Abstract
Activators of 5′-AMP-activated protein kinase (AMPK) 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), metformin, and exercise activate atypical protein kinase C (aPKC) and ERK and stimulate glucose transport in muscle by uncertain mechanisms. Here, in cultured L6 myotubes: AICAR- and metformin-induced activation of AMPK was required for activation of aPKC and ERK; aPKC activation involved and required phosphoinositide-dependent kinase 1 (PDK1) phosphorylation of Thr410-PKC-ζ; aPKC Thr410 phosphorylation and activation also required MEK1-dependent ERK; and glucose transport effects of AICAR and metformin were inhibited by expression of dominant-negative AMPK, kinase-inactive PDK1, MEK1 inhibitors, kinase-inactive PKC-ζ, and RNA interference (RNAi)-mediated knockdown of PKC-ζ. In mice, muscle-specific aPKC (PKC-λ) depletion by conditional gene targeting impaired AICAR-stimulated glucose disposal and stimulatory effects of both AICAR and metformin on 2-deoxyglucose/glucose uptake in muscle in vivo and AICAR stimulation of 2-[3H]deoxyglucose uptake in isolated extensor digitorum longus muscle; however, AMPK activation was unimpaired. In marked contrast to AICAR and metformin, treadmill exercise-induced stimulation of 2-deoxyglucose/glucose uptake was not inhibited in aPKC-knockout mice. Finally, in intact rodents, AICAR and metformin activated aPKC in muscle, but not in liver, despite activating AMPK in both tissues. The findings demonstrate that in muscle AICAR and metformin activate aPKC via sequential activation of AMPK, ERK, and PDK1 and the AMPK/ERK/PDK1/aPKC pathway is required for metformin- and AICAR-stimulated increases in glucose transport. On the other hand, although aPKC is activated by treadmill exercise, this activation is not required for exercise-induced increases in glucose transport, and therefore may be a redundant mechanism.
Keywords: tissue-specific atypical PKC activation by AMPK, primacy of AMPK in muscle atypical PKC activation, redundant activation of atypical PKC during exercise
5′-amp-dependent protein kinase (AMPK) senses 5′-AMP levels and regulates ATP supply (12). AMPK is activated physiologically by hypoxia and exercise, which increase cellular levels of 5′-AMP at the expense of ATP, and certain hormones, such as leptin and adiponectin (12). AMPK can also be activated by chemical agents that either increase 5′-AMP, e.g., by uncoupling mitochondrial oxidative phosphorylation, or mimic 5′-AMP, e.g., by 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), which is metabolized to AICAR-PO4 (ZMP), a 5′-AMP analog and by antidiabetic therapeutic agents such as thiazolidinediones (9) and metformin (9, 18, 28). AMPK activation in turn increases fatty acid oxidation in various tissues and stimulates glucose transport/uptake and glycolysis specifically in muscle, thereby increasing ATP generation (12). AMPK also diminishes expression and/or activation of sterol receptor element binding protein-1c (SREBP-1c) and other transcription factors in liver, thereby diminishing expression of mRNAs that produce enzymes that increase hepatic lipid synthesis and glucose production and release (8).
Agents that activate AMPK, such as AICAR and metformin, are important in the context of obesity and type 2 diabetes mellitus. In these insulin-resistant states, insulin stimulation of glucose transport in muscle and inhibition of gluconeogenesis in liver are understandably impaired, but hepatic lipogenesis, which insulin normally stimulates, is paradoxically increased. Activators of AMPK, on the other hand, increase glucose transport in muscle and diminish both gluconeogenesis and lipogenesis in liver. The ability of AMPK activation to have stimulatory effects on glucose transport in muscle and inhibitory effects on gluconeogenesis in liver similar to those of insulin, and simultaneously have inhibitory effects on lipogenesis in liver opposite to those of insulin, is unexplained but nevertheless therapeutically fortuitous. Moreover, AMPK appears to mediate many salutary gene expression effects of exercise (19). Thus agents that activate AMPK can serve as important adjuncts for preventing and treating obesity and type 2 diabetes.
The mechanism whereby AMPK activators increase glucose transport in muscle is only partly understood. Insulin stimulates glucose transport through activation of insulin receptor substrate-1 (IRS-1)-dependent phosphatidylinositol 3-kinase (PI3K), which, via increases in membrane levels of the acidic phospholipid phosphatidylinositol 3,4,5-trisphosphate (PIP3) activates both protein kinase B (PKB/Akt) and atypical protein kinase (aPKC) isoforms (ζ, λ, ι) by increasing the ability of phosphoinositide-dependent kinase-1 (PDK1) to interact with and phosphorylate threonine (Thr) residues in the activation loops (or T loops) of PKB/Akt (i.e., Thr308) and aPKCs (i.e., Thr410 in PKC-ζ, Thr411 in PKC-λ, and Thr403 in PKC-ι). In the case of aPKCs, in addition to activation loop phosphorylation, PIP3 also facilitates subsequent steps, viz., auto(trans)phosphorylation and allosteric alterations that are required for full enzyme activation (see Ref. 23). Together, PKB/Akt and aPKC increase the translocation of glucose transporters, in particular, Glut4 transporters in muscle cells and adipocytes, to the plasma membrane, thereby allowing glucose entry into the cell. It should be noted that PKC-ζ is the main aPKC in rat muscle, and PKC-λ is the main aPKC in mouse muscle and that aPKC isoforms function interchangeably in support of glucose transport (see Refs. 7, 20).
In part different from insulin, the AMPK activator AICAR does not activate IRS-1, PI3K, or PKB/Akt but nevertheless activates aPKC in rodent muscle by a mechanism that appeared to involve activation of the ERK pathway and phospholipase D (PLD)-dependent production of the acidic phospholipid phosphatidic acid (PA), which appears to function like PIP3 (5). Similarly, metformin increases aPKC activity in muscles of diabetic humans (16) and, like AICAR, stimulates glucose transport in L6 myocytes (13). However, a number of uncertainties remain, including 1) whether AICAR and metformin activate aPKC via AMPK, or whether aPKC activation precedes and leads to AMPK activation, as has been suggested to occur in endothelial cells (27); 2) whether AMPK activates aPKC via mechanisms requiring PDK1 and ERK; and 3) whether AMPK, PDK1, ERK, and/or aPKC are required for glucose transport effects of AICAR and metformin in muscle. Similarly, whereas muscle aPKC is activated during exercise (5), the functional role of aPKC in exercise-induced glucose transport is uncertain, because exercise alters signaling factors other than AMPK.
To evaluate these issues, we examined AMPK and aPKC requirements during actions of AICAR and metformin in L6 myotubes by selective inactivation of AMPK and PKC-ζ, the major aPKC in these rat-derived cells (20), and by muscle-specific inactivation of PKC-λ, the major aPKC in mouse muscle (7), which was depleted by Cre-LoxP methodology. We also examined the roles of PDK1 and ERK during activation of aPKC and glucose transport in L6 myotubes. Through these studies, we demonstrate that both AICAR and metformin, but not exercise, increase glucose transport in muscle through AMPK-dependent activation or actions of ERK, PDK1 and aPKC. However, different from muscle, AICAR and metformin activate AMPK but not aPKC in liver. These studies therefore define mechanisms that contribute importantly to the clinical usefulness of AMPK activators as therapeutic agents for treating insulin-resistant forms of diabetes and obesity.
MATERIALS AND METHODS
L6 Myotube Studies
L6 myotubes were cultured as described previously (5, 20), and, after differentiation, where indicated, we added to the medium [α-modified Eagle's medium (α-MEM)] the following: 1) 100 nM small silencing/interfering RNA (RNAi) targeting PKC-ζ or a control scrambled nontargeting RNAi, each along with Oligofectamine (used per instructions of Invitrogen) 72–96 h before experimental use to allow time to deplete aPKC, as described previously (20); 2) adenoviruses in indicated multiplicity of infection titers (MOI, ratio of viral particles per cell) 48 h before experimental use to allow for expression of indicated proteins, as described previously (5, 20); and/or 3) 2 mM metformin over 16 h, which elicits maximal increases in glucose transport (see Ref. 13). In initial studies, metformin treatment for 1–3 h elicited only modest increases in glucose transport, and we therefore conducted subsequent studies with the longer 16-h treatment period.
On the day of experimental usage, the cells were incubated for 3 h in fresh α-MEM containing 1 mg/ml bovine serum albumin (BSA) and, where indicated, 2 mM metformin. The cells were finally equilibrated in glucose-free Krebs Ringer phosphate (KRP) medium containing 1 mg/ml BSA and then incubated with or without 2 mM metformin or 50 μM AICAR for 40 min or 100 nM insulin for 30 min before measurement of 2-[3H]deoxyglucose uptake over 5 min. For studies of AMPK or aPKC activation similar treatment times were used for metformin and AICAR, viz., 16 h and 40 min, but for insulin an optimal treatment time of 15 min was used.
Rat Studies
Male rats weighing 250–300 g were obtained either from Harlan Industries and housed at the James A. Haley Veterans Hospital Vivarium in Tampa or from Janvier (Le Genest-St. Isle, France) and housed in the Vivarium at the Institut Federatif de Recherche in Nice. All experimental animal procedures in the US were fully approved by the Institutional Animal Care and Use Committee (IACUC) of the University of South Florida College of Medicine and the James A. Haley Veterans Administration Medical Center Research and Development Committee. All animal procedures in Nice, France conformed to current Guidelines for the Care and Use of Laboratory Animals of the National Institute of Health and Medical Research of France (INSERM) and were approved by the INSERM IACUC.
Muscle-Specific PKC-λ Knockout Mouse Studies
We previously reported (7) information on 1) introduction of loxP sites flanking the exon at nucleotides 110–233 in genomic mouse PKC-λ; 2) insertion of this floxed PKC-λ into the genome of mouse embryonic stem (ES) cells; and 3) effective deletion of this floxed PKC-λ by expression of Cre-recombinase in ES cells. ES cells containing the floxed PKC-λ allele were then used to generate mice with germ line transmitted floxed PKC-λ, and these mice were crossed with mice harboring a muscle creatine kinase (MCK)-regulated Cre-recombinase transgene to generate homozygous muscle-specific PKC-λ knockout mice and littermate wild-type control mice. As reported previously (7), these knockout mice have diminished levels and activity of total aPKC specifically in muscle (in accordance with the fact that PKC-λ is the major aPKC in mouse muscle), and this deficiency of aPKC is attended by impaired ability of insulin to stimulate muscle glucose transport both in vivo and in vitro. Of further note, muscle-specific PKC-λ knockout mice have 1) systemic insulin resistance and glucose intolerance, as determined in standard insulin and glucose tolerance tests, but normal fasting glucose levels; 2) diminished systemic glucose utilization specifically in muscle, as determined in hyperinsulinemic-euglycemic clamp studies; 3) other than for aPKC, no defects in insulin signaling to other factors, most notably, IRS-1-dependent PI3K and PKB/Akt, in muscle; and 4) no defects in insulin signaling or actions in either adipose or liver tissues.
Studies of glucose transport into isolated muscles or muscles of intact mice are described below. Continuous standardized treadmill exercise studies in intact mice were conducted as described previously (5), except that the uptake of glucose during the 20-min exercise period was simultaneously measured as described below.
Adenoviruses
Adenoviruses encoding wild-type or kinase-inactive (KI) dominant-negative forms of AMPKα1 and AMPKα2 have been described previously (26). Adenoviruses encoding KI PKC-ζ (5) and PDK1 (2) were also described previously.
RNAi Studies
As reported previously (20), to deplete PKC-ζ in L6 myotubes a SMART pool set of four 19-nt RNAi duplexes targeting four PKC-ζ mRNA sites and a scrambled control RNAi were obtained from Dharmacon and transfected with Oligofectamine (Invitrogen) into L6 myotubes over 96 h. This method depleted total aPKC levels [largely PKC-ζ with little or no PKC-λ in rat-derived L6 myotubes (20)] by ∼70–90%, and the specificity of the targeted mRNA for glucose transport studies was verified by showing that 1) insulin-stimulated PKB/Akt activation was intact and 2) insulin-stimulated glucose transport was fully rescued by adenovirus-mediated expression of PKC-λ, which was not affected by the RNAi that targeted PKC-ζ [note that PKC-λ functions interchangeably with PKC-ζ in supporting glucose transport (20)].
Lysate Preparations
Samples from cultured cells or from rat or mouse tissues were homogenized as described previously (5, 7, 20). Homogenizing buffer contained (in mM) 250 sucrose, 20 Tris · HCl (pH 7.5), 1.2 EGTA, 20 β-mercaptoethanol, 1 phenylmethylsulfonyl fluoride (PMSF), 1 Na3VO4, 1 NaF, and 1 Na4P2O7, with 10 μg/ml aprotinin, 20 μg/ml leupeptin, and 1 μM LR-microcystin. Homogenates were centrifuged for 10 min at 700 g to remove nuclei and cellular debris. Supernatants were then supplemented with 0.15 M NaCl, 1% Triton X-100, and 0.5% Nonidet to disrupt membranes and then used for immunoprecipitation of PKC-ζ/λ or AMPK.
aPKC Activation
Combined PKC-ζ plus PKC-λ (i.e., total aPKC) enzyme activity was measured as described previously (5, 7, 20). In brief, aPKCs were immunoprecipitated from salt/detergent-treated cell lysates with a rabbit polyclonal antiserum (Santa Cruz Biotechnology) that recognizes the COOH termini of both PKC-ζ and PKC-λ; [note that whereas rat-derived L6 myotubes, like rat muscle, contain primarily PKC-ζ, mouse muscle contains primarily PKC-λ (5, 7, 20)]. Precipitates were collected on Sepharose-AG beads (Santa Cruz Biotechnology) and incubated for 8 min at 30°C in 100 μl of buffer containing 50 mM Tris · HCl (pH 7.5), 100 μM Na3VO4, 100 μM Na4P2O7, 1 mM NaF, 100 μM PMSF, 4 μg of phosphatidylserine (Sigma), 50 μM [γ-32P]ATP (NEN Life Science Products, Boston, MA), 5 mM MgCl2, and, as substrate, 40 μM serine analog of the PKC-ε pseudosubstrate (BioSource), a preferred substrate for aPKCs. After incubation, 32P-labeled substrate was trapped on P-81 filter paper and counted.
In some experiments using the same assay system, instead of immunoprecipitated aPKC we measured the activity of recombinant glutathione S-transferase fusion protein forms of full-length human PKC-ζ incubated at 30°C for 30 min with or without full-length human PDK1 or constitutively active human M1-C312 AMPKα1 (all obtained from baculovirus/Sf9 insect expression systems and supplied by Cell Signaling Technology).
AMPK Activation
Immunoprecipitable AMPK (combined α1 and α2) activity was measured in lysates by the method of Wojtaszewski et al. (25) with rabbit polyclonal anti-AMPK antiserum (Cell Signaling Technologies) and SAMS peptide (Upstate Cell Signaling) as substrate. As in other kinase assays, blank activities were determined in cell lysate precipitates obtained with nonimmune serum.
ERK Activation
Immunoprecipitable ERK activity was measured with an enzymatic assay or by blotting for phospho-ERK as described previously (5).
Glucose Transport Studies in L6 Myotubes
After the initial treatments with RNAi (72–96 h), adenoviruses (48 h), and/or metformin (16 h) described above, unless otherwise indicated, myotubes were incubated in glucose-free KRP medium with or without 1) 2 mM metformin for 40 min, 2) 50 μM AICAR for 40 min, or 3) 100 nM insulin for 30 min, after which uptake of 2-[3H]deoxyglucose was measured over 5 min as described previously (5, 20).
In Vivo Glucose Transport Studies in Mice
As described previously (7), after an overnight 16- to 20-h fast 0.2 ml of physiological saline containing (each per g body wt) 0.05 μCi of 2-[3H]deoxyglucose (NEN/Life Science), 0.005 μCi of l-[14C]glucose (NEN), and, where indicated, 0.25 mg of AICAR or metformin was administered intraperitoneally 20 min (exercise studies) or 30 min (AICAR and metformin studies) before death. Uptake of total hexose (mainly glucose + tracer 2-[3H]deoxyglucose) in vastus lateralis muscle was measured by dividing the tissue [3H] cpm (corrected for nonspecific uptake as per l-[14C]glucose radioactivity) by the specific radioactivity of serum glucose, i.e., [3H] cpm/nmol glucose, as described previously (7).
In Vitro Glucose Transport Studies in Mouse Muscle
As described previously (7), uptake of 2-[3H]deoxyglucose was determined over 10 min in isolated tension-maintained extensor digitorum longus muscles (EDL) incubated under 95% O2-5% CO2 in glucose-free Krebs Ringer bicarbonate (KRB) medium containing 0.5 μM 2-deoxyglucose and tracer amounts of 2-[3H]deoxyglucose after treatment for 40 min with or without 2 mM AICAR.
Glut4/Glut1 Glucose Transporter Translocation Studies
As described previously (5, 7, 20), plasma membrane glucose transporter (Glut)1 and Glut4 levels were measured by purification of plasma membranes on a discontinuous sucrose gradient and Western analysis following incubation of L6 myotubes with or without metformin, AICAR, or insulin or in gastrocnemius muscles obtained from mice treated with or without AICAR or metformin.
AICAR Tolerance Test
Mice were subjected to AICAR tolerance testing as described previously (10). In brief, 250 mg/kg body wt AICAR was administered intraperitoneally and tail vein blood glucose levels were measured at 15-min intervals.
Western Blot Analyses
As described previously (5, 7, 20), lysates or plasma membranes were immunoblotted with 1) PKC-rabbit polyclonal antiserum (Santa Cruz Biotechnology) that recognizes COOH termini of both aPKCs, λ and ζ; 2) mouse monoclonal anti-Glut4 glucose transporter antibody (AbDserotec); 3) rabbit polyclonal anti-Glut1 glucose transporter antiserum (Santa Cruz Biotechnology); 4) rabbit polyclonal anti-AMPK antiserum (Cell Signaling Technologies); 5) rabbit polyclonal anti-phospho-Thr172-AMPK antiserum (Cell Signaling Technologies); 6) rabbit polyclonal anti-PDK1 antiserum (Cell Signaling Technologies); 7) rabbit polyclonal anti-phospho-Thr410-PKC-ζ antiserum (Cell Signaling Technologies); 8) rabbit polyclonal anti-phospho-ERK antiserum (Cell Signaling Technologies); and 9) rabbit polyclonal anti-phospho-Ser473-PKB/Akt (Cell Signaling Technologies).
Statistics
Means of two groups were analyzed by t-test. Means of three or more groups were analyzed by ANOVA and the least significant difference multiple comparison method. All experiments were conducted on at least two separate occasions.
RESULTS
Studies in L6 Myotubes
Glucose transport effects of AICAR, metformin, and insulin.
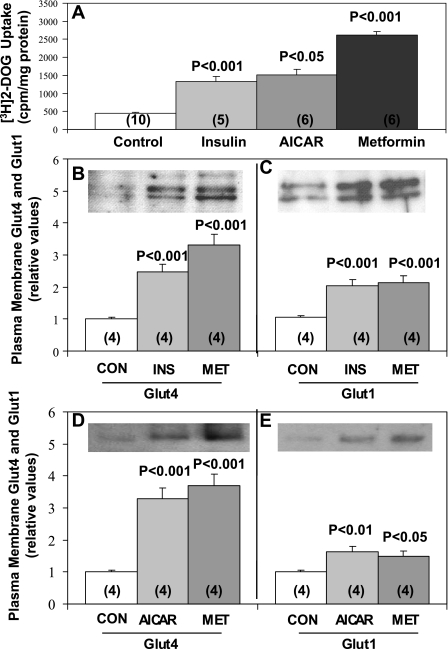
As reported previously (5), in L6 myotubes 50 μM AICAR, a maximally effective concentration, provoked two- to threefold increases in 2-[3H]deoxyglucose uptake within 40 min, i.e., comparable to those seen with maximal (100 nM) 30-min insulin treatment (Fig. 1A). As reported in previous studies of L6 myotubes (13), maximally effective 2 mM metformin treatment over 16 h provoked approximately four- to sixfold increases in 2-[3H]deoxyglucose uptake (Fig. 1A).
Fig. 1.
Effects of insulin (Ins), 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), and metformin (Met) on 2-[3H]deoxyglucose (DOG) uptake (A) and translocation of Glut4 (B and D) and Glut1 (C and E) glucose transporters to the plasma membrane in L6 myotubes. Myotubes were incubated with 2 mM metformin for 16 h and finally incubated in glucose-free Krebs Ringer phosphate (KRP) medium with or without 2 mM metformin for 40 min, 50 μM AICAR for 40 min, or 100 nM insulin for 30 min before measurement of 2-[3H]deoxyglucose uptake and isolation of plasma membranes for subsequent Western blot analyses of immunoreactive Glut4 and Glut1 levels. Values are mean ± SE results of n (in parentheses) determinations. Representative immunoblots of plasma membrane glucose transporter levels are also shown. P values indicate levels of significance of differences between treatment and control (Con) groups.
In addition to increasing 2-[3H]deoxyglucose uptake, 2 mM metformin treatment for 16 h provoked increases in the translocation of both Glut4 and Glut1 glucose transporters to the plasma membrane that were at least comparable in magnitude to those seen with either 30-min maximal insulin treatment (Fig. 1, B and C) or 40-min AICAR treatment (Fig. 1, D and E). It should be noted that total cellular contents of Glut4 and Glut1 glucose transporters in L6 myotubes were not altered by the 16-h 2 mM metformin treatment [Glut4: 1.01 ± 0.11 (mean ± SE; n = 4) in metformin treated vs. 1 ± 0.05 in control (mean ± SE; n = 4); Glut1: 1.02 ± 0.14 (mean ± SE; n = 4) in metformin treated vs. 1 ± 0.03 (mean ± SE; n = 4) in control] or by the 40-min treatment with 50 μM AICAR [Glut4: 0.98 ± 0.06 (mean ± SE; n = 4) in AICAR treated vs. 1 ± 0.05 (mean ± SE; N = 4) in control; Glut1: 1.05 ± 0.02 (mean ± SE; N = 4) in AICAR treated vs. 1 ± 0.03 (mean ± SE; n = 4) in control]. In addition to these findings in L6 myotubes, as seen below in Fig. 9, AICAR and metformin treatment in vivo over 30 min provoked acute increases in Glut4 and Glut1 translocation in gastrocnemius muscles of intact mice.
Fig. 9.
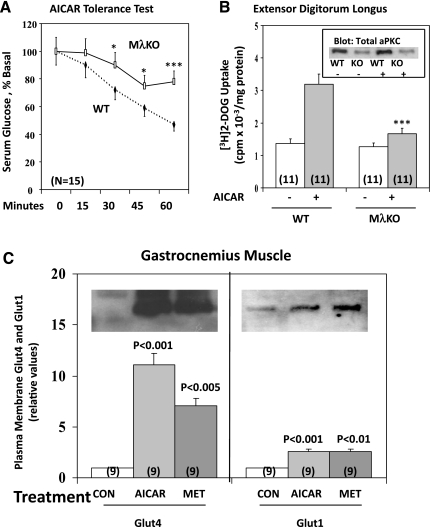
Effects of muscle-specific knockout of PKC-λ (MλKO) in mice on AICAR-stimulated glucose disposal in vivo (AICAR tolerance test; A) and 2-[3H]deoxyglucose uptake in vitro in isolated extensor digitorum longus muscle (EDL) (B). AICAR tolerance tests were conducted in 6-h-fasted mice with 10 males and 5 females in each group; initial blood glucose levels were 133 ± 4 and 134 ± 4 mg/dl (means ± SE; n = 15) in WT and knockout mice, respectively. EDL studies were conducted with muscles of fed mice, with 6 males and 5 females in each group. Results of males and females in both studies were indistinguishable and therefore pooled. Inset in B shows total aPKC levels in EDL muscles of WT and knockout (KO) mice treated with (+) or without (−) AICAR. *P < 0.05, ***P < 0.001 for comparison of indicated functions of MλKO and WT groups. Shown in C are representative immunoblots and levels of plasma membrane immunoreactive Glut4 and Glut1 glucose transporters in gastrocnemius muscles of WT treated with 0.9% saline (Con), 250 mg/kg body wt AICAR, or 250 mg/kg body wt metformin 30 min before death. Values indicate means ± SE of n (in parentheses) determinations. P values in C indicate levels of significance of differences between treatment and control (Con) groups.
AMPK activation by metformin and AICAR.
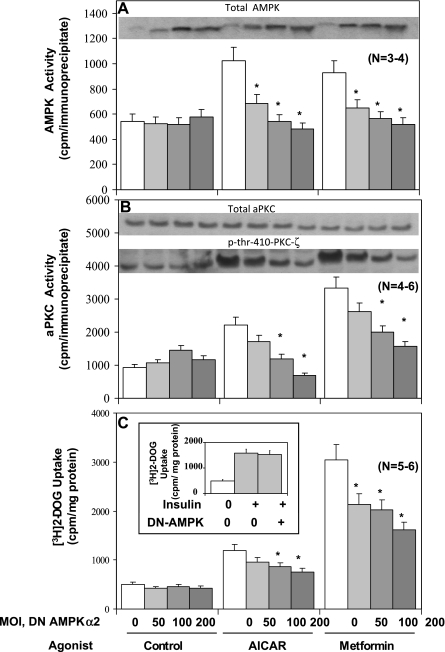
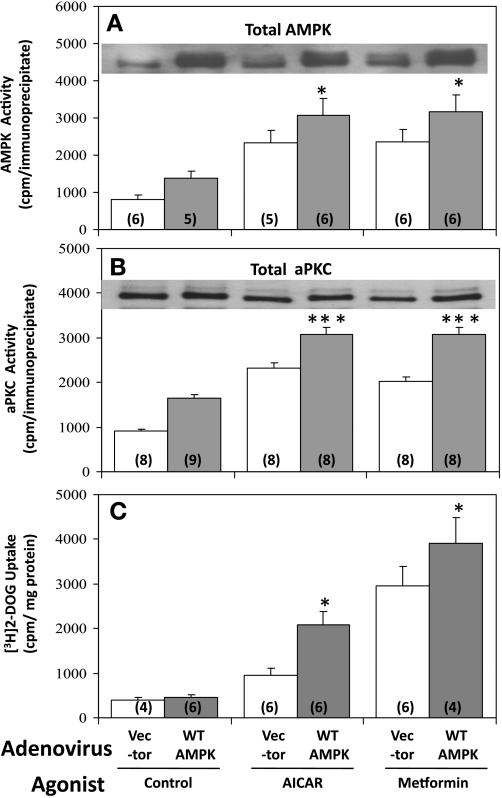
AMPK activity was comparably increased approximately twofold in response to 40-min AICAR treatment and 16-h metformin treatment (Fig. 2A and 3A). Stimulatory effects of both agents on AMPK activity were diminished by expression (see Fig. 2A, inset) of dominant-negative AMPKα2, with maximal inhibition occurring at ∼100–200 viral MOI (Fig. 2A). In contrast, stimulatory effects of both AICAR and metformin on AMPK activity were significantly enhanced by expression of wild-type AMPKα2 (Fig. 3A). The failure of expression of dominant-negative AMPKα2 to alter control, i.e., unstimulated, AMPK activity (Fig. 2A) may reflect a spuriously high basal activity owing to non-AMPK kinases coprecipitating with AMPK.
Fig. 2.
Dose-dependent effects of adenovirally mediated expression of dominant-negative (DN) 5′-AMP-activated protein kinase (AMPK)α2 on AICAR-stimulated and metformin-stimulated AMPK activity (A), atypical protein kinase C (aPKC) enzyme activity (B), phosphorylation of Thr410-PKC-ζ (B), and 2-[3H]deoxyglucose uptake (C) in L6 myotubes. Myotubes were incubated for 48 h with indicated multiplicity of infection (MOI) of adenovirus encoding DN AMPKα2 (note increases in expression of total AMPK with increasing MOI) or adenovirus vector (total adenovirus was kept constant at 200 MOI by varying the amount of vector) and then, where indicated, incubated with 2 mM metformin for 16 h and finally incubated in glucose-free KRP medium with or without 50 μM AICAR or 2 mM metformin for 40 min or insulin (100 nM) for 30 min, before measurement of AMPK activity, aPKC activity, p-Thr410-PKC-ζ immunoreactivity, and 2-[3H]deoxyglucose uptake. Values are means ± SE of n determinations. *P < 0.05 for DN AMPKα2-inhibited group vs. uninhibited (zero) group in AICAR and metformin series. Inset in A shows increases in levels of total AMPK on expression of DN AMPKα2 in various groups. Insets in B show levels of immunoreactivity of total aPKC and p-Thr410-PKC-ζ. Inset in C shows lack of effect of expression of DN AMPKα2 on insulin-stimulated 2-[3H]deoxyglucose uptake.
Fig. 3.
Effects of adenovirally mediated expression of wild-type (WT) AMPKα2 on AICAR- and metformin-stimulated AMPK activity (A), aPKC activity (B), and 2-[3H]deoxyglucose uptake (C) in L6 myotubes. Myotubes were incubated for 48 h with 200 MOI of adenovirus vector or adenovirus encoding WT AMPKα2 and, where indicated, with 2 mM metformin for 16 h, and finally incubated in glucose-free KRP medium with or without 50 μM AICAR or 2 mM metformin for 40 min, before measurement of AMPK activity, aPKC activity, and 2-[3H]deoxyglucose uptake. Values are means ± SE of n (in parentheses) determinations. *P < 0.05, ***P < 0.001 for WT AMPKα2 group vs. vector-treated group in AICAR and metformin series. Inset in A shows increases in total AMPK levels on expression of 200 MOI adenovirus encoding AMPKα2 in various groups. Inset in B shows lack of change in total aPKC levels with AICAR and metformin treatments, and upon expression of 200 MOI adenovirus encoding AMPKα2 in various groups (however, there appear to be slight shifts in mobility due to phosphorylations elicited by treatments).
Although not shown, dominant-negative AMPKα1 was slightly less effective than AMPKα2 for inhibiting AMPK activation, and combined addition of dominant-negative forms of AMPKα1 and AMPKα2 was no more effective than AMPKα2 alone for inhibiting AICAR- and metformin-induced increases in either AMPK activity or, as seen below, glucose transport. Subsequent studies were therefore conducted only with adenoviruses encoding dominant-negative and wild-type forms of AMPKα2.
AMPK is required for activation of glucose transport during actions of metformin and AICAR.
Both metformin and AICAR provoked increases in 2-[3H]deoxyglucose uptake, but, as noted above, metformin effects generally exceeded those of AICAR in L6 myotubes (Figs. 1A, 2C, and 3C). As with AMPK activity, expression of dominant-negative AMPKα2 inhibited effects of AICAR and metformin on 2-[3H]deoxyglucose uptake (Fig. 2C), and, in contrast, expression of wild-type AMPKα2 significantly enhanced the effects of both metformin and AICAR on 2-[3H]deoxyglucose uptake (Fig. 3C). Of further note, insulin effects on 2-[3H]deoxyglucose uptake were not altered by expression of dominant-negative AMPKα2 (Fig. 2C, inset); thus the inhibition of effects of AICAR and metformin on glucose transport by dominant-negative AMPKα2 were not due to nonspecific alterations in the transport process, and presumably reflected alterations in signaling pathways.
AMPK is required for activation of aPKC during actions of metformin and AICAR.
It was shown previously that AICAR activates aPKC in rat-derived cultured L6 myotubes and isolated rat EDL (5). Here we found that metformin and AICAR provoked increases in aPKC activity in L6 myotubes that were inhibited by expression of dominant-negative AMPKα2 (Fig. 2B) and enhanced by expression of wild-type AMPKα2 (Fig. 3B). It should be noted that total aPKC levels were not altered by either AICAR and metformin treatments or by expression of dominant-negative AMPKα2 treatments (Figs. 2B and 3B). On the other hand, as discussed further below, the phosphorylation of Thr410 in the activation loop (or T loop) of PKC-ζ was diminished by expression of dominant-negative AMPKα2 (Fig. 2B). These changes in aPKC activity and/or phosphorylation occurring with expression of dominant-negative and/or wild-type AMPK appeared to correlate reasonably well with changes in AMPK activity and glucose transport.
aPKC activation is required for metformin- and AICAR-induced increases in glucose transport.
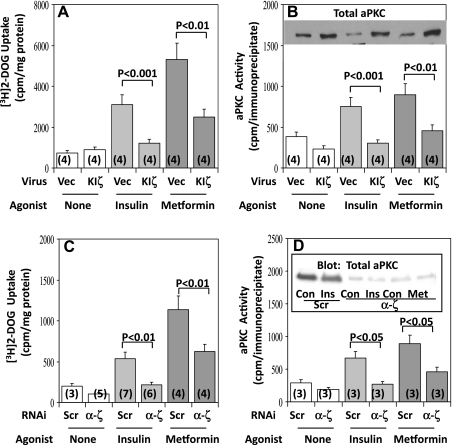
Adenovirally mediated expression of KI PKC-ζ has been found to inhibit AICAR- as well as insulin-stimulated 2-[3H]deoxyglucose uptake in L6 myotubes (5). Here we found that comparable adenovirally mediated expression of KI PKC-ζ (see 2- to 3-fold increases in total aPKC levels in Fig. 4B, inset) largely inhibited insulin effects and partially inhibited metformin effects on 2-[3H]deoxyglucose uptake (Fig. 4A) and aPKC activity (Fig. 4B) in these cells. Similarly, the knockdown (i.e., causing ∼80% depletion) of PKC-ζ by RNAi largely inhibited insulin effects and partially inhibited metformin effects on 2-[3H]deoxyglucose uptake (Fig. 4C) and aPKC activity (Fig. 4D). Whether residual aPKC or other factors accounted for the remaining effects of metformin on glucose transport in L6 myotubes treated with adenovirus encoding KI PKC-ζ or RNAi targeting PKC-ζ is uncertain.
Fig. 4.
Effects of adenovirally mediated expression of kinase-inactive (KI) PKC-ζ (KIζ, A and B) and small interfering RNA (RNAi) targeting PKC-ζ (α-ζ, C and D) on insulin- and metformin-stimulated glucose transport (A and C) and aPKC activity (B and D) in L6 myotubes. Myotubes were incubated for 96 h with 100 nM scrambled (Scr) control RNAi or RNAi targeting PKC-ζ (α-ζ) or for 48 h with 10 MOI of adenovirus vector (Vec) or adenovirus encoding KIζ and then, where indicated, treated with 2 mM metformin for 16 h and finally incubated in glucose-free KRP medium with or without 2 mM metformin for 40 min or 100 nM insulin for 30 min before measurement of uptake of 2-[3H]deoxyglucose and aPKC activity. Values are means ± SE of n (in parentheses) determinations. P values portray differences between indicated groups. Inset in B shows increases in total aPKC content on expression of KI PKC-ζ. Inset in D shows representative immunoblots that portray the depletion of total aPKC in cells treated with RNAi targeting PKC-ζ.
PDK1 is required for metformin- and AICAR-induced increases in aPKC activity and glucose transport.
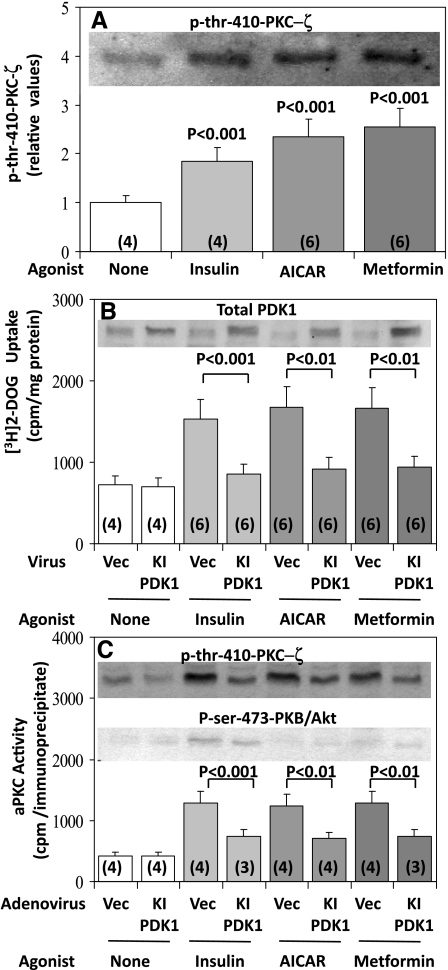
Studies of hypoxia-induced increases in AMPK activity in rat alveolar epithelial cells have shown that activation of the PKC-ζ in these cells is attended by an increase in phosphorylation of the activation loop (T loop) site, Thr410 (11); furthermore, because it was also found that recombinant constitutively active AMPK provoked increases in 32P incorporation into recombinant PKC-ζ in vitro, it was postulated that AMPK directly increases Thr410 phosphorylation (11). However, as discussed above, during insulin-induced aPKC activation the phosphorylation of Thr410 in PKC-ζ is mediated by PDK1 (see Refs. 2, 23), and this phosphorylation is facilitated by PI3K-derived PIP3 (23), which most likely binds to basic residues in the regulatory domain of aPKC, thereby inducing a dissociation of the pseudosubstrate sequence in the regulatory domain from the substrate-binding site in the catalytic domain, molecular unfolding, interaction of PDK1 with Thr410, auto(trans)phosphorylation of Thr560, and enhanced accessibility of substrate to the catalytic site (23). In this scheme, Thr410 phosphorylation is required, but alone is insufficient, for PKC-ζ activation by PIP3 (23).
With respect to AICAR action, we previously proposed (5) that the acidic phospholipid PA, produced by ERK-dependent PLD activation, acted analogously to the PI3K-derived acidic phospholipid PIP3, which presumably increases accessibility of the activation- or T-loop sites of both PKC-ζ (i.e., Thr410) and PKB/Akt (i.e., Thr308) to PDK1 during insulin action. In keeping with this idea, in L6 myotubes we found here that AICAR and metformin, like insulin, increased phosphorylation of Thr410 in PKC-ζ (Fig. 2B, Fig. 5, A and C). Moreover, as previously found in studies of insulin action in other cell types (2), adenovirally mediated expression of KI PDK1 (see Fig. 5B, inset) inhibited the increases in 1) enzymatic activation of aPKC (Fig. 5C); 2) Thr410 phosphorylation of PKC-ζ (Fig. 5C); and 3) increases in 2-deoxyglucose uptake (Fig. 5B) induced by AICAR and metformin, as well as insulin.
Fig. 5.
Increases in phosphorylation of Thr410-PKC-ζ following treatment with insulin, AICAR, and metformin (A) and effects of adenovirally mediated expression of KI phosphoinositide-dependent kinase 1 (PDK1) on insulin-, AICAR- and metformin-stimulated glucose transport (B) and aPKC phosphorylation and activity (C) in L6 myotubes. Myotubes were incubated for 48 h with 20 MOI of adenovirus vector or adenovirus encoding KI PDK1 and then, where indicated, treated with 2 mM metformin for 16 h and finally incubated in glucose-free KRP medium with or without 2 mM metformin or 50 μM AICAR for 40 min or 100 nM insulin for 30 min before measurement of [3H]2-deoxyglucose uptake and aPKC activity. Values are means ± SE of n (in parentheses) determinations. Inset in A shows representative increases in immunoreactive levels of phospho-Thr410-PKC-ζ on treatment with insulin, AICAR, and metformin. Inset in B shows increases in total cellular PDK1 content on expression of KI PDK1. Insets in C show representative immunoblots that portray levels of p-Thr410-PKC-ζ and p-Ser473-protein kinase B (PKB)/Akt in cells treated with adenovirus vector or adenovirus encoding KI PDK1. P values show levels of significance between treated and control groups in A and between indicated groups in B and C.
It may also be noted in Fig. 5C that insulin, but not AICAR or metformin, increased phosphorylation of Ser473 in PKB/Akt, and, moreover, as expected, expression of KI PDK1 blocked this phosphorylation of PKB/Akt, as well as the phosphorylation of Thr410 in PKC-ζ, induced by insulin. These results are in keeping with the idea that AICAR and metformin do not increase the enzyme activity, as such, of PDK1, since if PDK1 had been activated, it might be expected that PKB/Akt would have been phosphorylated at both Thr308 and Ser473, and thus activated, by these agents, which is clearly not the case. Accordingly, we believe that, like insulin, AICAR and metformin increase the accessibility of the PKC-ζ T-loop site, i.e., Thr410, to PDK; however, as discussed in more detail below, this increase in accessibility is mediated via PA during AICAR and metformin action and by PIP3 during insulin action. The ability of insulin, but not AICAR and metformin, to activate PKB/Akt most likely reflects that PIP3, but not PA, is able to interact with the PH domain in PKB/Akt that is required for T-loop phosphorylation.
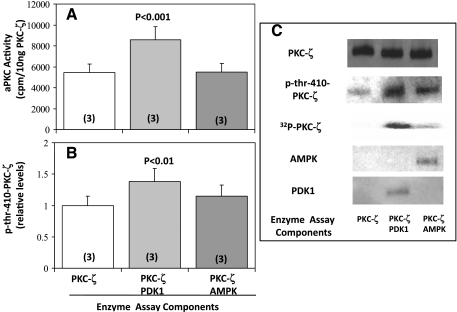
Because the above-described findings suggested that PDK1 is required for effects of AICAR and metformin on Thr410 phosphorylation and subsequent increases in PKC-ζ enzyme activity, it was of interest to compare the effects of recombinant forms of PDK1 and AMPK on recombinant PKC-ζ. As seen in Fig. 6C, Thr410 in recombinant PKC-ζ was already partially phosphorylated but could be further phosphorylated by incubation with wild-type PDK1, but not significantly by incubation with constitutively active AMPK (Fig. 6, B and C). Similarly, PDK1, but not AMPK, provoked increases in aPKC activity (Fig. 6A). It should also be noted that PDK1 provoked greater increases in 32P incorporation into PKC-ζ than AMPK (Fig. 6C).
Fig. 6.
Effects of incubation of recombinant forms of PDK1 and constitutively active AMPK with recombinant PKC-ζ on aPKC activity (A), phosphorylation of Thr410 in PKC-ζ (B and C), and increases in 32PO4 incorporation into PKC-ζ (C). C also shows levels of immunoreactive p-Thr410-PKC-ζ, PDK1, AMPK, and PKC-ζ in the reaction mixtures, which contained 10 ng of the indicated enzymes and all components of the aPKC assay system (see materials and methods). Values are means ± SE of n (in parentheses) determinations. P values show levels of significance between PDK1- and AMPK-treated vs. control group in which PKC-ζ was incubated alone.
ERK pathway activation is required for metformin- and AICAR-induced increases in aPKC activity and glucose transport.
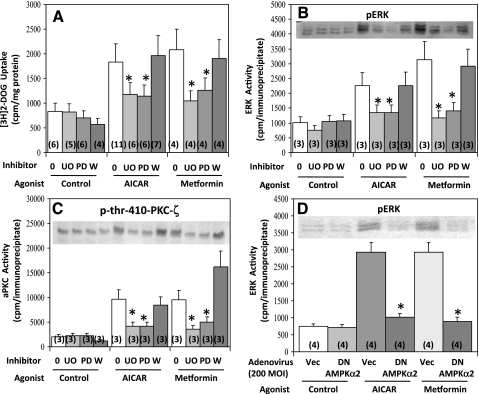
The above-described findings suggested that, in L6 myotubes, PDK1 is required for AMPK-induced increases in Thr410 PKC-ζ phosphorylation, PKC-ζ enzyme activity, and PKC-ζ-dependent glucose transport. Because it was previously found that inhibition of the ERK pathway and PLD blocked increases in aPKC activity and glucose transport induced by AMPK activation by dinitrophenol in L6 myotubes (5), it was presently of interest to find that two inhibitors of the ERK activator MEK1, viz., U-O126 and PD-98050, but not an inhibitor of the PI3K pathway, viz., wortmannin, inhibited effects of both AICAR and metformin on 2-[3H]deoxyglucose uptake (Fig. 7A) and PKC-ζ Thr410 phosphorylation and enzymatic activation (Fig. 7C), as well as ERK phosphorylation and enzymatic activation (Fig. 7B), in L6 myotubes. It should also be noted that expression of dominant-negative AMPKα2 inhibited the effects of AICAR and metformin on ERK phosphorylation and enzymatic activation (Fig. 4D), indicating that AMPK is upstream of ERK.
Fig. 7.
Effects of MEK1 inhibitors U-O126 (UO) and PD-98050 (PD), the phosphatidylinositol 3-kinase (PI3K) inhibitor wortmannin (W) (A–C), and DN AMPKα2 (D) on 2-[3H]deoxyglucose uptake (A), aPKC phosphorylation and enzyme activation (C), and ERK phosphorylation and enzyme activation (B and D) in L6 myotubes. Where indicated, myotubes were incubated for 48 h with 200 MOI of adenovirus vector or adenovirus encoding DN AMPKα2, then for 16 h without or with 2 mM metformin, then for 30 min without or with 25 μM UO-126, 50 μM PD-98050, or 100 nM wortmannin, and then for 40 min without (Control) or with 2 mM metformin or 50 μM AICAR, following which [3H]2-deoxyglucose uptake and phosphorylation and enzyme activities of aPKC and ERK were assessed. Values are means ± SE of n (in parentheses) determinations. *P < 0.05 for inhibitor-treated vs. uninhibited AICAR- and metformin-treated groups. Insets show representative immunoblots of phospho-ERK in B and D and phospho-Thr410-PKC-ζ in C.
Studies in Muscle and Liver of Intact Rats
We questioned whether metformin and AICAR activate aPKC, as well as AMPK, in tissues of intact rats. Comparison of the activation of these processes in muscle and liver seemed important, because aPKC mediates stimulatory effects of insulin on both glucose transport in muscle (7, 20) and lipogenesis in liver (17, 22, 24), but, as discussed above, AMPK activators, like insulin, stimulate glucose transport in muscle but, unlike insulin, diminish lipogenesis in liver (8, 12). Thus therapeutic usefulness of AMPK activators may be determined in part by differential effects on aPKC in various tissues.
Metformin activates AMPK and aPKC in rat muscle.
Within 60 min after subcutaneous injection of metformin (1 g/kg body wt) into intact Sprague-Dawley rats, the phosphorylation (Fig. 8A) and activity (Fig. 8C) of AMPK and the activity of aPKC (Fig. 8E) were increased ∼2- to 2.5-fold in the vastus lateralis muscle. Metformin-induced increases in muscle aPKC activity were in fact comparable in magnitude to those observed with insulin treatment (Fig. 8E). On the other hand, insulin, unlike metformin, did not increase muscle AMPK phosphorylation (Fig. 8A) or activity (Fig. 8C). It should be noted that AICAR has already been reported to activate aPKC in rat muscle (5).
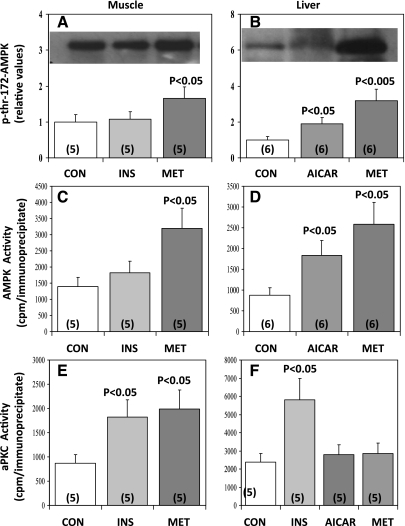
Fig. 8.
Effects of insulin, metformin, and AICAR on phosphorylation of Thr172 in AMPK (A and B) and enzyme activities of AMPK (C and D) and aPKC (E and F) in rat muscle (left) and liver (right). Rats were treated subcutaneously with 0.9% saline vehicle (Con) or insulin (1 U/kg body wt) for 30 min or metformin (250 mg/kg body wt) or AICAR (1 g/kg body wt) for 60 min. Values are means ± SE of n (in parentheses) determinations. P values indicate levels of significance of differences between treatment and Con groups. Representative blots of p-Thr172-AMPK are shown in insets of A and B.
AICAR and metformin activate AMPK, but not aPKC, in rat liver.
As in muscle, metformin increased AMPK phosphorylation (Fig. 8B) and AMPK activity (Fig. 8D) approximately threefold in rat liver, but, much differently from muscle, metformin failed to provoke an increase in hepatic aPKC activity (Fig. 8F). Similar to findings with metformin treatment, 60 min after subcutaneous injection of AICAR (1 g/kg body wt) AMPK phosphorylation (Fig. 8B) and AMPK activity (Fig. 8D) were increased approximately twofold in rat liver, but hepatic aPKC activity was not altered (Fig. 8F). In contrast to both metformin and AICAR, insulin activated aPKC in rat liver (Fig. 6F), presumably via PI3K (see Ref. 24).
Studies of AICAR and Metformin Action in Wild-Type and Muscle-Specific PKC-λ-Knockout Mice
AICAR tolerance testing.
As reported previously (10), administration of AICAR (250 mg/kg body wt) caused a steady decline of blood glucose over 60 min in wild-type mice (Fig. 9A). However, in muscle-specific PKC-λ-knockout mice, in which the level of total aPKC in various muscles is diminished (Figs. 9B, 10C, and 11B) and in which the ability of either AICAR or metformin to stimulate aPKC activity in muscle is largely abrogated (Fig. 10C), the effects of AICAR on blood glucose lowering were significantly reduced by ∼50% (Fig. 9A). Whether the remaining effects of AICAR on blood glucose lowering during the AICAR tolerance test in muscle-specific PKC-λ-knockout mice reflected decreases in hepatic glucose output, continued uptake of glucose in muscle as mediated by residual aPKC, or operation of other factors is presently unclear.
Fig. 10.
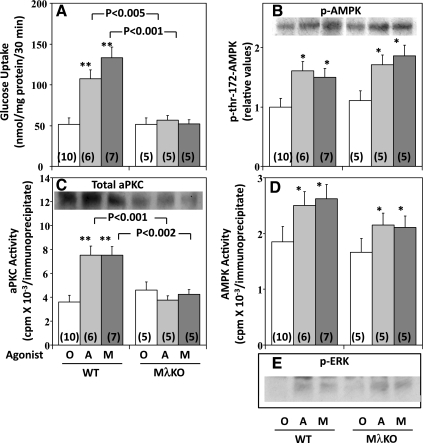
Effects of muscle-specific knockout of PKC-λ (MλKO) on AICAR- and metformin-induced increases in glucose uptake (A), aPKC activity (C), AMPK phosphorylation (B), AMPK enzyme activity (D) and phospho-ERK (E) in vastus lateralis muscle. WT and MλKO mice were injected intraperitoneally with tracer amounts of labeled d-2-deoxyglucose/l-glucose (see materials and methods) in 0.9% saline vehicle without (O) or with 250 mg/kg body wt AICAR (A) or 250 mg/kg body wt metformin (M) 30 min before death. Values are means ± SE of n (in parentheses) determinations. *P < 0.05, **P < 0.01 for comparisons of AICAR or metformin (A or M) treatment groups vs. control (O) group. Insets in B and C show representative immunoblots of p-Thr172-AMPK and total aPKC, respectively, in WT and MλKO muscles.
Fig. 11.
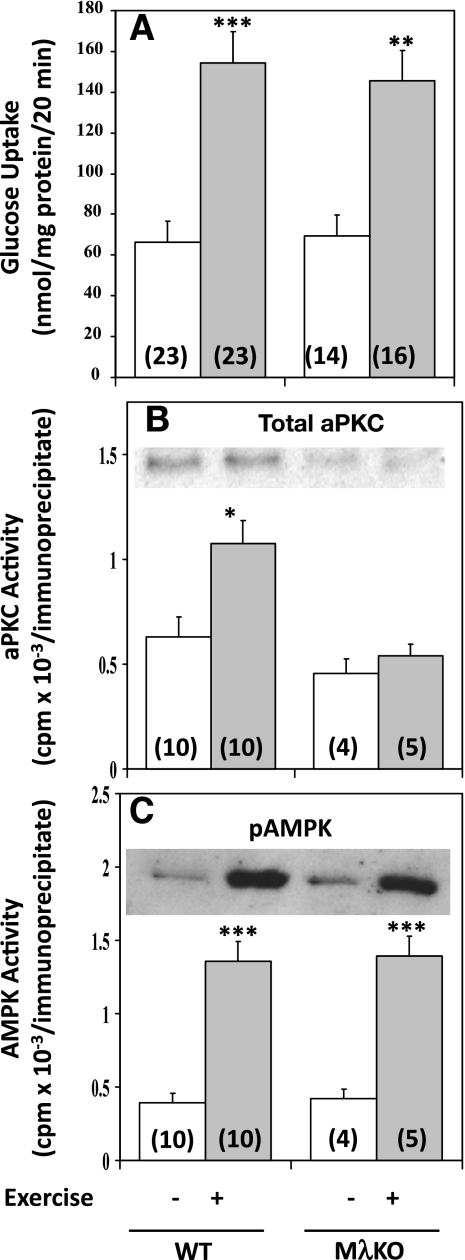
Effects of MλKO on treadmill exercise-induced increases in glucose uptake (A), aPKC activity (B), and AMPK activity (C) in vastus lateralis muscle. Mice were subjected to continuous treadmill exercise for 20 min, during which glucose uptake was measured as described in materials and methods. Values are means ± SE of n (in parentheses) determinations. *P < 0.05, **P < 0.01, ***P < 0.001 for comparisons of exercise and control/nonexercising groups. Representative blots of total aPKC and p-Thr172-AMPK are shown in insets of B and C, respectively.
Effects of AICAR on glucose transport in vitro.
Whereas AICAR provoked ∼2.5-fold increases in 2-[3H]deoxyglucose uptake in isolated EDL of wild-type mice, this effect was diminished by 70–80% in EDL of muscle-specific PKC-λ-knockout mice (Fig. 9B). These findings with AICAR are similar to those previously reported in studies of insulin-stimulated 2-[3H]deoxyglucose uptake, i.e., two- to threefold increases in wild-type muscle and marked impairment in muscles of muscle-specific PKC-λ-knockout mice (7).
Effects of treatment in vivo with AICAR and metformin on glucose transport, Glut4/1 glucose transporter translocation, and aPKC activity in mouse muscle.
Acute treatment in vivo over 30 min with AICAR (250 mg/kg body wt) or metformin (250 mg/kg body wt) provoked increases in 2-deoxyglucose/glucose uptake (Fig. 10A), Glut4 and Glut1 translocation (Fig. 9C), and aPKC activity (Fig. 10C) in hindlimb muscles of wild-type mice. However, in marked contrast, treatments with AICAR and metformin failed to significantly stimulate aPKC activity (Fig. 10C) and 2-deoxyglucose/glucose uptake (Fig. 10A) in muscles of muscle-specific PKC-λ-knockout mice.
Effects of AICAR and metformin on AMPK and ERK activation in vivo.
In contrast to the impairment in activating aPKC and glucose transport in muscles of muscle-specific PKC-λ-knockout mice in response to treatment with AICAR and metformin, there was no impairment in either the phosphorylation of Thr172 AMPK (Fig. 10B) or the enzymatic activation of AMPK (Fig. 10D) in muscles of these knockout mice. Similarly, increases in the phosphorylation of ERK following AICAR and metformin treatments in these muscles were not diminished by depletion of aPKC (Fig. 10E). Thus the activation of skeletal muscle AMPK and ERK by these agents appeared to be independent of aPKC.
Studies of Treadmill Exercise in Wild-Type and Muscle-Specific PKC-λ-Knockout Mice
It was shown previously that treadmill exercise provokes increases in aPKC activity in mouse muscle (5). Here we found that comparable treadmill exercise provoked approximately two- to threefold increases in glucose uptake (Fig. 11A) and AMPK activity (Fig. 11C), as well as approximately twofold increases in aPKC activity (Fig. 11B) in muscles of wild-type mice. In muscle-specific PKC-λ-knockout mice, however, despite a nearly complete loss of ability to increase aPKC activity (Fig. 11B), treadmill exercise continued to provoke increases in glucose transport (Fig. 11A) and AMPK activity (Fig. 11C) that were comparable in magnitude to those observed in wild-type littermates.
The failure of aPKC depletion in muscle-specific PKC-λ-knockout mice to diminish effects of exercise on glucose transport contrasts sharply with the inhibition of glucose transport effects of AICAR and metformin in muscle-specific PKC-λ-knockout mice. On the other hand, the failure to find a requirement for aPKC during exercise-induced increases in AMPK phosphorylation and activity in muscles of muscle-specific PKC-λ-knockout mice is similar to a comparable failure observed during AICAR and metformin treatment (cf. Fig. 10, C and D). Thus, with each of the agonists presently used, AMPK activation in muscle was not dependent on aPKC.
DISCUSSION
The present studies addressed several issues surrounding the mechanism of action of AMPK activators, including first, whether chemical agents that activate AMPK and aPKC in muscle, such as AICAR and metformin, activate aPKC through AMPK activation, or oppositely, whether AMPK is activated through aPKC, and second, whether aPKC is required for glucose transport effects of these AMPK activators. Germane to the first question, findings in both L6 myotubes and muscle-specific PKC-λ-knockout mice demonstrated that AICAR and metformin activate aPKC by a mechanism that is dependent on AMPK activation, and, conversely, AMPK activation by these agents is independent of aPKC. With respect to the second question, findings in both L6 myotubes and muscle-specific PKC-λ-knockout mice demonstrated that aPKC is required for glucose transport effects of both AICAR and metformin. Together, these findings show that AICAR and metformin stimulate glucose transport in muscle through sequential activation of AMPK and aPKC. This conclusion takes on added significance because metformin therapy in humans provokes increases in AMPK and aPKC activities in vastus lateralis muscle that accompany increases in rates of whole body glucose disposal in euglycemic-hyperinsulinemic clamp studies (16).
The present studies also addressed the question of whether exercise, which activates aPKC as well as AMPK in rodent (5) and human (3) muscle, requires aPKC for induction of increases in either AMPK activity or glucose transport. In this regard, it was particularly interesting to find in muscle-specific PKC-λ-knockout mice that effects of treadmill exercise on muscle glucose transport and AMPK phosphorylation/activation were fully or largely intact, despite nearly complete loss of ability to provoke increases in muscle aPKC activity. It therefore seems clear that exercise-induced increases in both AMPK activity and glucose transport occur independently of aPKC. On the other hand, our studies leave open the question of whether exercise activates muscle aPKC by a mechanism that is dependent on AMPK; in light of our findings in studies of AICAR and metformin action this possibility seems likely, but more definitive studies are needed.
The failure to find a requirement for aPKC during exercise-stimulated glucose uptake provided a sharp contrast for comparable studies of AICAR and metformin action in which a requirement for aPKC was apparent from studies in muscle-specific PKC-λ-knockout mice. Possible reasons for differences in aPKC requirements include the following: exercise, independently of AMPK, activates an aPKC pool that is not coupled to glucose transport; exercise, like AICAR and metformin, activates aPKC through AMPK, but coupling of AMPK-activated aPKC to glucose transport requires factors that are operative during actions of AICAR and metformin but not exercise; and, perhaps most plausible, exercise, like AICAR and metformin, activates aPKC through AMPK, but this signaling mechanism is redundant for increasing glucose transport during exercise. In this regard, it is relevant to note that AMPKα2 is required for effects of AICAR-induced, but not electrical contraction-induced, increases in glucose transport in muscle (10, 14). Thus AMPK-induced increases in aPKC may serve as a primary mechanism for increasing glucose transport during simple AMPK activation, but these increases in aPKC activity may be but one among two or more exercise-stimulated signaling mechanisms that are capable of activating glucose transport.
Whereas prolonged metformin treatment had stronger effects on glucose transport than more acute AICAR treatment in L6 myotubes, effects of metformin and AICAR on AMPK activity were similar. It may therefore be questioned whether the prolonged effects of metformin on glucose transport in L6 myotubes are mediated by AMPK-independent, as well as AMPK-dependent, mechanisms, or if the prolonged activation of AMPK during 16-h metformin treatment elicited increases in factors (? by gene expression) that favorably modulate glucose transport. Further studies are needed to answer these questions.
Although elicitation of full effects of metformin on glucose transport in L6 myotubes requires a relatively long treatment period (13), acute metformin treatment of both rats and mice in vivo for 30–60 min provoked increases in muscle aPKC activity and glucose uptake comparable to those seen with acute treatment with either AICAR or insulin (present results and Ref. 7). Thus, like AICAR and insulin, metformin can function as a rapid and potent activator of aPKC and glucose transport in muscles of intact rodents. However, here again, whether more prolonged treatment with metformin, or, for that matter, AICAR, in intact rodents provokes further increases in glucose transport beyond those seen in the acute studies, and the underlying mechanisms for such delayed increases, remain for future investigation.
In keeping with our previous report (5) indicating that AICAR-induced activation of aPKC in L6 myotubes requires activation of the ERK pathway, we found that ERK activation by both AICAR and metformin is dependent on AMPK and, furthermore, operation of the ERK pathway is required for AICAR- and metformin-induced increases in aPKC activity and glucose transport in L6 myotubes. Nevertheless, it remains uncertain how AMPK may activate the ERK pathway. In this regard, the nonreceptor tyrosine kinase PYK2 is activated by increases in intracellular Ca2+ or activation of other nonreceptor tyrosine kinases (1, 4, 15, 21), and PYK2 activates ERK and other MAP kinases that activate PLD and thereby generate the acidic phospholipid PA, which directly activates aPKC (5, 21). In this regard, it is interesting that ERK activation is seen during AICAR (6) and metformin (V. Luna, M. P. Sajan, R. V. Farese, unpublished observations) action in human muscle, but it is not clear whether AMPK mediates these activations of ERK (see Ref. 6).
Similar to findings in studies of hypoxia-induced AMPK activation in rat alveolar epithelial cells (11), we found that the activation of PKC-ζ by AICAR and metformin in L6 myotubes is attended by an increase in phosphorylation of the activation loop site in PKC-ζ, viz., Thr410. However, our findings further suggested that, as with insulin, the phosphorylation of Thr410 is mediated by PDK1 during actions of AICAR and metformin in intact L6 myotubes. Moreover, in incubations of recombinant enzymes, we found that PDK1, but not constitutively active AMPK, provoked significant increases in both Thr410 phosphorylation and enzyme activity of PKC-ζ. Further studies are needed to define the specific amino acid residues in PKC-ζ that are directly phosphorylated by AMPK, and to determine whether PDK1-induced Thr410 phosphorylation leads to an increase in phosphorylation of the auto(trans)phosphorylation site, i.e., Thr560, and/or other residues in PKC-ζ.
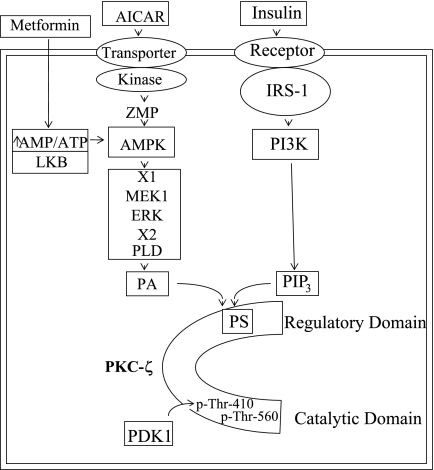
Although there are still many remaining uncertainties in how AMPK activators such as AICAR and metformin activate aPKCs, we believe that AICAR/metformin-dependent aPKC activation is mechanistically similar to insulin-dependent aPKC activation, particularly in the later activation steps. Thus, as portrayed in Fig. 12, in the case of insulin it is most likely that the acidic phospholipid PIP3, as generated by PI3K action, binds to still uncertain basic residues in or near the pseudosubstrate site in the regulatory domain, thereby causing a dissociation of the pseudosubstrate site from the substrate-binding site, opening of the molecule, increased access of PDK1 to the activation loop site (at Thr410 in PKC-ζ), and auto(trans)phosphorylation (at Thr560 in PKC-ζ). In the case of AMPK activators, it seems clear that MEK1-dependent activation of ERK is required for all aspects of aPKC activation, and our previous finding (5) indicating a requirement for PLD during AICAR action leads us to believe that PA, as generated by PLD operating downstream of ERK, acts analogously to PIP3 in activating aPKC.
Fig. 12.
Activation of PKC-ζ in L6 myotubes by AICAR and insulin. Increases in acidic phospholipids phosphatidylinositol 3,4,5-trisphosphate (PIP3) via phosphatidylinositol 3-kinase (PI3K) action and phosphatidic acid (PA) via phospholipase D (PLD) action are thought to bind to basic residues in or near the pseudosubstrate (PS) site in the regulatory domain and thereby open the major cleft and facilitate PDK1 access to the activation loop site, Thr410, and subsequent auto(trans)phosphorylation at Thr560, as well as allowing substrate access to the catalytic site. All alterations are needed to fully activate PKC-ζ. Note that AICAR is converted to AICAR-PO4 (ZMP), an analog of 5′-AMP, and metformin, like AICAR, activates AMPK by an as yet uncertain mechanism. See text for discussion of factors that couple AMPK to ERK (X1) and ERK to PLD (X2).
Importantly, despite activating AMPK in both muscle and liver, metformin and AICAR activated aPKC in muscle, but not in the liver, of intact rats. (We have similarly observed that AICAR activates AMPK, but not aPKC, in liver and isolated adipocytes of mice; data not shown.) The reason for tissue-specific differences in aPKC activation during AMPK activation is uncertain. Nevertheless, this fortuitous difference is in all probability relevant to the clinical usefulness of metformin, and potentially of AICAR, as therapeutic agent, since aPKC activation in liver would be expected to increase SREBP-1c expression (17, 22, 24) and hepatic lipid synthesis, and thereby promote tendencies to hepatosteatosis, hypertriglyceridemia, obesity, and insulin resistance. In this regard, metformin is known to diminish SREBP-1c expression, and we similarly found that 60-min metformin treatment diminished elevated hepatic SREBP-1c mRNA levels by 50–60% in type 2 diabetic Goto-Kakizaki rats (data not shown). It should also be noted that selective inhibition of hepatic aPKC by administration of adenovirus encoding KI aPKC elicits marked improvements in hepatosteatosis, hypertriglyceridemia, and insulin resistance in murine obesity models (22).
Together our findings show that, in skeletal muscle, AICAR and metformin activate aPKC via AMPK, ERK, and PDK1, and that aPKC is required for subsequent increases in glucose transport. Moreover, the ability of metformin and AICAR to activate aPKC selectively in muscle and thereby elicit increases in aPKC-dependent glucose transport, coupled with the ability to activate AMPK but not aPKC in liver, appear to be important determinants of the clinical usefulness of these and perhaps other AMPK activators for treating insulin-resistant forms of obesity and diabetes.
GRANTS
This work was supported by funds from the Department of Veterans Affairs Merit Review Program (R. V. Farese) and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-38079 (R. V. Farese) and DK-30136 (C. R. Kahn).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Andreev J, Galisteo ML, Kranenburg O, Logan SK, Chiu ES, Okigaki M, Cary LA, Moolenaar WH, Schlessinger J. Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J Biol Chem 276: 20130–20135, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert ML, Quon MJ, Lea-Currie R, Sen A, Farese RV. PKC-zeta mediates insulin effects on glucose transport in cultured preadipocyte-derived human adipocytes. J Clin Endocrinol Metab 87: 716–723, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose intolerance. Amelioration by rosiglitazone and exercise. Diabetes 52: 1926–1934, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Blaukatt A, Ivankovic-Dikic I, Gronroos E, Dolfi F, Tokiwa G, Vuori K, Dikic I. Adapter proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J Biol Chem 274: 14893–14901, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert ML, Farese RV, Jr, Farese RV. Activation of the ERK pathway and atypical protein kinase C isoforms in exercise- and AICAR-stimulated glucose transport. J Biol Chem 277: 23554–23562, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Cuthbertson DJ, Babraj JA, Mustard KJ, Towler MC, Green KA, Wackerhage H, Leese GP, Baar K, Thomason-Hughes M, Sutherland C, Hardie DG, Rennie MJ. 5-Aminoimidazole-4-carboxamide 1-beta-d-ribofuranoside acutely stimulates skeletal muscle 2-deoxyglucose uptake in healthy men. Diabetes 56: 2078–2084, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WJ, Jr, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M. Muscle-specific knockout of protein kinase C-λ impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 117: 2289–2301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferre P, Azzout-Marniche D, Foufelle F. AMP-activated protein kinase and hepatic genes involved in glucose metabolism. Biochem Soc Trans 31: 220–223, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Fryer LGD, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 277: 25226–25332, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Fuji N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolality-induced glucose transport in skeletal muscle. J Biol Chem 280: 39033–39041, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LE, Chandel NS, Sznajder JI. α1-AMPK-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase Cζ. Mol Cell Biol 29: 3455–3464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie DG. The AMP-activated protein kinase cascade. The key sensor of cellular energy status. Endocrinology 144: 5179–5183, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Hundal HS, Ramlal T, Reyes R, Leiter LA, Klip A. Cellular mechanism of metformin action involves glucose transporter translocation from an intracellular pool to the plasma membrane in L6 muscle cells. Endocrinology 131: 1165–1173, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen SB, Viollet B, Andreeli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not the alpha1 5′-AMPK-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279: 1070–1079, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature 31: 737–745, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Luna V, Casaubon L, Sajan MP, Gomez-Daspet J, Powe JL, Miura A, Rivas J, Standaert ML, Farese RV. Metformin improves atypical protein kinase C activation by insulin and phosphatidylinositol-3,4,5-(PO4)3 in muscle of diabetic subjects. Diabetologia 49: 375–382, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto M, Ogawa W, Akimoto K, Inoue H, Miyaki K, Furukawa K, Hayashi Y, Iguchi H, Marsuki Y, Hiramatsu R, Shimano H, Yamada N, Ohno S, Kasuga M, Noda T. PKCλ in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest 112: 935–944, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51: 2074–2081, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARδ agonists are exercise mimetics. Cell 134: 405–415, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajan MP, Rivas J, Pengfei Li Standaert ML, Farese RV. Repletion of atypical protein kinase C following RNAi-mediated depletion restores insulin-stimulated glucose transport. J Biol Chem 281: 17466–17473, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Sajan MP, Standaert ML, Bandyopadhyay G, Kanoh Y, Quon MJ, Reed BC, Dikic I, Farese RV. Sorbitol activates atypical protein kinase C and GLUT4 glucose transporter translocation/glucose transport through proline-rich tyrosine kinase-2, the extracellular signal-regulated kinase pathway and phospholipase D. Biochem J 362: 665–674, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sajan MP, Standaert ML, Nimal S, Varanasi U, Pastoor T, Mastorides S, Braun U, Leitges M, Farese RV. Critical role of atypical protein kinase C in activating hepatic SREBP-1c and NFκB in obesity. J Lipid Res 50: 1133–1145, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Standaert ML, Bandyopadhyay G, Kanoh Y, Sajan MP, Farese RV. Insulin and PIP3 activate PKC-ζ by mechanisms that are both dependent and independent of phosphorylation of activation loop T410 and autophosphorylation T560 sites. Biochemistry 40: 249–255, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Taniguchi CM, Kondo T, Sajan MP, Luo J, Bronson R, Asano T, Farese RV, Cantley LC, Kahn CR. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab 3: 343–353, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated kinase in human skeletal muscle. J Physiol 528: 221–226, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol 20: 6704–6711, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, Neumann D, Schlattner U, Zou MH. Activation of protein kinase C zeta by peroxynitrate regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem 281: 6366–6375, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fuji N, Musi N, Hirshman MF, Goodyear LJ, Moller DF. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]