Abstract
Impaired estrogen action is associated with the metabolic syndrome in humans. We sought to determine whether impaired estrogen action in female C57Bl6 mice, produced by whole body Esr1 ablation, could recapitulate aspects of this syndrome, including inflammation, insulin resistance, and obesity. Indeed, we found that global knockout (KO) of the estrogen receptor (ER)α leads to reduced oxygen uptake and caloric expenditure compared with wild-type (WT) mice. In addition, fasting insulin, leptin, and PAI-1 levels were markedly elevated, whereas adiponectin levels were reduced in normal chow-fed KO. Furthermore, ERα-KO mice exhibited impaired glucose tolerance and marked skeletal muscle insulin resistance that was accompanied by the accumulation of bioactive lipid intermediates, inflammation, and diminished PPARα, PPARδ, and UCP2 transcript levels. Although the relative glucose intolerance and insulin resistance phenotype in KO mice became more severe with high-fat feeding, WT mice were refractory to these dietary-induced effects, and this protection coincided with a marked increase in circulating adiponectin and heat shock protein 72 levels in muscle, liver, and fat. These data indicate that ERα is critical for the maintenance of whole body insulin action and protection against tissue inflammation during both normal chow and high-fat feeding.
Keywords: estrogen receptor-α, estrogen action, fatty acid metabolism, insulin action
insulin resistance is a central factor in the pathogenesis of type 2 diabetes and a defining feature of the metabolic syndrome, a constellation of abnormalities that includes obesity, hypertension, glucose intolerance, and dyslipidemia (21, 50). Prior to menopause, the incidence of type 2 diabetes is lower in women compared with men (50, 57). However, following menopause or ovariectomy this protection is lost, and a precipitous decline in insulin sensitivity coincides with increased fat mass and elevated circulating inflammatory markers [TNFα, IL-6, and plasminogen activator inhibitor-1 (PAI-1)], LDL, triglycerides, and fatty acids (13, 58, 66). Similarly, alterations in estrogen receptor (ER) expression in both sexes have been linked with increased prevalence of certain aspects of the metabolic syndrome (22, 53–54, 67). We hypothesize that ERα is important in the regulation of tissue substrate metabolism and inflammatory signaling and thus is critical in modulating insulin action and adiposity.
It is now widely accepted that impaired fatty acid metabolism and/or fatty acid oversupply cause heightened inflammatory signaling, and these are central contributors to whole body insulin resistance (3, 41, 84). Accumulation of lipid intermediates in insulin-responsive tissues can activate a host of “stress” kinases, and several of these have been shown to phosphorylate insulin receptor substrate (IRS)-1 on serine residues, leading to impaired insulin signaling and diminished glucose transport (14, 42, 79). Several lines of investigation in humans and rodents clearly show that inactivation of these stress kinases by pharmacological or genetic means leads to improved insulin action and reversal of diabetic complications (3, 41, 84). Similarly, 17β-estradiol supplementation has been shown to diminish inflammatory signaling (26–27, 34, 72) and improve insulin action (10, 46), but the link between these two pathways and whole body substrate metabolism is poorly defined.
Two forms of the ER have been identified, ERα (77) and ERβ (25), and each are encoded by separate genes, ESR1 and ESR2, respectively. Both receptors are expressed in a variety of cell types; however, the α-isoform is more highly expressed than the β-isoform in insulin-responsive tissues (20, 23, 25, 36, 77). Previous reports suggest that ERα is highly involved in estrogen-mediated regulation of substrate metabolism, since diminished ERα action has been shown to cause increased adiposity in humans and mice of both sexes (35, 67). This notion has recently been supported by work from Nilsson et al. (53) showing that ERα expression is diminished in adipose tissue from obese compared with lean subjects. Taken together, there is strong clinical evidence for a relationship between ERα expression levels and the incidence of the metabolic syndrome. These observations provide the clinical rationale for the current investigation, since little is known about the mechanisms causing reduced ERα expression levels in obese subjects or the specific tissues conferring ERα-mediated effects on metabolism, inflammation, and insulin sensitivity.
In the current investigation, we tested the hypothesis that whole body ablation of Esr1 can recapitulate aspects of the human metabolic syndrome, including impaired oxidative metabolism, glucose intolerance, tissue inflammation, obesity, and insulin resistance. In addition, we hypothesized that female mice lacking ERα would be more susceptible to high-fat (HF) diet-induced obesity and insulin resistance compared with ERα-replete animals. Herein, we show that loss of ERα leads to reduced oxygen uptake, tissue lipid accumulation, inflammation, impaired glucose tolerance, and insulin resistance. Furthermore, we find that ERα is essential in guarding against the adverse effects of high-fat diet in female mice, and this protection appears mediated in part by heightened adipogenic capacity, elevated circulating adiponectin, and increased abundance of a protective cellular chaperone protein, heat shock protein (HSP)72.
EXPERIMENTAL PROCEDURES
Animals.
Female wild-type (WT; n = 30) and ERα-knockout (KO; n = 30) mice were purchased at 8 wk of age from Jackson Laboratories (18). All procedures were performed in accordance with the Guide for Care and Use of Laboratory Animals of the National Institutes of Health (NIH) and were approved by the Animal Subjects Committee of the University of California Los Angeles. At age 18 wk, WT and KO animals were divided into two dietary groups. Twenty animals remained on normal chow (NC) diet, whereas 10 were fed a HF diet (HFD; 45% calories from fat, Harlan Teklad No. TD96132). Within the NC-fed groups, WT vs. KO, animals were divided further into two groups, basal vs. insulin-stimulated.
Circulating factors and glucose tolerance.
Blood was drawn from 6-h-fasted, 20-wk-old mice and analyzed for circulating factors: insulin (Lincoplex; Millipore), leptin (Lincoplex; Millipore), PAI-1 (Lincoplex; Millipore), adiponectin (Radioimmunoassay; Millipore), triglycerides (WAKO), cholesterol (WAKO), and nonesterified fatty acids (WAKO). Following a 2-wk recovery, glucose tolerance tests (GTTs; 1 g/kg dextrose) were performed on 6-h-fasted mice, as described previously (38–39).
Indirect calorimetry, feeding, and activity.
Animals were acclimated to the metabolic chambers (Oxymax; Columbus Instruments) over the first 24 h, and basal metabolic rate, V̇o2 and V̇co2, respiratory exchange ratio, feeding, and movement were recorded over the subsequent 48 h.
Hyperinsulinemic euglycemic clamp studies.
Two weeks after the GTT, dual catheters were surgically placed in the right jugular vein, and glucose clamp studies were performed 3 days postsurgery, as described previously (38–39). All animals were fasted for 6 h prior to the clamp and studied in the conscious state. Basal glucose turnover was determined by tracer dilution following a 90-min constant infusion (5.0 μCi/h, 0.12 ml/h) of d-[3-3H]glucose (PerkinElmer). After the basal period, glucose (50% dextrose; Abbott Laboratories) and insulin (12 mU · kg−1 · min−1; Novo Nordisk Pharmaceutical Industries) plus tracer (5.0 μCi/h) infusions were initiated simultaneously, and glucose levels clamped at euglycemia (∼120 mg/dl), using a variable glucose infusion rate (GIR). At steady state the total glucose disposal rate (GDR) measured by tracer dilution is equal to the sum of the rate of endogenous or hepatic glucose production (HGP) and the exogenous (cold) glucose infusion rate (GIR) (38–39, 68). The insulin-stimulated component of the total GDR (IS-GDR) is equal to the total GDR minus the basal glucose turnover rate.
Immunoblot analysis.
Skeletal muscle, adipose tissue, and liver samples were pulverized in liquid nitrogen and homogenized in RIPA lysis buffer containing protease and phosphatase inhibitors. All lysates were clarified, centrifuged, and resolved by SDS-PAGE. Samples were transferred to PVDF membranes and subsequently probed with the following antibodies for protein and phosphoprotein detection: ERα (Santa Cruz Biotechnology), actin (Sigma Chemical), tubulin (Sigma Chemical), glucose transporter-4 (GLUT4; Chemicon Millipore), HSP90 (Cell Signaling), HSP72 (Stressgen), IRS-1 (Santa Cruz Biotechnology)/phospho (p)-Ser307 (Upstate Millipore), Akt (Santa Cruz Biotechnology)/p-Ser473 (Cell Signaling), AMPK/p-Thr172 (Cell Signaling), JNK1/2/p-Thr183/Tyr185, and IKKα/β/p-Ser180/181 (Cell Signaling). To analyze tyrosine phosphorylation of IRS-1 and IRS-2, lysates were immunoprecipitated overnight with IRS-1 or IRS-2 antibody (Santa Cruz Biotechnology) and immobilized on protein A- (Upstate Millipore) or G-agarose beads (Santa Cruz Biotechnology). Phosphorylation was detected by immunoblotting with anti-pY20 antibody (BD Biosciences). Additionally, immunoprecipitates for IRS-1 were immunoblotted for phosphatidylinositol (PI) 3-kinase p85 subunit (Santa Cruz Biotechnology). Densitometric analysis was performed using NIH image-ready software, version 1.6, and BioRad Chemidoc Quantity One image software.
Muscle lipid intermediates.
Lipids were extracted from the quadriceps muscle by the Folch method (29). Triacylglycerol was saponified in an ethanol-KOH solution at 60°C and glycerol content determined fluorometrically. Diacylglycerol and ceramides were extracted and quantified as described previously (2, 29, 59).
Quantitative RT-PCR.
Skeletal muscle, liver, and adipose tissue RNA were isolated using tissue RNeasy kits (Qiagen, Valencia, CA) for fibrous and nonfibrous samples per the manufacturer's instructions. cDNA synthesis was performed using iScript cDNA synthesis kit from Bio-Rad (Hercules, CA). PCR reactions were prepared using iQ SYBR Green Supermix (Bio-Rad). All PCRs are performed in a Bio-Rad MyiQ real-time detection system (Bio-Rad). Quantification of a given gene, expressed as relative mRNA level compared with WT control, was calculated after normalization to a standard housekeeping gene (β-actin, GAPDH). We performed separate control experiments to ensure that the efficiencies of target and reference amplification are equal, as described in the User Bulletin 2 from Applied Biosystems. Primer pairs were designed using Primer Express 2.0 software or previously published sequences (39, 52, 82). Primer sets were selected spanning at least one exon-exon junction when possible and were checked for specificity using Basic Local Alignment Search Tool (National Center for Biotechnology Information). The specificity of the PCR amplification was confirmed by melting curve analysis, ensuring that a single product with its characteristic melting temperature was obtained. Primer sequences for the specific target genes analyzed are as follows (sequence 5′ to 3′): adiponectin: forward TGTTGGAATGACAGGAGCTGAA, reverse CACACTGAAGCCTGAGCGATAC; β-actin, forward AAGTCCCTCACCCTCCCAAAAG, reverse AAGCAATGCTGTCACCTTCCC; CCAAT/enhancer-binding protein (C/EBPα): forward CCCCCACTCAGCTTACAACAGG, reverse CACCCCACAAAGCCCAGAAAC; ERα: forward ATGAAAGGCGGCATACGGAAAG, reverse CACCCATTTCATTTCGGCCTTC; ERβ: forward GCCAACCTCCTGATGCTTCT, reverse TCGTACACCGGGACCACAT; fatty acid-binding protein 4 (FABP4): forward TTCGATGAAATCACCGCAGA, reverse GGTCGACTTTCCATCCCACTT; GAPDH: forward AATGTGTCCGTCGTGGATCT; reverse CATCGAAGGTGGAAGAGTGG; GLUT4: forward CCCCCGATACCTCTACATCATC, reverse GCATCAGACACATCAGCCCAG; leptin: forward GAGACCCCTGTGTCGGTTC, reverse CTGCGTGTGTGAAATGTCATTG; monocyte chemoattractant protein-1 (MCP-1): forward CAGCCAGATGCAGTTAACGC, reverse GCTGCTGGTGATCCTCTTG; neomycin resistance gene (NEO): forward GGAGAGGCTATTCGGCTATG, reverse GACAGGTCGGTCTTGACAAA; perilipin: forward ACAGCAGAATATGCCGCCAA, reverse GGCTGACTCCTTGTCTGGTG; peroxisome proliferator-activated receptor (PPAR)α: forward CCTGAACATCGAGTGTCGAATAT, reverse GGTCTTCTTCTGAATCTTGCAGCT; PPARδ: forward GCCTCGGGCTTCCACTAC, reverse AGATCCGATCGCACTTCTCA; PPARγ: forward GCCCTTTGGTGACTTTATGG, reverse CAGCAGGTTGTCTTGGATGT; sterol regulatory element-binding protein-1c (SREBP-1c): forward GGAGCCATGGATTGCACATT; reverse GCTTCCAGAGAGGAGCCCAG; TNFα: forward CACAAGATGCTGGGACAGTGA, reverse TCCTTGATGGTGGTGCATGA; uncoupling protein-2 (UCP2): forward TCCCCTGTTGATGTGGTCAA, reverse GATCCCAAGCGGAGAAAGG.
Statistics.
Values presented are expressed as means ± SE. Statistical analyses were performed using one- and two-way analysis of variance (ANOVA) with Tukey's post hoc comparison for identification of significance within and between groups (SPSS graduate pack; SPSS, Chicago, IL). Significance was set a priori at P < 0.05.
RESULTS
ERα deletion in skeletal muscle, liver, and adipose tissue.
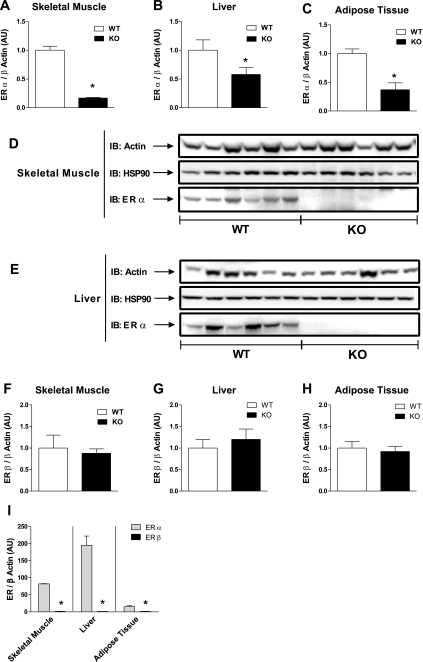
Quantitative RT-PCR and immunoblot analyses were performed to assess ERα and ERβ mRNA and protein expression levels in skeletal muscle, liver, and adipose tissue from NC-fed WT and ERα-KO mice. ERα mRNA was lower in ERα-KO mice in all tissues (Fig. 1, A–C). To ensure that residual ERα mRNA detected in KO mice was targeted, we also measured the amount of NEO sequence in tissues from both genotypes. The abundance of NEO sequence correlated well with total residual ERα in tissues from KO mice (r = 0.56, P = 0.01), and immunoblot analyses show the complete ablation of full-length (66 kDa) ERα protein from skeletal muscle and liver, the two primary tissues of focus (Fig. 1, D and E). Furthermore, additional RT-PCR analyses indicate no compensatory increase in ERβ in tissues devoid of ERα (Fig. 1, F–H). Our findings show that ERα is more highly expressed than ERβ in these three glucoregulatory tissues (Fig. 1I).
Fig. 1.
Wild-type (WT) estrogen receptor (ER)α transcript and protein are reduced in insulin target tissues from ERα-knockout (KO) mice. ERα mRNA/β-actin expression in skeletal muscle (A), liver (B), and adipose tissue (C) harvested from normal chow (NC)-fed WT (open bars; n = 12) and KO (black bars; n = 12) mice following a 6-h fast. WT expression levels were normalized to 1.0. Full-length (66 kDa) ERα protein is undetectable in skeletal muscle (D) and liver (E) from KO mice compared with WT. Representative blots are shown for ERα as well as loading controls, β-actin, and heat shock protein (HSP)90 (n = 5–6 mice/genotype). ERβ mRNA in skeletal muscle (F), liver (G), and adipose tissue (H) from WT (open bars; n = 12) and KO (black bars; n = 12) mice. No differences in ERβ mRNA/β actin expression levels were detected between the genotypes. I: ERα is the predominant ER transcript found in skeletal muscle, liver, and adipose tissue. Values for tissue RT-PCR and immunoblot (IB) analyses are represented as means ± SE and expressed in arbitrary units (AU). Mean differences were detected using ANOVA. *P < 0.05.
Body and tissue weights.
ERα-KO mice weighed significantly more (25–30%) than WT mice (Table 1) regardless of diet. A portion of this difference in body weight is explained by increased perigonadal fat pad weight (P = 0.001; Table 1). Dual-energy X-ray absorptiometry scanning confirmed that total body fat content as well as percent body fat were elevated by 67 (P = 0.016) and 48% (P = 0.0085), respectively, in KO mice compared with WT (Table 1). Whereas liver and heart weight were not different between the genotypes during NC feeding, both were elevated in KO mice following HFD (Table 1).
Table 1.
Animal characteristics following a 6-h fast in the basal and clamped state
| WT-NC | KO-NC | P Value | WT-HFD | KO-HFD | P Value | |
|---|---|---|---|---|---|---|
| n (Basal) | 12 | 12 | ||||
| n (Clamp) | 6 | 7 | 8 | 6 | ||
| Age, wk | 26 | 26 | 26 | 26 | ||
| Body weight, g | 22 ± 0.5 | 28 ± 0.2 | 0.001 | 24 ± 0.7 | 30 ± 0.4 | 0.04 |
| %Body fat | 12.9 ± 0.24 | 19.2 ± 2.5 | 0.0085 | ND | ND | |
| Total body fat, g | 2.39 ± 008 | 4.1 ± 0.5 | 0.016 | ND | ND | |
| Gonadal adipose weight, g | 0.22 ± 0.04 | 0.96 ± 0.07 | 0.001 | 0.45 ± 0.07 | 1.3 ± 0.13 | 0.0001 |
| Brown adipose weight, g | 0.086 ± 0.01 | 0.103 ± 0.004 | 0.028 | ND | ND | |
| Liver weight, g | 0.92 ± 0.03 | 1.0 ± 0.05 | 0.06 | 0.98 ± 0.04 | 1.21 ± 0.04 | 0.0017 |
| Heart weight, g | 0.1 ± 0.002 | 0.1 ± 0.004 | 0.22 | 0.1 ± 0.003 | 0.11 ± 0.003 | 0.026 |
| Fasting blood glucose, mg/dl | 117 ± 2 | 128 ± 2 | 0.009 | 129 ± 5 | 138 ± 3 | 0.2 |
| Clamp blood glucose, mg/dl | 122 ± 1 | 123 ± 1 | 0.34 | 116 ± 3 | 118 ± 2 | 0.64 |
| GIR, mg·kg−1·min−1 | 66 ± 4 | 44 ± 3.4 | 0.0014 | 57 ± 4.2 | 33 ± 2 | 0.002 |
| Fasting NEFA, mM | 0.69 ± 0.06 | 0.55 ± 0.04 | 0.12 | 1.08 ± 0.08 | 1.19 ± 0.07 | 0.38 |
| Clamp NEFA, mM | 0.28 ± 0.01 | 0.35 ± 0.03 | 0.048 | 0.35 ± 0.03 | 0.32 ± 0.04 | 0.45 |
| Estradiol, pg/ml | 25 ± 2.7 | 133 ± 8.6 | 0.0001 | ND | ND | |
| Fasting insulin, ng/ml | 0.31 ± 0.04 | 0.76 ± 0.08 | 0.001 | 0.42 ± 0.04 | 1.1 ± 0.1 | 0.0001 |
| Clamp insulin, ng/ml | 12 ± 2 | 16 ± 3 | 0.45 | 16 ± 2 | 20 ± 3 | 0.44 |
| Fasting triglyceride, mg/dl | 48 ± 4 | 75 ± 7 | 0.004 | ND | ND | |
| Fasting total cholesterol, mg/dl | 53 ± 1.5 | 72 ± 2.8 | 0.0003 | ND | ND | |
| Adiponectin, μg/ml | 27 ± 1 | 19 ± 2 | 0.001 | 41 ± 1 | 18 ± 1 | 0.0001 |
| Leptin, ng/ml | 0.75 ± 0.14 | 2.9 ± 0.2 | 0.0001 | 1.6 ± 0.4 | 25 ± 7.3 | 0.0004 |
| PAI-1, ng/ml | 0.72 ± 0.17 | 1.4 ± 0.1 | 0.04 | 1.1 ± 0.17 | 2.4 ± 0.13 | 0.0002 |
Body weights and biochemical parameters are represented as means ± SE; n = 6–12 animals/genotype, wild type (WT) vs. knockout (KO) and normal chow (NC) vs. high-fat diet (HFD). ND, not determined; GIR, glucose infusion rate; NEFA, nonesterified fatty acids; PAI-1, plasminogen activator inhibitor-1. Mean differences were detected using ANOVA, and exact P values are provided for each comparison. Metabolic parameters and circulating factors.
Blood metabolites, hormones, and adipokines.
Fasting blood glucose, triglyceride, and cholesterol levels were elevated by 10 (P = 0.009), 56 (P = 0.004), and 36% (P = 0.0003), respectively, in NC-fed KO mice (Table 1) despite the mice consuming a NC diet (Table 1). Fasting basal insulin, leptin, PAI-1, and estradiol levels were also all elevated in NC-fed KO mice compared with WT (Table 1). HF feeding was without effect on circulating insulin, leptin, or PAI-1 levels in WT mice but caused marked increases in all three parameters in KO mice (145%, P = 0.001; 286%, P = 0.00001; and 94%, P = 0.04, respectively; Table 1), whereas adiponectin was reduced by 30% vs. WT (P = 0.001; Table 1). Interestingly, following HF feeding, circulating adiponectin levels were markedly elevated by 52% (P = 0.001) in WT animals but were unchanged in KO mice (P = 0.67).
Reduced oxygen consumption, ambulatory movement, and energy expenditure in ERα-KO mice.
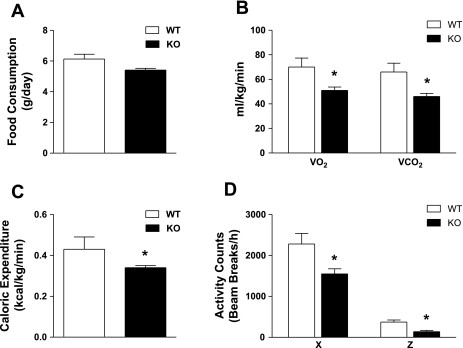
Despite the increased fat pad accumulation in ERα-KO mice, this alteration could not be explained by increased food consumption (P = 0.89; Fig. 2A). Oxygen consumption, CO2 production, and caloric expenditure and ambulatory movement were significantly reduced in KO vs. WT (P = 0.04, P = 0.018, P = 0.03, horizontal movement; P = 0.03, vertical movement, P =0.005; Fig. 2, B–D). These factors presumably contribute to the increased fat weight observed for KO mice even while they consume a NC diet.
Fig. 2.
Global ERα deletion is associated with reduced energy expenditure during consumption of a NC diet. Food consumption (A), oxygen uptake (B), caloric expenditure (C), and ambulatory movement (D; X, horizontal; Z, vertical) in NC-fed WT (open bars) vs. KO mice (black bars). Values are expressed as means ± SE. Mean differences between WT vs. KO were detected using ANOVA. *P < 0.05.
ERα deletion causes glucose intolerance and skeletal muscle insulin resistance in NC-fed mice.
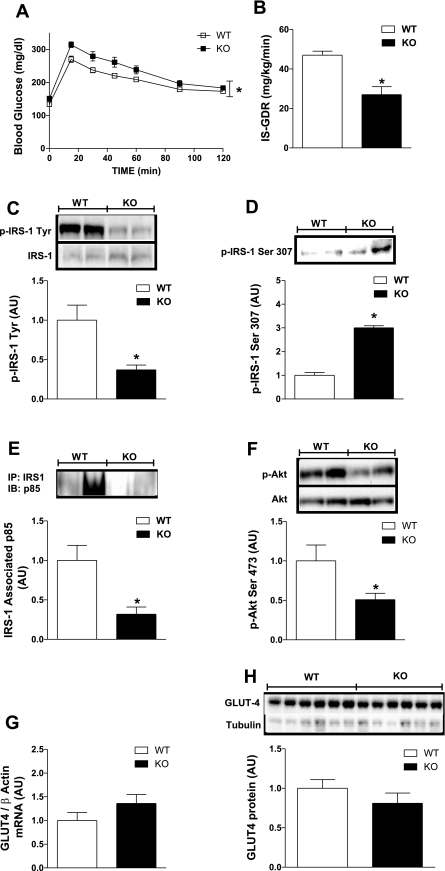
To investigate the impact of ERα ablation on glucose metabolism and insulin sensitivity, we performed GTTs and euglycemic hyperinsulinemic clamp studies. Following intraperitoneal injection of dextrose, the glucose curve for KO mice was significantly elevated compared with WT (P = 0.001; Fig. 3A), indicating impaired glucose tolerance for NC-fed ERα-KO mice. Next, we performed euglycemic hyperinsulinemic clamp studies to quantitatively determine the impact of ERα deletion on insulin sensitivity in skeletal muscle and liver. Despite a modest yet significant increase in fasting blood glucose in KO mice, no difference in basal glucose turnover rate was detected between the two genotypes (P = 0.26; data not shown). During clamp studies, circulating glucose and insulin concentrations were identical for both genotypes (P = 0.34 and P = 0.45, respectively; Table 1). The GIR required to maintain euglycemia was reduced by 33% (P = 0.0014) in KO mice compared with WT (Table 1). Radiolabeled glucose (d-[3-3H]glucose) was used to determine IS-GDR into peripheral tissues. The IS-GDR, which predominantly reflects skeletal muscle insulin sensitivity, was reduced by 40% (P = 0.001; Fig. 3B) in NC-fed KO compared with WT female mice.
Fig. 3.
ERα deletion causes impaired glucose tolerance and marked skeletal muscle insulin resistance. Glucose tolerance tests (GTTs; 1,000 mg/kg) and euglycemic hyperinsulinemic clamps (6-h fast, 12 mU · kg−1 · min−1 insulin infusate) were performed on NC-fed WT and KO mice at 26 wk of age. A: mean blood glucose concentrations ± SE during the GTT are shown for both WT (☐; n = 8) and KO (■; n = 8) mice. Repeated-measures ANOVA with Tukey's post hoc comparison was performed to detect mean differences between the 2 genotypes over time. *P < 0.05. B: skeletal muscle insulin sensitivity is represented by the insulin-stimulated glucose disposal rate (IS-GDR), and values are expressed as means ± SE for WT (open bars; n = 6) and KO (black bars; n = 7) mice during the euglycemic hyperinsulinemic clamp studies. Mean differences were detected using ANOVA. *P < 0.05. C–H: IB and RT-PCR analyses were performed to assess insulin signal transduction and glucose transporter 4 (GLUT4) glucose transport expression in female WT (open bars) and ERα-KO (black bars) mice (n = 6–12/group). Insulin receptor substrate-1 (IRS)-1 was immunoprecipitated and immunoblotted for tyrosine (C) and serine (D; Ser307) phosphorylation as well as phosphatidylinositol (PI) 3-kinase p85 subunit association (E). Additional IBs to detect Akt phosphorylation (F) as well as GLUT4 mRNA (G) and protein expression levels (H) were performed. Means ± SE are shown in AU for densitometric and RT-PCR analyses. Differences were detected by ANOVA. *P < 0.05.
Impaired skeletal muscle insulin signaling in ERα-KO mice.
We performed immunoblot analyses on soleus and quadriceps muscle to assess the protein levels of the insulin-responsive glucose transporter GLUT4 as well as levels of phosphorylation for insulin-signaling molecules downstream of the insulin receptor. Our data show that IRS-1 total protein was not different between KO [0.98 ± 0.13 arbitrary units (AU)] and WT (1.0 ± 0.12 AU) mice (P = 0.75; representative immunoblots Fig. 3C) but that tyrosine phosphorylation was diminished by 63% (P = 0.02; Fig. 3C). This coincided with increased IRS-1 Ser307 phosphorylation (P = 0.027; Fig. 3D), diminished IRS-1-associated PI 3-kinase p85 by 64% (P = 0.006; Fig. 3E), and a significant blunting in Akt1/2 Ser473 phosphorylation (P = 0.04; Fig. 3F) in quadriceps muscle from KO mice vs. WT. As with IRS-1 total protein, no difference in Akt1/2 total protein was detected between the two genotypes (P = 0.9; Fig. 3F). Whereas insulin signaling was markedly reduced, causing blunted glucose disposal in KO mice, no difference in skeletal muscle GLUT4 transcript or protein was detected in quadriceps (P = 0.28 and P = 0.20, respectively; Fig. 3, G and H) or soleus (P = 0.4 and P = 0.3, respectively; data not shown) between the genotypes.
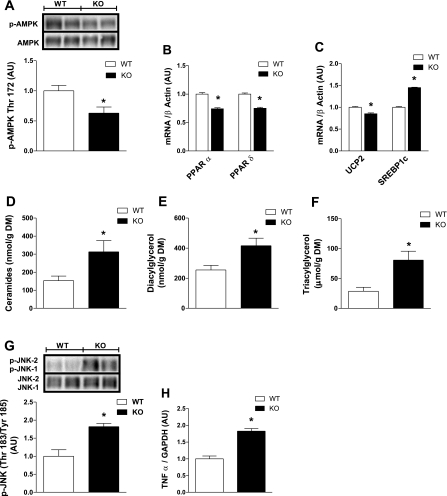
Reduction in markers of fatty acid oxidation and increased proinflammatory lipid intermediates in skeletal muscle from KO mice.
To investigate potential factors contributing to reduced energy expenditure and skeletal muscle insulin resistance, we performed additional immunoblot and RT-PCR analyses in muscle, focusing on muscle fatty acid handling and inflammation. Muscle AMPK activation, as reflected by AMPK phosphorylation (Thr172), was diminished by 37% in NC-fed KO muscle (P = 0.04; Fig. 4A). Furthermore, muscle expression levels of key transcriptional regulators of fatty acid oxidation, including PPARα (P = 0.03; Fig. 4B), PPARδ (P = 0.02; Fig. 4B), and UCP2 (P = 0.02; Fig. 4C), were concordantly reduced by 20–25%, whereas the expression of the central lipogenic regulator SREBP-1c was elevated by 54% (P = 0.016) in NC-fed KO mice (Fig. 4C).
Fig. 4.
ERα deletion causes reduced skeletal muscle fatty acid oxidation, accumulation of bioactive lipid intermediates, and inflammation during NC feeding. IB, RT-PCR, and biochemical analyses were performed on quadriceps muscle harvested from WT (open bars) and ERα-KO (black bars) mice (n = 6–12 animals/genotype). A: IB and densitometric analyses for phospho-AMP-activated protein kinase (p-AMPK). RT-PCR analyses were performed to quantify peroxisome proliferator-activated receptor (PPAR)α and PPARδ (B) as well as uncoupling protein-2 (UCP2) and sterol regulatory element-binding protein-1c (SREBP-1c) expression levels (C). Muscle lipid and lipid intermediates, ceramide (D), diacylglycerol, (E) and triacylglycerol (F) were quantified by biochemical assay. IB and RT-PCR analyses were performed to assess inflammatory signaling in quadriceps muscle: p-JNK (G) and TNFα/GAPDH mRNA (H) expression. Densitometric and RT-PCR analyses are presented as means ± SE in AU. Mean differences between WT vs. KO mice were detected using ANOVA. *P < 0.05.
As a likely consequence of reduced oxidative capacity, fatty acids were preferentially partitioned to storage. This shift in metabolism is well reflected by the marked accumulation of bioactive proinflammatory lipid intermediates, ceramides (P = 0.04; Fig. 4D), and diacylglycerol (P = 0.02; Fig. 4E) as well as triacylglycerol (elevated 2.6-fold, P = 0.03; Fig. 4F) in muscle from NC-fed KO vs. WT mice. Consistent with this, JNK phosphorylation, a marker of inflammatory signaling, was increased by 83% (P = 0.009; Fig. 4G) and TNFα expression approximately twofold (P = 0.005; Fig. 4H) in skeletal muscle from ERα-KO mice.
ERα-KO mice exhibit mild hepatic insulin resistance.
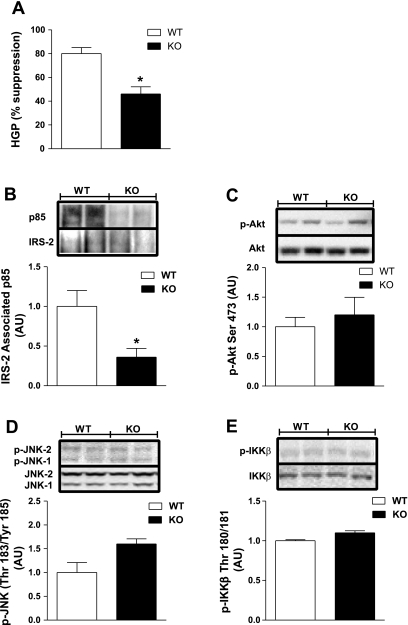
A portion of the reduction in GIR observed for KO mice during the glucose clamp studies can be explained by hepatic insulin resistance (Fig. 5A). HGP suppression was blunted by 20% (P = 0.037; data not shown) in NC-fed KO mice compared with WT. To confirm impaired hepatic insulin action, we performed immunoblot analyses on liver samples harvested following glucose clamp studies. IRS-2-associated PI 3-kinase p85 was diminished by 62% (P = 0.04) in KO mice (Fig. 5B). Surprisingly, we found no difference in Akt phosphorylation (Ser473; Fig. 5C), nor could we detect differences in the phosphorylation levels of inflammatory markers JNK and IKKβ (Fig. 5, D and E). These data suggest that defective ERα action leads to impaired insulin-mediated suppression of hepatic glucose production, but in contrast to muscle, heightened inflammation does not appear to be a causal factor during consumption of a NC diet.
Fig. 5.
ERα deletion causes hepatic insulin resistance independent of tissue inflammation. A: glucose clamps with [3-3H]glucose were performed on NC-fed WT (open bars; n = 6) and KO (black bars; n = 7) mice to assess the impact of ERα expression on hepatic glucose production (HGP %suppression) during hyperinsulinemia. IB and densitometric analyses were performed on liver samples to assess hepatic insulin action and inflammation. B: IRS-associated PI 3-kinase p85 levels. C: p-Akt. D: p-JNK. E: p-IKKβ. Values are expressed as means ± SE, and differences between WT vs. KO were detected using ANOVA. *P < 0.05.
ERα deletion leads to adipose tissue inflammation.
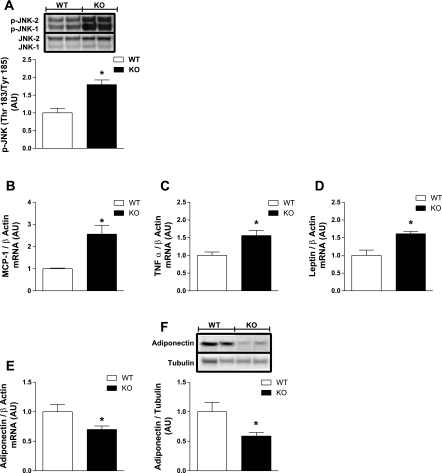
Similarly to muscle but in contrast to liver, inflammation was markedly elevated in white adipose tissue from NC-fed KO mice. JNK phosphorylation (Thr183/Tyr185) was increased by ∼90% (P = 0.007; Fig. 6A), MCP-1 expression was increased 2.6-fold (P = 0.037; Fig. 6B), and TNFα expression was increased by 56% (P = 0.04; Fig. 6C) in periovarian fat from KO vs. WT. Adipose tissue inflammation was paralleled by a 50% increase in leptin expression (P = 0.03) and a 30 (P = 0.03) and 50% (P = 0.02) reduction in adiponectin transcript and protein, respectively, in KO animals (Fig. 6, D–F).
Fig. 6.
Whole body ERα deletion leads to adipose tissue inflammation and reduced adiponectin expression. A: IB and densitometric analyses were performed on periovarian white adipose tissue samples harvested from NC-fed WT (open bars) and ERα-KO (black bars) mice to assess the inflammatory marker p-JNK. RT-PCR analyses were performed to determine monocyte chemoattractant protein-1 (MCP-1; B), TNFα (C), leptin (D), and adiponectin expression levels (E). F: reduced adipose tissue adiponectin total protein in KO mice was confirmed by immunoblotting. Values are expressed as means ± SE in AU. Mean differences between genotypes were detected using ANOVA. *P < 0.05.
Protection against HFD-induced insulin resistance is lost in female ERα-KO mice.
In contrast to previous findings for male mice (15, 39), we observed no increase in circulating insulin (P = 0.08), leptin (P = 0.08), or PAI-1 (P = 0.2) levels for WT female mice fed HFD vs. NC. In sharp contrast, these circulating factors were all elevated (insulin 45%, P = 0.04; leptin 8-fold, P = 0.007; PAI-1 71%, P = 0.001; Table 1) in ERα-KO animals following HFD.
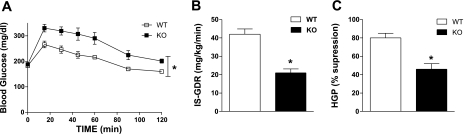
Glucose tolerance was also unaltered from NC in WT mice following HF feeding. However, this protection was absent in KO mice; thus, a further deterioration in glucose tolerance for KO mice was observed from the NC condition following HFD (P = 0.01; Fig. 3A vs. 7A, black squares). The glucose intolerance observed following 8-wk HFD is most likely attributable to the further decline in both muscle (IS-GDR, P = 0.045; Fig. 7B) and liver (%HGP suppression, P = 0.03; Fig. 7C) insulin sensitivity observed in KO mice. Herein, we also find no difference in skeletal muscle or hepatic insulin sensitivity for WT female mice fed a 45% HFD for 8 wk compared with NC-fed animals.
Fig. 7.
Whole body ERα deletion causes susceptibility to high-fat diet (HFD)-induced glucose intolerance and insulin resistance. GTT (1,000 mg/kg) and euglycemic hyperinsulinemic clamps (6-h fast, 12 mU · kg−1 · min−1 insulin infusate) were performed on WT and ERα-KO mice at 26 wk of age following consumption of a HFD (45% calories from fat) for 8 wk. A: mean blood glucose concentrations ± SE during the GTT are shown for both WT (☐; n = 6) and KO (■; n = 6). Repeated-measures ANOVA with Tukey's post hoc comparison was performed to detect mean differences between the 2 genotypes over time. *P < 0.05. Skeletal muscle insulin sensitivity as represented by IS-GDR (B) and hepatic glucose production (HGP %suppression; C) is expressed as means ± SE for WT (open bars; n = 8) and KO (black bars; n = 6) mice. Mean differences were detected using ANOVA. *P < 0.05.
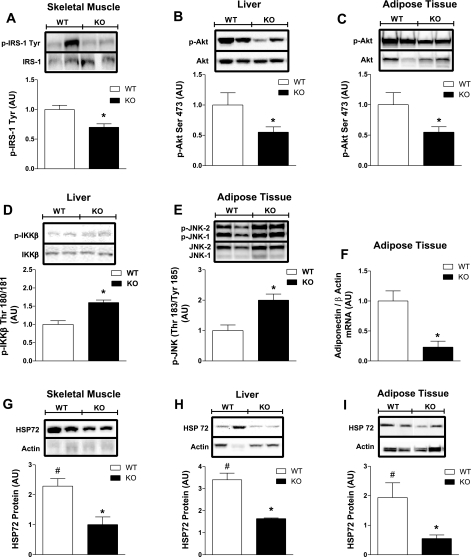
We performed immunoblot analyses on skeletal muscle, liver, and adipose tissue harvested from HF-fed mice following insulin stimulation to confirm impaired insulin signal transduction in KO vs. WT tissues (Fig. 8, A–C). Consistent with the notion that inflammation is an underlying cause of impaired insulin action during HF feeding, we detected elevated inflammatory signaling in liver (p-IKKβ, P = 0.0001; Fig. 8D) and adipose tissue (p-JNK, P = 0.007; Fig. 8E) from HF-fed KO mice.
Fig. 8.
Whole body ERα deletion causes tissue inflammation, impaired insulin action, and blunted HSP72 response to HFD. Tissues from female WT (open bars) and KO (black bars) mice fed a HFD for 8 wk were harvested following the clamp. IB and densitometric analyses were performed to quantify the phosphorylation of IRS-1 in muscle (immunoprecipitation: IRS-1, IB: pY; n = 6/genotype; A) and Akt (Ser473; n = 6/genotype; B and C) in liver and adipose. D and E: activation of proinflammatory serine kinases, as reflected by phosphorylation status of IKKβ (Ser180/181; n = 6/genotype) and JNK (Thr183/Tyr185; n = 6/genotype), was assessed in liver and adipose tissue, respectively. Densitometric analyses for the phosphorylation of IRS-1, Akt, IKKβ, and JNK are expressed as means ± SE in AU, and mean differences were detected by ANOVA. *P < 0.05. F: RT-PCR analyses of adipose tissue adiponectin expression relative to the housekeeping gene actin for HF-fed WT and KO mice. Values for gene expression are presented as means ± SE in AU, and differences were detected by ANOVA. *P < 0.05. HSP72 total protein levels in muscle (G), liver (H), and periovarian fat (I) were normalized to actin. Densitometric values for HF-fed WT mice are expressed relative to values for NC-fed WT mice, and in all 3 tissues HSP72 expression was significantly elevated 2- to 3-fold for female WT mice following HFD (open bars; #P < 0.05). Differences between densitometric values for HSP72 protein ± SE expressed in AU for HF-fed WT vs. KO mice were detected using ANOVA. *P < 0.05.
Given that inflammation was reduced and adipose tissue accretion less in WT vs. KO during HFD, we hypothesized that enhanced adipogenesis may be an important mechanism by which ERα-replete females accommodate an increased amount of fat while preventing deleterious effects on insulin action. To test this idea, we measured the expression levels of adipogenic markers in adipose tissue. In WT female mice, HF feeding caused a significant increase in C/EBPα (2.8-fold; P = 0.013), PPARγ (2.5-fold; P = 0.01), and FABP4 (3.4-fold; P = 0.015) compared with NC-fed mice (Table 2). In stark contrast, no change in adipogenic marker expression was detected in adipose tissue from HF-fed compared with NC-fed ERα-KO mice (Table 2).
Table 2.
Effect of HFD on adipose tissue gene expression
| WT-NC | KO-NC | P Value | WT-HFD | KO-HFD | P Value | |
|---|---|---|---|---|---|---|
| C/EBPα | 1.0 ± 0.37 | 1.1 ± 0.34 | 0.87 | 2.84 ± 0.1 | 0.7 ± 0.3 | 0.009 |
| PPARγ | 1.0 ± 0.28 | 0.85 ± 0.2 | 0.76 | 2.47 ± 0.1 | 0.68 ± 0.4 | 0.004 |
| PPARδ | ND | ND | 1.0 ± 0.22 | 0.2 ± 0.1 | 0.017 | |
| PPARα | ND | ND | 1.0 ± 0.2 | 0.54 ± 0.09 | 0.27 | |
| UCP2 | ND | ND | 1.0 ± 0.15 | 0.46 ± 0.09 | 0.02 | |
| FABP4 | 1.0 ± 0.31 | 1.6 ± 0.25 | 0.13 | 3.4 ± 0.33 | 1.46 ± 0.5 | 0.001 |
| Perilipin | ND | ND | 1.0 ± 0.25 | 0.11 ± 0.03 | 0.017 |
Means ± SE for CCAAT/enhancer-binding protein-α (C/EBPα), peroxisome proliferator-activated receptor (PPAR)γ, and fatty acid-binding protein 4 (FABP4) are expressed relative to WT-NC. Means ± SE for PPARδ, PPARα, uncoupling protein-2 (UCP2), and perilipin are expressed relative to WT-HFD, since analyses were not conducted on tissues from NC-fed mice. Total RNA was isolated from adipose tissue harvested from WT (n = 6–12) and KO (n = 6–12) mice following NC or HFD. RT-PCR analyses were performed using validated primer pairs for the indicated genes and normalized to the standard housekeeping gene β-actin. Mean differences were detected using 1- and 2-way ANOVA, and exact P values are provided for each comparison. Effects of HFD on adipose tissue gene expression.
In addition, we hypothesized that other sex-specific factors contributing to estrogen-mediated protection in WT females may be defective in mice devoid of ERα. Here, we show that HF feeding is associated with a 52% increase in circulating adiponectin (P = 0.0001; Table 1) in WT females, whereas no HFD-induced increase in adiponectin concentration was detected in KO mice (Table 1). Moreover, adiponectin expression in adipose tissue from HF-fed ERα-KO mice was reduced by 76% (P = 0.0002; Fig. 8F) compared with WT. Furthermore, whereas the levels of stress protein HSP72 were not different between the genotypes for NC (data not shown), HSP72 levels increased in skeletal muscle (129%, P = 0.003; Fig. 8G), liver (230%, P = 0.01; Fig. 8H), and adipose tissue (92%, P = 0.01; Fig. 8I) from WT mice following HFD. In contrast, no change in HSP72 protein was observed following HFD in KO animals for any of the three tissues analyzed.
DISCUSSION
The incidence of type 2 diabetes and the metabolic syndrome is shown to rise markedly in women following menopause (13, 43a). In addition, inactivating mutations or reductions in ERα expression are also associated with the presence of the metabolic syndrome in both sexes; however, the molecular underpinnings of these relationships are not well understood (22, 53, 67). Inflammation, insulin resistance, and increased adiposity are hallmark features of the metabolic syndrome, and in the present study we show that global loss of ERα recapitulates many of these features in mice, even when they are fed a NC diet. In circulation we find that triglycerides, total cholesterol, insulin, and leptin are all significantly elevated in ERα-deficient animals compared with WT. PAI-1, a surrogate index of systemic inflammation (45, 48), is nearly doubled, whereas adiponectin, an adipokine positively associated with insulin sensitivity (43, 73) and inflammation suppression (44, 56), is markedly decreased in KO mice.
In addition to alterations in circulating factors, we observed a significant impairment in glucose tolerance as well as markedly reduced liver and skeletal muscle insulin sensitivity in KO mice even when they were fed a NC diet. In contrast to Bryzgalova et al. (11), quantitatively we find that the difference in the glucose disposal rate between the two genotypes is due predominantly to impaired insulin action in skeletal muscle. These discrepancies likely arise due to differences in methodology; most notably, glucose clamp studies performed by Bryzgalova et al. (11) were conducted in anesthetized mice.
Importantly, our ex vivo findings are internally consistent with our observations during glucose clamp studies. Immunoblot analyses performed on lysates from soleus and quadriceps muscle show significant reductions in IRS-1 tyrosine phosphorylation, IRS-1-associated PI 3-kinase (p85 subunit), and Akt phosphorylation (Ser473). Diminished IRS-1 tyrosine phosphorylation was paralleled by markedly increased IRS-1 Ser307 levels in NC-fed KO mice compared with WT, and this molecular event has previously been shown to impair downstream signal transduction and diminish glucose transport (47, 79). We quantitatively examined the impact of ERα on total muscle GLUT4 transcript and protein in a large cohort of mice and found no differences between the groups. This is in sharp contrast to unquantified claims by Barros and colleagues (6, 7) suggesting that GLUT4 transcript and protein are diminished in muscle from ERα-KO mice. These findings by Barros and colleagues (6, 7) are indeed surprising in that GLUT4 is regulated by redundant transcriptional pathways (51, 85). Given that total GLUT4 transcript and protein are not reduced in humans or rodents even in the context of insulin resistance, obesity, and type 2 diabetes, it is reasonable to speculate that, in the absence of ERα, other transcription factors compensate to maintain GLUT4 levels (5, 8, 9, 32, 33, 40). We have recently investigated this relationship between ERα and GLUT4 expression further in muscle from lean and insulin resistant ob/ob mice, and we find that, whereas muscle ERα transcript levels are reduced with obesity (50%; P = 0.001), muscle GLUT4 transcript and protein levels are identical to those of lean controls (unpublished observations). Therefore, taken together, we are quite confident that total GLUT4 expression is not the central mechanism underlying skeletal muscle insulin resistance in female ERα-KO mice.
Alternatively, as we hypothesized, our ex vivo immunoblot and RT-PCR analyses show heightened inflammatory signaling as reflected by increased JNK phosphorylation and TNFα transcript in quadriceps muscle from NC-fed ERα-KO mice. A likely contributing factor to skeletal muscle inflammation is the marked increase in bioactive, proinflammatory lipid intermediates diacylglycerol and ceramides found in ERα-KO muscle. The mechanistic link between the accumulation of lipid intermediates, activation of inflammatory signaling cascades, and impaired insulin action is well shown in cells and rodents, and indeed these factors are observed concurrently in human subjects of obesity and type 2 diabetes (1, 14, 42, 71, 78, 81).
Concomitant with the increase in skeletal muscle proinflammatory lipid intermediates, we show reduced PPARα, PPARδ, and UCP2 message as well as diminished AMP-activated protein kinase (AMPK) activation, which taken together is suggestive of impaired skeletal muscle oxidative metabolism in ERα-KO mice. AMPK is known as a master metabolic switch regulating fatty acid metabolism in skeletal muscle (28, 74), and its activity correlates well with insulin sensitivity (4, 28). Recent work by Rogers et al. (65) shows that estrogen-induced AMPK activation is ERα mediated; thus, loss of estrogen action due to receptor ablation is a likely cause for reduced AMPK activity in skeletal muscle from ERα-KO mice. In addition, it is also plausible that inflammation is a secondary and indirect contributor to impaired oxidative metabolism (49, 62, 78), but regardless of whether cellular oxidative metabolism is reduced by a direct or indirect mechanism, our findings in muscle are in agreement with our in vivo observation of reduced whole body oxygen uptake in ERα-KO mice, as measured by indirect calorimetry. In total, these data are congruent with the published actions of estrogen to activate AMPK and stimulate PPARα/PPARδ expression while downregulating genes that control lipogenesis, including SREBP-1c (12, 19, 65, 70), and are entirely consistent with our findings of increased lipid accumulation in muscle from ERα-KO mice.
In liver, loss of ERα led to a modest impairment in hepatic insulin sensitivity, as measured by tracer dilution during glucose clamp studies. This impairment was confirmed by ex vivo immunoblotting of liver samples from clamped mice that showed reduced IRS-PI 3-kinase p85 association. Unexpectedly and in contrast to our findings in muscle, we found no difference in Akt phosphorylation or inflammatory signaling in liver from ERα-KO mice compared with WT. Given that inflammation is not a causal mechanism for hepatic insulin resistance in KO animals and given that STAT3 is a known ERα target, an alternative and plausible explanation for elevated HGP in KO mice during insulin stimulation is impaired STAT3 suppression of key enzymes (phosphoenolpyruvate carboxykinase and glucose-6-phosphatase) regulating glucose output by the liver (31, 60). Clearly, the role of ERα in regulating hepatocyte substrate metabolism and insulin action requires further elucidation.
In adipose tissue, ERα is shown to exert important effects on adipocyte cell size and total adiposity (35). Herein, we show that loss of ERα in adipose tissue of female mice leads to increased fat weight gain and total percent body fat, similar to findings for male mice, as described previously by Heine et al. (35). These data are also consistent with findings in women where reduced ERα expression is highly associated with adiposity (53). Our data support the hypothesis that the dramatic adipose phenotype seen in ERα-KO mice may manifest as a result of impaired adipocyte fatty acid handling and inflammation as well as reduced fatty acid oxidation by skeletal muscle. In support of this first notion, under both NC- and HF-fed conditions, inflammation was increased significantly in adipose tissue from ERα-KO mice compared with WT; however, given that adipose tissue is comprised of a heterogeneous mix of cell type, the source of this inflammation remains to be determined. A major limitation of this study is the inability to dissect apart the specific tissue contributions to the overall phenotype. Given that adipose tissue is major endocrine organ shown to secrete various adipokines and cytokines involved in regulating substrate metabolism, insulin action, and inflammation in other glucoregulatory tissues, in future studies it will be important to determine directly the role of ERα in the various adipose tissue cell constituents as well as the metabolic consequences of adipocyte-specific ERα deletion on whole body insulin sensitivity.
Although a 40–50% reduction in insulin-mediated glucose disposal is observed consistently in male mice following 8–16 wk of HF feeding (15, 39), estrogen-replete females are often protected against chronic dietary or acute fatty acid-induced insulin resistance (24, 30, 37). Consistent with these published findings, herein we show that WT ERα, estrogen-replete female mice are refractory to HFD-induced insulin resistance. The induction of HSP72 expression in skeletal muscle, liver, and adipose tissue during HF feeding may in part provide protection against cellular stress due to lipid oversupply, because we have shown recently that HSP72 overexpression prevents fat-induced inflammation and insulin resistance in male mice (16). Furthermore, previous reports suggest that this protective chaperone protein is more highly expressed in females vs. males (55, 76). Our current findings are consistent with the notion that HSP72 levels are modulated by estrogen and or ERα since a HFD-induced increase in HSP72 expression was observed in tissues from WT mice but was absent in ERα-KO.
In addition to increased HSP72 expression, HF feeding also caused a significant two- to threefold increase in expression of markers of adipogenesis and a 52% increase in circulating adiponectin not seen in HF-fed ERα-KO mice. During lipid oversupply, adipocytes expand in both size and number to accommodate increased fatty acid delivery (75). Importantly, increasing cell number while maintaining smaller-size adipocytes is associated with insulin sensitivity (80). Thus our work suggests that, in addition to increased oxidative function, estrogenized ERα-replete mice possess an enhanced adipogenic capacity allowing for the maintenance of smaller adipocytes, and this may represent an important mechanism by which female mice accommodate excessive amounts of fatty acids without deleterious effects to insulin sensitivity. Collectively, these data provide compelling evidence that ERα is critical for the induction of protective mechanisms to combat the cellular stress of overnutrition.
There is mounting evidence supporting that normally cycling females are protected against metabolic perturbations known to cause insulin resistance in males (24, 30, 37). Women show enhanced insulin sensitivity compared with men when normalized to lean mass, and this is a likely contributor to the reduced incidence of type 2 diabetes in premenopausal women (57, 83). Furthermore, in rodents, whereas male Zucker Leprfa/crl rats become diabetic with age, females rarely convert from impaired glucose tolerance to frank diabetes (17). Although sex differences in metabolism and disease susceptibility are well documented, the molecular mechanism(s) explaining these sexual dimorphisms is not well understood. We hypothesize that females with intact estrogen signaling may be uniquely equipped to upregulate specific pathways to better combat metabolic insult and cellular stress. Herein, we have provided evidence in support of three ERα-dependent mechanisms involved in the protection against inflammation and diet-induced insulin resistance. It is worth noting that in recent years the metabolic syndrome has been more prevalent in men than in women, but this disparity is quickly diminishing, especially in women under 40 yr of age in which the relative prevalence of this syndrome has increased by 76% (63, 64, 69). The cause for this marked change in the incidence of the metabolic syndrome in young women is unknown, but it is reasonable to speculate that impaired ERα action leading to loss of estrogen-mediated protective mechanisms underlies in part these clinical observations.
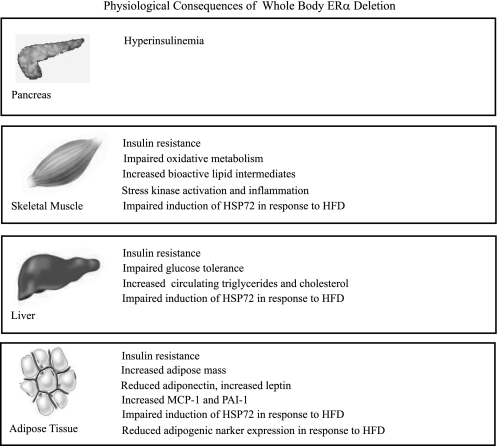
In conclusion, ERα deletion manifests in many of the clinical outcomes that characterize the metabolic syndrome, including increased adiposity, elevated levels of circulating and tissue inflammatory markers, impaired glucose tolerance, and insulin resistance (Fig. 9). With recent findings linking estrogen receptor dysfunction to chronic disease, our data suggest that a critical look beyond hormone levels to hormone receptor conformation and action is essential in understanding type 2 diabetes susceptibility in women following menopause.
Fig. 9.
Schematic overview summarizing the effects of whole body ERα deletion on metabolism, inflammation, and insulin action. Global ERα deletion causes reduced fatty acid oxidation and the accumulation of triglyceride and bioactive lipid intermediates in skeletal muscle. These changes in metabolism are associated with heightened inflammatory signaling and impaired insulin action in glucoregulatory tissues. In addition, female ERα-KO mice are more susceptible to the deleterious effects of HFD, and we presume that this results from an inability to mount an appropriate stress response (increase circulating adiponectin levels, activate adipogenesis, and upregulate HSP72 expression levels in skeletal muscle, liver, and adipose tissue) in the face of lipid oversupply. PAI-1, plasminogen activator inhibitor-1.
GRANTS
These studies were supported in part by research grants from the UCLA Department of Medicine, the National Institute of Diabetes and Digestive and Kidney Diseases (DK-060484, DK-073227 to A. L. Hevener), and the Iris Cantor UCLA Women's Health Foundation.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We are grateful to Jerrold Olefsky for continued support of our work. We give special thanks to Dr. Ken Korach for generous contributions to the Hevener Laboratory. We appreciate the technical contributions of Ui Jeong Yun, Teo Soleymani, and Pedram Daraei for their assistance with tissue preparation. In addition, we thank Peter Tontonoz, Mark Febbraio, and Nai-Wen Chi for their constructive comments in the preparation of this article and Rima Boyadijan for performing plasma adipokine and cytokine analyses.
REFERENCES
- 1.Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Allred JB, Guy DG. Determination of coenzyme A and acetyl CoA in tissue extracts. Anal Biochem 29: 293–299, 1969 [DOI] [PubMed] [Google Scholar]
- 3.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11: 191–198, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes 55: 2277–2285, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Banks EA, Brozinick JT, Jr, Yaspelkis BB, 3rd, Kang HY, Ivy JL. Muscle glucose transport, GLUT-4 content, and degree of exercise training in obese Zucker rats. Am J Physiol Endocrinol Metab 263: E1010–E1015, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JÅ. Participation of ERα and ERβ in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab 297: E124–E133, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Barros RP, Machado UF, Warner M, Gustafsson JÅ. Muscle GLUT4 regulation by estrogen receptors ERβ and ERα. Proc Natl Acad Sci USA 103: 1605–1608, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brozinick JT, Jr, Etgen GJ, Jr, Yaspelkis BB, 3rd, Ivy JL. Glucose uptake and GLUT-4 protein distribution in skeletal muscle of the obese Zucker rat. Am J Physiol Regul Integr Comp Physiol 267: R236–R243, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Brozinick JT, Jr, Etgen GJ, Jr, Yaspelkis BB, 3rd, Kang HY, Ivy JL. Effects of exercise training on muscle GLUT-4 protein content and translocation in obese Zucker rats. Am J Physiol Endocrinol Metab 265: E419–E427, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Brussaard HE, Gevers Leuven JA, Frölich M, Kluft C, Krans HM. Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia 40: 843–849, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, Khan A. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49: 588–597, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Campbell SE, Mehan KA, Tunstall RJ, Febbraio MA, Cameron-Smith D. 17beta-estradiol upregulates the expression of peroxisome proliferator-activated receptor alpha and lipid oxidative genes in skeletal muscle. J Mol Endocrinol 31: 37–45, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88: 2404–2411, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys 419: 101–109, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Choi CS, Fillmore JJ, Kim JK, Liu ZX, Kim S, Collier EF, Kulkarni A, Distefano A, Hwang YJ, Kahn M, Chen Y, Yu C, Moore IK, Reznick RM, Higashimori T, Shulman GI. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest 117: 1995–2003, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA 105: 1739–1744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corsetti JP, Sparks JD, Peterson RG, Smith RL, Sparks CE. Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female rats. Atherosclerosis 148: 231–241, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol 9: 1441–1454, 1995 [DOI] [PubMed] [Google Scholar]
- 19.D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 280: 35983–35991, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Dechering K, Boersma C, Mosselman S. Estrogen receptors alpha and beta: two receptors of a kind? Curr Med Chem 7: 561–576, 2000 [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care 15: 318–368, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Deng HW, Li J, Li JL, Dowd R, Davies KM, Johnson M, Gong G, Deng H, Recker RR. Association of estrogen receptor-alpha genotypes with body mass index in normal healthy postmenopausal Caucasian women. J Clin Endocrinol Metab 85: 2748–2751, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest 116: 561–570, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, Kelly DP. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator- activated receptor alpha- deficient mice. J Clin Invest 102: 1083–1091, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjold M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab 82: 4258–4265, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Evans MJ, Eckert A, Lai K, Adelman SJ, Harnish DC. Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ Res 89: 823–830, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Evans MJ, Lai K, Shaw LJ, Harnish DC, Chadwick CC. Estrogen receptor alpha inhibits IL-1beta induction of gene expression in the mouse liver. Endocrinology 143: 2559–2570, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 282: E18–E23, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Frayn KN, Maycock PF. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. J Lipid Res 21: 139–144, 1980 [PubMed] [Google Scholar]
- 30.Frias JP, Macaraeg GB, Ofrecio J, Yu JG, Olefsky JM, Kruszynska YT. Decreased susceptibility to fatty acid-induced peripheral tissue insulin resistance in women. Diabetes 50: 1344–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, Gustafsson JA, Dahlman-Wright K. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol 20: 1287–1299, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Garvey WT, Maianu L, Hancock JA, Golichowski AM, Baron A. Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM. Diabetes 41: 465–475, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest 101: 2377–2386, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol 25: 2957–2968, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA 97: 12729–12734, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87: 905–931, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Hevener A, Reichart D, Janez A, Olefsky J. Female rats do not exhibit free fatty acid-induced insulin resistance. Diabetes 51: 1907–1912, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat Med 9: 1491–1497, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, Folian B, Subramaniam S, Gonzalez FJ, Glass CK, Ricote M. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest 117: 1658–1669, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hevener AL, Reichart D, Olefsky J. Exercise and thiazolidinedione therapy normalize insulin action in the obese Zucker fatty rat. Diabetes 49: 2154–2159, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005–2011, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a.Kalyani RR, Franco M, Dobs AS, Ouyang P, Vaidya D, Bertoni A, Gapstur SM, Golden SH. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab 94: 4127–4135, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopp HP, Krzyzanowska K, Möhlig M, Spranger J, Pfeiffer AF, Schernthaner G. Effects of marked weight loss on plasma levels of adiponectin, markers of chronic subclinical inflammation and insulin resistance in morbidly obese women. Int J Obes (Lond) 29: 766–771, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Kressel G, Trunz B, Bub A, Hülsmann O, Wolters M, Lichtinghagen R, Stichtenoth DO, Hahn A. Systemic and vascular markers of inflammation in relation to metabolic syndrome and insulin resistance in adults with elevated atherosclerosis risk. Atherosclerosis 202: 263–271, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Lee CC, Kasa-Vubu JZ, Supiano MA. Differential effects of raloxifene and estrogen on insulin sensitivity in postmenopausal women. J Am Geriatr Soc 51: 683–688, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem 278: 2896–2902, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Mertens I, Verrijken A, Michiels JJ, Van der Planken M, Ruige JB, Van Gaal LF. Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int J Obes (Lond) 30: 1308–1314, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Milanesi L, Russo de Boland A, Boland R. Expression and localization of estrogen receptor alpha in the C2C12 murine skeletal muscle cell line. J Cell Biochem 104: 1254–1273, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J 149: 33–45, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Murgia M, Jensen TE, Cusinato M, Garcia M, Richter EA, Schiaffino S. Multiple signalling pathways redundantly control glucose transporter GLUT4 gene transcription in skeletal muscle. J Physiol 587: 4319–4327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Nilsson M, Dahlman I, Rydén M, Nordström EA, Gustafsson JA, Arner P, Dahlman-Wright K. Oestrogen receptor alpha gene expression levels are reduced in obese compared to normal weight females. Int J Obes (Lond) 31: 900–907, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int J Obes Relat Metab Disord 27: 1020–1027, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Papaconstantinou AD, Goering PL, Umbreit TH, Brown KM. Regulation of uterine hsp90alpha, hsp72 and HSF-1 transcription in B6C3F1 mice by beta-estradiol and bisphenol A: involvement of the estrogen receptor and protein kinase C. Toxicol Lett 144: 257–270, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Park PH, Huang H, McMullen MR, Mandal P, Sun L, Nagy LE. Suppression of lipopolysaccharide-stimulated tumor necrosis factor-alpha production by adiponectin is mediated by transcriptional and post-transcriptional mechanisms. J Biol Chem 283: 26850–26858, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 163: 427–436, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev 23: 90–119, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Preiss J, Loomis CR, Bishop WR, Stein R, Niedel JE, Bell RM. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem 261: 8597–8600, 1986 [PubMed] [Google Scholar]
- 60.Ramadoss P, Unger-Smith NE, Lam FS, Hollenberg AN. STAT3 targets the regulatory regions of gluconeogenic genes in vivo. Mol Endocrinol 23: 827–837, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Razmara A, Sunday L, Stirone C, Wang XB, Krause DN, Duckles SP, Procaccio V. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J Pharmacol Exp Ther 325: 782–790, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Regitz-Zagrosek V, Lehmkuhl E, Mahmoodzadeh S. Gender aspects of the role of the metabolic syndrome as a risk factor for cardiovascular disease. Gend Med 4, Suppl B: S162–S177, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol 95: 136–147, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Rogers NH, Witczak CA, Hirshman MF, Goodyear LJ, Greenberg AS. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem Biophys Res Commun 382: 646–650, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sites CK, Toth MJ, Cushman M, L'Hommedieu GD, Tchernof A, Tracy RP, Poehlman ET. Menopause-related differences in inflammation markers and their relationship to body fat distribution and insulin-stimulated glucose disposal. Fertil Steril 77: 128–135, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331: 1056–1061, 1994 [DOI] [PubMed] [Google Scholar]
- 68.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82: 420–430, 1959 [DOI] [PubMed] [Google Scholar]
- 69.Steinbaum SR. The metabolic syndrome: an emerging health epidemic in women. Prog Cardiovasc Dis 46: 321–336, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 4: 465–474, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 45: 42–72, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Sun WH, Keller ET, Stebler BS, Ershler WB. Estrogen inhibits phorbol ester-induced I kappa B alpha transcription and protein degradation. Biochem Biophys Res Commun 244: 691–695, 1998 [DOI] [PubMed] [Google Scholar]
- 73.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99: 16309–16313, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100: 328–341, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest 97: 2553–2561, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voss MR, Stallone JN, Li M, Cornelussen RN, Knuefermann P, Knowlton AA. Gender differences in the expression of heat shock proteins: the effect of estrogen. Am J Physiol Heart Circ Physiol 285: H687–H692, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, Chambon P. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA 82: 7889–7893, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 283: E413–E422, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Yamauchi T, Kamon J, Waki H, Murakami K, Motojima K, Komeda K, Ide T, Kubota N, Terauchi Y, Tobe K, Miki H, Tsuchida A, Akanuma Y, Nagai R, Kimura S, Kadowaki T. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem 276: 41245–41254, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab 297: E211–E224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell 126: 801–810, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Yki-Jarvinen H. Sex and insulin sensitivity. Metabolism 33: 1011–1015, 1984 [DOI] [PubMed] [Google Scholar]
- 84.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293: 1673–1677, 2001 [DOI] [PubMed] [Google Scholar]
- 85.Zorzano A, Palacin M, Guma A. Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol Scand 183: 43–58, 2005. [DOI] [PubMed] [Google Scholar]