Abstract
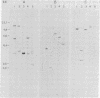
A variety of genes have been identified that specify the synthesis of the components of guanine nucleotide-binding proteins (G proteins). Eight different guanine nucleotide-binding alpha-subunit proteins, two different beta subunits, and one gamma subunit have been described. Hybridization of cDNA clones with DNA from human-mouse somatic cell hybrids was used to assign many of these genes to human chromosomes. The retinal-specific transducin subunit genes GNAT1 and GNAT2 were on chromosomes 3 and 1; GNAI1, GNAI2, and GNAI3 were assigned to chromosomes 7, 3, and 1, respectively; GNAZ and GNAS were found on chromosomes 22 and 20. The beta subunits were also assigned--GNB1 to chromosome 1 and GNB2 to chromosome 7. Restriction fragment length polymorphisms were used to map the homologues of some of these genes in the mouse. GNAT1 and GNAI2 were found to map adjacent to each other on mouse chromosome 9 and GNAT2 was mapped on chromosome 17. The mouse GNB1 gene was assigned to chromosome 19. These mapping assignments will be useful in defining the extent of the G alpha gene family and may help in attempts to correlate specific genetic diseases with genes corresponding to G proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley P. L., Ellison J., Sullivan K. A., Bourne H. R., Cox D. R. Chromosomal assignment of the murine Gi alpha and Gs alpha genes. Implications for the obese mouse. J Biol Chem. 1987 Nov 5;262(31):15299–15301. [PubMed] [Google Scholar]
- Barnes D. M. How cells respond to signals. Science. 1986 Oct 17;234(4774):286–288. doi: 10.1126/science.2429365. [DOI] [PubMed] [Google Scholar]
- Beals C. R., Wilson C. B., Perlmutter R. M. A small multigene family encodes Gi signal-transduction proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7886–7890. doi: 10.1073/pnas.84.22.7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt C., Harper M. E., Franchini G., Nesbitt M. N., Simon M. I. Chromosomal mapping of murine c-fes and c-src genes. Mol Cell Biol. 1984 May;4(5):978–981. doi: 10.1128/mcb.4.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bégin-Heick N. Absence of the inhibitory effect of guanine nucleotides on adenylate cyclase activity in white adipocyte membranes of the ob/ob mouse. Effect of the ob gene. J Biol Chem. 1985 May 25;260(10):6187–6193. [PubMed] [Google Scholar]
- Codina J., Yatani A., Grenet D., Brown A. M., Birnbaumer L. The alpha subunit of the GTP binding protein Gk opens atrial potassium channels. Science. 1987 Apr 24;236(4800):442–445. doi: 10.1126/science.2436299. [DOI] [PubMed] [Google Scholar]
- Didsbury J. R., Snyderman R. Molecular cloning of a new human G protein. Evidence for two Gi alpha-like protein families. FEBS Lett. 1987 Jul 13;219(1):259–263. doi: 10.1016/0014-5793(87)81228-0. [DOI] [PubMed] [Google Scholar]
- Démant P., Iványi D., van Nie R. The map position of the rds gene on the 17th chromosome of the mouse. Tissue Antigens. 1979 Jan;13(1):53–55. doi: 10.1111/j.1399-0039.1979.tb01136.x. [DOI] [PubMed] [Google Scholar]
- Farfel Z., Brickman A. S., Kaslow H. R., Brothers V. M., Bourne H. R. Defect of receptor-cyclase coupling protein in psudohypoparathyroidism. N Engl J Med. 1980 Jul 31;303(5):237–242. doi: 10.1056/NEJM198007313030501. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fong H. K., Amatruda T. T., 3rd, Birren B. W., Simon M. I. Distinct forms of the beta subunit of GTP-binding regulatory proteins identified by molecular cloning. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3792–3796. doi: 10.1073/pnas.84.11.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H. K., Yoshimoto K. K., Eversole-Cire P., Simon M. I. Identification of a GTP-binding protein alpha subunit that lacks an apparent ADP-ribosylation site for pertussis toxin. Proc Natl Acad Sci U S A. 1988 May;85(9):3066–3070. doi: 10.1073/pnas.85.9.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier R. E., Moran R. G. Complementation mapping in microcell hybrids: localization of Fpgs and Ak-1 on Mus musculus chromosome 2. Somatic Cell Genet. 1983 Jan;9(1):69–84. doi: 10.1007/BF01544049. [DOI] [PubMed] [Google Scholar]
- Gao B., Gilman A. G., Robishaw J. D. A second form of the beta subunit of signal-transducing G proteins. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6122–6125. doi: 10.1073/pnas.84.17.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Fong H. K., Teplow D. B., Dreyer W. J., Simon M. I. Isolation and characterization of a cDNA clone for the gamma subunit of bovine retinal transducin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6948–6952. doi: 10.1073/pnas.81.22.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H., Kozasa T., Nagata S., Nakamura S., Katada T., Ui M., Iwai S., Ohtsuka E., Kawasaki H., Suzuki K. Molecular cloning and sequence determination of cDNAs for alpha subunits of the guanine nucleotide-binding proteins Gs, Gi, and Go from rat brain. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3776–3780. doi: 10.1073/pnas.83.11.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Lalley P. A., Minna J. D., Francke U. Conservation of autosomal gene synteny groups in mouse and man. Nature. 1978 Jul 13;274(5667):160–163. doi: 10.1038/274160a0. [DOI] [PubMed] [Google Scholar]
- Lem J., Fournier R. E. Assignment of the gene encoding cytosolic phosphoenolpyruvate carboxykinase (GTP) to Mus musculus chromosome 2. Somat Cell Mol Genet. 1985 Nov;11(6):633–638. doi: 10.1007/BF01534728. [DOI] [PubMed] [Google Scholar]
- Lerea C. L., Somers D. E., Hurley J. B., Klock I. B., Bunt-Milam A. H. Identification of specific transducin alpha subunits in retinal rod and cone photoreceptors. Science. 1986 Oct 3;234(4772):77–80. doi: 10.1126/science.3529395. [DOI] [PubMed] [Google Scholar]
- Lochrie M. A., Hurley J. B., Simon M. I. Sequence of the alpha subunit of photoreceptor G protein: homologies between transducin, ras, and elongation factors. Science. 1985 Apr 5;228(4695):96–99. doi: 10.1126/science.3856323. [DOI] [PubMed] [Google Scholar]
- Lochrie M. A., Simon M. I. G protein multiplicity in eukaryotic signal transduction systems. Biochemistry. 1988 Jul 12;27(14):4957–4965. doi: 10.1021/bi00414a001. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Itoh H., Kozasa T., Kaziro Y. Sequence analysis of cDNA and genomic DNA for a putative pertussis toxin-insensitive guanine nucleotide-binding regulatory protein alpha subunit. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5384–5388. doi: 10.1073/pnas.85.15.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel T., Winslow J. W., Smith J. A., Seidman J. G., Neer E. J. Molecular cloning and characterization of cDNA encoding the GTP-binding protein alpha i and identification of a related protein, alpha h. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7663–7667. doi: 10.1073/pnas.83.20.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau J. H., Taylor B. A. Lengths of chromosomal segments conserved since divergence of man and mouse. Proc Natl Acad Sci U S A. 1984 Feb;81(3):814–818. doi: 10.1073/pnas.81.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakafuku M., Itoh H., Nakamura S., Kaziro Y. Occurrence in Saccharomyces cerevisiae of a gene homologous to the cDNA coding for the alpha subunit of mammalian G proteins. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2140–2144. doi: 10.1073/pnas.84.8.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J., Michel T., Eddy R., Shows T., Seidman J. G. Genes for two homologous G-protein alpha subunits map to different human chromosomes. Hum Genet. 1987 Nov;77(3):259–262. doi: 10.1007/BF00284481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukada T., Tanabe T., Takahashi H., Noda M., Haga K., Haga T., Ichiyama A., Kanagawa K., Hiranaga M., Matsuo Primary structure of the alpha-subunit of bovine adenylate cyclase-inhibiting G-protein deduced from the cDNA sequence. FEBS Lett. 1986 Mar 3;197(1-2):305–310. doi: 10.1016/0014-5793(86)80347-7. [DOI] [PubMed] [Google Scholar]
- Peterson T. C., Killary A. M., Fournier R. E. Chromosomal assignment and trans regulation of the tyrosine aminotransferase structural gene in hepatoma hybrid cells. Mol Cell Biol. 1985 Sep;5(9):2491–2494. doi: 10.1128/mcb.5.9.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Buckler C. E. Statistical considerations for linkage analysis using recombinant inbred strains and backcrosses. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1423–1427. doi: 10.1073/pnas.83.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Simon M., Cohn V. H., Fournier R. E., Lem J., Klisak I., Heinzmann C., Blatt C., Lucero M., Mohandas T. Assignment of the human and mouse prion protein genes to homologous chromosomes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7358–7362. doi: 10.1073/pnas.83.19.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Liao Y. C., Alborzi A., Beiderman B., Chang F. H., Masters S. B., Levinson A. D., Bourne H. R. Inhibitory and stimulatory G proteins of adenylate cyclase: cDNA and amino acid sequences of the alpha chains. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6687–6691. doi: 10.1073/pnas.83.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nie R., Iványi D., Démant P. A new H-2-linked mutation, rds, causing retinal degeneration in the mouse. Tissue Antigens. 1978 Aug;12(2):106–108. doi: 10.1111/j.1399-0039.1978.tb01305.x. [DOI] [PubMed] [Google Scholar]