Abstract
Suboptimal developmental environments program offspring to lifelong metabolic problems. The aim of this study was to determine the impact of protein restriction in pregnancy on maternal liver lipid metabolism at 19 days of gestation (dG) and its effect on fetal brain development. Control (C) and restricted (R) mothers were fed with isocaloric diets containing 20 and 10% of casein. At 19 dG, maternal blood and livers and fetal livers and brains were collected. Serum insulin and leptin levels were determinate in mothers. Maternal and fetal liver lipid and fetal brain lipid quantification were performed. Maternal liver and fetal brain fatty acids were quantified by gas chromatography. In mothers, liver desaturase and elongase mRNAs were measured by RT-PCR. Maternal body and liver weights were similar in both groups. However, fat body composition, including liver lipids, was lower in R mothers. A higher fasting insulin at 19 dG in the R group was observed (C = 0.2 ± 0.04 vs. R = 0.9 ± 0.16 ng/ml, P < 0.01) and was inversely related to early growth retardation. Serum leptin in R mothers was significantly higher than that observed in C rats (C = 5 ± 0.1 vs. R = 7 ± 0.7 ng/ml, P < 0.05). In addition, protein restriction significantly reduced gene expression in maternal liver of desaturases and elongases and the concentration of arachidonic (AA) and docosahexanoic (DHA) acids. In fetus from R mothers, a low body weight (C = 3 ± 0.3 vs. R = 2 ± 0.1 g, P < 0.05), as well as liver and brain lipids, including the content of DHA in the brain, was reduced. This study showed that protein restriction during pregnancy may negatively impact normal fetal brain development by changes in maternal lipid metabolism.
Keywords: programming, development, docosahexaenoic acid, arachidonic acid
human epidemiological (28, 31) and experimental animal studies (16, 23, 25) have shown that a suboptimal environment either in the womb or early in the neonatal life alters growth and predisposes individuals to lifelong health problems. Maternal dietary deficiencies in pregnancy result in multiple adverse outcomes in the offspring (3, 12). Fetal growth depends mostly on the amount and type of nutrients obtained from the mother. Therefore, the mother must adapt her metabolism to support this continuous draining of substrates. The effects of an altered intrauterine environment can be passed transgenerationally by epigenetic mechanisms involving changes in gene expression (41). During late gestation, maternal liver plays a central role in whole body lipid metabolism. Maternal triglycerides (TG) are not transported intact across the placenta, whereas free fatty acids (FAs), including long-chain polyunsaturated fatty acids (LC-PUFAs), can be transported (32). Therefore, a deficient maternal FA intake, particularly essential FAs (EFAs), may have important consequences on fetal maturation and postnatal development.
Arachidonic acid (AA) and docosahexaenoic acid (DHA) constitute the major LC-PUFAs in brain tissue and are important structural components of the central nervous system. Low content of AA and DHA is associated with abnormal prenatal and postnatal development of retina and brain (24). These FAs transferred across the placenta are accumulated in brain during fetal development (34). AA and DHA are formed from the dietary linoleic acid (LA) and α-linolenic acid (LNA), respectively, by a series of alternating desaturation and elongation reactions to form LC-PUFAs (37). Maternal liver is probably the main source for fetal brain LC-PUFAs, since no measurable activity of fetal liver and placenta desaturases has been found (30). In the rat, downregulation of Δ6 desaturase (Δ6D) in the liver of maternal protein deficiency during pregnancy has been reported (9), and a reduction in maternal dietary protein intake in pregnancy resulted in a lower concentration of DHA in maternal liver and plasma and impaired accumulation of DHA into fetal brain phospholipids (5). In the sheep, maternal nutrient restriction (50% of regular food intake) from early to midgestation modifies the profiles of LC-PUFAs in fetal tissues (43). However, to our knowledge, little attention has been paid to studying the effects of nutrient restriction on maternal liver lipid metabolism during pregnancy. We hypothesized that maternal dietary protein restriction in the absence of any change, either qualitative or quantitative, in the fat content of the diet would lead to lowered DHA and AA concentrations in the maternal liver, the key site of LC-PUFA synthesis. Since the fetus has a low ability to synthesize these essential fats, we further hypothesized that fetal brain concentrations would also be reduced. Although brain development spans the fetal and neonatal period in both altricial species such as rodents and precocial species such as man, early development is a period marked by myelination in critical areas of the brain such as the cortex.
METHODS
Care and Maintenance of Animals
All procedures were approved by the Animal Experimentation Ethics Committee of the Instituto Nacional de Ciencias Medicas y Nutricion, Salvador Zubiran (INNSZ). Details of maternal diet, breeding, and management of the experimental groups of offspring have been published in detail (42). Briefly, mothers were virgin female albino Wistar rats aged 11 ± 1 wk and weighing 240 ± 20 g, obtained from the INNSZ. Female rats with regular cycles were maintained on Purina 5001 rodent diet and under controlled lighting (lights on from 0700 to 1900 at 22–23°C). Eighteen female rats were mated overnight with proven male breeders, and the day on which spermatozoa were present in a vaginal smear was designated as the day of conception (day 0). Only rats that were pregnant within 5 days of introduction of the male were retained in the study. Pregnant rats were transferred to individual cages and allocated at random to one of two groups to be fed either a 20% casein [control diet (C); n = 7] or 10% casein isocaloric diet [restricted diet (R); n = 6] (42). Rats were weighed daily and during the study had free access to the experimental diet and water. Food was provided in the form of large, flat biscuits that were retained behind a grill through which the rats nibbled the food. The amount of food provided each day was weighed, as was the amount remaining after 24 h. Food intake was also measured in six female age-matched nonpregnant rats. At 19 days of gestation (dG) (19 was chosen as a day representative of late gestation but before enough of the occurrence of events leading to parturition), at 6 AM, food was removed from pregnant rats. On this day, between 10 and 11 AM (4-h fasting), pregnant rats were rapidly euthanized by decapitation by experienced personnel trained in the procedure using a rodent guillotine (Thomas Scientific). To ensure homogeneity of study subjects, litters of more than 12 or less than eight pups were excluded from the study. Trunk blood was collected into polyethylene tubes and allowed to clot at 4°C for 1 h and centrifuged at 1,500 g for 15 min at 4°C; after that, serum was stored at −20°C until assayed. Maternal livers were dissected, cleaned, weighed, and frozen at −75°C. Abdominal midline incision was performed, the uterine horns were exposed, and embryos were delivered alive, counted, and weighed. Each placenta was cleaned and weighed, and the diameter was measured with calipers. Fetal brain and liver were isolated, weighed, and pooled per litter and immediately frozen in liquid nitrogen for further determinations. The need to pool tissues from all fetuses in the litter precluded the ability to analyze outcomes according to offspring sex.
Biochemical Analyses
Carcass components.
After being weighed (wet weight), each carcass was chopped into small pieces, placed in a tared beaker, and dried at 60°C to constant weight. The weight lost is considered to be body water. The dried carcasses were ground up, and aliquots were taken for lipid determination by the Soxhlet method (1).
Leptin radioimmunoassay.
Maternal serum leptin concentration was determined by radioimmunoassay (RIA) using a commercial rat kit from Linco Research (St. Charles, MO), cat. no. RL-83K, with detection limit 0.5 ng/ml, using 100-μl samples. Each serum sample was assayed in duplicate. The intra- and interassay coefficients of variations were <4 and <5%, respectively.
Insulin RIA.
Serum insulin concentration was determined by RIA using commercial rat kits from Linco Research, cat. no. RI-13K. Each serum sample was determined in duplicate. The intra- and interassay coefficients of variations were <4 and <6%, respectively.
Blood glucose measurement.
Serum glucose concentration was determined spectrophotometrically using the enzymatic hexokinase method (Beckman Coulter, Fullerton, CA). Intra- and interassay coefficients of variations were <2 and <3%, respectively.
Lipid measurements.
Serum TG, HDL, LDL, VLDL, and cholesterol were determined enzymatically with the Synchron CX auto analyzer (Beckman Coulter).
FA Analysis
Total lipids were extracted from maternal liver by Soxhlet method (1) and fetus brain and liver according to the method used by Folch et al. (13), with some modifications. This sample was homogenized with 500 μl of 0.9% NaCl and 1 ml of chloroform-methanol (2:1). After that, extraction of FAs was carried out with the addition of chloroform (3 × 2 ml). The organic phase was pooled, and 120–150 μl of methanol was added until organic phase turned transparent, and then 1 g of Na2SO4 was added and vortexed. The organic phase was transferred in a new tube and evaporated under a stream of nitrogen.
Preparation of FA Methyl Esters
Two milliliters of methanol, 100 μl of toluene, and 40 μl of 2% methanolic sulfuric acid were added to the above residue and heated at 90°C for 2 h. After that the tubes were placed on ice, and 1 ml of 5% NaCl was added. FA methyl esters (FAME) were extracted with hexane (3 × 2 ml), and mixture was centrifuged at 1,500 g for 1 min. The organic phase was pooled and evaporated under stream of nitrogen. Two hundred microliters of hexane was added to the dark-colored residue and then centrifuged at 1,500 g for 5 min. The clear solution was injected in the chromatograph. The FA analysis was carried out in an Agilent model 6850 gas chromatograph equipped with a flame ionization detector, and automatic split injection was carried out using an Agilent 6850 autosampler. The chromatographic column was an HP-INNOWax capillary column (30 m, 0.25 mm, 0.25 μm) (J & W Scientific). One hundred twenty-five micrograms of heptadecanoic acid as internal standard was added to 100 mg of tissue. A sample of 1 μl was injected in split mode (50:1) at 250°C. The carrier gas was helium with a constant linear velocity of 24 cm/s, and the interface temperature was kept at 280°C. The oven temperature was raised from 50 to 230°C (33). Identification of the FAME was based upon retention times obtained for methyl ester standards from Poly Science, and each one was expressed as percentage of total FA in the sample.
Isolation of Total RNA and Northern Blot Analysis
Total RNA was isolated from maternal liver of rats at 19 dG using the method of Chomczynski and Sacchi (8). Fifteen micrograms of RNA was electrophoresed in a 1% agarose gel containing 37% formaldehyde, transferred to a nylon membrane filter (Hybond-N+), and cross-linked with an ultraviolet cross-linker (Amersham). RNA integrity and location of the 28S and 18S ribosomal RNA bands were determined under ultraviolet light. Δ5D and Δ6D mRNA expression in the pregnant rat liver were analyzed by Northern blot. The Δ5D cDNA probe was a 688-bp PCR product amplified from rat liver cDNA. The forward and reverse primers used for the PCR reaction were 5′-TCTTGCCCACGATGCCACGAC-3′ and 5′-CTTTGCCCCGCCTGCTTCTGA-3′, respectively. The Δ6D cDNA probe was a 925-pb PCR product. The forward and reverse primers were 5′-TGCCTTCCGTGCCTTCCAC-3′ and 5′-GTGCCCGCTGAACCAGTCATT-3′, respectively. The PCR products were purified with the high pure PCR product purification kit (Roche) and labeled with Redivue [32P]deoxycytidine triphosphate (110 TBq/mmol) by using the Rediprime DNA labeling kit (Amersham). Membranes were prehybridized with rapid-hyb buffer at 65°C for 1 h and then hybridized with the cDNA probe (53.3 MBq/l) for 2.5 h at 65°C. Membranes were washed once with 2× citrate saline solution (SSC; 1× SSC = 0.15 mol/l sodium chloride and 15 mol/l sodium citrate) and 0.1% SDS (wt/vol) at room temperature for 20 min and then twice for 15 min with 0.1 × SSC/0.1% SDS (wt/vol) at 65°C. Digitized images and quantification of radioactivity (dpm) of the bands were carried out using the Instant Imager (Packard Instrument, Meriden, CT). Membranes were also exposed to Ecktascan film (Kodak) at −70°C with an intensifying screen.
Analysis of mRNA Expression by Real-time Quantitative RT-PCR
For mRNA expression of stearoyl-CoA desaturase-1 (SCD-1), Δ5D and Δ6D by real-time RT-PCR, 300 ng of total RNA was subjected to reverse transcription and then amplified by PCR using Taqman Universal Master Mix (Applied Biosystems). Parallel nontemplate control reactions were run in the absence of RNA to assess the degree on any nucleic acid contamination in the reaction mixture. TaqMan fluorogenic probes and oligonucleotide primers were obtained from Applied Biosystems. TaqMan PCR assays for each target gene were carried out by triplicate in 96-well optical plates with the ABI prism 7000 sequence detection system (PerkinElmer Applied Biosystems). PCR was performed using 1.4 μl of cDNA, 0.6 μl of TaqMan assay mix containing 200 nM sense and antisense primers and 100 nM TaqMan fluorogenic probe, 6 μl of TaqMan Universal PCR Master Mix, and 4 μl of H2O. The protocol used for PCR amplifications was as follows: one cycle at 50°C for 2 min, one cycle at 95°C for 10 min, and 40 cycles at 95°C for 15 s and at 60°C for 1 min. The relative amount of each mRNA was calculated by using the comparative threshold cycle method (user bulletin no. 2; PerkinElmer Applied Biosystems). The probes and primers for rat genes were obtained from PerkinElmer Applied Biosystems (predeveloped TaqMan assay reagent control kits). The probes and primers for rat genes were obtained from PE Applied Biosystems (predeveloped TaqMan Assay Reagent Control kits). The assay numbers for each gene were Rn00594894-g1 (SCD-1), Rn00584915_m1 (Δ5D), and Rn00580220_m1 (Δ6D). Amplification was then performed by 45 cycles at 95°C for 15 s and at 60°C for 60 s. The cDNA quantity in each sample was normalized with the 18S. Real-time PCR was carried out in triplicate for each sample.
For elongase-2 and -5 (Elovl-2 and -5) by RT-PCR, relative mRNA levels of target genes and invariant transcript β-actin were determined using cDNA preparation for tissues and HC11 cells. Synthesized cDNA was mixed with LightCycler Fast Start DNA MasterPLUS SYBR Green I (Roche, Indianapolis, IN) and with various sets of gene-specific forward and reverse primers as follows and subjected to real-time PCR quantification using the Light Cycler 3.5 detection System (Roche): for Elovl-2, forward GGA AGA AAT ACC TCA CGC AG and reverse TGG CTT TTT TCG GTA TGT C; for Elovl-5, forward CTC AAC CTG CTG TCT CTC TA and reverse ATC TGG TGG TTG TTC TTA CG; for β-actin, forward TGG AAT CCT GTG GCA TCC ATG and reverse TAA AAC GCA GCT CAG TAA CAG. PCR amplification was performed in a total volume of 20 μl containing 2 μl of the cDNA sample, 40 pmol of each primer, and 4 μl of MasterPLUS SYBR Green. For each reaction, the polymerase was activated by preincubation at 95°C for 10 min. Amplification was then performed by 35 cycles at 95°C for 10 s and at 60°C or 62°C for 7 s. All reactions were performed in triplicate. Relative amounts of mRNA were calculated using the comparative threshold cycle method (cycle method, LightCycler software version 4.0; Roche). All results were normalized to the housekeeping gene abundance of β-actin mRNA.
Statistical Analysis
All data are presented as means ± SE. Fetal body weight and liver and brain weight, as well as placenta diameter and weight, are the average of the whole litter. Statistical analysis was performed using unpaired Student's t-test to compare C and R mothers. Real time RT-PCR results (mRNA gene/18S or β-actin normalized ratio) are expressed relative to C group. Correlations for maternal serum insulin and Δ5D liver gene expression and maternal serum insulin and fetal weight were calculated using a Pearson correlation. P < 0.05 was considered significant.
RESULTS
Maternal Parameters at 19 dG
At the beginning of pregnancy, both groups of pregnant rats, C and R, increased their food intake (C = 38.7 ± 3.0, R = 42.9 ± 2.0 g/day) compared with age-matched nonpregnant rats (22.8 ± 1.03 g/day). Food intake was rising during pregnancy, and there were no differences between C and R groups except at 19 dG, in which both groups decreased their food intake, and this drop was more evident in the R group (C = 18.9 ± 0.7, R = 16.4 ± 0.8 g/day, P < 0.05). Table 1 shows that, at 19 dG, rats fed a low-protein diet have similar body weights compared with C pregnant rats. However, the R group showed a significant decrease in fat body composition. In both groups, the liver weights were similar; however, a significant decrease in maternal liver lipids was observed in the R group.
Table 1.
Maternal characteristics at 19 dG in rats fed control (20% protein) or restricted (10% protein) diet during pregnancy
| Control (n = 7) | Restricted (n = 6) | |
|---|---|---|
| Body weight, g | 314 ± 13 | 305 ± 12 |
| Body fat, g | 9.2 ± 0.44 | 6.8 ± 0.3† |
| Liver weight, g | 12.1 ± 0.7 | 11.4 ± 0.5 |
| Liver lipids, mg/100 mg | 6.3 ± 0.04 | 4.7 ± 0.01* |
Results are means ± SE. dG, days of gestation.
P < 0.05;
P < 0.001 vs. control.
Biochemical Maternal Serum Parameters at 19 dG in Rats Fed Protein-Restricted Diet
There were no differences in glucose, TG, HDL, LDL, VLDL, and cholesterol serum levels between C and R (Table 2). However, serum leptin levels were significantly higher in the R than in the C group. Serum insulin concentrations were 3.6 times higher in the R group compared with C mothers (Table 2).
Table 2.
Maternal biochemical parameters at 19 dG in rats fed control (20% protein) or restricted (10% protein) diet during pregnancy
| Control (n = 7) | Restricted (n = 6) | |
|---|---|---|
| Glucose, mg/dl | 64.5 ± 3.5 | 54.5 ± 11.7 |
| Insulin, ng/ml | 0.19 ± 0.04 | 0.88 ± 0.16† |
| Leptin, ng/ml | 5.05 ± 0.13 | 6.9 ± 0.7* |
| Triglycerides, mg/dl | 237 ± 32 | 239 ± 13 |
| Cholesterol, mg/dl | 44 ± 3.9 | 42 ± 1.6 |
| HDL, mg/dl | 23 ± 0.8 | 24 ± 2.3 |
| LDL, mg/dl | 16.4 ± 2.9 | 13.2 ± 1.5 |
| VLDL, mg/dl | 4.6 ± 0.1 | 4.8 ± 0.5 |
Results are means ± SE.
P < 0.05;
P < 0.01 vs. control.
Percentage of FAs in Maternal Liver at 19 dG
In the R group, the percentage of stearic acid in maternal liver was higher compared with C. In contrast, oleic acid, arachidic acid, AA, and DHA percentages were lower in the R group (Table 3).
Table 3.
%Fatty acids in the maternal liver at 19 dG in rats fed control (20% protein) or restricted (10% protein) diet during pregnancy.
| %Fatty Acid | Control | Restricted |
|---|---|---|
| C12:0 Lauric | 0.2 ± 0.06 | ND |
| C14:0 Myristic | 1.5 ± 0.5 | 1.6 ± 0.7 |
| C16:0 Palmitic | 33.3 ± 3.0 | 33.9 ± 1.1 |
| C16:1 Palmitoleic | 2.4 ± 0.2 | 2.3 ± 0.3 |
| C18:0 Stearic | 21.4 ± 1.1 | 26.1 ± 1.0* |
| C18:1 Oleic | 26.0 ± 1.4 | 21.8 ± 1.3* |
| C18:2 Linoleic | 8.1 ± 0.7 | 9.1 ± 0.4 |
| C18:3 Linolenic | 0.158 ± 0.02 | 0.188 ± 0.02 |
| C20:3 Arachidic | 0.3 ± 0.01 | 0.2 ± 0.01* |
| C20:4 Arachidonic | 5.3 ± 1.6 | 3.9 ± 0.6* |
| C20:5 Eicosapentaenoic | 0.2 ± 0.1 | 0.3 ± 0.01 |
| C22:6 Docosahexaenoic | 1.2 ± 0.06 | 0.8 ± 0.07* |
Results are means ± SE; n = 5. ND, not detectable.
P < 0.05 vs. control.
Maternal Liver Desaturase and Elongase Gene Expression at 19 dG
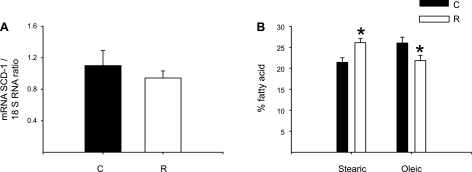
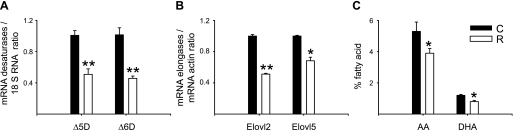
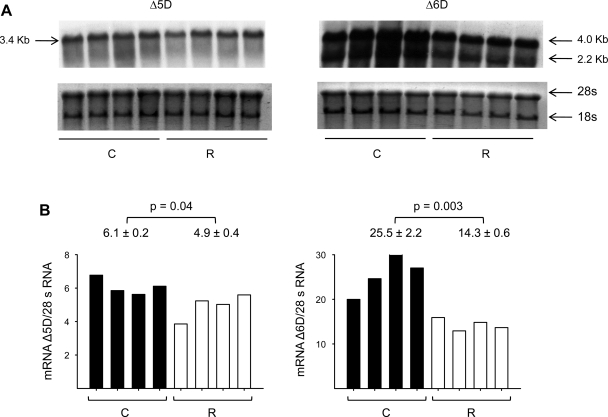
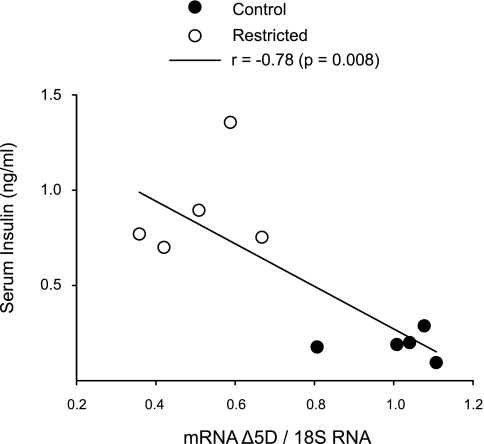
No differences in SCD-1 mRNA relative abundance between C and R groups were observed (Fig. 1), but there was a significant decrease in Δ5D, Δ6D, and Elovl-2 and -5 mRNAs in the R group (Fig. 2). By Northern blots (Fig. 3), maternal liver Δ6D mRNA was in both groups significantly more abundant than Δ5D mRNA, and as expected, there was a significant negative correlation (r = −0.78, P < 0.001) between maternal insulin serum concentrations and liver Δ5D mRNA (Fig. 4).
Fig. 1.
Maternal liver stearoyl-CoA desaturase-1 (SCD-1) relative abundance gene expression by real-time PCR assay (A) and %fatty acid (stearic and oleic) by gas chromatography (B) at 19 days of gestation (dG) of rats fed control (C; 20% protein) or restricted (R; 10% protein) diet during pregnancy. Means ± SE; n = 5. *P < 0.05 vs. C.
Fig. 2.
Maternal liver desaturases (A), elongase relative abundance gene expression by real time RT-PCR (B), and %fatty acid [arachidonic acid (AA) and docosahexaeonic acid (DHA)] by gas chromatography (C) at 19 dG of rats fed C (20% protein) or R (10% protein) diet during pregnancy. Means ± SE; n = 5. **P < 0.001; *P < 0.05 vs C. Δ5D and Δ6D, Δ5 and Δ6 desaturase; Elovl-2 and -5, elongase-2 and -5.
Fig. 3.
Northern blot analysis of Δ5D and Δ6D (A) and relative abundance or mRNA/ribosomal 28S of maternal liver (B) at 19 dG of rats fed C (20% protein) or R (10% protein) diet during pregnancy. Means ± SE; n = 4.
Fig. 4.
Maternal liver Δ5D gene expression as a function of serum insulin at 19 dG of rats fed C (20% protein) or R (10% protein) diet during pregnancy. Pearson correlation, P = 0.008 and r = −0.78.
Fetal Characteristics
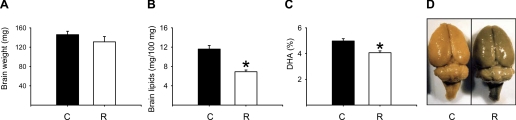
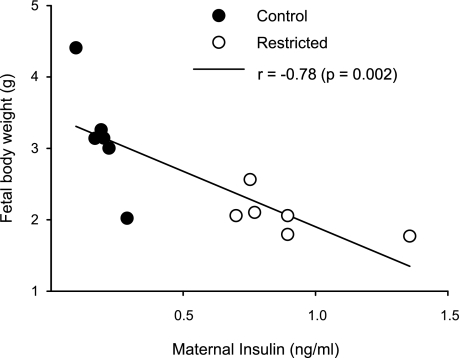
Fetal weight and placenta weight and diameter at 19 dG are summarized in Table 4. Mean fetal weight was significantly lower in the R group than in the C group, with no differences in number of fetuses per dam and in placenta weight and diameter. There were also no changes in brain weight; however, color and texture were different, brains from the R group were darker (many dark spots) compared with C brains (Fig. 5D), and the texture for C brains was tougher than R. In contrast, liver weight, fetal liver, and brain lipids were lower in the R group (Fig. 5). The percentage of DHA from total FA in fetal brain was significantly lower in the R group (Fig. 5). In addition, there was a negative correlation between fetal body weight and maternal serum insulin concentration at 19 dG (r = −0.78, P < 0.01; Fig. 6).
Table 4.
Fetal and placenta characteristics at 19 dG in rats fed control (20% protein) or restricted (10% protein) diet during pregnancy
| Control (n = 7) | Restricted (n = 6) | |
|---|---|---|
| No. of fetuses/dam | 10.3 ± 0.9 | 9.2 ± 0.6 |
| Placenta weight, g | 0.42 ± 0.02 | 0.40 ± 0.02 |
| Placenta diameter, mm | 0.99 ± 0.05 | 0.96 ± 0.06 |
| Fetal weight, g | 3.1 ± 0.3 | 2.0 ± 0.1* |
| Liver weight, mg | 175 ± 19 | 140 ± 3* |
| Liver lipids, mg/100 mg | 35.8 ± 1.8 | 18.7 ± 2.7† |
Results are means ± SE. Fetuses or placentas were averaged per litter.
P < 0.05;
P < 0.001 vs. control.
Fig. 5.
Fetal brain weight (A), lipids (B; mg/100 mg), %DHA (C), and photograph at 19 dG (D) of rats fed C (20% protein) or R (10% protein) diet during pregnancy. Means ± SE (n = 6–7 litters/group). *P < 0.05 vs. C.
Fig. 6.
Fetal body weight as a function of maternal serum insulin at 19 dG. Pearson correlation, P = 0.002 and r = −0.78 (n = 6 mothers or litters).
DISCUSSION
Many investigators have examined the effect of maternal malnutrition during pregnancy and lactation on the offspring; however, few studies have taken into consideration the impact of protein restriction on maternal lipid metabolism and its implication in fetal brain formation during gestation. Early malnutrition affects susceptibility to chronic diseases in adulthood (2), and maternal low-protein diet can program enzyme activity in offspring (26). However, previous studies indicated that offspring from rats fed a low-protein diet during pregnancy had higher susceptibility to develop chronic diseases (40, 41), and their mothers underwent metabolic adaptations to maintain an adequate fetal development. During the first half of normal pregnancy, progressive accumulation of maternal fat depots occurs due to increased adipose tissue lipogenesis and glycerolneogenesis (27). In this study, although there were not differences in maternal body and liver weights, there was a significant decrease in maternal body and liver lipid content as well as on fetal body weight; similar results have been reported with severe protein restriction (6%) (21).
Numerous animal studies have investigated the effect of maternal low-protein diet on glucose metabolism in the offspring (12, 40); however, little is known on the maternal responses derived from this condition. Herein, no differences in maternal serum glucose and TG concentrations were seen in C and R rats, which may be the result of an increased insulin-mediated glucose uptake by peripheral tissues and by the ability of insulin to suppress hepatic glucose output.
Hyperinsulinemia is a common finding in pregnancy (12). Insulin resistance is responsible for both decline in adipose tissue lipoprotein lipase and enhanced adipose tissue lipolytic activities. This condition results in high TG synthesis by increasing FA and glycerol uptake by the liver. In this study, although protein restriction during gestation did not affect maternal serum glucose levels, high insulin serum concentrations were observed, suggesting metabolic adaptations in glucose metabolism at the end of pregnancy. In addition, high insulin concentrations at 19 dG in R rats were negatively correlated to fetal weight. These results were in agreement with those reported previously in pregnant women (38).
Leptin is a hormone that regulates food intake and energy expenditure. Serum leptin concentrations usually increase from the middle to the end of pregnancy (10, 11). During this period, the high levels of leptin do not mediate a great inhibitory effect on food intake (36). In this study, leptin serum concentrations were on the range of pregnant rats (3–7 ng/ml). However, fasting serum leptin concentrations in R rats were higher than those observed in C pregnant rats; despite the lower body fat in this group, leptin in the R mothers may alter the energy balance in contribution to fetal development.
Protein restriction significantly affected the production of maternal liver LC-PUFAs. There are several potential ways in which low protein (and hence, amino acid) intake in the maternal diet may affect maternal liver PUFA synthesis. The most likely is an effect on availability of amino acids for enzyme synthesis. Enzyme activity could also be impacted by altered histone acetylation and methylation resulting from changes such as decreased one-carbon cycle components. Indeed, maternal liver contained an increased stearic to oleic acid ratio. Although a nonsignificant difference in hepatic SCD-1 mRNA relative abundance, an enzyme that catalyzes oleic acid formation from stearic acid, which is the rate-limiting step for cellular synthesis of monosaturated FA from saturated FA, was observed in R rats compared with controls, alterations in the expression of SCD-1 cannot be ruled out and deserve further investigation.
In this study, maternal protein restriction resulted in a negative effect, particularly on the capacity of maternal liver to desaturate LA and LNA. A significant decrease in maternal hepatic AA and DHA was observed in the R group, which correlated with low expression levels of Δ5D, Δ6D, Elovl-2, and Elovl-5. In line with this observation, Mercuri et al. (22) showed that maternal severe protein restriction (5% protein) during gestation decreased Δ6D activity in the maternal liver, and they suggested that this could affect the normal supply of LC-PUFAs for the normal fetus growth and tissue development. In fact, in the present study, mild protein restriction during pregnancy reduced significantly the concentration of DHA in maternal liver and fetal brain. Before birth, most if not all fetal FAs originate from the maternal circulation (17). After birth, milk, maternal liver (29), and diet later in life (17) represent the main sources of FAs. Whether these maternal liver changes in lipid metabolism could be expressed transgenerationally cannot be ascertained from the results presented in this study. Ozanne et al. (26) demonstrated in 3-mo-old offspring from R pregnant mothers a decrease in hepatic Δ5D activity and DHA concentrations, suggesting the establishment of a particular gene expression pattern, such as that involving metabolic lipid enzymes, as seen in this study.
The last period of intrauterine life in the rat (17–21 dG) is demarcated by an increase in body and brain weight together with an active neurogenesis stage. During this period, a rapid accumulation of DHA, unparalleled to other FAs, takes place (35). The supply of EFA and LC-PUFAs is critical for normal development of the fetus (6, 15, 20, 24). Indeed, nutritional status of the mother during gestation has been positively associated with fetal growth. In general, reduced maternal nutritional status, particularly regarding EFAs, has been correlated with reduced neonatal growth and head circumference in humans (7). In fact, at birth, AA status in preterm infants has been correlated with body weight (18, 19), and it has been proposed that it is related to intrauterine growth rather than to postnatal growth (39).
The brain is one of the richest organs in lipids (4). Our results showed that a protein-restricted diet during gestation significantly reduced fetal body weight and liver weight, with no significant changes in fetal brain weight. However, the content of fetal brain lipids, particularly DHA, was significantly lower compared with C rats. This last finding correlates with data reported previously by Burdge et al. (5) in which DHA concentration in fetal brain at 20 dG from 50% of protein restriction was lower compared with controls. As described in methods, the need to pool tissue from all fetuses in a litter removes the possibility of an analysis by fetal sex. We are currently studying the effect of the observed changes in brain composition on offspring behavioral phenotype.
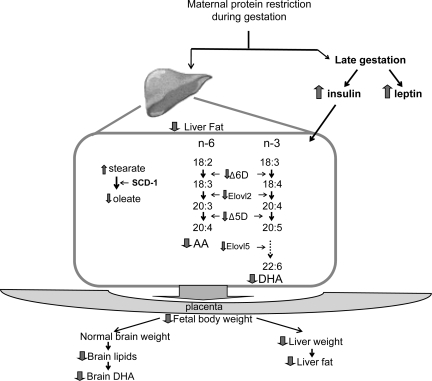
In conclusion, there is evidence that small changes in maternal dietary fat intake modify the composition of brain membranes during fetal growth; however, few studies deal with the negative impact of maternal protein restriction during gestation on fetal brain development. The present study showed that moderated protein restriction during pregnancy significantly reduced the concentration of maternal liver AA and DHA and maternal liver desaturase and elongase gene expression. These results may negatively impact fetal development, including the brain (Fig. 7).
Fig. 7.
Adaptative mechanisms proposed in the pregnant rat subjected to isocaloric protein restriction diet during pregnancy.
GRANTS
C. J. Bautista is a graduate student from Doctorado en Ciencias Biomedicas, Universidad Nacional Autónoma de México. This work was partially supported by Consejo Nacional de Ciencia y Tecnología (48839), Mexico, and the National Institute of Child Health and Human Development (HD-21350).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.AOAC (Association of Official Analytical Chemists) Official Methods of Analysis of AOAC International ( 17th ed.), edited by Harwitz W. Gaithersburg, MD: AOAC International, 2002, p. 920.05–920.39. [Google Scholar]
- 2.Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol 561: 355–377, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bautista CJ, Boeck L, Larrea F, Nathanielsz PW, Zambrano E. Effects of a maternal low protein isocaloric diet on milk leptin and progeny serum leptin concentration and appetitive behavior in the first 21 days of neonatal life in the rat. Pediatr Res 63: 358–363, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bourre JM. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J Nutr Health Aging 8: 163–174, 2004 [PubMed] [Google Scholar]
- 5.Burdge GC, Dunn RL, Wootton SA, Jackson AA. Effect of reduced dietary protein intake on hepatic and plasma essential fatty acid concentrations in the adult female rat: effect of pregnancy and consequences for accumulation of arachidonic and docosahexaenoic acids in fetal liver and brain. Br J Nutr 88: 379–387, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev 4: 121–129, 1980 [DOI] [PubMed] [Google Scholar]
- 7.Clandinin MT, Jumpsen J, Suh M. Relationship between fatty acid accretion, membrane composition, and biologic functions. J Pediatr 125: S25–S32, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 9.De Tomas ME, Mercuri O, Serres C. Effect of cross-fostering rats at birth on the normal supply of essential fatty acids during protein deficiency. J Nutr 113: 314–319, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Du F, Higginbotham DA, White BD. Food intake, energy balance and serum leptin concentrations in rats fed low-protein diets. J Nutr 130: 514–521, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Ehrhardt RA, Slepetis RM, Bell AW, Boisclair YR. Maternal leptin is elevated during pregnancy in sheep. Domest Anim Endocrinol 21: 85–96, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Twinn DS, Wayman A, Ekizoglou S, Martin MS, Hales CN, Ozanne SE. Maternal protein restriction leads to hyperinsulinemia and reduced insulin-signaling protein expression in 21-mo-old female rat offspring. Am J Physiol Regul Integr Comp Physiol 288: R368–R373, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 15.Foreman-van Drongelen MM, van Houwelingen AC, Kester AD, Hasaart TH, Blanco CE, Hornstra G. Long-chain polyunsaturated fatty acids in preterm infants: status at birth and its influence on postnatal levels. J Pediatr 126: 611–618, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Guzman C, Cabrera R, Cardenas M, Larrea F, Nathanielsz PW, Zambrano E. Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol 572: 97–108, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J Pediatr 143: S1–S8, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Koletzko B, Braun M. Arachidonic acid and early human growth: is there a relation? Ann Nutr Metab 35: 128–131, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Leaf AA, Leighfield MJ, Costeloe KL, Crawford MA. Factors affecting long-chain polyunsaturated fatty acid composition of plasma choline phosphoglycerides in preterm infants. J Pediatr Gastroenterol Nutr 14: 300–308, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Leaf AA, Leighfield MJ, Costeloe KL, Crawford MA. Long chain polyunsaturated fatty acids and fetal growth. Early Hum Dev 30: 183–191, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Macêdo GS Ferreira CL, Menegaz A, Arantes VC, Veloso RV, Carneiro EM, Boschero AC, Oller do Nascimento CM, Latorraca MQ, Gomes-da-Silva MH. Correlation of serum leptin and insulin levels of pregnant protein-restricted rats with predictive obesity variables. Braz J Med Biol Res 41: 519–525, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Mercuri O, Elena de Tomas M, Itarte H. Prenatal protein depletion and delta 9, delta 6 and delta 5 desaturases in the rat. Lipids 14: 822–825, 1979 [PubMed] [Google Scholar]
- 23.Nathanielsz PW. Animal models that elucidate basic principles of the developmental origins of adult diseases. ILAR J 47: 73–82, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Neuringer M, Connor WE. n-3 fatty acids in the brain and retina: evidence for their essentiality. Nutr Rev 44: 285–294, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature 427: 411–412, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Ozanne SE, Martensz ND, Petry CJ, Loizou CL, Hales CN. Maternal low protein diet in rats programmes fatty acid desaturase activities in the offspring. Diabetologia 41: 1337–1342, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Palacin M, Lasuncion MA, Asuncion M, Herrera E. Circulating metabolite utilization by periuterine adipose tissue in situ in the pregnant rat. Metabolism 40: 534–539, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr 70: 811–816, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Cruz M, Tovar AR, Palacios-González B, Del Prado M, Torres N. Synthesis of long-chain polyunsaturated fatty acids in lactating mammary gland: role of Delta5 and Delta6 desaturases, SREBP-1, PPARalpha, and PGC-1. J Lipid Res 47: 553–560, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez A, Sarda P, Nessmann C, Boulot P, Leger CL, Descomps B. Delta6- and delta5-desaturase activities in the human fetal liver: kinetic aspects. J Lipid Res 39: 1825–1832, 1998 [PubMed] [Google Scholar]
- 31.Roseboom TJ, van der Meulen JH, Ravelli AC, van Montfrans GA, Osmond C, Barker DJ, Bleker OP. Blood pressure in adults after prenatal exposure to famine. J Hypertens 17: 325–330, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Ruyle M, Connor WE, Anderson GJ, Lowensohn RI. Placental transfer of essential fatty acids in humans: venous-arterial difference for docosahexaenoic acid in fetal umbilical erythrocytes. Proc Natl Acad Sci USA 87: 7902–7906, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandra P, David F. High-throughput capillary gas chromatography for the determination of polychlorinated biphenyls and fatty acid methyl esters in food samples. J Chromatogr Sci 40: 248–253, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res 24: 69–176, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Schiefermeier M, Yavin E. n-3 Deficient and docosahexaenoic acid-enriched diets during critical periods of the developing prenatal rat brain. J Lipid Res 43: 124–131, 2002 [PubMed] [Google Scholar]
- 36.Seeber RM, Smith JT, Waddell BJ. Plasma leptin-binding activity and hypothalamic leptin receptor expression during pregnancy and lactation in the rat. Biol Reprod 66: 1762–1767, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Sprecher H, Luthria DL, Mohammed BS, Baykousheva SP. Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J Lipid Res 36: 2471–2477, 1995 [PubMed] [Google Scholar]
- 38.Waterland RA, Garza C. Potential mechanisms of metabolic imprinting that lead to chronic disease. Am J Clin Nutr 69: 179–197, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Woltil HA, van Beusekom CM, Schaafsma A, Muskiet FA, Okken A. Long-chain polyunsaturated fatty acid status and early growth of low birth weight infants. Eur J Pediatr 157: 146–152, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Zambrano E, Bautista CJ, Deás M, Martínez-Samayoa PM, González-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol 571: 221–230, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zambrano E, Martínez-Samayoa PM, Bautista CJ, Deás M, Guillén L, Rodríguez-González GL, Guzmán C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol 566: 225–236, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zambrano E, Rodríguez-González GL, Guzmán C, García-Becerra R, Boeck L, Díaz L, Menjivar M, Larrea F, Nathanielsz PW. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol 563: 275–284, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y, Nijland M, Miller M, Ford S, Nathanielsz PW, Brenna JT. The influence of maternal early to mid-gestation nutrient restriction on long chain polyunsaturated fatty acids in fetal sheep. Lipids 43: 525–531, 2008. [DOI] [PubMed] [Google Scholar]