Abstract
Glucagon-like peptide-2 (GLP-2) is a neuropeptide secreted from endocrine cells in the gut and neurons in the brain. GLP-2 stimulates intestinal crypt cell proliferation and mucosal blood flow while decreasing gastric emptying and gut motility. However, a GLP-2-mediated signaling network has not been fully established in primary cells. Since the GLP-2 receptor mRNA and protein were highly expressed in the mouse hippocampus, we further characterized that human 125I-labeled GLP-21–33 specifically bound to cultured hippocampal neurons with Kd = 0.48 nM, and GLP-2 acutely induced subcellular translocalization of the early gene c-Fos. Using the whole cell patch clamp, we recorded barium currents (IBa) flowing through voltage-gated Ca2+ channels (VGCC) in those neurons in the presence of GLP-2 with and without inhibitors. We showed that GLP-2 (20 nM) enhanced the whole cell IBa mediated by L-type VGCC that was defined using an L-type Ca2+ channel blocker (nifedipine, 10 μM). Moreover, GLP-2-potentiation of L-type VGCC was abolished in neurons pretreated with a PKA inhibitor (PKI14–22, 1 μM). Finally, using a fluorescent nonmetabolized glucose analog (6-NBDG) tracing imaging, we showed that glucose was taken up directly by cultured neurons. GLP-2 increased 2-deoxy-d-[3H]glucose uptake that was dependent upon dosage, activation of PKA, and potentiation of L-type VGCC. We conclude that GLP-2 potentiates L-type VGCC activity through activating PKA signaling, partially stimulating glucose uptake by primary cultured hippocampal neurons. The potentiation of L-type VGCC may be physiologically relevant to GLP-2-induced neuroendocrine modulation of neurotransmitter release and hormone secretion.
Keywords: glucagon-like peptide-2, glucagon-like peptide-2 receptor, L-type calcium channel, whole cell patch clamp
glucagon-like peptide-2 (GLP-2) is a neuropeptide secreted from endocrine cells in the gut and neurons in the brain. Through a specific G protein-coupled receptor, GLP-2R, GLP-2 stimulates intestinal crypt cell proliferation and mucosal blood flow to promote nutrient absorption while decreasing gastric emptying and gut motility to inhibit food intake (38, 58). The GLP-2R is expressed not only in distinct gastrointestinal cells (such as enteric neurons, enteroendocrine cells, subepithelial myofibroblasts, and pancreatic α-cells) but also in specific regions of the central nervous system (CNS, such as the hippocampus and hypothalamus) (38, 58). Currently, there is much interest in the central effects of GLP-1/2 in the neuroendocrine control of food intake. Besides the hypothalamus, the hippocampus is sensitive to hunger and satiety signals, thus influencing appetitive behavior through inhibitory learning and memory processes (22, 23, 67). Moreover, neurohormonal hypothalamic circuits are integrated for the control of energy homeostasis with high-order hippocampal learning and memory processes (22). However, the GLP-2-mediated cellular action and signaling network are largely unknown in the CNS.
Calcium entry through voltage-gated Ca2+ channels (VGCC) in hippocampal neurons is important for neurotransmitter release, synaptic plasticity, gene transcription, and neuronal survival (3). Increases in intracellular Ca2+ concentrations can activate key kinases or phosphatases, which modulate ion channels, transcription factors, and regulatory proteins that are involved in synaptic plasticity and memory formation (13). For example, Ca2+ acting on the synaptic vesicle protein synaptotagmin I triggers rapid exocytosis of neurotransmitters (15, 16). In addition, Ca2+ entry through L-type VGCC induces long-term potentiation in the hippocampal pyramidal neurons, which is an important form of neuronal plasticity in learning and memory processing (61).
The evidence that GLP-2R is expressed exclusively in these excitable cells suggests that GLP-2 signal transduction may mediate electrical activity of the plasma membrane, thus inducing neurotransmitter release from neurons and hormone secretion from endocrine cells. For example, GLP-2 stimulates glucagon secretion from pancreatic α-cells through unidentified molecular mechanisms (43). Furthermore, GLP-1, coproduced with GLP-2 from neurons or endocrine cells, augments barium currents flowing through L-type VGCC through a cAMP-dependent mechanism in pancreatic β-cells (40, 57). This may play a crucial role in inducing insulin secretion and neurotransmitter release. Moreover, the activity of the L-type VGCC is enhanced in neurons by cAMP-dependent protein kinase A (PKA). It is well established that GLP-2R activation increases intracellular cAMP concentration and enhances PKA-dependent signaling in hippocampal neurons (39). However, it is unknown whether GLP-2 potentiates L-type VGCC activity in hippocampal neurons.
In the mammalian brain, ∼50% of the total energy consumption is associated with neural signaling, including maintenance of resting potentials and neurotransmitter recycling (2, 46). In the brain, glucose is the predominant substrate for energy metabolism. Delivery of glucose from the blood to the brain requires transport across the endothelial cells of the blood-brain barrier and the plasma membranes of neurons and glia. Previous studies show that GLP-2 enhances glucose uptake by enterocytes through promoting cellular translocation of ATP-dependent Na+/glucose cotransporter 1 (17, 25). However, it is unknown whether GLP-2 affects glucose uptake by neurons where ATP-independent, facilitative glucose transporters (such as GLUT3) are expressed. A recent study indicates that Ca2+ influx is an important modulator of insulin-mediated glucose uptake through Ca2+/calmodulin, which acts in GLUT4 translocation to the plasma membrane (35, 62). Therefore, we wanted to determine whether GLP-2 facilitates glucose uptake by enhanced activity of L-type VGCC in neurons.
Our present study shows that GLP-2 potentiates the activation of L-type Ca2+ channels associated with stimulated glucose uptake in primary cultured hippocampal neurons. This L-type Ca2+ channel activation may play an important role in triggering neurotransmitter release and hormone secretion from GLP-2R-positive neurons and endocrine cells, respectively.
METHODS
All experiments were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. The C57BL/6J mice were fed ad libitum and given free access to water.
Localization of GLP-2R.
The expression of glp2r mRNA in the mouse brain was performed by in situ hybridization (36) and quantified by an automated image processing technique (11). The cellular distribution of glp2r mRNA abundance was denoted by a digital false-color map representing the original expression pattern, on which it was painted in yellow, blue, and red, respectively, for the weak, moderate, and strong expressions. The localization of the GLP-2R protein was confirmed by immunohistochemistry and defined on cultured hippocampal neurons by use of immunocytochemistry. Methods in details are provided in Supplementary materials (Supplementary materials are found in the online version of this paper.).
Primary culture of hippocampal neurons.
Neurons were obtained from 1-day-old neonatal mice. The hippocampus was dissected in F-12 medium, digested with L15-NeurobasalA medium containing 0.1% trypsin and 0.1% DNAase at 37°C for 30 min, triturated with a pipette tip, and centrifuged at 1,000 rpm for 5 min. Dissociated cells were seeded onto slides precoated with poly-l-lysine for 30 min and cultured for 5–6 days in NeurobasalA medium supplemented with B27, 10% FBS, 10 ng/ml fibroblast growth factor-β (FGFβ), 0.5 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μg/ml Primocin (Amaxa). Cytosine-β-d-arabinofuranoside (10 μM; Sigma) was added 48 h postplating to inhibit proliferation of glial cells in the primary culture.
Intact cell binding assay.
To determine GLP-2-specific binding, using a modified protocol (8), we incubated primary neurons on DIV 6 in 24-well plates of 0.2 ml each with 0–1.0 nM 125I-labeled human GLP-2 (1–33; Phoenix Pharmaceuticals) with or without 0.5–10 μM unlabeled human GLP-2 (1–33; American Peptide).
Whole cell voltage clamp.
Whole cell voltage clamp recordings were performed in cultured hippocampus neurons (63). Barium currents (IBa) flowing through Ca2+ channels were recorded using an extracellular solution containing 140 mM TEA-Cl, 2 mM MgCl2, 3 mM BaCl2, 10 mM glucose, 10 mM HEPES (pH 7.4 adjusted with TEA-OH, osmolarity 320), and a pipette solution containing 120 mM CsCl, 1 mM MgCl2, 10 mM HEPES, 10 mM EGTA, 4 mM Mg-ATP, and 0.3 mM Na-GTP (pH 7.2 adjusted with CsOH, osmolarity 300 mOsm).
2-deoxy-d-[3H]glucose uptake.
2-Deoxy-d-[3H]glucose ([3H]2-DG) uptake was determined as described (7). Hippocampal neurons on DIV 6 were fasted with serum-free NeurobasalA for 4 h and exposed to GLP-2 at 20 nM for 30 min at 37°C in KRH buffer (125 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 2.6 mM MgSO4, 5 mM HEPES, pH 7.4) containing 500 μM 2-DG and [1,2-3H]2-DG (30–60 Ci/ mmol, MP Biomedicals). When tested, 10 μM nifedipine (Sigma) or 1 μM PKA inhibitor amide 14–22 (PKI, Calbiochem) was added 30 min prior to GLP-2 treatment. Cytochalasin B (50 μM) was added for the background subtraction of nonspecific glucose uptake.
Glucose uptake by 6-NBDG tracing.
Fluorescent nonmetabolized 6-NBDG {6-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-6-deoxyglucose} was used in place of [3H]2-DG (64, 65). Neurons on coverslips were loaded with 300 μM 6-NBDG for 5 (or 15) min, washed out, incubated in KRH buffer for 15 min, washed, and incubated in KRH buffer for 15 min. Labeled living neurons were detected under a fluorescence confocal microscopy.
Statistical analysis.
Data were analyzed by ANOVA (SAS version 9.1; SAS Institute, Cary, NC). Data are expressed as means ± SE. P values < 0.05 or 0.01 were considered statistically significant.
RESULTS
GLP-2R functionally expressed in primary hippocampal neurons.
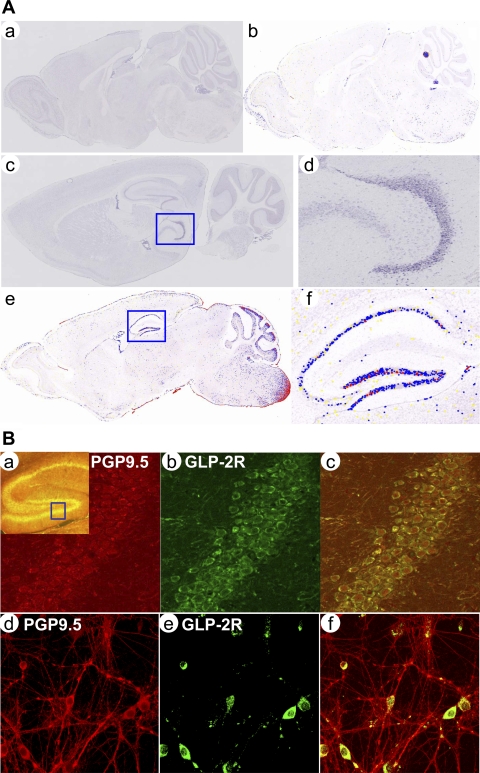
To identify spatial expression patterns of glp2r mRNA in the brain, we performed in situ hybridization at a cellular resolution. We found that the glp2r mRNA was most abundantly expressed in the hippocampus (Fig. 1 A, c–f) compared with the cortex, cerebellum, hypothalamus, and brainstem regions (data not shown), where glp2r mRNA is localized in rat and mouse brain (38, 39, 58). This glp2r-specific expression pattern might indicate that GLP-2R plays a distinct role in the regulation of spatial metabolism and synaptic transmission. By immunohistochemistry, GLP-2R protein was expressed in the hippocampus as well (as indicated in yellow in Fig. 1B, a). At the cellular level, GLP-2R (in green in Fig. 1B, b) was expressed on the surface of cellular membranes and colocalized with neurons [labeled by a neuron-specific protein, i.e., protein gene product 9.5 (PGP9.5), as shown in red in Fig. 1B a and merged in yellow in Fig. 1B, c]. Furthermore, GLP-2R was expressed mainly in neuronal soma (in green in Fig. 1B, e) but not in dendrites in primary cultured of hippocampal neurons.
Fig. 1.
A: glucagon-like peptide-2 receptor (GLP-2R) mRNA expressed in the mouse brain. First, the spatial pattern for glp2r mRNA expression was revealed using a GLP-2R cRNA antisense probe by in situ hybridization in the mouse sagittal section. The positive signal for GLP-2R mRNA in blue was detected in the hippocampus marked in a square (c) and further magnified to cellular resolution (d). Second, levels of glp2r mRNA abundance was quantified by an automated image processing technique and painted in yellow, blue, and red, respectively, for the weak, moderate, and strong expression on a digital false-color map (e and f) representing the original expression pattern. [NB: images a (unpainted) and b (painted) were negative controls for in situ hybridization using a GLP-2R cRNA sense probe.] B: GLP-2R protein expressed in hippocampal neurons. The expression pattern for the GLP-2R protein was confirmed in confocal immmunohistochemistry (a–c) or immunocytochemistry (d–f). GLP-2R protein was stained in green (b and e) and neuronal marker PGP9.5 in red (a and d). GLP-2R protein was expressed in the hippocampus (in yellow) and marked in a square (a) and localized on the plasma membrane of the neuronal body when further magnified in b. Furthermore, GLP-2R protein was expressed in hippocampal neurons cultured on DIV 5 (d–f). [NB: negative controls were performed without primary antibody against GLP-2R in the incubation (images not shown)].
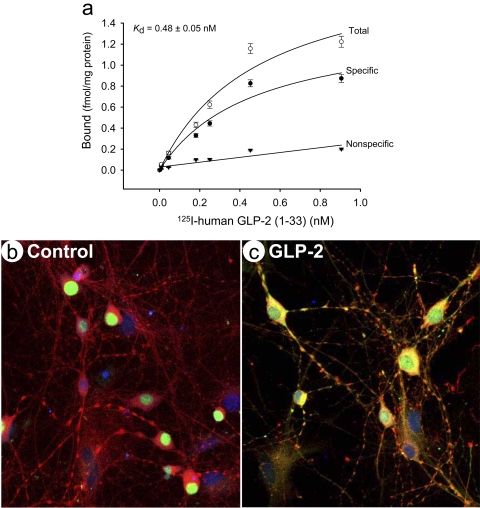
We wanted to characterize further whether the endogenous GLP-2R functionally responded to GLP-2, its native ligand. The intact cell binding assay was used to test the GLP-2 binding curve. Primary hippocampal neurons showed a saturable and specific binding to the 125I-labeled human GLP-2 (Fig. 2a). By regression analysis, the specific binding at the top plateau, i.e., the maximal number of binding sites (Bmax) was 1.42 fmol/mg protein and Kd = 0.48 ± 0.05 nM, similar to a high-affinity binding (0.57 nM) of 125I-Tyr34 human GLP-21-34 in COS cells stably transfected with rat GLP-2R cDNA, which has a maximal binding at 1,839 fmol/mg protein (45). This saturation binding indicated that the affinity of human GLP-2 for mouse endogenous GLP-2R was comparable to that of overexpressed rat GLP-2R. However, the binding site density was lower in the intact primary neurons.
Fig. 2.
GLP-2 induced specific binding and c-Fos translocalization. The function of endogenous GLP-2R was characterized in primary cultured hippocampal neurons. First, GLP-2 specific binding was estimated in an intact-cell binding assay. The fitting curve showed specific and saturable binding with an affinity constant (Kd) of 0.48 ± 0.05 nM 125I-GLP-2 (a). Second, neuronal activation was characterized using expression of an early response gene c-fos by confocal immunocytochemistry, shown in the absence (b) and presence (c) of GLP-2 at 20 nM for 30 min. c-Fos protein was stained in green, neuronal marker PGP9.5 in red, and nuclei DNA in blue with TO-PRO3. NB: c-Fos protein was localized exclusively to neuronal nuclei in a quiescent status (b) but translocalized to the cytoplasm compartment upon GLP-2 stimulation (c).
Next, we wanted to test whether primary neurons acutely responded to GLP-2 treatment. c-fos, an immediate early gene widely used as a functional marker of activated neurons (33), regulates expression of target genes that can influence neuronal excitability and survival in the hippocampus (68). Under basal condition, the c-Fos protein was expressed and localized exclusively to the nucleus in scattered neurons (Fig. 2b). Upon GLP-2 stimulation for 30 min, the c-Fos protein was revealed not only in the nucleus but also in the cytoplasm and dendrites (Fig. 2c), indicating c-Fos protein transient translocation from the nucleus to the cytoplasm (and dendrites) or reverse. Although the c-fos gene can be rapidly induced within 5 min and 2 h (4, 32), c-Fos protein is unstable in the cytoplasm through ubiquitin- and/or proteosome-dependent degradation pathways. This spatiotemporal regulation of c-Fos may be mediated by the GLP-2R-activated cAMP pathway (53, 55), altering neuronal activity in the hippocampus.
GLP-2-enhnaced Ba2+ currents predominately through L-type VGCC potentiation.
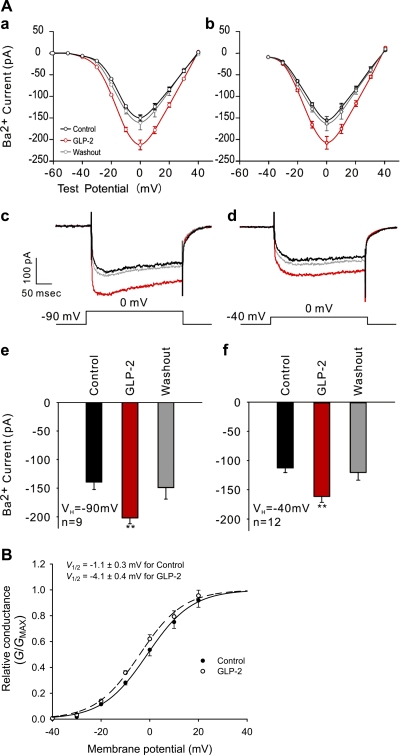
Because Ca2+ influx can directly increase neuronal excitation through synaptic receptors and voltage-gated channels (37), we wanted to examine whether GLP-2 stimulation could modulate activity of VGCC in primary neurons. To minimize the confounding effects of Ca2+ on the kinetics of VGCC, Ba2+ currents (IBa) were recorded flowing through all subtypes of VGCC in primary neurons. Ba2+ currents were recorded through a voltage step protocol (from −90 to 40 mV). The I-V curve showed reverse potentials and peaks (at ∼40 mV) as typical values for Ba2+ currents (Fig. 3 A, a and b). No shift was found in the voltage dependence of Ca2+ channel activation, indicating that IBa was increased throughout the voltage range. Moreover, the peak of the whole cell IBa was also achieved at 0 mV. GLP-2 potentiated the whole cell IBa in cultured hippocampal neurons (Fig. 3A, e, P < 0.01). The peak of IBa was 139 ± 13 pA for neurons under the basal condition, whereas it was 202 ± 10 pA for neurons in the presence of 20 nM GLP-2, indicating that GLP-2 enhanced VGCC activity by 45%. This effect was reversed after washout of GLP-2.
Fig. 3.
A: GLP-2 increased Ba2+ currents mainly attributed to potentiated activity of L-type Ca2+ channels in hippocampal neurons. Holding potentials (VH) at −90 and −40 mV were applied to trace IBa flowing through all types and L-types, respectively, of voltage-gated Ca2+ channels (VGCC) using the whole cell patch clamp. Representative I-V curves are shown using voltage step protocols from −90 to 40 mV in a step of 10 mV (a) and from −40 to 40 mV in a step of 10 mV (b). NB: no shift was observed in the voltage dependence of Ca2+ channel activation. Peak IBa were reached at 0 mV test potential (a–d). Averaged peak IBa (reached at test voltage of 0 mV) are represented for holding potentials of −90 (e) or −40 mV (f). Curves or bars in black, red, and gray represent recording procedures for control, GLP-2 stimulation (20 nM), and washout, respectively. **P < 0.01. B: GLP-2 enhanced activation of L-type Ca2+ channels in hippocampal neurons. The relationship between relative conductance (G/Gmax) and membrane potential (test voltage) was best fitted by Boltmann sigmoid regression. Normalized G-V curves in solid and dotted lines represent recording procedures for control and GLP-2 stimulation (20 nM), respectively.
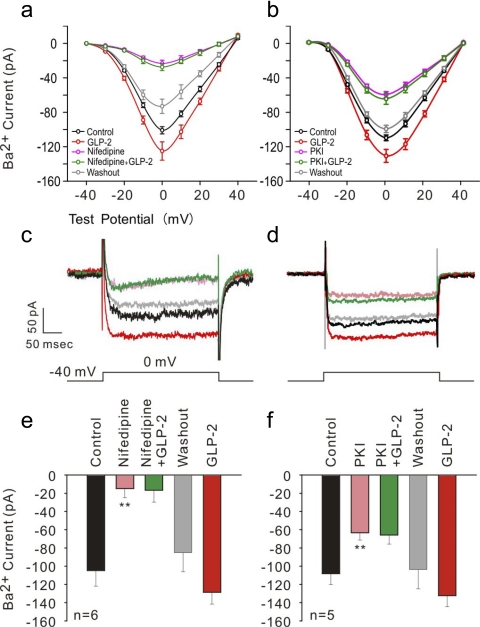
L-type VGCC are predominant VGCC in hippocampal neurons. We further characterized a GLP-2-mediated increase in the peak of Ba2+ inward currents flowing through L-type VGCC by isolating it electrophysiologically (at a holding potential of −40 mV shown in Fig. 3A, b, d, and f) (18, 60); and defined it pharmacologically using an L-type Ca2+ channel blocker, nifedipine (at 10 μM) (Fig. 4, a, c, and e). Approximately 80% of the peak IBa was attributed to GLP-2-mediated enhancement of L-type VGCC activation under the basal condition or under the stimulatory condition, indicating that the peak IBa (reached at 0 mV) evoked at the holding potential of −40 mV was flowing substantially through L-type VGCC. Importantly, ∼85% of peak IBa was inhibited pharmacologically in the presence of nifedipine at 10 μM, representing that recorded IBa was mainly through L-type channels. Moreover, the peak IBa was not increased in GLP-2-treated neurons pretreated with nifedipine. The average peak IBa was at −15 ± 10 pA for nifedipine-treated neurons and −17 ± 13 pA for nifedipine-preloaded, GLP-2-treated neurons. However, after a washout procedure, it increased again (−129 ± 13 pA, P < 0.01) by 51% in the presence of GLP-2 compared with that of −85 ± 11 pA under the washout condition. These data suggest that GLP-2-enhanced whole cell IBa was mediated mainly by potentiating L-type VGCC activity in primary neurons.
Fig. 4.
GLP-2 increased L-type IBa by activating PKA signaling pathway. Holding potential (VH) at −40 mV was applied to trace IBa flowing through L-types of VGCC by whole cell patch clamp. Representative I-V curves are shown using the voltage step protocol from −40 to 40 mV in a step of 10 mV (a and b). NB: no shift was observed in voltage dependence of Ca2+ channel activation. Peak IBa were reached at 0 mV test potential (a–d). Averaged peak IBa (reached at test voltage of 0 mV) are represented for the holding potential of −40 mV (e and f). Curves or bars in black, red, pink, green, and gray represent recording procedures for control, GLP-2 stimulation (20 nM), L-type VGCC blocker nifedipine (10 μM), or PKA inhibitor PKI (1 μM), GLP-2 + nifedipine or PKI, and washout, respectively. **P < 0.01.
GLP-2-enhanced L-type IBa in a PKA-dependent manner.
In the present study, we focused on GLP-2-mediated L-type VGCC activity by recording Ba2+ currents in primary neurons, removing the complication of Ca2+-induced inactivation of L-type VGCC. Therefore, we further analyzed the activation kinetics from the G-V curve (Fig. 3B). The V1/2 was at −1.06 ± 0.32 mV for the controlled neurons and at −4.11 ± 0.38 mV for the GLP-2-treated neurons. This was a shift (P < 0.01) toward a more hyperpolarized activation, indicating that an enhanced activation of L-type VGCC might be attributed to GLP-2-increased inward IBa. Hyperpolarization shift in the G-V curve reflects an improved coupling between gating charge movement and channel opening.
PKA is shown to enhance activation of L-type VGCC by phosphorylating the α1C subunit (of Cav1.2) (20, 24). It has been demonstrated that GLP-2 increases intracellular cAMP accumulation and enhances PKA activity in HEK cells overexpressing GLP-2R. Thus, GLP-2-induced potentiation of L-type VGCC activation might be mediated through activating cAMP-dependent PKA signaling pathways. To assess this possibility, we tested whether GLP-2-mediated potentiation of L-type VGCC activity was attenuated in neurons pretreated with the membrane-permeable PKA inhibitor PKI14–22. We found that GLP-2 treatment did not increase the peak IBa in neurons that were preloaded with PKI 14–22 (at 1 μM) (Fig. 4, b, d, and f). There was no difference in the peak IBa (−64 ± 8 pA for PKI14–22-treated neurons and −66 ± 10 pA for PKI14–22-preloaded, GLP-2-treated neurons). After a washout procedure, however, the peak IBa was increased in the presence of GLP-2 as described above.
GLP-2-mediated stimulation in glucose uptake.
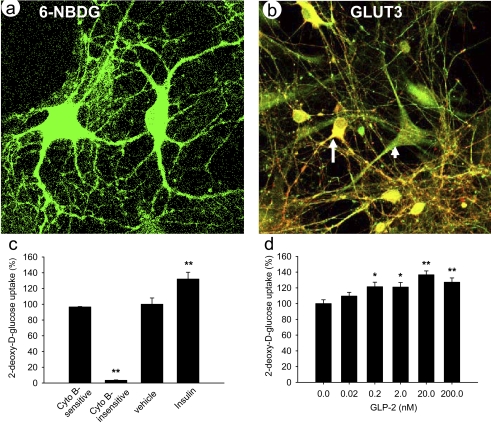
To determine whether hippocampal neurons directly take up glucose, we first used 6-NBDG as a fluorescent, nonhydrolyzable glucose analog to trace glucose uptake and transport (31, 64). Neurons could directly take up and utilize glucose as energy sources in vitro (Fig. 5a), which is in agreement with recent reports (31, 52). Furthermore, we detected the protein expression of the facilitative glucose transporter (GLUT3) mainly responsible for neuronal glucose uptake, which may play a key role in synaptic energy supply and neurotransmitter synthesis (19). GLUT3 (labeled in green in Fig. 5b) was expressed not only in neurons (identified by a neuron-specific marker PGP9.5 in red), but also in glial cells. However, the abundance of GLUT3 protein did not increase in neurons after treatment with GLP-2 at 20 nM for 30 min (data not shown). These data are in agreement with reports that expression of the GLUT3 protein does not acutely increase within 3 h in neurons treated with brain-derived neurotrophic factor (6, 51). However, we cannot rule out the possibility that acute GLP-2-stimulated glucose uptake might be attributed to subcellular translocation of GLUT3.
Fig. 5.
GLP-2 dose-dependently stimulated glucose uptake by hippocampal neurons. a: primary neurons were traced while taking up glucose directly using fluorescent nonmetabolized glucose analog 6-NBDG {6-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-6-deoxyglucose} imaging method. b: protein of GLUT3 (predominant isoform of facilitative GLUTs) was expressed in hippocampal neurons cultured on DIV 5, where neurons were identified by neuronal marker PGP9.5 (in red) and GLUT3 immunostained (in green). c: glucose uptake was quantified by 2-deoxy-d-[3H]glucose ([3H]2-DG) uptake assay. Approximately 96.5% of 2-DG uptake was attributed to cytochalasin B-sensitive, i.e., mainly through facilitative GLUT-mediated uptake. d: glucose uptake was stimulated in a dose-dependent manner by GLP-2 treatment for 30 min and maximized at 20 nM. Experiments were repeated independently 3 times. *P < 0.05 and **P < 0.01.
Moreover, we quantified whether GLP-2 modulates glucose uptake in primary neurons by the [3H]2-DG uptake assay. 2-DG is a glucose analog that is transported specifically by ATP-independent, facilitative glucose transporters, but not by ATP-dependent Na+/glucose cotransporters. In the presence of cytochalasin B (50 μM), a competitive inhibitor of glucose transport through facilitative glucose transporters, 2-DG uptake was at 3.5%. Thus 96.5% of 2-DG uptake was cytochalasin B sensitive, mainly through facilitative glucose transporter- mediated uptake (Fig. 5c). Compared with the baseline, insulin (50 nM) stimulated 2-DG uptake. Insulin regulates glucose uptake by hippocampal neurons by promoting GLUT3 translocation in the presence of 40 mM KCl (59). 2-DG uptake by primary neurons increased in a dose-dependent manner with increasing concentrations of GLP-2 (Fig. 5d) and reached a plateau at 20 nM.
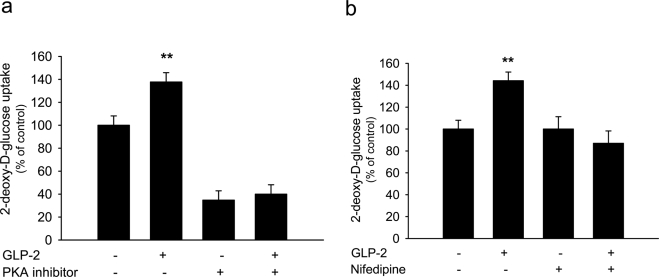
We further characterized cellular mechanisms by which GLP-2 stimulated glucose uptake in primary neurons. Previous studies showed that GLP-2 activates cAMP-dependent PKA signaling to promote neuronal survival. We wanted to examine whether GLP-2-mediated stimulation of 2-DG uptake was mediated partially through the activation of cAMP-dependent PKA. Although primary neurons were preloaded with PKI14–22 (1 μM) 30 min prior to GLP-2 treatment, GLP-2-mediated stimulation in 2-DG uptake was abolished completely by PKI14–21 (Fig. 6a). To examine whether GLP-2-stimulated uptake of glucose was associated with the potentiation of L-type VGCC activity, hippocampal neurons were treated with GLP-2 in the presence of nifedipine (10 μM). GLP-2-mediated stimulation on 2-DG uptake was completely blocked by nifedipine (Fig. 6b). Moreover, the basal uptake of glucose was suppressed by the PKA inhibitor but not by the L-type VGCC blocker. (Note that the abundance of GLUT3 protein was not increased in neurons treated with GLP-2 at 20 nM for 30-min. Thus, the abundance of GLUT3 protein might not be attributed to GLP-2-acutely mediated stimulation of glucose uptake.) Together, these data indicate that GLP-mediated stimulation of glucose uptake might involve the potentitaion of L-type VGCC activity.
Fig. 6.
GLP-2-stimulated glucose uptake was mediated by activating PKA signaling pathway (a) and associated with potentiated activity of L-type Ca2+ channels (b). Glucose uptake was determined in hippocampal neurons cultured on DIV 6 using a 2-DG uptake assay. GLP-2 (at 20 nM) mediated stimulation of glucose uptake, but this stimulation was abolished in the presence of PKA inhibitor PKI (1 μM) or L-type VGCC blocker nifedipine (10 μM). (NB: basal uptake of glucose was suppressed by PKI but not by nifedipine.) Experiments were repeated independently 3 times. **P < 0.01.
DISCUSSION
In the present study, we characterized functionally the primary culture model of mouse hippocampal neurons that expressed endogenous GLP-2R, and we showed that GLP-2 increased Ba2+ currents through potentiated activation of L-type Ca+ channels by using the whole cell voltage clamp. This potentiation was mediated by activating the cAMP-dependent PKA signaling pathway. Moreover, GLP-2 stimulated glucose uptake by primary neurons, and this stimulation was not only dependent upon activation of PKA, but also associated with potentiation of L-type Ca+ channels. We speculate that GLP-2-mediated potentiation of L-type Ca+ channel activity may play an important role in Ca2+-triggered neurotransmitter release and hormone secretion from GLP-2R-positive neurons and endocrine cells, respectively. In addition, GLP-2-mediated potentiation of L-type VGCC activity would induce long-term potentiation in the hippocampal pyramidal neurons, which may play a critical role in coupling cellular signal transduction to learning and memory (61) or neuroprotection (39).
Characterization of endogenous GLP-2R in primary neurons.
GLP-2R is expressed in endocrine cells, neurons, and myofibroblasts. GLP-2R function and signaling have been studied mainly in cell lines overexpressing the receptor, and functional expression of endogenous GLP-2R has not been characterized in primary cells. We found that GLP-2R was expressed not only in primary hippocampal neurons but was also specifically and functionally responsible for GLP-2-mediated stimulation. We were the first to characterize binding properties of endogenous GLP-2R and showed a saturated curve with the binding affinity (Kd) similar to that in GLP-2R-overexpressed membrane preparation. Moreover, subcellular translocalization of the early gene c-fos might reflect an acute GLP-2-mediated action. Therefore, this characterization of endogenous GLP-2R would validate the usefulness of working with primary cultures of hippocampal neurons in further studies of GLP-2 function and signaling.
GLP-2 potentiates activation of L-type VGCC.
One of the major findings in the present study is that GLP-2 increased the peak IBa (by ∼50%) flowing through VGCC, which was attributed to the potentiation of L-type VGCC activation in primary hippocampal neurons. Potentiation of the L-type subtype was concluded from the following data. 1) L-type Ca2+ channels are predominantly and functionally expressed in the somatodendritic compartment of hippocampal neurons cultured for ∼5 days (48). Moreover, L-type Ca2+ channels are functionally present in CA1 pyramidal neurons, contributing to spinal Ca2+ influx (29). 2) L-type currents in neurons are attributed mainly to the activation of Cav1.2 and Cav1.3, which are evoked within −40 to 0 mV (14). Barium currents were recorded at a holding potential of −40 mV in voltage step protocols in the present study. 3) GLP-2-mediated potentiation was completely blocked by the L-type Ca2+ channel blocker nifedipine, providing evidence for GLP-2-mediated increases in IBa flowing through L-type VGCC in hippocampal neurons. L-type Ca2+ channels (including Cav1.2 and Cav1.3 in endocrine cells and neurons) serve as key transducers of plasma membrane potential changes into local intracellular Ca2+ transients. This L-type Ca2+ current initiates distinct signaling pathways in regulation of hormone secretion, neurotransmitter release, synaptic plasticity, and gene transcription (9, 13, 14, 27). For example, potentiation of L-type VGCC may increase Ca2+ influx into hippocampal neurons, which is associated with late-phase, long-term potentiation underlying learning and memory (28, 44).
GLP-2-mediated potentiation of L-type VGCC activity through activating PKA signaling.
GLP-2 increased IBa flowing through L-type VGCC as a result of enhanced activation of those channels indicated by a significant leftward shift in the G-V curve. Furthermore, GLP-2-mediated potentiation of L-type VGCC activity was blocked by a PKA inhibitor, suggesting that GLP-2-mediated potentiation was dependent on activation of the PKA signaling. GLP-2 increases intracellular cAMP production by activating GLP-2R-coupled Gs protein. The cAMP-dependent PKA regulates a wide array of cellular functions, including modulating the activity of the L-type VGCC in hippocampal neurons (20, 21, 29). L-type VGCC is composed minimally of pore-forming α1-subunits along with accessory β- and α2β-subunits. L-type Ca2+ channel (Cav1.2) is associated with the G protein-coupled receptor in hippocampal neurons (20). Importantly, the activity of Cav1.2 can be enhanced in hippocampal neurons by β-adrenergic ligands, which is mediated by PKA-dependent phosphorylation of the α1-subunit (at Ser1928) (20, 21, 24, 26, 29, 47). Thus, we speculate that GLP-2 modulates α1-subunit phosphorylation and facilitates L-type VGCC activation, causing a left shift in the conductance-voltage relationship and potentiating peak IBa at hyperpolarized membrane potentials. However, we cannot exclude the possibility that suppression of the inactivation process of the L-type Ca2+ channel is involved in the GLP-2-mediated increase in Ba2+ currents.
Glucose uptake in hippocampal neurons.
In the present study, we have shown that glucose can be taken up directly by primary neurons, as indicated by the intracellular accumulation of 6-NBDG. In addition, the GLUT3 protein was highly expressed in primary neurons transporting glucose across the plasma membrane. It has been hypothesized that neurons obtain their energy source from glial lactate, especially during neuronal activation (56). However, this concept is challenged by a recent model that indicates that the neuron is the primary site of glucose uptake and utilization under both steady-state and stimulated conditions (56). Neuronal uptake of glucose is mediated by facilitative glucose transporters such as GLUT3 (19, 41). Insulin promotes GLUT3 translocation to the plasma membrane, enabling neurons to respond to energy demand induced by increased neuronal activity (59). However, glucose transport (via GLUT3) is largely independent of insulin in the CNS (42). In neurons, phosphorylated CREB (cAMP regulatory element-binding) can bind the glut3 gene, trans-activating its expression (49). However, we could not find any increase in the abundance of GLUT3 protein or subcellular translocation of GLUT3 protein in hippocampal neurons treated with GLP-2 for 30 min. We cannot exclude the involvement of other isoforms (e.g., GLUT1, -4, and -8) in GLP-2-mediated stimulation of glucose uptake. We speculate that insulin-stimulated glucose uptake in the present study might be attributed to insulin-responsive GLUT4 and -8 that are also expressed in hippocampal neurons (1, 5, 10, 50, 54). The novel role of GLP-2-stimulated glucose uptake in neurons might be important, since cellular glucose uptake is predominantly insulin independent in the brain. Further studies are warranted to confirm which isoforms of facilitative glucose transporters in neurons are specifically regulated by GLP-2.
Another major finding in the present study is that GLP-2-mediated stimulation of glucose uptake was involved with the potentiation of L-type VGCC activation in primary neurons. Previous studies suggest that Ca2+ influx through L-type VGCC is involved in insulin-stimulated glucose transport in skeletal muscle (66). Thus, we hypothesized that GLP-2-mediated enhancement of Ca2+ channel activity would alter glucose uptake in neurons. Interestingly, GLP-2-mediated stimulation of glucose uptake was completely blocked in the presence of nifedipine, an L-type Ca2+ channel inhibitor, suggesting that this metabolic action is mediated partially by enhanced L-type Ca2+ channel activity. Recent studies show that increased influx of Ca2+ across the plasma membrane is associated with insulin-mediated increase in glucose uptake (34, 35), possibly through facilitating docking, fusion, and/or insertion of GLUT4 in the membrane via Ca2+ sensor (such as synaptotagmin I) in the neurons (34, 35, 62) or through modulating calcium-calmodulin-dependent kinase II signaling (30). However, nifedipine did not affect the basal level of 2-DG uptake. This is in agreement with studies that a solitary decrease in Ca2+ influx is not enough to alter basal glucose uptake (12, 35, 62).
The novel function of GLP-2-mediated potentiation of L-type Ca+ channel activity might be physiologically relevant to its neuroendocrine modulation of neurotransmitter release and hormone secretion. As mentioned previously, it is assumed that GLP-2 executes cellular actions and metabolic effects mainly through secondary mediators such as neurotransmitters (e.g., nitric oxide, serotonin, and vasoactive intestinal peptide) and hormones (e.g., glucagon, IGF-I, and KGF). GLP-2-mediated enhancement of L-type Ca+ channel activity would increase cellular Ca2+ influx, resulting in Ca2+-triggered release of neurotransmitters and secretion of hormones. Moreover, GLP-2-mediated potentiation of L-type Ca+ channel activity would induce long-term potentiation, which is highly relevant to key functions (e.g., learning and memory) of the hippocampus. Finally, GLP-2-stimulated uptake of glucose might be equally relevant to energetic demand for neuronal activities and regulation of glucose metabolism in the brain. This regulatory process might be physiologically important, because uptake and utilization of glucose in the brain is insulin independent. However, further studies are warranted to define physiological significance of GLP-2-mediated neuronal action and signaling by using neuron-specific GLP-2R deficiency mouse models.
In summary, we showed that GLP-2-mediated potentiation of L-type Ca2+ channel activity was mediated through the activation of PKA. GLP-2-mediated stimulation of glucose uptake was not only dependent upon activation of PKA, but also coupled with potentiated activation of L-type Ca2+ channels. GLP-2-potentiated activation of L-type Ca2+ channels may be physiologically relevant to neurotransmitter release, synaptic plasticity, and energetic demand for memory formation in the hippocampus. However, further studies are warranted to establish whether GLP-2-potentiated activation of L-type Ca2+ channels initiates Ca2+-triggered neurotransmitter release and hormone secretion from GLP-2R-positive neurons and endocrine cells and to define whether GLP-2R activation modulates phosphorylation of α1-subunits of L-type VGCC in those cells.
GRANTS
This work is supported by the U. S. Department of Agriculture, Agricultural Research Service (USDA/ARS) under Cooperative Agreement No. 6250-51000-043, National Institute of Diabetes and Digestive and Kidney Diseases Grant K01 DK-75489, and the National Natural Science Foundation of China (NSFC) 30728016 (X. Guan). This work is a publication of the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children's Hospital, Houston, TX. The contents of this publication do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
DISCLOSURES
No conflicts of interest are reported by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Douglas Burrin, Lawrence Chan, and Aijun Zhang at Baylor College of Medicine for scientific and technical support.
REFERENCES
- 1.Apelt J, Mehlhorn G, Schliebs R. Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J Neurosci Res 57: 693–705, 1999 [PubMed] [Google Scholar]
- 2.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21: 1133–1145, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 260: 181–186, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Basbous J, Jariel-Encontre I, Gomard T, Bossis G, Piechaczyk M. Ubiquitin-independent- versus ubiquitin-dependent proteasomal degradation of the c-Fos and Fra-1 transcription factors: is there a unique answer? Biochimie 90: 296–305, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Benomar Y, Naour N, Aubourg A, Bailleux V, Gertler A, Djiane J, Guerre-Millo M, Taouis M. Insulin and leptin induce Glut4 plasma membrane translocation and glucose uptake in a human neuronal cell line by a phosphatidylinositol 3-kinase-dependent mechanism. Endocrinology 147: 2550–2556, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Burkhalter J, Fiumelli H, Allaman I, Chatton JY, Martin JL. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J Neurosci 23: 8212–8220, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkhalter J, Fiumelli H, Allaman I, Chatton JY, Martin JL. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J Neurosci 23: 8212–8220, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bylund DB, Deupree JD, Toews ML. Radioligand-binding methods for membrane preparations and intact cells. Methods Mol Biol 259: 1–28, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Calin-Jageman I, Lee A. Ca(v)1 L-type Ca2+ channel signaling complexes in neurons. J Neurochem 105: 573–583, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, Devaskar SU, Moley KH. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci USA 97: 7313–7318, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carson JP, Eichele G, Chiu W. A method for automated detection of gene expression required for the establishment of a digital transcriptome-wide gene expression atlas. J Microsc 217: 275–281, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Cartee GD, Briggs-Tung C, Holloszy JO. Diverse effects of calcium channel blockers on skeletal muscle glucose transport. Am J Physiol Regul Integr Comp Physiol 263: R70–R75, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron 59: 882–901, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57: 411–425, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Chapman ER. Synaptotagmin: a Ca(2+) sensor that triggers exocytosis? Nat Rev Mol Cell Biol 3: 498–508, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem 77: 615–641, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Cheeseman CI. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am J Physiol Regul Integr Comp Physiol 273: R1965–R1971, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Chernevskaya NI, Obukhov AG, Krishtal OA. NMDA receptor agonists selectively block N-type calcium channels in hippocampal neurons. Nature 349: 418–420, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Choeiri C, Staines W, Messier C. Immunohistochemical localization and quantification of glucose transporters in the mouse brain. Neuroscience 111: 19–34, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1. 2 Science 293: 98–101, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Davare MA, Dong F, Rubin CS, Hell JW. The A-kinase anchor protein MAP2B and cAMP-dependent protein kinase are associated with class C L-type calcium channels in neurons. J Biol Chem 274: 30280–30287, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus 19: 235–252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav 86: 731–746, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Gao T, Yatani A, Dell'Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron 19: 185–196, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Guan X, Stoll B, Lu X, Tappenden KA, Holst JJ, Hartmann B, Burrin DG. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology 125: 136–147, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, McKnight GS, Hell JW. Critical role of cAMP-dependent protein kinase anchoring to the L-type calcium channel Cav1.2 via A-kinase anchor protein 150 in neurons. Biochemistry 46: 1635–1646, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Hell JW, Yokoyama CT, Breeze LJ, Chavkin C, Catterall WA. Phosphorylation of presynaptic and postsynaptic calcium channels by cAMP-dependent protein kinase in hippocampal neurons. EMBO J 14: 3036–3044, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helton TD, Xu W, Lipscombe D. Neuronal L-type calcium channels open quickly and are inhibited slowly. J Neurosci 25: 10247–10251, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoogland TM, Saggau P. Facilitation of L-type Ca2+ channels in dendritic spines by activation of beta2 adrenergic receptors. J Neurosci 24: 8416–8427, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Illario M, Monaco S, Cavallo AL, Esposito I, Formisano P, D'Andrea L, Cipolletta E, Trimarco B, Fenzi G, Rossi G, Vitale M. Calcium-calmodulin-dependent kinase II (CaMKII) mediates insulin-stimulated proliferation and glucose uptake. Cell Signal 21: 786–792, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Itoh Y, Abe T, Takaoka R, Tanahashi N. Fluorometric determination of glucose utilization in neurons in vitro and in vivo. J Cereb Blood Flow Metab 24: 993–1003, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Kang DK, Kim KO, Lee SH, Lee YS, Son H. c-Fos expression by dopaminergic receptor activation in rat hippocampal neurons. Mol Cell 10: 546–551, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Kovacs KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol 20: 665–672, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Lanner JT, Bruton JD, Katz A, Westerblad H. Ca(2+) and insulin-mediated glucose uptake. Curr Opin Pharmacol 8: 339–345, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Lanner JT, Katz A, Tavi P, Sandstrom ME, Zhang SJ, Wretman C, James S, Fauconnier J, Lannergren J, Bruton JD, Westerblad H. The role of Ca2+ influx for insulin-mediated glucose uptake in skeletal muscle. Diabetes 55: 2077–2083, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Limback-Stokin K, Korzus E, Nagaoka-Yasuda R, Mayford M. Nuclear calcium/calmodulin regulates memory consolidation. J Neurosci 24: 10858–10867, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lovshin J, Estall J, Yusta B, Brown TJ, Drucker DJ. Glucagon-like peptide (GLP)-2 action in the murine central nervous system is enhanced by elimination of GLP-1 receptor signaling. J Biol Chem 276: 21489–21499, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Lovshin JA, Huang Q, Seaberg R, Brubaker PL, Drucker DJ. Extrahypothalamic expression of the glucagon-like peptide-2 receptor is coupled to reduction of glutamate-induced cell death in cultured hippocampal cells. Endocrinology 145: 3495–3506, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Ma X, Bruning J, Ashcroft FM. Glucagon-like peptide 1 stimulates hypothalamic proopiomelanocortin neurons. J Neurosci 27: 7125–7129, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Maher F, Simpson IA. The GLUT3 glucose transporter is the predominant isoform in primary cultured neurons: assessment by biosynthetic and photoaffinity labelling. Biochem J 301: 379–384, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maher F, vies-Hill TM, Simpson IA. Substrate specificity and kinetic parameters of GLUT3 in rat cerebellar granule neurons. Biochem J 315: 827–831, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier JJ, Nauck MA, Pott A, Heinze K, Goetze O, Bulut K, Schmidt WE, Gallwitz B, Holst JJ. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology 130: 44–54, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, Stiess M, Marais E, Schulla V, Lacinova L, Goebbels S, Nave KA, Storm DR, Hofmann F, Kleppisch T. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci 25: 9883–9892, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboj RK, Chen H, McCallum K, Sumner- Smith M, Drucker DJ, Crivici A. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA 96: 1569–1573, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nehlig A, Coles JA. Cellular pathways of energy metabolism in the brain: is glucose used by neurons or astrocytes? Glia 55: 1238–1250, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron 55: 261–275, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pravettoni E, Bacci A, Coco S, Forbicini P, Matteoli M, Verderio C. Different localizations and functions of L-type and N-type calcium channels during development of hippocampal neurons. Dev Biol 227: 581–594, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Rajakumar A, Thamotharan S, Raychaudhuri N, Menon RK, Devaskar SU. Trans-activators regulating neuronal glucose transporter isoform-3 gene expression in mammalian neurons. J Biol Chem 279: 26768–26779, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Reagan LP, Rosell DR, Alves SE, Hoskin EK, McCall AL, Charron MJ, McEwen BS. GLUT8 glucose transporter is localized to excitatory and inhibitory neurons in the rat hippocampus. Brain Res 932: 129–134, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322: 1551–1555, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322: 1551–1555, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Roux P, Blanchard JM, Fernandez A, Lamb N, Jeanteur P, Piechaczyk M. Nuclear localization of c-Fos, but not v-Fos proteins, is controlled by extracellular signals. Cell 63: 341–351, 1990 [DOI] [PubMed] [Google Scholar]
- 54.Sankar R, Thamotharan S, Shin D, Moley KH, Devaskar SU. Insulin-responsive glucose transporters-GLUT8 and GLUT4 are expressed in the developing mammalian brain. Brain Res Mol Brain Res 107: 157–165, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Sasaki T, Kojima H, Kishimoto R, Ikeda A, Kunimoto H, Nakajima K. Spatiotemporal regulation of c-Fos by ERK5 and the E3 ubiquitin ligase UBR1, and its biological role. Mol Cell 24: 63–75, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27: 1766–1791, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suga S, Kanno T, Nakano K, Takeo T, Dobashi Y, Wakui M. GLP-I(7–36) amide augments Ba2+ current through L-type Ca2+ channel of rat pancreatic beta-cell in a cAMP-dependent manner. Diabetes 46: 1755–1760, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Tang-Christensen M, Larsen PJ, Thulesen J, Romer J, Vrang N. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat Med 6: 802–807, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Uemura E, Greenlee HW. Insulin regulates neuronal glucose uptake by promoting translocation of glucose transporter GLUT3. Exp Neurol 198: 48–53, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Zheng H, Liu C, Zhu C, Wang W, Li Z. Ciliary neurotrophic factor-treated astrocyte conditioned medium regulates the L-type calcium channel activity in rat cortical neurons. Neurochem Res 33: 826–832, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Westenbroek RE, Ahlijanian MK, Catterall WA. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature 347: 281–284, 1990 [DOI] [PubMed] [Google Scholar]
- 62.Whitehead JP, Molero JC, Clark S, Martin S, Meneilly G, James DE. The role of Ca2+ in insulin-stimulated glucose transport in 3T3-L1 cells. J Biol Chem 276: 27816–27824, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Wu ZZ, Pan HL. High voltage-activated Ca(2+) channel currents in isolectin B(4)-positive and -negative small dorsal root ganglion neurons of rats. Neurosci Lett 368: 96–101, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Yamada K, Nakata M, Horimoto N, Saito M, Matsuoka H, Inagaki N. Measurement of glucose uptake and intracellular calcium concentration in single, living pancreatic beta-cells. J Biol Chem 275: 22278–22283, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Yamada K, Saito M, Matsuoka H, Inagaki N. A real-time method of imaging glucose uptake in single, living mammalian cells. Nat Protoc 2: 753–762, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Young JC, Balon TW. Role of dihydropyridine sensitive calcium channels in glucose transport in skeletal muscle. Life Sci 61: 335–342, 1997 [DOI] [PubMed] [Google Scholar]
- 67.Yu Y, Wang Q, Huang XF. Energy-restricted pair-feeding normalizes low levels of brain-derived neurotrophic factor/tyrosine kinase B mRNA expression in the hippocampus, but not ventromedial hypothalamic nucleus, in diet-induced obese mice. Neuroscience 160: 295–306, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Zhang J, Zhang D, McQuade JS, Behbehani M, Tsien JZ, Xu M. c-fos regulates neuronal excitability and survival. Nat Genet 30: 416–420, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.