Abstract
The kisspeptins are neuropeptides that stimulate the hypothalamo-pituitary-gonadal (HPG) axis. The smallest endogenous kisspeptin, kisspeptin-10 (KP-10), binds to the receptor KISS1R with a similar affinity to the full-length peptide, kisspeptin-54 (KP-54), but is less effective in vivo, possibly because of increased enzymatic breakdown or clearance. The kisspeptin system may have therapeutic potential in the treatment of reproductive disorders and endocrine cancers. We have rationally modified the structure of KP-10 and tested the binding affinity of these analogs for the KISS1R. Those analogs that bound with relatively high affinity to KISS1R were tested for ability to stimulate ERK1/2 phosphorylation in vitro and for their ability to stimulate the HPG axis in vivo. One analog, [dY]1KP-10, bound to KISS1R with lower affinity to KP-10 and exhibited similar bioactivity in vitro. However, in vivo peripheral administration of [dY]1KP-10 increased plasma LH and testosterone more potently than KP-10 itself at 20 min postinjection in mice. In addition, 60 min postinjection, 0.15 nmol [dY]1KP-10 significantly increased total testosterone levels in mice whereas the same dose of KP-10 had no significant effect. Should manipulation of the kisspeptin/KISS1R signaling system prove therapeutically useful, long-lasting analogs such as [dY]1KP-10 may have greater therapeutic potential than endogenous forms of kisspeptin.
Keywords: Kiss-1, KISS1R, agonist, luteinizing hormone, testosterone
the kisspeptins are potent neuropeptide stimulators of the hypothalamo-pituitary-gonadal (HPG) axis, acting via the G protein-coupled receptor KISS1R (also known as GPR54) (11, 17, 23). Absence of kisspeptin signaling in rodents and humans results in hypogonadotrophic hypogonadism (HH) and lack of sexual maturation (4, 29). Conversely, administration of exogenous KP-10 to immature rats stimulates sexual maturation and induces precocious puberty (21). Furthermore, kisspeptin stimulates the release of LH, FSH, and testosterone when administered centrally or peripherally to male rats or primates (7, 14, 18–20, 30, 33), and peripheral administration of kisspeptin stimulates gonadotrophin release in male and female humans (5, 6). Studies have shown that kisspeptin is likely to have its main action at the level of the hypothalamus (7, 10, 15, 20, 30, 33), although direct actions on the pituitary have been suggested (20).
The KiSS-1 gene that encodes kisspeptins was first discovered as an antimetastasis gene (12), and kisspeptins have been suggested as a possible treatment for endocrine-related cancers (22). Manipulation of the kisspeptin signaling system has therapeutic potential. Drugs based on the kisspeptin molecule are potential treatments for delayed puberty and HH, as well as metastatic cancer (3, 13, 26). However, the kisspeptins themselves may be of limited clinical utility because of their short circulating half-life (32). The endogenous kisspeptins are cleaved from a 145-amino acid precursor protein. All share the common COOH-terminal decapeptide sequence YNWNSFGLRF-NH2, which comprises KP-10, the smallest endogenous kisspeptin that has significant bioactivity at the KISS1R (1, 17). Rational modification of the KP-10 molecule may result in a kisspeptin analog with increased bioefficacy and may also identify points within the kisspeptin-10 molecule that are susceptible to enzymatic degradation.
Previous studies have investigated the effect of rational modification of the KP-10 molecule on activity at the KISS1R. The five COOH-terminal amino acids of KP-10, in particular residues 6, 8, 9, and 10, appear critical to agonistic activity at the human KISS1R (22, 24, 34) Gutierrez-Pascual et al. (8) investigated the effect of modifications to rat KP-10 on activity at the rat Kiss1r and concluded that residues 6 and 10 are also crucial for bioactivity in this system. Roseweir et al. (27) screened a number of human KP-10 analogs for agonistic and antagonistic activity at the human KISS1R, determining that specific amino acid substitutions at positions 1, 5, and 8 could produce a high-affinity KISS1R antagonist.
All the kisspeptins bind to KISS1R with similar affinity (11). KP-54 has been reported to have a more potent effect than KP-10 and KP-14 on gonadotrophin release following peripheral administration in rats, possibly because of its resistance to enzymatic breakdown, and correspondingly longer half-life (32), although other data suggest that they have a similarly potent effect in mice (16). Thus it is important to study the effect of rational modifications to the structure of KP-10 in vivo as well as in vitro.
We designed and synthesized 21 analogs of KP-10. All analogs were tested for in vitro receptor binding and in vitro bioactivity. In vivo studies were then carried out on those analogs showing a high level of in vitro activity, or those that bound strongly to the KISS1R. This approach has determined that a modified form of KP-10, [dY]1KP-10, has a more potent stimulatory effect on the HPG axis in vivo than the endogenous peptide.
MATERIALS AND METHODS
KP-10 Analog Design
Sequential modifications were made to the human kisspeptin molecule. First, an alanine scan replaced each amino acid in turn with the neutral amino acid alanine. Analogs with specific amino acids exchanged for a structurally similar alternative, or for the equivalent d-amino acid, were also designed (see Table 1 for sequences). Peptides were synthesized by the F-moc solid phase peptide synthesis technique (2) by The MRC Peptide Synthesis Core Facility, Imperial College London.
Table 1.
Sequences and binding affinities of KP-10 and analogs
| Peptide | Sequence | IC50, nM |
|---|---|---|
| KP-10 | YNWNSFGLRF-NH2 | 1.0 + 0.3 |
| Alanine screened analogs | ||
| ANA1 | ANWNSFGLRF-NH2 | 23.2 + 14.3 |
| ANA2 | YAWNSFGLRF-NH2 | 145.1 + 138.0 |
| ANA3 | YNANSFGLRF-NH2 | 2.7 + 0.7 |
| ANA4 | YNWASFGLRF-NH2 | 43.5 + 15.5 |
| ANA5 | YNWNAFGLRF-NH2 | 0.8 + 0.4 |
| ANA6 | YNWNSAGLRF-NH2 | 109.5 + 89.8 |
| ANA7 | YNWNSFALRF-NH2 | 4.6 + 1.5 |
| ANA8 | YNWNSFGARF-NH2 | 77.4 + 10.0 |
| ANA9 | YNWNSFGLAF-NH2 | 42.3 + 6.7 |
| ANA10 | YNWNSFGLRA-NH2 | |
| Amino acid exchange analogs | ||
| ANA11 | FNWNSFGLRF-NH2 | 3.5 + 2.1 |
| ANA12 | YDWNSFGLRF-NH2 | 29.1 + 13.5 |
| ANA13 | YNWDSFGLRF-NH2 | 140.2 + 60.6 |
| ANA14 | YNWNTFGLRF-NH2 | 2.3 + 1.6 |
| ANA15 | YNWNSYGLRF-NH2 | 87.5 + 30.7 |
| ANA16 | YNWNSFGIRF-NH2 | 39.9 + 8.1 |
| ANA17 | YNWNSFGLKF-NH2 | 9.8 + 2.6 |
| ANA18 | YNWNSFGLRH-NH2 | 378.4 + 266.0 |
| Enantiomer exchange analogs | ||
| ANA19 ([dY]1KP-10) | [dY]NWNSFGLRF-NH2 | 3.6 + 0.3 |
| ANA20 | YNWNS[dF]GLRF-NH2 | 252.9 + 144.0 |
| ANA21 | YNWNSFGLR[dF]-NH2 | 447.2 + 173.0 |
IC50 in nM are means ± SE (n = 3–5). KP, kisspeptin; ANA, analog. Binding assays were carried out against [125I]KP-54 binding to KISS1R in CHO-KISS1R membrane preparations. Each receptor binding assay was carried out in triplicate. Changes to the sequence of KP-10 are indicated in bold.
Preparation of CHO-KISS1R Membranes
Chinese hamster ovary (CHO) cells, which had been stably transfected with the human KISS1R (CHO-KISS1R cells) (kindly donated by Prof. M. Parmentier, IRIBHN, Brussels, Belgium) were cultured in GIBCO Ham's F12 medium (Invitrogen, Paisley, UK) containing 10% fetal bovine serum and 100 IU/ml penicillin and 100 μg/ml streptomycin. Membranes were prepared by homogenizing and differential centrifugation, as previously described (25). Briefly, cells were scraped into PBS, added to 100 ml of 1 nM pH 7.4 HEPES buffer, and centrifuged for 15 min at 1,450 g. The resultant pellet was resuspended in 50 nM HEPES buffer, by use of a motorized homogenizer (IKA). The mixture was then centrifuged as previously described, and the resulting supernatant was ultracentrifuged (Sorvall, Stevenage, UK) at 105,000 g at 4°C for 1 h. The resulting membrane was used for receptor binding assays as described below.
Receptor Binding Assay Using CHO-KISS1R Membranes
Assays were carried out in siliconized Eppendorf tubes in 500 μl of buffer (2 mM MgCl2, 6.5 mM CaCl2, 20 mM HEPES pH 7.4, and 1% BSA). Unlabeled peptide (at a final concentration of 200, 20, 5, 1, 0.2, 0.02 and 0.002 nM) was incubated with 50 μl radiolabeled [125I]KP-54 iodinated by the iodogen method (25), and 100 μg CHO-KISS1R membrane protein at 30°C for 30 min. Following incubation, tubes were centrifuged at 16,000 g for 3 min and the supernatant was discarded.
The pellets were washed in 500 μl of assay buffer and centrifuged at 16,000 g for a further 3 min, and the supernatant was discarded. Pellets were counted in a gamma counter (model NE1600, Thermo Electron) for 240 s.
Effect of KP-10 and Analogs on ERK1/2 Phosphorylation In Vitro
In the CHO-KISS1R cells used, KISS1R receptor activation results in increased ERK1/2 phosphorylation (23). The CASE kit (tebu-bio, Northampton, UK) uses a cell-based ELISA technique to determine relative levels of phosphorylated ERK1/2 within plated cells. CHO-KISS1R cells were plated into 96-well plates (Nunc) and allowed to grow to confluency overnight. Cells were then washed with 100 μl of serum-free Ham's F12 media containing 100 IU/ml penicillin and 100 μg/ml streptomycin (GIBCO). In initial experiments, cells were treated with 100–1,000 nM KP-10 or 1,000 nM analogs in 100 μl for 5 min. Subsequently, the dose-response effects of KP-10 and [dY]1KP-10 (ANA19) were compared by incubating cells in 0.1, 1, 10, 100, 1,000, or 10,000 nM of either peptide for 5 min. Following treatment, the medium was then removed and the cells were fixed with 4% formaldehyde buffer and stored at 4°C until measurement of ERK1/2 phosphorylation.
The ELISA was carried out according to the manufacturer's instructions. Briefly, fixed cells were incubated with 50 μl of supplied anti-phosphoERK or anti-panERK primary antibody solution at room temperature for 1 h. They were then treated with secondary antibody and finally treated with a color-changing substrate. The absorbance of each well was measured at 450 nm via a spectrophotometer (Labsystems Multiskan MS, Cambridge, UK). Finally cells were stained with the supplied cell-staining solution to determine relative cell number in each well, and absorbance was read at 595 nm. The 450-nm reading was then normalized by dividing it by the 595-nm reading of the same well to reflect the cell number.
Animals
Studies were carried out on adult male C57Bl/6 mice. Animals weighed between 20 and 25 g at the time of the study and were caged in groups of 8–11. All animals were maintained under controlled temperature (21–23°C) and a 12:12-h light-dark cycle (lights on at 0700) with food (RMI diet, SDS) and water available ad libitum. Animal procedures carried out in the department were conducted under the British Home Office Animals Scientific Procedures Act 1986 (Project Licence 70/6402) and were authorized by the Home Office after successful completion of the local ethical review process.
LH and Total Testosterone Response Following ip Administration of KP-10 in Mice
Mice were injected intraperitoneally (ip) at time 0 on the study day with either saline or KP-10 at 0.3, 1, 10, or 30 nmol. At t = 20 min, mice were euthanized by CO2 inhalation, and blood was collected immediately by cardiac puncture. Blood was stored on ice until centrifugation at 15,600 g for 7 min, and plasma was stored at −80°C until assay. Plasma was assayed by RIA for LH, FSH, and total testosterone.
Initial Study To Determine In Vivo Effect of Selected Kisspeptin Analogs
Under the protocol described above, male C57Bl/6 mice were ip injected at t = 0 either with 0.5 nmol KP-10 or with kisspeptin analogs with an IC50 < 10 nM in our initial receptor binding studies. Analogs were administered at doses chosen to reflect their binding affinity to KISS1R relative to KP-10 itself, as determined in initial receptor binding studies [dose in nmol = (analog IC50/KP-10 IC50) × 0.5]. At t = 20, mice were euthanized, plasma was collected, and LH and testosterone were measured by RIA.
During the course of these studies, a study investigating the effect of a number of KP-10 analogs on a pheromone-responsive LacZ reporter gene system in yeast was published (22). The study did not test the analogs for receptor binding affinity or in vivo efficacy at stimulating gonadotrophin or testosterone release. However, their data did suggest that [A]5KP-10 (ANA5 in our studies) might act as a KISS1R superagonist and that [A]8KP-10 (ANA8 in our studies) did not activate KISS1R in their system. Given that ANA8 did bind to KISS1R in our experiments, we hypothesized that it might act as a KISS1R antagonist. Therefore, as previously described, C57Bl/6 mice were ip injected at t = 0 with 0.5 nmol KP-10, or with kisspeptin ANA5 at 0.5 nmol or ANA8 at 0.5 or 10 nmol. At t = 20 min, mice were euthanized, blood was collected and separated, and LH and testosterone were measured by RIA.
Comparing the Dose-Response Effect of ip [dY]1KP-10 and KP-10 on Plasma LH and Testosterone
As previously described, male C57BLl/6 mice were ip injected at t = 0 with either KP-10, or with kisspeptin analog 19 ([dY]1KP-10) at 0.015, 0.05, or 0.15 nmol. At t = 20, mice were euthanized, blood was collected and separated, and LH and testosterone were measured by RIA.
Comparing the Time Course of the Effect of ip [dY]1KP-10 and KP-10 on Plasma LH and Testosterone
As previously described, male C57Bl/6 mice were ip injected at t = 0 with either KP-10, or with [dY]1KP-10 at 0.5 nmol. At t = 20, t = 60, mice were euthanized, blood was collected and separated, and LH and testosterone were measured by RIA. A subsequent study was performed using doses of 0.15 nmol of KP-10 and [dY]1KP-10 and including an additional t = 120 time point.
RIAs
LH and FSH plasma levels were measured by use of reagents and methods obtained from the National Hormone and Pituitary program (Dr. A. Parlow, University of California, Harbor Medical Center, Los Angeles, CA), and the radiolabeled peptides were prepared by the chloramine-T method (28). The RIAs were prepared in 0.06 M phosphate EDTA buffer (pH 7.4) and left to incubate for 3 days at 4°C before separation by immunoprecipitation. Results were calculated in terms of a National Institute of Diabetes and Digestive and Kidney Diseases standard preparation. The intra- and interassay coefficients of variation were 8.2 and 13.6%, respectively, for LH and 8.3 and 12.4%, respectively, for FSH. Plasma total testosterone was measured by using a commercially available kit (Siemens Biomedical). Assays were carried out according to the manufacturers’ instructions. Briefly, standards ranging from 0 to 55 ng/ml, as provided, and plasma samples were added to antibody coated tubes (as provided) in 50 μl volumes. Radiolabeled 125I-total testosterone was added to tubes in 1-ml volumes (∼1,000 cps/tube). The tubes were then vortexed and incubated at 37°C for 3 h before counting and data reduction. The intra- and interassay coefficients of variation were < 10%.
Statistical Analysis
Curves and IC50 values were calculated by use of the Prism 4 program (GraphPad Software, San Diego, CA). IC50s were determined as means ± SE of three or more independent assays carried out in triplicate. All ELISA and in vivo studies were analyzed by one-way ANOVA with Tukey's post hoc test. In all cases, P < 0.05 was considered significant.
RESULTS
Initial Receptor Binding Screen
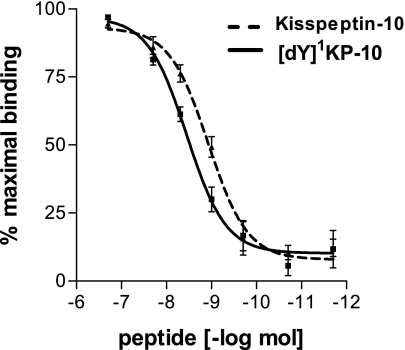
Initial receptor binding studies were carried out on analogs using membranes prepared from CHO-KISS1R cells. Binding studies produced an IC50 of 1.00 ± 0.34 nmol for KP-10 (Fig. 1). Initial analog binding studies showed that only one analog, ANA5, had an IC50 lower than that of KP-10 itself. Other analogs had IC50s ranging from 3.2 to 447 nM, and ANA10 did not bind at concentrations up to 200 nM. Subsequent studies suggested that ANA19 ([dY]1KP-10) was a KISS1R super agonist, and the receptor binding curve for [dY]1KP-10 is thus shown (Fig. 1).
Fig. 1.
Competition binding curves for kisspeptin-10 (KP-10) and [dY]1KP-10 against [125I]KP-54 to the receptor KISS1R in Chinese hamster ovary (CHO) cells that had been stably transfected with the human KISS1R (CHO-KISS1R cells) cell membrane preparations. Binding assays were carried out in triplicate and repeated 3–5 times.
Effect of KP-10 and Analogs on ERK1/2 Phosphorylation In Vitro
The effect of KP-10 on ERK1/2 activation was established. Treatment of plated cells with KP-10 at 100 or 1,000 nM induced phosphorylation of ERK1/2 in a dose-dependent manner at 5 min [0.521 ± 0.014 arbitrary units (AU) (control), 0.752 ± 0.07.32 AU (KP-10 100 nM), 0.931 ± 0.0789 AU* (KP-10 1,000 nM); n = 3; *P < 0.001].
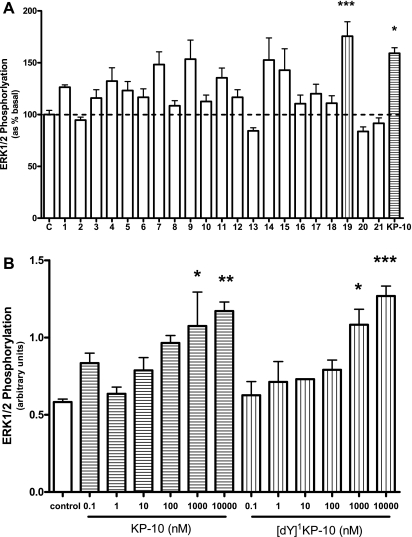
All analogs were tested for their ability to induce ERK1/2 phosphorylation at 1,000 nM. The experiments were carried out on 3 separate study days, and the data are therefore represented as % basal response. [dY]1KP-10 and KP-10 significantly increased ERK phosphorylation compared with control. The effect of KP-10 compared with [dY]1KP-10 was not significantly different. Although several other analogs tested increased ERK phosphorylation, these changes did not reach statistical significance (Fig. 2A).
Fig. 2.
A: ERK1/2 phosphorylation following treatment of CHO-KISS1R cells with KP-10 analogs or KP-10 at 1,000 nM for 5 min. Data are from 3 separate studies and results are shown as percentage of basal effect; n = 4–5. B: ERK1/2 phosphorylation following treatment of CHO-KISS1R cells with KP-10 or [dY]1KP-10 at 0.1, 1, 10, 100, 1,000, or 10,000 nM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control (ANOVA with post hoc Tukey's adjustment); n = 3–4.
Following this study, a dose-response study was carried out to compare the effects of [dY]1KP-10 and KP-10 on ERK phosphorylation. Treatment with KP-10 or [dY]1KP-10 at 1,000 or 10,000 nM doses significantly increased ERK phosphorylation compared with control (Fig. 2B). There was no significant difference between the effects of KP-10 and [dY]1KP-10 at equal doses.
Studies to Determine Efficacy of Analogs In Vivo
To determine a dose-response for ip KP-10 in mice.
Administration of 10 or 30 nmol KP-10 significantly increased plasma LH and administration of 1, 10, or 30 nmol KP-10 significantly increased plasma testosterone at t = 20 (Table 2). Because a dose of 1 nmol KP-10 appeared to result in a maximal stimulation of testosterone, a lower dose of 0.5 nmol was used for future analog studies.
Table 2.
Effect of intraperitoneal administration of KP-10 on plasma levels of LH and testosterone in male mice 20 min postinjection in vivo
| Treatment | LH, ng/ml | Testosterone |
|---|---|---|
| Saline | 0.451 + 0.046 | 0.955 + 0.234 |
| KP-10 0.3 nmol | 0.705 + 0.078 | 10.430 + 4.495 |
| KP-10 1 nmol | 2.727 + 0.516 | 36.893 + 1.270* |
| KP-10 10 nmol | 3.124 + 1.049* | 36.728 + 6.956* |
| KP-10 30 nmol | 4.949 + 0.130* | 37.067 + 4.028* |
Results are means ± SE.
P < 0.01 vs. saline (ANOVA with post hoc Tukey's adjustment), n = 6.
Plasma FSH was not significantly changed by KP-10 administration at any dose at this time point (data not shown).
In vivo screen of analogs with IC50 < 10 nM.
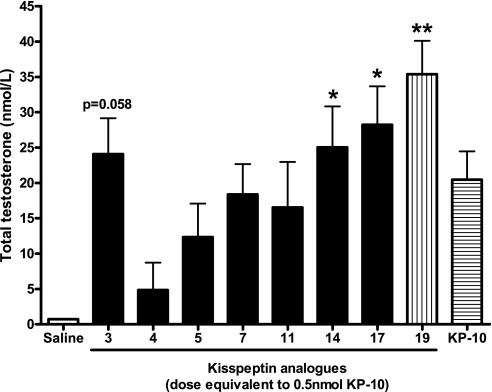
Administration of all analogs and KP-10 increased total testosterone at t = 20 min, but the relatively low n number meant that only the effects of ANA14, ANA17, and ANA19 ([dY]1KP-10) were statistically significant (Fig. 3).
Fig. 3.
Effect of intraperitoneal (ip) administration of KP-10 or KP-10 analogs on plasma total testosterone in male mice at 20 min postinjection. *P < 0.05, **P < 0.01 vs. saline (ANOVA with post hoc Tukey's adjustment); n = 6.
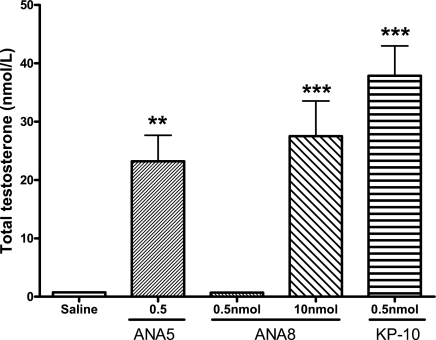
ANA5 and ANA8 had previously been tested in vitro for their ability to activate KISS1R in a LacZ reporter gene assay in yeast (22). ANA5 had been reported to have superagonistic activity in vitro, and, in contrast, ANA8 had been reported to have no agonistic activity at a concentration of 10 μm. Since our studies suggested that ANA8 did bind to the KISS1R, and that ANA8 was thus a potential KISS1R antagonist, we investigated the effect of both ANA5 and ANA8 in vivo.
ANA5 stimulated the release of testosterone at 20 min. However, this effect was not as great as that of KP-10. At a dose of 0.5 nmol, ANA8 did not influence testosterone release. However, at a higher dose of 10 nmol, ANA8 significantly stimulated testosterone release (Fig. 4), suggesting that ANA8 is a weak agonist of the KISS1R, rather than an antagonist.
Fig. 4.
Effect of ip administration of analogs ANA5 and ANA8 on plasma total testosterone in male mice at 20 min postinjection. **P < 0.01, ***P < 0.01 vs. saline (ANOVA with post hoc Tukey's adjustment); n = 5–6.
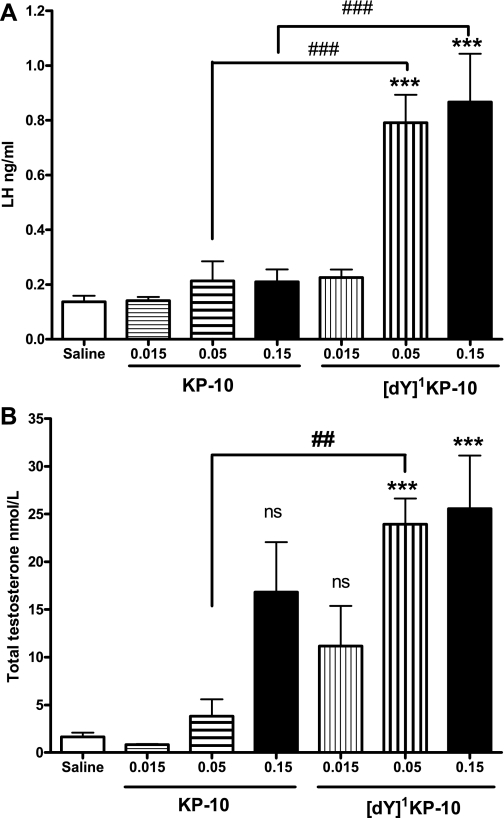
Comparing the dose-response effect of ip [dY]1KP-10 and KP-10 on plasma LH and testosterone.
The initial screening experiments suggested that ANA19 ([dY]1KP-10) might act as a KISS1R super agonist. A dose-response study of [dY]1KP-10 vs. KP-10 at 20 min demonstrated that [dY]1KP-10 had a more potent effect on total testosterone than KP-10. Doses of 0.05 and 0.15 nmol [dY]1KP-10 significantly increased LH compared with saline and KP-10 at the same dose (Fig. 5A). Testosterone was also significantly increased at these doses of [dY]1KP-10 compared with saline and significantly increased at 0.05 nmol [dY]1KP-10 compared with 0.05 nmol KP-10 (Fig. 5B).
Fig. 5.
Effect of ip administration of [dY]1KP-10 and KP-10 on plasma LH (A) and plasma total testosterone (B) in male mice at 20 min postinjection in vivo. ***P < 0.001 vs. saline; ##P < 0.01 vs. equimolar KP-10; ###P < 0.001 vs. equimolar KP-10 (ANOVA with post hoc Tukey's adjustment); n = 8.
Comparing the time course of the effect of ip [dY]1KP-10 and KP-10 on plasma LH and testosterone.
[dY]1KP-10 has a lower binding affinity for the KISS1R than KP-10, suggesting that its increased potency in vivo could be due to increased longevity in the circulation.
Thus we compared the time course of the effects of 0.5 nmol KP-10 and [dY]1KP-10 at 20 and 60 min postinjection on LH and total testosterone levels in mice. At 20 min postinjection, [dY]1KP-10 significantly increased LH. LH was elevated following KP-10 at 20 min postinjection, but this did not reach statistical significance. However, both KP-10 and [dY]1KP-10 significantly increased testosterone at 20 min [t = 20, plasma LH: 0.649 ± 0.203 ng/ml (saline), 2.535 ± 0.777 ng/ml (KP-10), 3.115 ± 0.758 ng/ml* ([dY]1KP-10); testosterone: 3.373 ± 1.101 nmol/l (saline), 74.499 ± 17.903 nmol/l* (KP-10), 85.185 ± 30.860 nmol/l* ([dY]1KP-10); n = 4–6; *P < 0.05 vs. saline]. At 60 min postinjection, neither KP-10 or [dY]1KP-10 significantly raised LH, but [dY]1KP-10 did significantly raise testosterone [t = 60, plasma LH: 0.720 ± 0.110 ng/ml (saline), 1.049 ± 0.316 ng/ml (KP-10), 1.401 ± 0.226 ng/ml ([dY]1KP-10); testosterone: 3.815 ± 0.789 nmol/l (saline), 50.116 ± 22.474 nmol/l (KP-10), 94.572 ± 29.471 nmol/l* ([dY]1KP-10); n = 4–6; *P < 0.05 vs. saline].
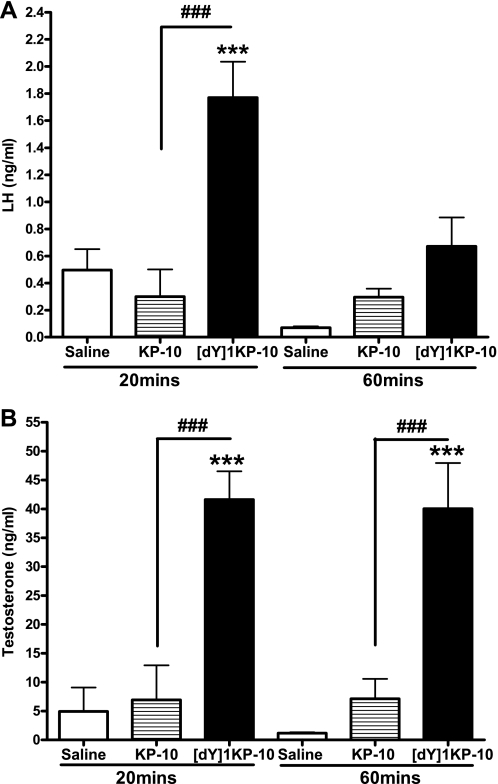
To better examine potential differences between the effects of KP-10 and [dY]1KP-10, we compared the time course of the effects of a lower dose of 0.15 nmol KP-10 and [dY]1KP-10, at 20, 60, and 120 min postinjection on LH and total testosterone levels in mice.
At 20 min postinjection [dY]1KP-10 significantly increased LH, but KP-10 did not. There was a trend toward an increase in LH following [dY]1KP-10 at 60 min postinjection, which did not reach statistical significance (Fig. 6A). At 120 min, plasma LH had returned to basal in all groups (data not shown).
Fig. 6.
Effect of ip administration of 0.15 nmol [dY]1KP-10 and KP-10 on plasma LH (A) and plasma total testosterone (B) in male mice at 20 and 60 min postinjection in vivo. ***P < 0.001 vs. saline, ###P < 0.001 vs. equimolar KP-10 (ANOVA with post hoc Tukey's adjustment); n = 4–7.
[dY]1KP-10 significantly increased plasma testosterone levels at 20 and 60 min postinjection. Testosterone was raised in response to KP-10, but this change did not achieve statistical significance at any time point investigated (Fig. 6B). At 120 min, plasma testosterone had returned to basal in all groups (data not shown).
DISCUSSION
Investigating the in vitro and in vivo bioactivity of a series of KP-10 analogs has identified [dY]1KP-10 as a KISS1R agonist with greater in vivo bioactivity than KP-10. The kisspeptins stimulate the HPG axis and thus offer a target for therapies to treat reproductive disorders. KP-10 has a similar effect in vitro to its longer counterpart, KP-54. However, KP-10 is less potent than KP-54 in vivo, possibly because it is broken down more quickly in the circulation (32). This limits the potential clinical utility of kisspeptin-10 itself. KP-54 is a much longer peptide, and consequently more expensive to produce, making it less economically viable as a potential therapy. Rational modification of the KP-10 structure to increase bioactivity may produce a molecule with increased therapeutic potential.
Initial receptor binding studies identified the residues in KP-10 critical for receptor binding. The phenylalanine (F) residues at position 6 and position 10 appear critical for KISS1R binding. Exchanging these phenylalanines for alanine, a similar amino acid , or d-phenylalanine greatly reduced the molecule's affinity for KISS1R.
During the course of these studies, several papers were published investigating the effect of sequential manipulation of KP-10 on in vitro and in vivo activity (8, 22, 24, 27). Niida et al. (22) investigated the effects of a number of the human KP-10 analogs that we were also studying, specifically ANA1–10 (which they also designated ANA1–10) and 19–21 (ANA11, 16, and 20, respectively, in their studies) on the in vitro activation of human KISS1R using a LacZ reporter gene system in yeast. Orsini et al. (24) also investigated the binding and agonistic activity of ANA1–10 and ANA15–17 at the human KISS1R. We have also investigated the receptor binding affinity and the ability of these analogs to stimulate intracellular signaling pathways, although in our case by measuring phosphorylation of ERK1/2. In addition, we have investigated the in vivo activity of those analogs that bound relatively well to the KISS1R. Our data are generally in accord with those described by Niida et al. and Orsini et al. However, unlike Niida et al., although in accord with Orsini et al., we found that although ANA5 bound with higher affinity to the KISS1R than KP-10, it was not more potent than KP-10 in vitro. We have also presented data that suggests that ANA5 is less potent than KP-10 in vivo. Thus while ANA5 may act as a KISS1R super agonist in specific in vitro systems, it does not appear to have greater activity than KP-10 in vivo.
Roseweir et al. (27) determined the binding and activity of a number of human KP-10 analogs at the human KISS1R in a series of studies to design an effective KISS1R antagonist. Their results also highlighted the importance of the five COOH-terminal amino acids to receptor activation. Because of the systematic design of these experiments, many of the analogs investigated incorporate a number of different substitutions. Other evidence suggests that the conformation of the Phe6-Gly7 peptide bond is important for bioactivity in human KP-10 (34). Specific amino acid substitutions at positions 1, 5, and 8 resulted in a high-affinity KISS1R antagonist, suggesting that these positions are important for the bioactivity of KP-10 (27).
Gutiérrez-Pascual et al. (8) investigated the rat analogs equivalent to the human analogs we designated ANA1–10, replacing sequential residues in the rat KP-10 molecule with alanine. Our findings are broadly in agreement with theirs. Their study also suggests that residues 6 and 10 are crucial for bioactivity of rat KP-10 at the rat Kiss1r. They suggest that the low agonist activity of ANA10 may reflect the importance of residue 10 in binding to the Kiss1r, but that this requires experimental confirmation. Binding data from our study and from Orsini et al. (24) strongly suggests that this is the case and that residue 10 of human KP-10 is critical for receptor binding. It would be interesting to determine the effects of a rodent [dY]1KP-10 analog on the HPG axis, and in particular whether such an analog would have greater bioactivity at the human KISS1R. Our studies did identify a novel potential super agonist for KISS1R. Interestingly, in the studies by Niida et al. (22), [dY]1KP-10 did not demonstrate any activation of KISS1R. This may reflect the different reporter systems used. In our studies, [dY]1KP-10 bound to the KISS1R with approximately fourfold lower affinity than KP-10. However, the increase in in vitro bioactivity exhibited by this peptide led to it being further investigated in vivo. Despite its lower affinity, it demonstrated a similar potency in vitro and greater potency in vivo than KP-10. The difference in concentrations required for the effects may be due to the use of an artificial reporter system of CHO-KISS1R cells. [dY]1KP-10 appeared to have a more potent effect on LH and testosterone than KP-10 itself in vivo. No effect was seen on FSH, which may reflect that a higher dose was required. Endogenous kisspeptin has been reported to have an EC50 for its stimulatory effect on FSH ten times greater than that for its effect on LH (31).
Further experiments are required to determine whether the increased potency of [dY]1KP-10 in vivo reflects increased longevity of the molecule in circulation. [dY]1KP-10 had a lower affinity for the human KISS1R in vitro, suggesting that its increased effects may not be due to improved receptor binding. The tyrosine at position 1 of endogenous KP-10 may therefore be a target for proteolytic cleavage. Alternatively, altering the molecule at this point may alter its confirmation sufficiently to impair proteolytic cleavage at other sites. It may be possible to further increase the longevity of the effects of [dY]1KP-10 extending the molecule to convert it to an analog of KP-54. Although this would increase the cost of synthesis, the improved longevity may be sufficient to justify this cost. It is interesting that ip administration of KP-10 to male mice at doses of up to 0.15 nmol did not significantly increase LH levels at 20 min following injection. We have previously found that relatively high doses of human KP-10 are required to stimulate the HPG axis in rats following peripheral administration (32, 33). Mikkelsen et al. (16) have compared the effects of ip administration of mouse and human KP-10 on testosterone in mice and suggest that mouse KP-10 is more potent than human KP-10 at stimulating testosterone release in this model. The lack of effect we observed may therefore reflect this lower effectiveness of human KP-10 in mice.
The utility of manipulating the kisspeptin system as a disease treatment is still to be demonstrated. Several groups have suggested that this approach may have clinical utility in the treatment of reproductive disorders, such as delayed puberty, prostatic cancer, and metastatic cancer (9, 22, 28, 35). Our studies have identified and tested a number of KP-10 analogs, one of which, [dY]1KP-10, has greater in vivo bioactivity than KP-10 itself. Further studies are required to explore the therapeutic potential of KISS1R super agonists.
GRANTS
K. G. Murphy is supported by a Biotechnology and Biological Sciences Research Council New Investigator Award. J. H. Cooke is supported by the Medical Research Council (MRC). This research is funded by programme grants from the MRC (G7811974) and Wellcome Trust (072643/Z/03/Z) and by an European Union FP6 Integrated Project Grant LSHMCT-2003-503041. We are also grateful for support from the National Institute for Health Research Biomedical Research Centre funding scheme and an Integrative Mammalian Biology Capacity building award.
DISCLOSURES
The authors have nothing to disclose.
ACKNOWLEDGMENTS
The authors thank the Hypothalamic Group for help with the in vivo studies detailed in this paper.
REFERENCES
- 1.Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, Malli R, Sharabi A, Hiden U, Graier W, Knofler M, Andreae F, Wagner O, Quaranta V, Desoye G. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci 117: 1319–1328, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Chang CD, Meienhofer J. Solid-phase peptide synthesis using mild base cleavage of N alpha-fluorenylmethyloxycarbonylamino acids, exemplified by a synthesis of dihydrosomatostatin. Int J Pept Protein Res 11: 246–249, 1978 [DOI] [PubMed] [Google Scholar]
- 3.Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28: 8691–8697, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100: 10972–10976, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 90: 6609–6615, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab 92: 3958–3966, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145: 4073–4077, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Gutiérrez-Pascual E, Leprince J, Martinez-Fuentes AJ, Segalas-Milazzo I, Pineda R, Roa J, Duran-Prado M, Guilhaudis L, Desperrois E, Lebreton A, Pinilla L, Tonon MC, Malagon MM, Vaudry H, Tena-Sempere M, Castano JP. In vivo and in vitro structure-activity relationships and structural conformation of Kisspeptin-10-related peptides. Mol Pharmacol 76: 58–67, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Harms JF, Welch DR, Miele ME. KISS1 metastasis suppression and emergent pathways. Clin Exp Metastasis 20: 11–18, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80: 264–272, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le PE, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276: 34631–34636, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst 88: 1731–1737, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Makri A, Pissimissis N, Lembessis P, Polychronakos C, Koutsilieris M. The kisspeptin (KiSS-1)/GPR54 system in cancer biology. Cancer Treat Rev 34: 682–692, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun 320: 383–388, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102: 1761–1766, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikkelsen JD, Bentsen AH, Ansel L, Simonneaux V, Juul A. Comparison of the effects of peripherally administered kisspeptins. Regul Pept 152: 95–100, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 276: 28969–28975, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145: 4565–4574, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology 146: 1689–1697, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology 146: 156–163, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol 561: 379–386, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niida A, Wang Z, Tomita K, Oishi S, Tamamura H, Otaka A, Navenot JM, Broach JR, Peiper SC, Fujii N. Design and synthesis of downsized metastin (45–54) analogs with maintenance of high GPR54 agonistic activity. Bioorg Med Chem Lett 16: 134–137, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411: 613–617, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Orsini MJ, Klein MA, Beavers MP, Connolly PJ, Middleton SA, Mayo KH. Metastin (KiSS-1) mimetics identified from peptide structure-activity relationship-derived pharmacophores and directed small molecule database screening. J Med Chem 50: 462–471, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Owji AA, Smith DM, Coppock HA, Morgan DG, Bhogal R, Ghatei MA, Bloom SR. An abundant and specific binding site for the novel vasodilator adrenomedullin in the rat. Endocrinology 136: 2127–2134, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Roa J, Castellano JM, Navarro VM, Handelsman DJ, Pinilla L, Tena-Sempere M. Kisspeptins and the control of gonadotropin secretion in male and female rodents. Peptides 30: 57–66, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29: 3920–3929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF., Jr Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology 147: 2122–2126, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 349: 1614–1627, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102: 2129–2134, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tena-Sempere M. GPR54 and kisspeptin in reproduction. Hum Reprod Update 12: 631–639, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Thompson EL, Murphy KG, Patterson M, Bewick GA, Stamp GW, Curtis AE, Cooke JH, Jethwa PH, Todd JF, Ghatei MA, Bloom SR. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am J Physiol Endocrinol Metab 291: E1074–E1082, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 16: 850–858, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Tomita K, Niida A, Oishi S, Ohno H, Cluzeau J, Navenot JM, Wang ZX, Peiper SC, Fujii N. Structure-activity relationship study on small peptidic GPR54 agonists. Bioorg Med Chem 14: 7595–7603, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Tomita K, Oishi S, Ohno H, Fujii N. Structure-activity relationship study and NMR analysis of fluorobenzoyl pentapeptide GPR54 agonists. Biopolymers 90: 503–511, 2008. [DOI] [PubMed] [Google Scholar]