Abstract
Urocortin 3 (Ucn 3) is a corticotropin-releasing factor (CRF)-related peptide with high affinity for the type 2 CRF receptor (CRFR2). Central administration of Ucn 3 stimulates the hypothalamic-pituitary-adrenal axis, suppresses feeding, and elevates blood glucose levels, suggesting that activation of brain CRFR2 promotes stress-like responses. Several CRFR2-expressing brain areas, including the ventromedial hypothalamus (VMH) and the posterior amygdala (PA), may be potential sites mediating the effects of Ucn 3. In the present study, Ucn 3 or vehicle was bilaterally injected into the VMH or PA, and food intake and plasma levels of ACTH, corticosterone, glucose, and insulin were determined. Food intake was greatly reduced in rats following Ucn 3 injection into the VMH. Ucn 3 injection into the VMH rapidly elevated plasma levels of glucose and insulin but did not affect ACTH and corticosterone secretion. Injection of Ucn 3 into the PA did not alter any of the parameters measured. We determined that the majority of CRFR2-positive neurons in the VMH were excitatory glutamatergic, and a subset of these neurons project to the arcuate nucleus of the hypothalamus (ARH). Importantly, stimulation of CRFR2 in the VMH increased proopiomelanocortin mRNA expression in the ARH. In conclusion, the present study demonstrates that CRFR2 in the VMH mediates some of the central effects of Ucn 3, and the ARH melanocortin system may be a downstream target of VMH CRFR2 neurons.
Keywords: corticotrophin-releasing factor, urocortin, feeding, hypothalamus, blood glucose, hypothalamic-pituitary-adrenal axis
urocortin 3 (Ucn) 3 is a member of the CRF peptide family identified in humans and rodents (20, 31). It displays high affinity for the type 2 CRF receptor (CRFR2) with minimal affinity for the type 1 CRF receptor (CRFR1) (20, 31). Thus Ucn 3 has been considered as an endogenous ligand for CRFR2. In the brain, Ucn 3-expressing neurons are located primarily in the hypothalamus and the amygdala (31, 33). Within the hypothalamus, the majority of Ucn 3-positive neurons are found in the rostral part of the perifornical area with moderate levels of Ucn 3-positive cells found in the median preoptic nucleus (33). Ucn 3-immunoreactive nerve fibers and terminals are distributed mostly in the forebrain areas, including the hypothalamus and limbic system, where prominent Ucn 3 fibers are found in the lateral septum, amygdala, and the bed nucleus of stria terminalis (BNST) (33). In the hypothalamus, Ucn 3 fibers predominately innervate the ventromedial nucleus (VMH), with moderate levels of innervation found in medial preoptic area and the ventral premammillary nucleus (33). These anatomical features suggest that central Ucn 3 may be involved in multiple physiological and behavioral regulations.
Similar to other members of the CRF family, central administration of Ucn 3 results in a number of effects that are often associated with stress responses. These effects include suppression of feeding (14, 39, 43), stimulation of sympathetic nervous system (SNS) activity and elevation of blood glucose levels (22). Infusion of Ucn 3 into rat brains has been shown to stimulate the hypothalamo-pituitary adrenal (HPA) axis to elevate levels of plasma ACTH and corticosterone (22), although this effect is less pronounced in mice (43, 56). On the other hand, central injection of Ucn 3 does not induce stress-like behaviors (39, 43, 56). The lack of behavioral effects of Ucn 3 suggests that CRFR2 in the brain plays a role in mediating autonomic aspects of stress-associated responses.
Several brain areas, including the VMH and the posterior part of the amygdala (PA) may potentially mediate the effects of Ucn 3 as these areas express high levels of CRFR2 (6) and receive Ucn 3 innervation (33). The VMH has been shown to play an important role in the regulation of feeding (1, 3, 5, 18, 23, 29, 49), SNS activity (26), blood glucose levels (48, 50) and the HPA axis activity (52). Importantly, activation of CRFR2 in the VMH has been shown to suppress feeding in several animal models (14, 40). Furthermore, a study by McCrimmon et al. (36) showed that injection of CRF and related peptides into the VMH blunted the hypoglycemia-induced counterregulatory response and elevated plasma corticosterone levels 60 min after the injection. This supports a potential role of CRF receptors in the VMH in glucose homeostasis and HPA hormonal secretion. However, it remains to be determined whether stimulation of CRFR2 in the VMH has an acute effect in modulating blood glucose levels and/or HPA hormone secretion. To address this issue, we determined the time course of plasma glucose and hormone levels in animals receiving bilateral Ucn 3 injections into the VMH. In addition to VMH, the PA has been linked to the regulation of appetite and HPA axis activity (11, 27, 28, 46), raising the possibility that CRFR2 in the PA may mediate effects of Ucn 3 in the brain. In the present study, we examined this question using site-specific injection of Ucn 3 into the PA to determine effects on food intake and plasma levels of glucose and hormones indicative of stress and metabolic status.
Although the mechanism by which VMH regulates energy homeostasis remains to be fully appreciated, recent research suggests a novel link between the VMH and the arcuate nucleus of the hypothalamus (ARH). Using a sophisticated electrophotostimulation technique, Sternson et al. (51) has shown that the VMH provides a direct excitatory afferent input to proopiomelanocortin (POMC) neurons in the ARH, suggesting that POMC neurons are a downstream target of VMH. ARH POMC neurons have been recognized as a key anorectic system in the brain (12), and these neurons also play an important role in regulating blood glucose levels (19, 41, 57). It is conceivable that activation of CRFR2 neurons in the VMH leads to stimulation of ARH POMC neurons, which in turn regulate feeding and glucose homeostasis. Two anatomical studies were carried out to address this possibility. First, a double label in situ hybridization was performed to simultaneously detect mRNAs for CRFR2 and vesicular glutamate transporter isoform-2 (VGLUT2), a marker for glutamatergic neurons (16, 17, 53), to determine if CRFR2 is expressed in excitatory glutamatergic neurons in the VMH. Second, retrograde tracing was used to determine whether CRFR2 neurons in the VMH project to the ARH. Finally, POMC mRNA levels in the ARH were examined by in situ hybridization in rats following bilateral VMH or PA injection of Ucn 3 or vehicle to assess whether activation of CRFR2 in these regions by Ucn 3 stimulates POMC gene expression in the ARH.
MATERIALS AND METHODS
Animals.
Male adult (300–350 g) Spraque-Dawley rats (Charles River, Hollister, CA) were group-housed in a 12:12-h light-dark cycle (lights on 0600) humidity- and temperature-controlled vivarium (22°C) with standard rodent chow and water available ad libitum. Animals were acclimated to the vivarium for 1 wk before experiments and handled during the week before the start of testing. Surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee of The Salk Institute.
Cannulation.
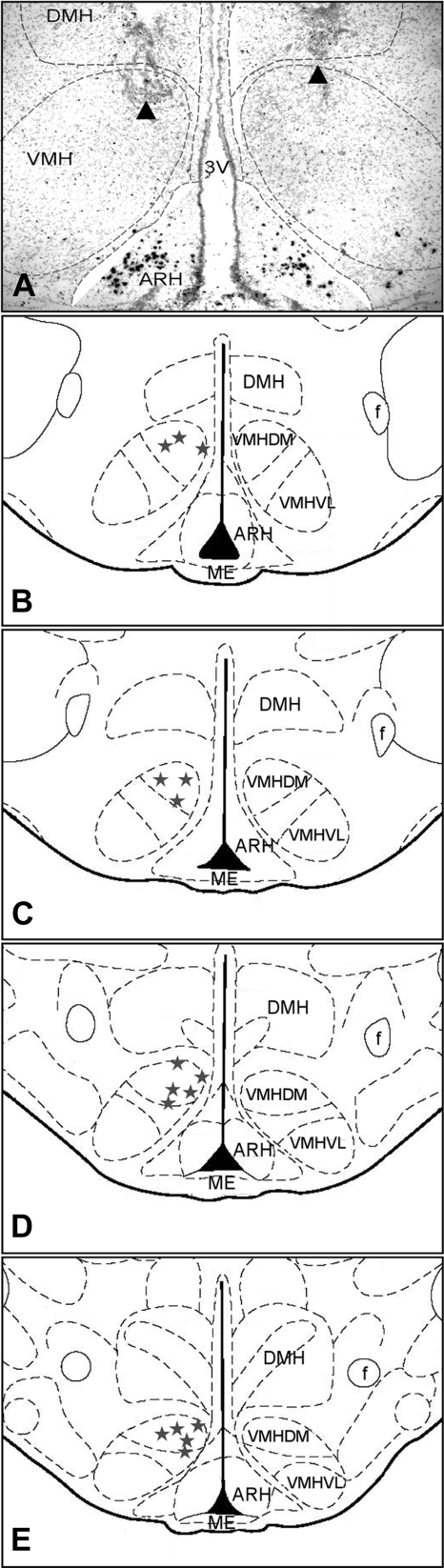
Rats were anesthetized with rodent cocktail and placed in a stereotaxic apparatus. For VMH cannulation, a double-guide cannula (28 gauge; center-to-center, 1.0 mm; tubing length below pedestal, 9.6 mm; Plastics One, Roanoke, VA) was inserted near the VMH [coordinates: 3.3 mm caudal, 0.5 mm lateral to bregma, and 8.4 mm ventral to the dura, according to the atlas of Paxinos and Watson (42)]. The guide cannula was secured to the skull with acrylic dental cement and anchored with stainless-steel bone screws. A dummy cannula with tip extending 0.5 mm below the guide cannula was inserted in the guide cannula to maintain patency until the time of injections. An additional group of rats received bilateral cannulation in the posterior part of the amygdala (PA). The procedure was similar to VMH cannulation except that two single cannulas were implanted in each site of the PA (coordinates: 3.6 mm caudal, 3.8 mm lateral to bregma, and 9.4 mm ventral to the dura). Rats were handled daily during the recovery period, and only animals displaying normal food intake and weight gain after surgeries were used. At the end of experiment, brains were sectioned to determine the placement of cannulas. As shown in Fig. 1, only animals with the tip of the cannula located near the dorsomedial part of the VMH were included in the analyses.
Fig. 1.
A: representative photomicrograph showing cannula placement in the ventromedial hypothalamus (VMH). Tips of cannulas are indicated by arrowheads. B–D: schematic drawings showing the placement of cannulas in the VMH of rats included in this study. 3V, third ventricle; ARH, arcuate nucleus of hypothalamus; DMH, dorsomedial nucleus of hypothalamus; ME, median eminence; VMH, ventromedial nucleus of hypothalamus; VMHDM, dorsomedial division of VMH; VMHVL, ventrolateral division of VMH.
Effect of Ucn 3 on plasma insulin, glucose, ACTH, and corticosterone levels.
Before the experiment (2 days), jugular vein catheters (PE-50; Becton Dickinson, Sparks, MD) were inserted into the right atrium of the rats under light halothane anesthesia. The catheter was filled with sterile heparinized saline, passed through a subcutaneous tunnel, and exteriorized at the back of the neck. After the catheterization, rats were housed in individual cages. Rats were fasted overnight before the experiment. On the day of the experiment, the rats were transferred to opaque sampling cages, and the jugular vein catheter was connected to a sampling tube to allow for remote sequential blood sampling. After a period (2–3 h) of acclimation, experiments were started at 0900–1000. A blood sample was collected before the start of the treatment. Ucn 3 (0.1 nmol in 0.4 μl per side per animal, n = 8) or vehicle [0.4 μl double-filtered sterile artificial cerebrospinal fluid (aCSF; n = 8)] was injected into the VMH or PA of rats through an internal cannula (32 gauge; tip extended 0.5 mm below the guide cannula) that was connected via PE-50 tubing to a Hamilton syringe. Solutions were manually injected over a 1-min period, and then the internal cannula remained in situ for 1 min after the injection. Blood samples were collected at 5, 15, 30, 60, 120, and 240 min following the injection. The samples were immediately centrifuged and stored at −80°C until assay for glucose and hormones. After the experiment, animals were returned to home cages and were allowed to recover for 10 days before the feeding study. Plasma insulin (Linco, St. Louis, MO), ACTH (Nichols Institute Diagnostics, San Juan Capistrano, CA), and corticosterone (MP Biomedicals, Solon, OH) levels were determined using commercial immunoassay kits, and plasma glucose levels were measured by Trinder assay (Sigma, St. Louis, MO).
Effect of Ucn 3 on food intake.
Rats were remotely injected in the VMH or PA at 15 min before lights off with mouse Ucn 3 (0.1 nmol in 0.4 μl per side per animal; n = 8) or aCSF (n = 8). Immediately after the injections, preweighted rat chow was placed in cages. The dose of Ucn 3 used was based on literature reports as causing a maximal suppression of food intake when given intracerebroventricularly or directly in specific brain nuclei (14). Food and water intake was measured 2 and 4 h after injection. At the completion of the experiment, animals were euthanized, and the brains were collected and stored at −80°C.
Retrograde tracer injection.
Animals were anesthetized and placed in a stereotaxic apparatus. A glass micropipette with a tip diameter of 25–35 μm was filled with a retrograde tracer, fluorogold (FG, 2% wt/vol, in physiological saline), and inserted in the main portion of the ARH, an area containing a high density of POMC neurons. Injection coordinates were 3.2 mm caudal, 0.25 mm lateral to the bregma, and 9.75 mm below the dura, according to the atlas of Paxinos and Watson (42). FG was injected by iontophoresis with 2 μA current and pulsed at 7-s intervals for 10 min. The glass pipette was left in situ for an additional 10 min to avoid the spread of tracer along the pipette track. Animals were perfused with fixation 7 days after the injection. Brains were removed and sectioned. Animals with an injection site near the target area (n = 5) were used for combined immunohistochemistry and in situ hybridization to visualize FG and CRFR2 mRNA simultaneously.
In situ hybridization.
For POMC mRNA, rat brains were sectioned into one-in-four 20-μm coronal sections with a cryostat. POMC cRNA probe was transcribed from linearized POMC cDNA as template in the presence of the 35S-labeled UTP (Perkin Elmer, Boston, MA). The specific activity of the probe ranged from 1 to 5 × 105 cpm/μl of hybridization buffer. The brain sections were fixed in 4% paraformaldehyde, treated with a fresh solution containing 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0), followed by a rinse in 2X saline-sodium citrate buffer (SSC), dehydrated through a graded series of alcohol, delipidated in chloroform, rehydrated through a second series of alcohol, and then air dried. The slides were exposed to POMC cRNA probe overnight in moist chambers at 55°C. After the incubation, the slides were washed in SSC with increasing stringency, in RNase A, in 0.1X SSC at 60°C, and dehydrated through graded series of alcohol and dried. Slides were dipped in NTB emulsion (Kodak, New Haven, CT), exposed for 7 days at 4°C, and developed. Following development, the slides were counterstained with creysl violet to identify anatomical landmarks.
Single label in situ hybridization for VGLUT2 mRNA was first performed using cRNA probes described by Fremeau et al. (16) to validate the cRNA probe for detecting VGLUT2 mRNA (data not shown). Double-label in situ hybridization for CRFR2 and VGLUT2 mRNAs was performed as previously described (7). Briefly, VGLUT2 cRNA probe was labeled with digoxigenin-UTP (Roche Diagnostics, Indianapolis, IN). CRFR2 cRNA probe was labeled with [33P]UTP. The concentration of digoxigenin-labeled VGLUT2 cRNA in the hybridization mixture was 0.1 μg/ml. The slides were exposed to the mixture of VGLUT2 and CRFR2 cRNA probes in moist chambers for 15 h at 55°C. After incubation and posthybridization washes, slides were incubated in alkaline phosphatase (AP)-conjugated goat anti-digoxigenin antibody (1:1,000; Roche Diagnostics) overnight at 4°C. The AP complexes were visualized with a mixture of nitroblue tetrazolium and 5-bromo-4-chloro-3-inodyl phosphate toluidinum. Slides were then dipped in 3% parlodion followed by NTB emulsion, exposed for 14 days at 4°C, and developed.
For double-label immunohistochemistry and in situ hybridization to detect FG and CRFR2 mRNA, rat brain sections were rinsed in 0.05 M potassium phosphate-buffered saline (KPBS) followed by treatment with 1% NaBH4-KPBS solution. Sections were incubated with FG antibodies raised in rabbit (1:30,000; Millipore) in KPBS with 0.4% Triton X-100 at room temperature for 1 h, followed by 4°C for 48 h. After incubation, sections were rinsed in KPBS and incubated in biotinylated donkey anti-rabbit IgG (1:600; Jackson Immunoresearch) in KPBS with 0.4% Triton X-100 for 1 h at room temperature, followed by a 1-h incubation at room temperature in avidin (A)-biotin (B) complex solution (4.5 μl of A and B each/ml of KPBS-0.4% Triton X-100; Vectastain ABC Elite Kit; Vector Laboratories). The antibody-peroxidase complex was visualized with a mixture of 3,3-diaminobenzidine (0.2 mg/ml) and 3% H2O2 (0.83 μl/ml) in 0.05 M Tris buffer-saline solution. Following FG staining, brain sections were washed with KPBS and exposed to [33P]UTP-labeled CRFR2 cRNA probe overnight in moist chambers at 55°C. Sections were then washed, mounted on gelatin-coated slides, and dipped in NTB emulsion. The slides were exposed for 10 days at 4°C and developed.
Results were examined and captured by a Nikon 80i microscope coupled with a QImaging CCD camera (BioVision, Exton, PA). Images were analyzed and quantified with iVision software (BioVision). VGLUT2-CRFR2 double-labeled cells in the VMH were identified and counted. A VGLUT2-labeled neuron was considered to be double-labeled with CRFR2 if the number of silver grains over the cell was greater than three times the background level.
Statistical analysis.
All values are expressed as means ± SE. Statistical analysis of data was performed using one-way ANOVA, or two-way ANOVA on repeated measures with time and treatment as the factors, followed by Scheffé's F post hoc test. P < 0.05 was accepted as statistically significant.
RESULTS
Effect of Ucn 3 in the VMH on plasma hormones and glucose levels.
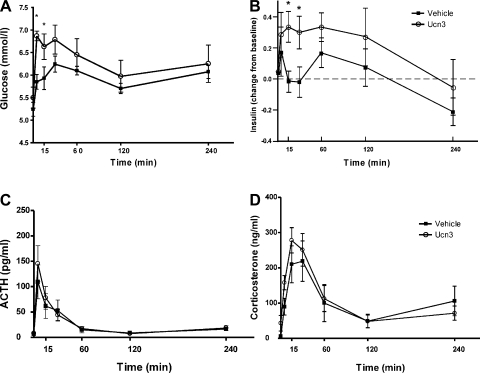
Ucn 3 injection in the VMH significantly elevated blood glucose levels 5 min after injection compared with controls. Glucose levels remained elevated at 30 min after the injection and gradually returned to basal levels (Fig. 2A). Ucn 3 administered in the VMH elicited a small increase in plasma insulin levels 15 min after the injection compared with control animals (Fig. 2B).
Fig. 2.
Effect of Urocortin 3 (Ucn 3) injection into the VMH on plasma levels of glucose (A), insulin (B), ACTH (C), and corticosterone (D). Note that both vehicle and Ucn 3 injection resulted in a small elevation of ACTH and corticosterone levels compared with the baseline levels (time 0). *P < 0.05 vs. vehicle control.
Injection of either vehicle or Ucn 3 in the VMH induced a small increase in plasma ACTH and corticosterone (Fig. 2, C and D) compared with the pretreatment baseline levels, but no significant differences were found between Ucn 3 and vehicle-treated animals.
Effect of Ucn 3 in the VMH on food intake.
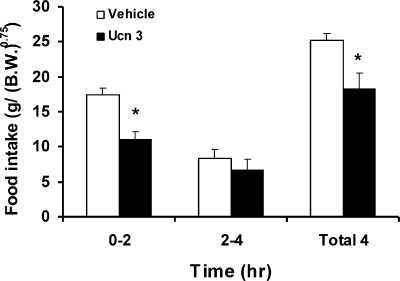
Ucn 3 or vehicle was administered to the VMH bilaterally before night-time feeding. As shown in Fig. 3, rats treated with Ucn 3 ate significantly less compared with vehicle-treated rats. The suppression was most significant in the first 2 h following injection. The later half of the food intake was also attenuated, albeit not statistically significant. Total accumulated food intake during the 4-h postinjection period was significantly less in Ucn 3-treated rats compared with the vehicle controls.
Fig. 3.
Food intake of rats receiving VMH injection of Ucn 3 or vehicle. Ucn 3 or vehicle was injected into free-moving rats 15 min before the lights were turned off, and food intake was monitored for 4 h. Ucn 3-injected rats ate significantly less than vehicle-treated rats in the first 2 h after the injection; total food intake measured during the 4-h postinjection period was also reduced. *P < 0.05 vs. vehicle control.
Effect of Ucn 3 injection in the VMH on POMC expression in the ARH.
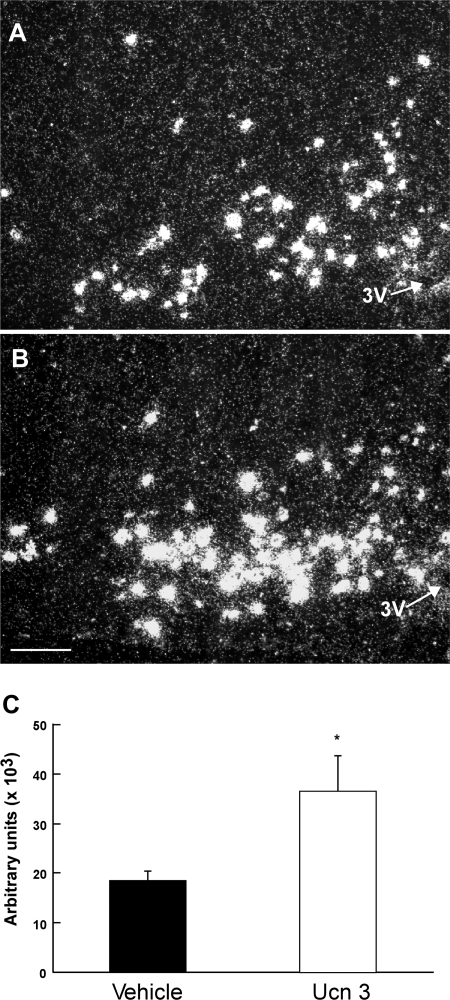
Analysis of POMC mRNA content in the ARH revealed that POMC mRNA levels were significantly elevated in the ARH of rats treated with Ucn 3 compared with the vehicle control (Fig. 4). The increase in POMC mRNA in response to the injection of Ucn 3 in the VMH was observed throughout the ARH.
Fig. 4.
Representative photomicrographs showing proopiomelanocortin (POMC) mRNA signals in vehicle (A)- and Ucn 3 (B)-treated rats in the arcuate nucleus of the hypothalamus (ARH). C: summary of POMC mRNA levels in the ARH in rats injected with either vehicle or Ucn 3 into the VMH. Scale bar = 10 μm. 3V, 3rd ventricle. *P < 0.05. vs. vehicle control.
Colocalization of CRFR2 and VGLUT2 mRNAs in the VMH.
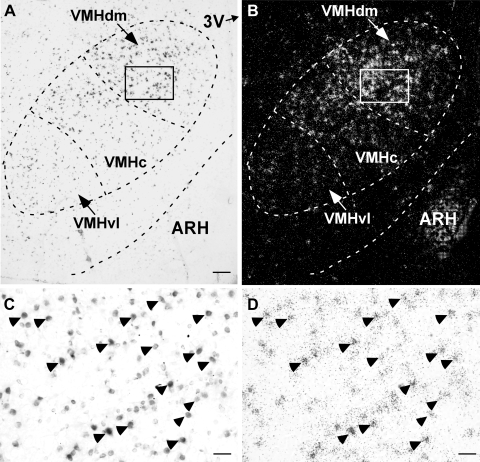
To determine if CRFR2-positive neurons in the VMH are excitatory neurons, double-labeled in situ hybridization was performed to simultaneously visualize mRNAs for CRFR2 and VGLUT2, a marker for glutamatergic cells. As shown in Fig. 5, both VGLUT2 mRNA and CRFR2 mRNA hybridization signals were observed in the VMH. The two materials had a slightly different pattern of distribution within the nucleus. Whereas VGLUT2 mRNA signals were observed throughout the VMH (Fig. 5A), CRFR2 mRNA signals were found mostly in the dorsomedial and central divisions of the VMH, with only a few scattered signals found in the ventrolateral part of the nucleus (Fig. 5B). Semiquantitative analysis showed that nearly all CRFR2 mRNA-positive neurons found in the VMH also expressed VGLUT2 mRNA (Fig. 5, C and D, and Table 1). CRFR2 mRNA-positive cells are clearly a subpopulation of VGLUT2 neurons in the VMH, as many VGLUT2 neurons in the central and ventrolateral divisions of the VMH did not express CRFR2 mRNA and overall 38% of VGLUT2 cells in the VMH expressed CRFR2 mRNA (Table 1).
Fig. 5.
Double-label in situ hybridization showing the colocalization of type 2 corticotropin-releasing factor receptor (CRFR2) and vesicular glutamate transporter isoform-2 (VGLUT2) in the VMH. Representative photomicrographs showing brain sections probed with digoxigenin-labeled VGLUT2 cRNA probe (A) and 33P-labeled CRFR2 cRNA probe (B). C and D: high-magnification brightfield photomicrograph of the boxed area in A and B showing colocalization of VGLUT2 mRNA signals (C, dark cells) and CRFR2 mRNA signals (D, black dot clusters) in the VMH. Representative double-label neurons are indicated by arrowheads. Scale bar = 150 μm (A and B) and 20 μm (C and D). VMHc, ventromedial nucleus of hypothalamus central division; VMHdm, dorsomedial division of VMH; VMHvl, ventrolateral division of VMH.
Table 1.
Average number of VGLUT2-positive and CRFR2-positive and double-labeled cells identified in the VMH and percentage of double-labeled cells with respect to total VGLUT2-positive cells and to total CRFR2-positive cells
| VGLUT2-Positive, cells/section | CRFR2-Positive, cells/section | Double-Label Cells, cells/section | Double-Label Cells in Single VGLUT2 Neurons, % | Double-Label Cells in Single CRFR2 Neurons, % |
|---|---|---|---|---|
| 597.2 ± 48.5 | 257.6 ± 19.1 | 248.2 ± 18.5 | 37.97 ± 2.1 | 90.71 ± 3.59 |
Data are means ± SE. VGLUT2, vesicular glutamate transporter isoform-2; CFRF2, type 2 corticotropin-releasing factor receptor; VMH, ventromedial hypothalamus.
Afferent input to the ARH from CRFR2 neurons in the VMH.
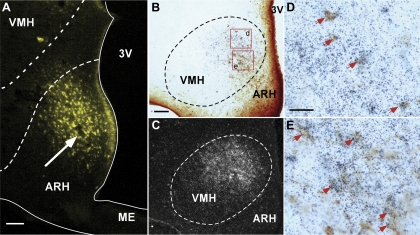
A retrograde tracing experiment was conducted using FG as the tracer to determine if CRFR2 neurons in the VMH project directly to the ARH. Only animals with an FG injection site in the ARH were included for the colocalization study. As shown in Fig. 6A, the unilateral injection of FG was mostly confined to the region of the ARH. Abundant FG-positive neurons were observed in the VMH, especially in the dorsomedial portion of the nucleus (Fig. 6B). A few scattered FG-positive cells were also noted in the ventrolateral division of the VMH (Fig. 6B). Most of the FG-positive neurons in the dorsomedial part of the VMH were also CRFR2 mRNA-positive (Fig. 6, D and E), indicating that CRFR2 neurons in the VMH provide direct afferent input in the ARH. In addition to FG/CRFR2 double-labeled neurons, noticeable single-labeled CRFR2 hybridization signals were also observed in the dorsomedial part of the VMH (Fig. 6, D and E).
Fig. 6.
Afferent input into the ARH from CRFR2 neurons in the VMH. A: representative fluorescence micrograph showing fluorogold (FG) injection site (white arrow) in the ARH. B and C: representative photomicrographs showing FG-positive cells (B, dark brown color cells) and CRFR2 mRNA-positive signals (C, silver grain clusters) in the VMH. D and E: high-power magnification of boxed areas in B showing colocalization of FG immunoreactivity (brown color cells) and CRFR2 mRNA signals (black dot clusters). Representative double-labeled cells are indicated by red arrows. Scale bar = 125 (A), 100 (B), 25 (D) μm. ME, median eminence.
Effect of Ucn 3 injection in the PA.
Bilateral injection of Ucn 3 in the PA did not affect plasma levels of hormones (ACTH, insulin, corticosterone) and glucose, food intake, and POMC gene expression in the ARH compared with vehicle controls (data not shown).
DISCUSSION
Central Ucn 3 injection in the brain has been shown to induce a number of physiological responses, including suppression of appetite, stimulation of the HPA axis, and elevation of blood glucose levels (22, 29, 43). In the present study, site-specific injection was used to investigate possible regions that mediate the effects of Ucn 3 in the brain. Ucn 3 injection into the VMH, but not into the PA, significantly suppressed feeding and rapidly elevated blood glucose levels compared with vehicle controls. Ucn 3 did not alter ACTH or corticosterone levels compared with the controls, regardless of the injection site. These results argue that CRFR2 in the VMH mediates the effect of Ucn 3 on feeding and glucose homeostasis. The effect of Ucn 3 on HPA axis activity may be mediated by other CRFR2-positive brain areas. A number of CRFR2-expressing areas, including the paraventricular nucleus of hypothalamus (PVH), are potential sites for this regulation.
The present study confirms earlier reports (14, 40) that activation of CRFR2 in the VMH suppresses food intake, indicating that CRFR2 in this brain nucleus plays an important role in modulating feeding. Importantly, the hypophagic effect is specific to VMH CRFR2, since stimulation of CRFR2 in other brain areas, including the PA (present study) and the PVH (40), does not modify food intake. Although the exact role of the VMH in regulating feeding has been controversial (26), recent studies have redefined the importance of this brain nucleus in feeding. Mice deficient in steroidogenic factor-1, a transcription factor expressed predominately in the VMH, have abnormal VMH development and are obese (35). Leptin receptor (13), glucokinase (24, 34), and ATP-sensitive K channel (2, 37) are also expressed in the VMH, allowing VMH neurons to sense peripheral metabolic signals. Similar to Ucn 3, injection of leptin directly in VMH significantly suppresses feeding (21). Moreover, gene knockout studies have shown that deletion of leptin receptor in the VMH leads to hyperphagia and obesity (4, 9). The present work not only confirms that the VMH mediates the hypophagic effect of Ucn 3, but also reinforces the notion that the VMH is a critical brain area in regulating feeding and energy balance.
In addition to the modulation of feeding, extensive studies have demonstrated that the VMH plays an important role in glucose sensing and glucose homeostasis (25, 47). Consistent with this notion, Ucn 3 injection into the VMH results in a rapid elevation of plasma glucose levels. This indicates that the VMH is a downstream target of Ucn 3 in regulating blood glucose levels. The rapid hyperglycemic effect of Ucn 3 is likely due, at least in part, to activation of SNS activity, since central injection of Ucn 3 has been shown to elevate plasma epinephrine and noriepinephrine levels (22). In the VMH, two major classes of glucose-sensitive neurons have been identified (24, 25, 34): glucose-excited (GE) neurons increase neuronal activity in response to increased levels of glucose, whereas glucose-inhibited (GI) neurons decrease neuronal activity in response to elevated levels of glucose. It has been suggested that GE neurons are involved in inhibiting, whereas GI neurons are involved in enhancing, sympathetic activity (30). Taken together, it is conceivable that CRFR2 is expressed in GI neurons, and activation of CRFR2 by Ucn 3 stimulates sympathetic system activity and subsequently elevates blood glucose levels. This is supported by a preliminary study in which electrophysiological recordings showed the neuronal activity of CRFR2-positive neurons in the VMH was suppressed by high glucose concentrations (10).
In contrast to the rapid hyperglycemic effect observed in the present study, McCrimmon and coworkers (36) have shown that, under insulin-induced hypoglycemia, injection of urocortin peptides into the VMH blunted hypoglycemia-induced secretion of glucagon and epinephrine and reduced the elevation of blood glucose levels in response to hypoglycemia. This report concludes that, under hypoglycemic conditions, activation of CRFR2 in the VMH has an inhibitory effect on the hypoglycemia-induced counterregulatory response by suppressing secretion of glucagon and epinephrine. It should be noted that their work was conducted under constant hypoglycemia induced by insulin infusion while animals in our study were normglycemic. Moreover, McCrimmon et al. (36) measured the plasma levels of glucagon and epinephrine 60 min after the injection of urocortins into the VMH while we observed rapid elevation of blood glucose levels from 5 to 30 min after Ucn 3 injection into the nucleus. Present and previous studies indicate that VMH CRFR2 signaling is closely involved in the central regulation of glucose homeostasis and its function may be context and time dependent. Clearly, more investigations are needed to resolve this issue.
The present study demonstrates that Ucn 3 injection into the VMH results in a small elevation of plasma insulin levels compared with vehicle controls. Acute electrical or chemical stimulation of VMH neurons in most cases did not significantly modify plasma levels of insulin (8, 48, 50). On the other hand, VMH lesion leads to chronic hyperinsulinemia (44, 45, 54), and vagotomy attenuates the elevation of insulin levels. These studies suggest that VMH neurons do not acutely modulate insulin secretion, but, under chronic conditions, the VMH plays a profound modulatory role in plasma insulin levels through regulation of parasympathetic outflow. Thus it is likely the acute elevation of insulin secretion observed in the present study was a secondary response to Ucn 3-induced hyperglycemia.
The functional role of VMH in regulating the HPA axis is less defined. A lesion study suggested that the VMH is involved in glucocorticoid feedback regulation of basal ACTH secretion (52). In the present study, injection of either vehicle or Ucn 3 in the VMH resulted in a small increase in plasma ACTH and corticosterone compared with pretreatment baseline levels. A similar vehicle effect of the VMH on corticosterone has been reported elsewhere (38). The levels of ACTH and corticosterone induced by both vehicle and Ucn 3 injection are small compared with that induced by central CRF peptides injection or restraint stress (22). These results suggest CRFR2 in the VMH does not play a major role in stimulating HPA axis and corticosterone secretion. It has been shown that microinjection of CRF, Ucn 1, and Ucn 2, but not Ucn 3, into the VMH results in an elevation of plasma corticosterone (36). Both CRF and Ucn 1 bind CRFR1 with high affinity. Ucn 2 at high concentrations has been shown to activate cAMP production via CRFR1 (31). On the other hand, Ucn 3 has minimal affinity for CRFR1 and does not activate adenylate cyclase in cells expressing CRFR1 (31). It is plausible that CRFR1, but not CRFR2, in the VMH is involved in modulating the HPA axis.
CRFR2 is expressed in the PA (6, 55). In the present study, bilateral injection of Ucn 3 in the PA fails to modify any of the parameters measured. Although the amygdala has been suggested to play a role in the regulation of feeding and the HPA axis, our results do not support a role of CRFR2 in this area in regulating feeding, blood glucose levels, and the HPA axis. A recent study has shown that social defeat stress activates amygdaloid neurons that express CRFR2 (15), suggesting CRFR2 neurons in the amygdala may be involved in stress-associated behaviors.
The detailed mechanism and neurocircuit by which VMH neurons regulate feeding and blood glucose levels remains to be determined. A recent study by Sternson and coworkers (51) suggests that anorectic POMC neurons in the ARH are a direct downstream target of the VMH (51). Using a sophisticated electrophotostimulation technique, Sternson et al. (51) showed ARH POMC neurons receive direct excitatory stimulation from neurons in the medial part of the VMH. In contrast, the orexigenic neuropeptide Y neurons in the ARH receive no direct input from the VMH and only weak inhibitory input from within the ARH. The present studies determined that the majority of CRFR2 in the VMH is expressed in excitatory glutamatergic neurons. Using retrograde tracing, we demonstrate CRFR2 neurons in the VMH provide direct afferent input into the ARH. It is noteworthy that FG injection in the ARH labeled approximately half of the CRFR2 neurons in the dorsomedial part of the VMH, suggesting that a subpopulation of CRFR2 neurons in the VMH project to the ARH. Alternatively, the number of CRFR2 neurons labeled by the retrograde tracer may be proportional to the amount deposited in the ARH. In the present study, the tracer was delivered in the ARH by iontophoresis with a small current (2 μA) to limit the spread of FG outside of the nucleus. This might result in partial labeling of neurons in the VMH that project to the ARH.
In the present study, we also show that activation of CRFR2 in the VMH by Ucn 3 results in elevation of POMC mRNA levels compared with vehicle-treated rats. This finding, together with the anatomical data, extends earlier reports by identifying the origin (VMH CRFR2/VGLUT2-positive neurons), ending (ARH POMC neurons), and neurochemical phenotype (glutamatergic) of the VMH excitatory input to ARH. Recently, we identified Ucn 3-positive neurons residing in the posterior part of the BNST and the medial amygdala as major Ucn 3 afferent inputs to the VMH (32). Importantly, Ucn 3 expression in these two areas was suppressed by fasting (22 and Li, unpublished observations). Consistent with this observation, the strength of VMH excitatory input to POMC neurons is diminished by fasting (51). Taken together, these data suggest a novel Ucn 3 neural circuit involving VMH CRFR2 neurons and ARH POMC cells in the regulation of appetite and blood glucose levels.
In conclusion, the present work shows that the VMH is a target of Ucn 3 in modulating food intake and blood glucose levels. Activation of CRFR2 in the VMH is not required for central Ucn 3-induced ACTH and corticosterone secretion. CRFR2 neurons in the VMH are glutamatergic and project to the ARH. Activation of CRFR2 in the VMH by Ucn 3 results in an increase of POMC mRNA levels in the ARH, thus suggesting that ARH POMC neurons are a potential downstream target of CRFR2 neurons in the VMH.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants P01 DK-026741-29 (W. Vale) and R01 DK-078049 (C. Li).
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. This work is partially supported by the Adler Foundation, the Kleberg Foundation, and the Clayton Medical Research Foundation (CMRF), Inc. W. Vale is a CMRF Senior Investigator.
ACKNOWLEDGMENTS
We thank Dr. J. Rivier (Salk Institute, La Jolla, CA) for providing the peptides used in the study. We also thank M. Digruccio for technical assistance.
REFERENCES
- 1.Aravich PF, Beltt BM. Perifornical fiber system mediates VMH electrically-induced suppression of feeding. Physiol Behav 29: 195–200, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci 11: 97–118, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Avrith D, Mogenson GJ. Reversible hyperphagia and obesity following intracerebral microinjections of colchicine into the ventromedial hypothalamus of the rat. Brain Res 153: 99–107, 1978 [DOI] [PubMed] [Google Scholar]
- 4.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 149: 2138–2148, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown FD, Fessler RG, Rachlin JR, Mullan S. Changes in food intake with electrical stimulation of the ventromedial hypothalamus in dogs. J Neurosurg 60: 1253–1257, 1984 [DOI] [PubMed] [Google Scholar]
- 6.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expresssion to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci 15: 6340–6350, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Li C, Haskell-Luevano C, Cone RD, Smith MS. Altered expression of agouti-related protein and its colocalization with neuropeptide Y in the arcuate nucleus of the hypothalamus during lactation. Endocrinology 140: 2645–2650, 1999 [DOI] [PubMed] [Google Scholar]
- 8.de Jong A, Strubbe JH, Steffens AB. Hypothalamic influence on insulin and glucagon release in the rat. Am J Physiol Endocrinol Metab Gastrointest Physiol 233: E380–E388, 1977 [DOI] [PubMed] [Google Scholar]
- 9.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49: 191–203, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Digruccio M, Chen P, Cheng J, Moenter S, Li C. Generation of transgenic mice expressing Cre recombinase in type 2 corticotropin-releasing factor receptor (CRFR2) neurons in the brain. Soc Neurosci Abst: 198.111, 2007 [Google Scholar]
- 11.Dunn JD, Whitener J. Plasma corticosterone responses to electrical stimulation of the amygdaloid complex: cytoarchitectural specificity. Neuroendocrinology 42: 211–217, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Ellacott KL, Cone RD. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog Horm Res 59: 395–408, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395: 535–547, 1998 [PubMed] [Google Scholar]
- 14.Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szucs A, Koob GF, Zorrilla EP. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology 32: 1052–1068, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fekete EM, Zhao Y, Li C, Sabino V, Vale WW, Zorrilla EP. Social defeat stress activates medial amygdala cells that express type 2 corticotropin-releasing factor receptor mRNA. Neuroscience 162: 5–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fremeau RT, Jr, royer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 31: 247–260, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci 21: RC181, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat Rec 78: 149–161, 1940 [Google Scholar]
- 19.Hochgeschwender U, Costa JL, Reed P, Bui S, Brennan MB. Altered glucose homeostasis in proopiomelanocortin-null mouse mutants lacking central and peripheral melanocortin. Endocrinology 144: 5194–5202, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med 7: 605–611, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Jacob RJ, Dziura J, Medwick MB, Leone P, Caprio S, During M, Shulman GI, Sherwin RS. The effect of leptin is enhanced by microinjection into the ventromedial hypothalamus. Diabetes 46: 150–152, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Jamieson PM, Li C, Kukura C, Vaughan J, Vale W. Urocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoids. Endocrinology 147: 4578–4588, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kalra PS, Dube MG, Xu B, Kalra SP. Increased receptor sensitivity to neuropeptide Y in the hypothalamus may underlie transient hyperphagia and body weight gain. Regul Pept 72: 121–130, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55: 412–420, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53: 549–559, 2004 [DOI] [PubMed] [Google Scholar]
- 26.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav 87: 221–244, 2006 [DOI] [PubMed] [Google Scholar]
- 27.King BM, Cook JT, Dallman MF. Hyperinsulinemia in rats with obesity-inducing amygdaloid lesions. Am J Physiol Regul Integr Comp Physiol 271: R1156–R1159, 1996 [DOI] [PubMed] [Google Scholar]
- 28.King BM, Cook JT, Rossiter KN, Rollins BL. Obesity-inducing amygdala lesions: examination of anterograde degeneration and retrograde transport. Am J Physiol Regul Integr Comp Physiol 284: R965–R982, 2003 [DOI] [PubMed] [Google Scholar]
- 29.King BM, Zansler CA, Tatford AC, 3rd, Neville KL, Sam H, Kass JM, Dallman MF. Level of corticosterone replacement determines body weight gain in adrenalectomized rats with VMH lesions. Physiol Behav 54: 1187–1190, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: role in obesity and diabetes. Am J Physiol Regul Integr Comp Physiol 276: R1223–R1231, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA 98: 7570–7575, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Vaughan J, Vale W. Urocortin III afferents into the ventromedial hypothalamaus of the rat. Soc Neurosci Abst 396: 320, 2003 [Google Scholar]
- 33.Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci 22: 991–1001, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch RM, Tompkins LS, Brooks HL, Dunn-Meynell AA, Levin BE. Localization of glucokinase gene expression in the rat brain. Diabetes 49: 693–700, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology 143: 607–614, 2002 [DOI] [PubMed] [Google Scholar]
- 36.McCrimmon RJ, Song Z, Cheng H, McNay EC, Weikart-Yeckel C, Fan X, Routh VH, Sherwin RS. Corticotrophin-releasing factor receptors within the ventromedial hypothalamus regulate hypoglycemia-induced hormonal counterregulation. J Clin Invest 116: 1723–1730, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 4: 507–512, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Narita K, Nishihara M, Takahashi M. Concomitant regulation of running activity and metabolic change by the ventromedial nucleus of the hypothalamus. Brain Res 642: 290–296, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Ohata H, Shibasaki T. Effects of urocortin 2 and 3 on motor activity and food intake in rats. Peptides 25: 1703–1709, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Ohata H, Suzuki K, Oki Y, Shibasaki T. Urocortin in the ventromedial hypothalamic nucleus acts as an inhibitor of feeding behavior in rats. Brain Res 861: 1–7, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449: 228–232, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Paxions G, Watson C. The Rat Brain in Stereotaxic Coordinates San Diego, CA: Academic, 1998 [Google Scholar]
- 43.Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides 25: 659–666, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Penicaud L, Kinebanyan MF, Ferre P, Morin J, Kande J, Smadja C, Marfaing-Jallat P, Picon L. Development of VMH obesity: in vivo insulin secretion and tissue insulin sensitivity. Am J Physiol Endocrinol Metab 257: E255–E260, 1989 [DOI] [PubMed] [Google Scholar]
- 45.Rohner F, Dufour AC, Karakash C, Le Marchand Y, Ruf KB, Jeanrenaud B. Immediate effect of lesion of the ventromedial hypothalamic area upon glucose-induced insulin secretion in anaesthetized rats. Diabetologia 13: 239–242, 1977 [DOI] [PubMed] [Google Scholar]
- 46.Rollins BL, King BM. Amygdala-lesion obesity: what is the role of the various amygdaloid nuclei? Am J Physiol Regul Integr Comp Physiol 279: R1348–R1356, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Routh VH. Glucosensing neurons in the ventromedial hypothalamic nucleus (VMN) and hypoglycemia-associated autonomic failure (HAAF). Diabetes Metab Res Rev 19: 348–356, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Shimazu T, Ishikawa K. Modulation by the hypothalamus of glucagon and insulin secretion in rabbits: studies with electrical and chemical stimulations. Endocrinology 108: 605–611, 1981 [DOI] [PubMed] [Google Scholar]
- 49.Shimizu N, Oomura Y, Plata-Salaman CR, Morimoto M. Hyperphagia and obesity in rats with bilateral ibotenic acid-induced lesions of the ventromedial hypothalamic nucleus. Brain Res 416: 153–156, 1987 [DOI] [PubMed] [Google Scholar]
- 50.Steffens AB. The modulatory effect of the hypothalamus on glucagon and insulin secretion in the rat. Diabetologia Suppl 20: 411–416, 1981 [PubMed] [Google Scholar]
- 51.Sternson SM, Shepherd GMG, Friedman JM. Topographic mapping of VMH –> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci 8: 1356–1363, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Suemaru S, Darlington DN, Akana SF, Cascio CS, Dallman MF. Ventromedial hypothalamic lesions inhibit corticosteroid feedback regulation of basal ACTH during the trough of the circadian rhythm. Neuroendocrinology 61: 453–463, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2). J Neurosci 21: RC182, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tokunaga K, Fukushima M, Kemnitz JW, Bray GA. Effect of vagotomy on serum insulin in rats with paraventricular or ventromedial hypothalamic lesions. Endocrinology 119: 1708–1711, 1986 [DOI] [PubMed] [Google Scholar]
- 55.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Venihaki M, Sakihara S, Subramanian S, Dikkes P, Weninger SC, Liapakis G, Graf T, Majzoub JA. Urocortin III, a brain neuropeptide of the corticotropin-releasing hormone family: modulation by stress and attenuation of some anxiety-like behaviours. J Neuroendocrinol 16: 411–422, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes 53: 1959–1965, 2004. [DOI] [PubMed] [Google Scholar]