subjected to lipolytic conditions such as the infusion of a catecholamine.
GRANTS
This work was supported by the National Institutes of Health (Road Map Grant 5-R-33-DK-070291 and Grant 5-R01-DK-069752) and the Cleveland Mt. Sinai Health Care Foundation.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank the Case Western Reserve University Mouse Metabolic Phenotyping Center for help with rat surgery.
REFERENCES
- 1.Bougnères PF, Saudubray JM, Marsac C, Bernard O, Odièvre M, Girard J. Fasting hypoglycemia resulting from hepatic carnitine palmitoyl transferase deficiency. J Pediatr 98: 742–746, 1981 [DOI] [PubMed] [Google Scholar]
- 2.Chen JL, Peacock E, Samady W, Turner SM, Neese RA, Hellerstein MK, Murphy EJ. Physiologic and pharmacologic factors influencing glyceroneogenic contribution to triacylglyceride glycerol measured by mass isotopomer distribution analysis. J Biol Chem 280: 25396–25402, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Deng S, Zhang GF, Kasumov T, Roe CR, Brunengraber H. Interrelations between C4-ketogenesis, C5-ketogenesis and anaplerosis in the perfused rat liver. J Biol Chem 284: 27799–27807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diltoer M, Camu F. Glucose homeostasis and insulin secretion during isoflurane anesthesia in humans. Anesthesiology 68: 880–886, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Evans K, Clark ML, Frayn KN. Effects of an oral and intravenous fat load on adipose tissue and forearm lipid metabolism. Am J Physiol Endocrinol Metab 276: E241–E248, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Fielding BA, Samra JS, Ravell CL, Frayn KN. Metabolism of individual fatty acids during infusion of a triacylglycerol emulsion. Lipids 34: 535–541, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Halperin ML, Cheema-Dhadli S. Renal and hepatic aspects of ketoacidosis: a quantitative analysis based on energy turnover. Diabetes Metab Rev 5: 321–336, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Kinman RP, Kasumov T, Jobbins KA, Thomas KR, Adams JE, Brunengraber LN, Kutz G, Brewer WU, Roe CR, Brunengraber H. Parenteral and enteral metabolism of anaplerotic triheptanoin in normal rats. Am J Physiol Endocrinol Metab 291: E860–E866, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Leclerc J, Des Rosiers C, Montgomery JA, Brunet J, Ste-Marie L, Reider MW, Fernandez CA, Powers L, David F, Brunengraber H. Metabolism of R-β-hydroxypentanoate and of β-ketopentanoate in conscious dogs. Am J Physiol Endocrinol Metab 268: E446–E452, 1995 [DOI] [PubMed] [Google Scholar]
- 10.McGarry JD, Foster DW. The regulation of ketogenesis from octanoic acid. The role of the tricarboxylic acid cycle and fatty acid synthesis. J Biol Chem 246: 1149–1159, 1971 [PubMed] [Google Scholar]
- 11.Mitchell GA, Gauthier N, Lesimple A, Wang SP, Mamer O, Qureshi I. Hereditary and acquired diseases of acyl-coenzyme A metabolism. Mol Genet Metab 94: 4–15, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Mochel F, DeLonlay P, Touati G, Brunengraber H, Kinman RP, Rabier D, Roe CR, Saudubray JM. Pyruvate carboxylase deficiency: clinical and biochemical response to anaplerotic diet therapy. Mol Genet Metab 84: 305–312, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Nordenstrom J, Neeser G, Olivecrona T, Wahren J. Effect of medium- and long-chain triglyceride infusion on lipoprotein and hepatic lipase in healthy subjects. Eur J Clin Invest 21: 580–585, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Nye CK, Hanson RW, Kalhan SC. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J Biol Chem 283: 27565–27574, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oyama T, Latto P, Holaday DA. Effect of isoflurane anaesthesia and surgery on carbohydrate metabolism and plasma cortisol levels in man. Can Anaesth Soc J 22: 696–702, 1975 [DOI] [PubMed] [Google Scholar]
- 16.Paik MJ, Park KH, Park JJ, Kim KR, Ahn YH, Shin GT, Lee G. Patterns of plasma fatty acids in rat models with adenovirus infection. J Biochem Mol Biol 40: 119–124, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Roe CR, Ding JH. Mitochondrial fatty acid oxidation disorders. In: The Metabolic and Molecular Bases of Inherited Diseases, edited by Scriver CR, Beaudet AL, Sly WS, Valle D. New York: McGraw-Hill, 2001, p. 2297–2326 [Google Scholar]
- 18.Roe CR, Mochel F. Anaplerotic diet therapy in inherited metabolic disease: therapeutic potential. J Inherit Metab Dis 29: 332–340, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest 110: 259–269, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roe CR, Yang BZ, Brunengraber H, Roe DS, Wallace M, Garritson BK. Carnitine palmitoyltransferase II deficiency: successful anaplerotic diet therapy. Neurology 71: 260–264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samra JS, Giles SL, Summers LK, Evans RD, Arner P, Humphreys SM, Clark ML, Frayn KN. Peripheral fat metabolism during infusion of an exogenous triacylglycerol emulsion. Int J Obes Relat Metab Disord 22: 806–812, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Saudubray JM, Martin D, De Lonlay P, Touati G, Poggi-Travert F, Bonnet D, Jouvet P, Boutron M, Slama A, Vianey-Saban C, Bonnefont JP, Rabier D, Kamoun P, Brivet M. Recognition and management of fatty acid oxidation defects: a series of 107 patients. J Inherit Metab Dis 22: 488–502, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Williamson DH, Lund P, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 103: 514–527, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wollenberger A, Ristau O, Schoffa G. A simple technic for extremely rapid freezing of large pieces of tissue. Pflugers Arch Gesamte Physiol Menschen Tiere 270: 399–412, 1960 [PubMed] [Google Scholar]
- 25.Ziegler A, Zaugg CE, Buser PT, Seelig J, Kunnecke B. Non-invasive measurements of myocardial carbon metabolism using in vivo 13C NMR spectroscopy. NMR Biomed 15: 222–234, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Zuurbier CJ, Keijzers PJ, Koeman A, van Wezel HB, Hollmann MW. Anesthesia's effects on plasma glucose and insulin and cardiac hexokinase at similar hemodynamics and without major surgical stress in fed rats. Anesth Analg 106: 135–142, 2008. [DOI] [PubMed] [Google Scholar]