Abstract
Pioglitazone preserves pancreatic β-cell morphology and function in diabetic animal models. In this study, we investigated the molecular mechanisms by which pioglitazone protects β-cells in diabetic db/db mice. In addition to the morphological analysis of the islets, gene expression profiles of the pancreatic islet were analyzed using laser capture microdissection and were compared with real-time RT-PCR of db/db and nondiabetic m/m mice treated with or without pioglitazone for 2 wk or 2 days. Pioglitazone treatment (2 wk) ameliorated dysmetabolism, increased islet insulin content, restored glucose-stimulated insulin secretion, and preserved β-cell mass in db/db mice but had no significant effects in m/m mice. Pioglitazone upregulated genes that promote cell differentiation/proliferation in diabetic and nondiabetic mice. In db/db mice, pioglitazone downregulated the apoptosis-promoting caspase-activated DNase gene and upregulated anti-apoptosis-related genes. The above-mentioned effects of pioglitazone treatment were also observed after 2 days of treatment. By contrast, the oxidative stress-promoting NADPH oxidase gene was downregulated, and antioxidative stress-related genes were upregulated, in db/db mice treated with pioglitazone for 2 wk, rather than 2 days. Morphometric results for proliferative cell number antigen and 4-hydroxy-2-noneal modified protein were consistent with the results of gene expression analysis. The present results strongly suggest that pioglitazone preserves β-cell mass in diabetic mice mostly by two ways; directly, by acceleration of cell differentiation/proliferation and suppression of apoptosis (acute effect); and indirectly, by deceleration of oxidative stress because of amelioration of the underlying metabolic disorder (chronic effect).
Keywords: β-cell dysfunction, pioglitazone, oxidative stress, cell proliferation, cell apoptosis
type 2 diabetes mellitus is a progressive disease that is caused by both impaired insulin secretion and insulin resistance (4, 33). Impairment of insulin secretion in type 2 diabetes is assumed to be associated with functional abnormalities in pancreatic β-cells because of genetic alterations. However, many aspects of the molecular mechanisms of impaired β-cell function remain unclear. Prevention of the progression of pancreatic β-cell dysfunction in patients with diabetes mellitus is critical to the long-term management of this disease.
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor γ (PPARγ) agonists, very effectively improve glycemic control in type 2 diabetics (24). Furthermore, when compared with a placebo, pioglitazone treatment reduced the rate of permanent use of insulin in patients with type 2 diabetes (7). Reportedly, TZDs also prevent diabetes. The troglitazone in the prevention of diabetes (TRIPOD) study revealed that troglitazone treatment reduced the incidence of type 2 diabetes by 55% in women with a history of gestational diabetes (1, 2). Other TZDs also significantly reduced the risk of incident diabetes (9). Moreover, in prediabetic Otsuka Long-Evans Tokushima fatty (OLETF) rats, pioglitazone treatment completely prevented the development of diabetes (3). Thus the results of these studies raise the possibility that PPARγ agonists might prevent the development of diabetes.
A large body of evidence suggests that TZDs both protect against β-cell damage and preserve β-cell function (3, 13, 15, 22, 36). In obese Zucker rats, rosiglitazone maintains β-cell proliferation and prevents loss of β-cells (11). Similarly, in animal models of diabetes, pioglitazone preserves pancreatic islet structure, β-cell mass, and insulin secretory function (5, 23). The previous study reported that treatment with pioglitazone restored β-cell insulin secretion in obese diabetic db/db mice by preservation of β-cell mass resulting from a reduction in oxidative stress (14). Furthermore, pioglitazone both protects human β-cells against apoptosis or loss of function after exposure to interleukin-1β or high-glucose concentrations in vitro (39) and enhances glucose-sensitive insulin secretion (40). Recently, TZD treatment was reported to improve β-cell function, which is strongly correlated with glycemic control, in patients with type 2 diabetes mellitus (12). We previously reported that early pioglitazone treatment preserved islet morphology and β-cell function in obese diabetic db/db mice (17). Thus, although previous studies clearly demonstrate that TZDs prevent β-cell damage in the diabetic state, the precise mechanism of this effect remains to be elucidated.
The purpose of the present study was to identify the molecular mechanisms by which pioglitazone prevents pancreatic β-cell damage in db/db mice. The results of the present study clearly show that pioglitazone preserves β-cell mass in diabetic mice not only by promotion of cell differentiation/proliferation and suppression of apoptosis (acute effect) but also by reduction of oxidative stress because of amelioration of the underlying metabolic disorder (chronic effect).
MATERIALS AND METHODS
Animals.
Six-week-old male BKS.Cg-+Leprdb/+Leprdb/Jcl (db/db) mice and BKS.Cg-m +/m+/Jcl (m/m) control mice were obtained from Clea Japan (Tokyo, Japan). All animals were housed in the animal facility of the Kawasaki Medical School on a 12:12-h light-dark cycle. The animals were provided free access to standard feed (MF; Oriental Yeast, Tokyo, Japan) and tap water and were maintained at 25°C. These studies were approved by the Animal Use Committee of Kawasaki Medical School (no. 07–089) and were conducted in compliance with the Animal Use Guidelines of the Kawasaki Medical School.
Intervention protocol.
For the long-term study, 10-wk-old db/db mice were divided into the following two groups and treated for two weeks: the pioglitazone group (n = 5) received pioglitazone (30 mg·kg body wt−1·day−1, oral), and the control group (n = 5) was administered vehicle. Normal control m/m mice of the same age were also treated with pioglitazone (30 mg·kg body wt−1·day−1, oral). The pioglitazone was provided by Takeda Pharmaceuticals (Osaka, Japan) and was prepared as an emulsion in distilled water containing 0.5% carboxymethylcellulose. Both the pioglitazone and vehicle were administered orally using a stomach probe. The pioglitazone dosage was chosen based on a previous report (20).
To assess the direct, short-term effects of pioglitazone, independent of any improvements in metabolism, 10-wk-old db/db and m/m mice were treated for 2 days with either pioglitazone (30 mg·kg body wt−1·day−1, oral; n = 5) or vehicle (n = 5) for 2 days. The animal care procedures were the same as those used in the long-term study.
Blood biochemical markers.
Blood was collected from the tail vein at 10 and 12 wk of age (before and after intervention). Blood glucose was measured immediately, and plasma was separated and stored at −80°C until assay for insulin, triglyceride (TG), nonesterified fatty acid (NEFA), and adiponectin. Blood glucose was measured by the enzyme electrode method using a Free Style kit (Kissei Pharmaceutical, Nagano, Japan). The concentration of insulin in plasma was measured using an ELISA kit (Morinaga Institute of Biological Science, Yokohama, Japan). Plasma TG and NEFA concentrations were measured enzymatically using the Triglyceride E-Test Wako and NEFA C-Test Wako kits (Wako Pure Chemical Industries, Osaka, Japan). The concentration of plasma adiponectin concentration was measured using the mouse adiponectin ELISA kit (Circulex, Nagano, Japan).
Measurement of TG and insulin content in pancreatic islets.
Pancreatic islets were isolated by collagenase digestion, as previously reported (18). Briefly, Hanks’ balanced salt solution (HBSS) containing 1.5 mg/ml collagenase (Collagenase P, Roche, Switzerland) and 10% FBS was infused in the bile duct. The excised pancreas was immersed in HBSS and centrifuged three times (1,100 rpm, 2 min). The resulting pellet was passed through a metal filter (500 μm gauge), and the filtrate was centrifuged using Histopaque-1077 (Sigma, St. Louis, MO). The islets were stored at −80°C until use in the TG and insulin measurement.
Isolated 45–60 pancreatic islets were washed two times in PBS. A high-salt buffer (50 μl; 2 M NaCl, 2 mM EDTA, and 50 mM sodium phosphate) was added, followed by sonication for 1 min to disrupt the pancreatic islets. After centrifugation at 12,000 rpm for 5 min, 10 μl of the supernatant were mixed with 10 μl of t-butanol and 50 μl of Triton X-100-methyl alcohol (1:1). The TG content in the pancreatic islets was measured using a commercially available kit (Triglyceride, E-Test Wako). For insulin measurement, the islets were dissolved in acid ethanol, and insulin content was measured by ELISA, as described above.
Glucose-stimulated insulin secretion from isolated pancreatic islets.
Size-matched pancreatic islets were prepared (5 pancreatic islets/tube) and preincubated in Krebs-Ringer-bicarbonate-HEPES buffer (containing 5 mg/ml BSA, pH 7.4, saturated 95% O2-5% CO2, 37°C, 60 min). The supernatant was replaced with either a 3 or 16.7 mM glucose solution, and the mixture was incubated for an additional 60 min. The supernatant was recovered and stored at −80°C until it could be used in the insulin assay.
Immunohistochemistry.
The pancreas was cut into 4-μm tissue sections, which were then stained with hematoxylin-eosin. To reduce the background staining intensity, sections were immersed in methanol containing 3% hydrogen peroxide, a blocking agent of endogenous peroxidase activity, for 15 min. After being rinsed with PBS (10 mM, pH 7.0), the sections were incubated with either a mixture of antibodies (rabbit anti-glucagon antibody: anti-somatostatin antibody = 1:1; Nichirei) at 25°C for 1 h or a mouse anti-insulin antigen monoclonal antibody, anti-proliferative cell number antigen (PCNA) monoclonal antibody (Nichirei, Tokyo, Japan), or rabbit Ki67 monoclonal antibody (Epitomics), and mouse anti-4-hydroxy-2-noneal modified protein (4-HNE) monoclonal antibody (25 μg/ml; Japan Institute for the Control of Aging, Shizuoka, Japan) at 4°C for 14 h. After rinsing with PBS, a Simple Stain DAB solution (Nichirei) was added, and the mixture was incubated at 25°C for 7 min. For counterstaining, the sections were stained with hematoxylin.
Morphometric analysis.
The image analysis software NIH Image version 1.61 was used to calculate whole pancreas area, islet area, and insulin-positive and -negative cells and to determine the relative islet area and the relative β-cell area. Using a total of 15 sections (5 sections from 3 different areas of the pancreas) for each group of mice, β-cell mass was estimated using the following formula: cell mass (mg) = islet area/(whole pancreas area × pancreas weight) (mg). Cells positive for PCNA and Ki67 staining were quantified by the presence of a dark brown nuclear stain. Observations were made from a minimum of 50 islets and, when quantified, were expressed as a percentage of the total number of islet cells. Using a previously reported method (31), the intensity of the 4-HNE immunoreactivity in the islets was graded semiquantitatively as follows: negative (score 0); weakly positive (lightly stained, but clearly differentiated from negative background, score 1); moderately positive (strongly stained area = 2 places, score 2); and strongly positive (strongly stained area = 3 places, score 3). Scores from a minimum of 50 islets were averaged for each experimental animal.
Laser capture microdissection.
The mice were anesthetized intraperitoneally with pentobarbital sodium (0.05 mg/g). The pancreas was excised, placed in optimum cutting temperature compound, and cryopreserved. Frozen slices of 8 μm thickness were prepared using a cryostat and pasted on slide glasses. Slides were immediately stained and subjected to laser capture microdissection (LCM) or stored at −80°C until staining. After immersion in 70% ethanol and diethyl pyrocarbonate (DEPC)-processed water for 30 s each, the slides were stained with hematoxylin for several seconds and then immersed in DEPC-processed water, followed by 70, 95, and 100% ethanol for 30 s each and, finally, xylene for 5 min. After tissue staining, the islets were irradiated with a laser using a PixCell system (Arcturus, Mountain View, CA). The peripheral area was removed first from slices 30 μm in length, and then the β-cell-rich core area was collected.
Real-time PCR.
RNA samples from the islet core tissue obtained by LCM were extracted using a PicoPure RNA Isolation Kit (PN 12206-01; Arcturus). TaqMan Reverse Transcription Reagents (N808-0234; Applied Biosystems) were used for reverse transcription. Random hexamers were used as the primers for cDNA synthesis. The primers were designed using Primer Express, based on mRNA sequences downloaded from the GenBank nucleotide database.
A reaction mixture was prepared by combining 0.5 μl of sample, 1 μl of 50 nM primers, 5 μl of SYBR Green PCR Master Mix (Applied Biosystems) solution, and 3.5 μl of diluent solution. Dissociation curve analysis was performed in all experiments to determine the dissociation temperature, and the size of the PCR products was confirmed using agarose gel electrophoresis. To quantify gene expression, 2−ΔCT was calculated, using 18S rRNA as an internal control and compared with the mRNA value for each control group of mice.
Statistical analysis.
All data are means ± SE. A Mann-Whitney U-test was used for comparison among multiple groups, and P < 0.05 was regarded as significant. Statistical analyses were performed using StatView version 5 (SAS).
RESULTS
Changes in body weight and biochemical markers.
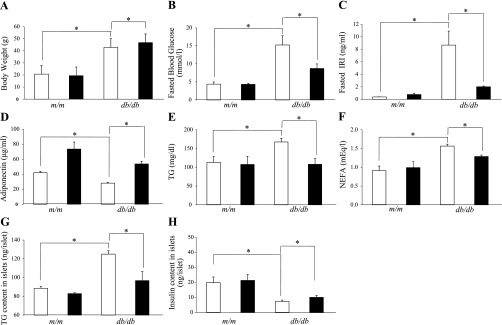
At baseline (10 wk of age), body weight, fasting blood glucose (FBG) and fasting plasma insulin (FIRI) concentrations were similar between the control and pioglitazone groups for either db/db or m/m mice (data not shown). At 12 wk of age, body weight, FBG, FIRI, TG, and NEFA were significantly greater, and plasma adiponectin concentration was lower in db/db mice compared with m/m mice (Fig. 1, A–F).
Fig. 1.
Changes in body weight (A), fasting blood glucose (B), fasting plasma insulin (C), serum adiponectin (D), triglyceride (TG; E), nonesterified fatty acid (NEFA; F), islet TG content (G), and islet insulin content (H) in nondiabetic m/m mice and diabetic db/db mice treated with or without pioglitazone for 2 wk (long-term study). The daily dose of pioglitazone was 30 mg/kg body wt, and the control group was administered vehicle. Open bars, control group; filled bars, pioglitazone group. Data are means ± SE for 5 mice/group. *P < 0.05
In the long-term study, pioglitazone increased the body weight of db/db mice (Fig. 1A). FBG and plasma insulin concentrations were significantly reduced in pioglitazone-treated mice compared with controls (Fig. 1, B and C). Plasma adiponectin concentrations were significantly increased after 2 wk of pioglitazone treatment (Fig. 1D). Pioglitazone significantly lowered plasma TG and NEFA concentrations (Fig. 1, E and F). By contrast, in m/m mice, pioglitazone had no effect on body weight or biochemical parameters. The TG content in the pancreatic islets of db/db mice was significantly greater than for m/m mice, and pioglitazone lowered the islet TG content level in db/db mice (Fig. 1G). At the age of 12 wk, the islet insulin content in db/db mice was less than that in m/m mice, and pioglitazone increased the insulin content in db/db but not in m/m mice (Fig. 1H).
In the short-term study, 2 days of pioglitazone treatment did not affect any of the metabolic parameters, such as body weight, FBG, FIRI, plasma adiponectin concentration, and islet TG and insulin contents in either m/m or db/db mice [Supplemental. Fig. S1 (Supplemental data for this article can be found on the American Journal of Physiology: Endocrinology and Metabolism website.)].
Morphological changes in pancreatic islets.
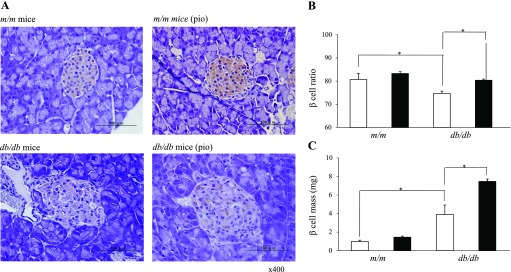
Figure 2 shows typical immunostaining patterns of pancreatic islet tissues from m/m and db/db mice treated with or without pioglitazone for 2 wk. The islets of db/db mice appeared much larger and more varied in size than m/m mice. Moreover, the islet architecture of db/db mice was disrupted, whereas that of m/m mice appeared normal. The intensity of immunostaining for insulin in islets from db/db mice was less than that in m/m mice.
Fig. 2.
Effects of 2 wk of pioglitazone treatment on islet morphology (at 12 wk of age). A: representative immunostaining for insulin in pancreatic islet tissue sections from m/m and db/db mice with or without pioglitazone treatment. Effect of pioglitazone on the β-cell ratio (B) and β-cell mass (C). The β-cell ratio and cell mass of each group at 12 wk of age are shown. The β-cell ratio was calculated as a percentage of β-cells to whole islet cells. Open bars, control group; filled bars, pioglitazone group. Data are means ± SE for 5 mice/group. P < 0.05 vs. m/m mice (*) and vs. control group (†).
Pioglitazone increased β-cell mass in db/db mice.
In the long-term study, the ratio of β-cells to total islet cells in the islets was lower in db/db mice than in m/m mice, and pioglitazone treatment increased the β-cell ratio in db/db mice (Fig. 2B). In the previous study, we demonstrated that the β-cell mass in db/db mice increased to compensate hyperglycemia and then decreased significantly with age (17). At the age of 12 wk, the β-cell mass in db/db mice was still greater than that in m/m mice. Pioglitazone treatment further increased the β-cell mass in db/db mice by enlarging islet size and increasing the β-cell ratio. In m/m mice, the β-cell ratio and β-cell mass appeared to be slightly increased in the pioglitazone treatment group but were not significantly different from the untreated control group (Fig. 2C).
Glucose-stimulated insulin secretion from isolated pancreatic islets.
In the long-term study, basal insulin secretion assessed by addition of 3 mM glucose was significantly lower in db/db mice than in the m/m mice, and no significant difference was observed between pioglitazone-treated and -untreated control mice. A high-concentration glucose challenge (16.7 mM) induced marked insulin secretion in both strains of mice, but insulin secretion in db/db mice remained less than that in m/m mice. Of note, insulin secretion in db/db mice was restored with pioglitazone treatment (Table 1).
Table 1.
Glucose-stimulated insulin secretion from pancreatic islets of m/m and db/db mice treated with or without pioglitazone for 2 wk (long-term study)
| 3 mM | 16.7 mM | |
|---|---|---|
| m/m control | 0.60 ± 0.07 | 5.68 ± 0.38 |
| m/m pio-treated | 0.41 ± 0.08 | 6.73 ± 1.34 |
| db/db control | 0.18 ± 0.05* | 1.51 ± 0.21* |
| db/db pio-treated | 0.20 ± 0.09* | 3.92 ± 0.83*† |
Data are means ± SE ng/ml/islet (5 independent experiments per group).
: P < 0.05 versus control m/m mice.
: P < 0.05 versus control db/db mice.
In the short-term study, addition of glucose at a high concentration (16.7 mM) significantly potentiated the insulin response in both m/m and db/db mice. However, pioglitazone failed to restore the reduced insulin response in db/db mice (data not shown).
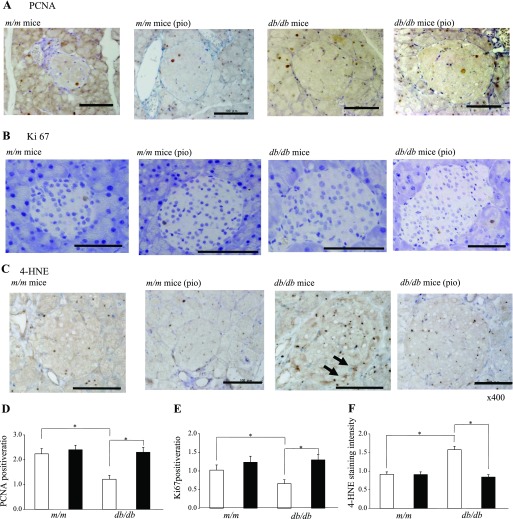
Morphometric analysis of islet cells.
Positive staining for PCNA and Ki67, markers of cell proliferation, was found exclusively in the nuclei of β-cells in the islet (Fig. 3, A and B). The ratio of PCNA- and Ki67-positive cells in the islets of db/db mice was less than that observed in m/m mice, and pioglitazone treatment for 2 wk significantly increased the intensity of staining in db/db mice (Fig. 3, D and E). Positive staining for 4-HNE-modified proteins, a marker of oxidative stress-related lipid peroxidation products, was evident in the cytoplasm of islets from untreated db/db mice (Fig. 3C). The intensity of staining was significantly decreased by pioglitazone treatment for 2 wk in db/db mice (Fig. 3F). These observations were not observed in mice treated for 2 days (data not shown).
Fig. 3.
Effect of pioglitazone on cell proliferation and oxidative stress markers in β-cells. Representative immunostaining for proliferative cell nuclear antigen (PCNA; A), Ki67 (B), and 4-hydroxy-2-noneal modified protein (4-HNE; C) in pancreatic tissue sections. Bars = 100 μm. D–F: PCNA, Ki67, and 4-HNE expression were analyzed semiquantitatively. Open bars, control group; filled bars, pioglitazone group. Data are means ± SE for 5 mice/group. *P < 0.05.
Gene expression profiles in pancreatic islets.
Gene expression profiles in the islet core area were directly analyzed using LCM and real-time RT-PCR. In the long-term experiment (Table 2), PPARγ was significantly downregulated in both m/m and db/db mice treated with pioglitazone (P < 0.05). Pioglitazone decreased expression of the insulin II gene in both strains of mice. The cell differentiation-promoting genes Pdx-1, NeuroD, and Nkx2–2 were significantly upregulated in pioglitazone-treated db/db mice compared with controls. Interestingly, upregulation of the NeuroD and Nkx2–2 genes was also observed in pioglitazone-treated m/m mice. Pioglitazone significantly increased the expression of genes that promote cell proliferation [cyclin D1, cyclin E1, and extracellular signal-regulated kinase 1 (ERK1)] in both m/m and db/db mice. The effects of pioglitazone on expression of these cell kinetics-associated genes were more pronounced in db/db mice. On the other hand, the apoptosis-related caspase-activated DNase (CAD) gene, which was expressed at high levels in db/db mice, was significantly downregulated, and the anti-apoptotic bcl-2 and ICAD-S genes were upregulated in pioglitazone-treated db/db mice. The oxidative stress-related genes such as NADPH oxidase, catalase, and glutathione peroxidase 1 (GSHPx) were highly expressed in db/db but not in m/m mice. NADPH oxidase gene expression was not detected any longer after pioglitazone treatment for 2 wk. Catalase, GSHPx, and superoxide dismutase 2 gene expressions, which were associated with anti-oxidative stress, were significantly increased with pioglitazone treatment in db/db mice but not in m/m mice. Expression of the ER stress-related X-box binding protein-1 gene was downregulated with pioglitazone treatment in db/db mice but not in m/m mice. Sterol regulatory element-binding protein 1c and tumor necrosis factor gene expression, which were elevated in db/db mice, were significantly reduced by pioglitazone treatment.
Table 2.
Effects of pioglitazone on gene expression in the islet core area measured by LCM and real-time RT-PCR
| Gene Name | Abbreviation | ID | m/m cont | pio | db/db cont | pio |
|---|---|---|---|---|---|---|
| Pancreatic hormones | ||||||
| InsulinI | InsulinI | X04725 | 1 | 0.97 | 1 | 0.69 |
| InsulinII | InsulinII | NM 008384 | 1 | 0.68 | 1 | 0.39* |
| Glucagon | Glucagon | NM 008100 | nd | nd | 1 | 0.82 |
| Somatostatin | Somatostatin | NM 009215 | nd | nd | 1 | 0.75 |
| Pancreatic polypeptide | PP | NM 008918 | nd | nd | 1 | 1.23 |
| Amylin | Amylin | NM 010491 | 1 | 0.78 | 1 | 0.69 |
| PPARs | ||||||
| Peroxisome proliferator activated receptor gamma | PPARγ | NM 011146 | 1 | 0.06* | 1 | 0.07* |
| Cell differentiation | ||||||
| Pancreatic and duodenal homeobox 1 | PDX-1 | NM 008814 | 1 | 1.00 | 1 | 4.00* |
| Hairy and enhancer of split 1 | Hes1 | NM 008235 | 1 | 0.65 | 1 | 0.49 |
| Neurogenic differentiation 1 | NeuroD | NM 010894 | 1 | 4.00* | 1 | 4.15* |
| NK2 transcription factor related, locus 2 | Nkx2–2 | NM 010919 | nd | + | 1 | 3.00* |
| Cell proliferation | ||||||
| Cyclin D1 | Ccnd1 | NM 007631 | nd | + | 1 | 2.08* |
| Cyclin E1 | Ccne1 | NM 007633 | nd | + | nd | + |
| Extracellular signal-regulated kinase 1 | ERK1 | NM 011952 | nd | + | 1 | 5.04* |
| Apoptosis | ||||||
| β-Cell leukemia/lymphoma2 | Bcl-2 | NM 177410 | 1 | 1.56 | 1 | 4.61* |
| Caspase-activated DNase | CAD | AB009377 | nd | nd | 1 | 0.01* |
| Inhibitor of caspase-activated DNase | ICAD-S | AB009376 | nd | nd | nd | + |
| Oxidative stress and ER stress | ||||||
| Nicotinamide adenine dinucleotide phosphate oxidase | NADPH oxidase | NM 172203 | nd | nd | 1 | nd |
| Catalase | Catalase | BC047126 | nd | nd | 1 | 2.56* |
| Glutathione peroxidase 1 | GSHPx | NM 008160 | nd | nd | 1 | 3.00* |
| Superoxide dismutase 2 | SOD2 | NM 013671 | 1 | 0.68 | 1 | 6.02* |
| X-box binding protein-1 | XBP-1 | NM 013842 | 1 | 0.90 | 1 | nd |
| Lipid synthesis | ||||||
| Fatty acid synthase | FAS | NM 007988 | nd | nd | 1 | 0.80 |
| Fatty acid transporter 4 | FATP4 | NM 011989 | nd | nd | 1 | 0.97 |
| Sterol regulatory element-binding protein-1c | SREBP1c | AL669954 | nd | nd | 1 | 0.45* |
| Inflammation | ||||||
| Tumor necrosis factor | TNF | NM 013693 | nd | nd | 1 | 0.25* |
The nondiabetic m/m and diabetic db/db mice were treated with or without pioglitazone for 2 wk (long-term study). The data are the mRNA levels of pioglitazone-treated mice relative to mRNA of control mice. cont: control group, pio: pioglitazone group. Each result is the mean of 5 experiments per group. The RNA sample for each experiment was collected from the islet core area in one pancreas. When a significant level of mRNA was detected ≤2 experiments out of 5 total experiments, the result was reported as not detected (nd). +: mRNA was detected ≥3 out of 5 total experiments, but the ratio was not calculated.
: P < 0.05 versus control mice.
In the short-term study (Supplemental Table S1), PPARγ gene expression in β-cells was significantly reduced with pioglitazone treatment in both strains of mice. In db/db mice, pioglitazone significantly upregulated genes related to both cell differentiation (Pdx-1 and Nkx2–2) and cell proliferation (cyclin E1 and ERK1). The bcl-2 gene was upregulated, and the CAD gene was downregulated, after 2 days of pioglitazone treatment. On the other hand, expression of oxidative stress-related and lipid synthesis-related genes was not affected by pioglitazone in the short-term intervention.
To confirm the LCM data, we further examined mRNA levels of several representative islet genes indicated below in isolated islets from m/m and db/db mice treated with or without pioglitazone for 2 wk (Table 3). Gene expression profiles of insulin II, PPARγ, Pdx-1, ERK1, and bcl-2 were not different significantly from those in the islet core observed in the LCM study, supporting an availability of this method.
Table 3.
Effects of pioglitazone on gene expression in the islet from m/m and db/db mice treated with or without pioglitazone for 2 wk
| Gene Name | Abbreviation | ID | m/m cont | pio | db/db cont | pio |
|---|---|---|---|---|---|---|
| Pancreatic hormones | ||||||
| InsulinI | InsulinI | X04725 | 1 | 0.89 | 1 | 0.60 |
| InsulinII | InsulinII | NM 008384 | 1 | 0.78 | 1 | 0.29* |
| Amylin | Amylin | NM 010491 | 1 | 1.02 | 1 | 0.60 |
| PPARs | ||||||
| Peroxisome proliferator activated receptor gamma | PPARγ | NM 011146 | 1 | 0.12* | 1 | 0.16* |
| Cell differentiation | ||||||
| Pancreatic and duodenal homeobox 1 | PDX-1 | NM 008814 | 1 | 1.20 | 1 | 3.56* |
| Hairy and enhancer of split 1 | Hes1 | NM 008235 | 1 | 0.55 | 1 | 0.40 |
| Neurogenic differentiation 1 | NeuroD | NM 010894 | 1 | 3.15* | 1 | 3.89* |
| NK2 transcription factor related, locus 2 | Nkx2–2 | NM 010919 | 1 | 1.56 | 1 | 2.56* |
| Cell proliferation | ||||||
| Cyclin D1 | Ccnd1 | NM 007631 | nd | + | 1 | 2.56* |
| Cyclin E1 | Ccne1 | NM 007633 | nd | + | nd | + |
| Extracellular signal-regulated kinase 1 | ERK1 | NM 011952 | nd | + | 1 | 4.56* |
| Apoptosis | ||||||
| B-cell leukemia/lymphoma2 | Bcl-2 | NM 177410 | 1 | 1.68 | 1 | 3.98* |
| Caspase-activated DNase | CAD | AB009377 | nd | nd | 1 | 0.12* |
| Inhibitor of caspase-activated DNase | ICAD-S | AB009376 | 1 | 1.88 | nd | + |
| Oxidative stress and ER stress | ||||||
| Nicotinamide adenine dinucleotide phosphate oxidase | NADPH oxidase | NM 172203 | nd | nd | 1 | nd |
| Catalase | Catalase | BC047126 | nd | nd | 1 | 3.01* |
| Glutathione peroxidase 1 | GSHPx | NM 008160 | nd | nd | 1 | 2.96* |
| Superoxide dismutase 2 | SOD2 | NM 013671 | 1 | 0.98 | 1 | 4.56* |
| X-box binding protein-1 | XBP-1 | NM 013842 | 1 | 0.88 | 1 | nd |
| Lipid synthesis | ||||||
| Fatty acid synthase | FAS | NM 007988 | nd | nd | 1 | 0.75 |
| Fatty acid transporter 4 | FATP4 | NM 011989 | nd | nd | 1 | 0.65 |
| Sterol regulatory element-binding protein-1c | SREBP1c | AL669954 | nd | nd | 1 | 0.31* |
| Inflammation | ||||||
| Tumor necrosis factor | TNF | NM 013693 | nd | nd | 1 | 0.15* |
The data are the mRNA levels of pioglitazone-treated mice relative to mRNA of control mice. cont: control group, pio: pioglitazone group. Each result is the mean of 5 experiments per group. The nd (not detected) and + symbols were defined as described for long-term study (Table 2).
P < 0.05 versus control mice.
DISCUSSION
Numerous studies have shown that TZDs preserve both β-cell function and cell mass in animal models of diabetes (3, 11, 13, 15, 22, 23, 36). In this study, we also demonstrated that pioglitazone improved glucose-stimulated insulin secretion from the islets and increased the β-cell mass in diabetic db/db mice. Recent studies reported that the protective effect of TZDs on β-cells was related to enhanced proliferation and to protection against apoptosis and oxidative stress (35). However, the effects of TZDs have been studied mostly at the morphological and functional levels. Regarding the molecular mechanisms of these compounds, rosiglitazone reportedly regulates IPF1 and Foxa2 gene expression in isolated rat islets and MIN6 cells (27), but the molecular mechanisms by which TZD affects β-cells remains to be determined.
The results of the present study strongly suggested that pioglitazone preserves β-cell mass in diabetic mice via two different molecular mechanisms. In the first, pioglitazone directly promotes β-cell differentiation/proliferation and suppresses cell apoptosis by acting as a PPARγ agonist. In the second mechanism, pioglitazone indirectly reduces oxidative stress-related cell apoptosis through its metabolic effects. Pioglitazone effect on β-cell gene expression related to cell kinetics was observed in both the diabetic db/db mice and the nondiabetic m/m mice, irrespective of the treatment duration. In addition, short-term pioglitazone treatment downregulated apoptosis-promoting CAD gene expression in db/db mice and upregulated the anti-apoptotic bcl-2 gene in both m/m and db/db mice. On the other hand, expression of antioxidative stress- and ER stress-related genes was regulated only in diabetic db/db mice after long-term treatment with pioglitazone. These results were consistent with the immunohistological analysis of PCNA, Ki67, and 4-HNE.
Long-term pioglitazone treatment restored the β-cell ratio and increased β-cell mass in db/db mice. Interestingly, insulin II gene expression was rather decreased by pioglitazone treatment for 2 wk, but not for 2 days, suggesting a reduced secretory demand of insulin by an increase in insulin sensitivity (17). Based on the gene expression profiles, a major molecular mechanism by which pioglitazone preserves β-cells in diabetic mice may be suppression of oxidative stress-induced cell apoptosis, acceleration of β-cell kinetics, and deceleration of apoptosis. It is well known that pancreatic β-cells are very sensitive to oxidative stress, which is accelerated by glucolipotoxicity in the diabetic state (16, 28). It is widely accepted that the metabolic effects of TZDs on β-cells are due to a decrease in both lipotoxicity and glucotoxicity (21). In our study, long-term pioglitazone treatment improved hyperglycemia and hypertriglyceridemia, and reduced islet TG content, ameliorating glucolipotoxicity in db/db mice. Therefore, the effects of pioglitazone were clearly evident only in diabetic db/db mice, but not in nondiabetic m/m mice, in which the oxidative stress is reduced and apoptotic pathways are inactivated.
The β-cell dysfunction in type 2 diabetes may result from impaired insulin secretion and/or a decrease in β-cell mass (37). Several pathophysiological mechanisms have been identified as potential contributors to β-cell stress and subsequent dysfunction, including glucotoxicity, lipotoxicity, and increased secretory demand resulting from insulin resistance. In addition, disturbances in secretion of various adipose tissue-secreted factors or cytokines derived from the innate immune system might also play a causal role (6, 10, 21, 28, 32). Furthermore, both hyperglycemia and hyperlipidemia are associated with induction of oxidative stress in β-cells, which in turn causes defective insulin gene expression and increased apoptosis. Increased β-cell apoptosis may be an important factor leading to loss of β-cells and the onset of type 2 diabetes (21, 26, 28).
A previous report suggested that, in db/db islets, chronic hyperglycemia progressively reduced mRNA levels of many genes that play a role in β-cell glucose sensing; several transcription factors important for the maintenance of β-cell differentiation were also reduced (19). In this study, no significant difference in expression of genes related to β-cell glucose sensing was observed between pioglitazone-treated and -untreated db/db mice (data not shown).
The function of PPARγ in human β-cells (8, 38) remains controversial. Dubois et al. (8) reported that PPARγ was present in all three endocrine cell types studied (α-, β-, and δ-cells) using immunohistochemistry. These results support the hypothesis that PPARγ agonists have a direct effect on human pancreatic endocrine cells (8). In fact, in MIN6 cells, pioglitazone suppressed β-cell lipoapoptosis through a reduction in intracellular TG content and oxidative stress (30). Another study demonstrated that overexpression of PPARγ suppressed glucose-stimulated insulin secretion from MIN6 cells, and pioglitazone augmented this inhibitory effect, suggesting a negative role of PPARγ on pancreatic β-cell function (25). On the other hand, troglitazone reportedly stimulates insulin secretion from cultured pancreatic islets (29). Thus the actions of both PPARγ itself and its agonists, TZDs, on pancreatic islet cell function remain incompletely understood.
The results of the present study show that pioglitazone significantly decreased PPARγ expression in db/db and m/m mice. We speculate that significant activation of PPARγ by pioglitazone may reduce its gene expression in a negative-feedback mechanism. Of note, a previous report demonstrated that β-cell-selective elimination of PPARγ resulted in significant islet hyperplasia (29). These observations suggest a relationship between PPARγ activation and β-cell proliferation. On the other hand, troglitazone treatment decreased endogenous PPARγ expression and TG content in the liver of db/db mice, suggesting a relationship between PPARγ expression and TG accumulation (34). In the present study, both islet TG content and PPARγ expression were reduced by pioglitazone. The precise role of PPARγ in the molecular mechanism by which piolglitazone affects β-cell kinetics and function remains to be elucidated.
In conclusion, the current study employed an animal model of obese type 2 diabetes (db/db mice) to investigate the effects of pioglitazone treatment on both morphological and functional changes that occur during the early stages of pancreatic β-cell dysfunction and the molecular mechanisms of these changes. Our results demonstrate that pioglitazone prevented morphological damage to the islets and preserved β-cell function mainly due to both deceleration of oxidative stress-induced cell apoptosis (chronic effect) and acceleration of β-cell differentiation/proliferation (acute and direct effect). The present findings provide a greater understanding of the mechanism by which pioglitazone improves type 2 diabetes in clinical studies.
GRANTS
This study was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (18591008; K. Kaku) and Research Project Grants from Kawasaki Medical School (17-502, 18-501, 19-502; K. Kaku).
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Fumiko Takaki and Miho Kobayashi for excellent technical assistance.
This paper qas presented in abstract form at the 50th annual meeting of the Japan Diabetes Society (Sendai, 2007) and at the 43rd annual meeting of the European Association for the Study of Diabetes (Amsterdam, 2007).
REFERENCES
- 1. Azen SP, Peters RK, Berkowitz K, Kjos S, Xiang A, Buchanan TA. TRIPOD (TRoglitazone In the Prevention Of Diabetes): a randomized, placebo-controlled trial of troglitazone in women with prior gestational diabetes mellitus. Control Clin Trials 19: 217–231, 1998. [DOI] [PubMed] [Google Scholar]
- 2. Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 51: 2796–2803, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Choi SH, Zhao ZS, Lee YJ, Kim SK, Kim DJ, Ahn CW, Lim SK, Lee HC, Cha BS. The different mechanisms of insulin sensitizers to prevent type 2 diabetes in OLETF rats. Diabetes Metab Res Rev 23: 411–418, 2007. [DOI] [PubMed] [Google Scholar]
- 4. DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care 15: 318–368, 1992. [DOI] [PubMed] [Google Scholar]
- 5. Diani AR, Sawada G, Wyse B, Murray FT, Khan M. Pioglitazone preserves pancreatic islet structure and insulin secretory function in three murine models of type 2 diabetes. Am J Physiol Endocrinol Metab 286: E116–E122, 2004. [DOI] [PubMed] [Google Scholar]
- 6. Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M. Mechanisms of beta-cell death in type 2 diabetes. Diabetes 54, Suppl 2: S108–S113, 2005. [DOI] [PubMed] [Google Scholar]
- 7. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366: 1279–1289, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Dubois M, Pattou F, Kerr-Conte J, Gmyr V, Vandewalle B, Desreumaux P, Auwerx J, Schoonjans K, Lefebvre J. Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in normal human pancreatic islet cells. Diabetologia 43: 1165–1169, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Durbin RJ. Thiazolidinedione therapy in the prevention/delay of type 2 diabetes in patients with impaired glucose tolerance and insulin resistance. Diabetes Obes Metab 6: 280–285, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 52: 1–8, 2003. [DOI] [PubMed] [Google Scholar]
- 11. Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, Buckingham RE. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes 50: 1021–1029, 2001. [DOI] [PubMed] [Google Scholar]
- 12. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 292: E871–E883, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Hull RL, Shen ZP, Watts MR, Kodama K, Carr DB, Utzschneider KM, Zraika S, Wang F, Kahn SE. Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes 54: 2235–2244, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Ishida H, Takizawa M, Ozawa S, Nakamichi Y, Yamaguchi S, Katsuta H, Tanaka T, Maruyama M, Katahira H, Yoshimoto K, Itagaki E, Nagamatsu S. Pioglitazone improves insulin secretory capacity and prevents the loss of beta-cell mass in obese diabetic db/db mice: Possible protection of beta cells from oxidative stress. Metabolism 53: 488–494, 2004. [DOI] [PubMed] [Google Scholar]
- 15. Juhl CB, Hollingdal M, Porksen N, Prange A, Lonnqvist F, Schmitz O. Influence of rosiglitazone treatment on beta-cell function in type 2 diabetes: evidence of an increased ability of glucose to entrain high-frequency insulin pulsatility. J Clin Endocrinol Metab 88: 3794–3800, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Kaneto H, Matsuoka TA, Nakatani Y, Kawamori D, Miyatsuka T, Matsuhisa M, Yamasaki Y. Oxidative stress, ER stress, and the JNK pathway in type 2 diabetes. J Mol Med 83: 429–439, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Kawasaki F, Matsuda M, Kanda Y, Inoue H, Kaku K. Structural and functional analysis of pancreatic islets preserved by pioglitazone in db/db mice. Am J Physiol Endocrinol Metab 288: E510–E518, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Kitamura T, Kido Y, Nef S, Merenmies J, Parada LF, Accili D. Preserved pancreatic beta-cell development and function in mice lacking the insulin receptor-related receptor. Mol Cell Biol 21: 5624–5630, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kjorholt C, Akerfeldt MC, Biden TJ, Laybutt DR. Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of beta-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes 54: 2755–2763, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Larsen PJ, Jensen PB, Sorensen RV, Larsen LK, Vrang N, Wulff EM, Wassermann K. Differential influences of peroxisome proliferator-activated receptors gamma and -alpha on food intake and energy homeostasis. Diabetes 52: 2249–2259, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 23: 201–229, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Lin CY, Gurlo T, Haataja L, Hsueh WA, Butler PC. Activation of peroxisome proliferator-activated receptor-gamma by rosiglitazone protects human islet cells against human islet amyloid polypeptide toxicity by a phosphatidylinositol 3′-kinase-dependent pathway. J Clin Endocrinol Metab 90: 6678–6686, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Matsui J, Terauchi Y, Kubota N, Takamoto I, Eto K, Yamashita T, Komeda K, Yamauchi T, Kamon J, Kita S, Noda M, Kadowaki T. Pioglitazone reduces islet triglyceride content and restores impaired glucose-stimulated insulin secretion in heterozygous peroxisome proliferator-activated receptor-gamma-deficient mice on a high-fat diet. Diabetes 53: 2844–2854, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Mudaliar S, Henry RR. New oral therapies for type 2 diabetes mellitus: The glitazones or insulin sensitizers. Annu Rev Med 52: 239–257, 2001. [DOI] [PubMed] [Google Scholar]
- 25. Nakamichi Y, Kikuta T, Ito E, Ohara-Imaizumi M, Nishiwaki C, Ishida H, Nagamatsu S. PPAR-gamma overexpression suppresses glucose-induced proinsulin biosynthesis and insulin release synergistically with pioglitazone in MIN6 cells. Biochem Biophys Res Commun 306: 832–836, 2003. [DOI] [PubMed] [Google Scholar]
- 26. Piro S, Anello M, Di Pietro C, Lizzio MN, Patane G, Rabuazzo AM, Vigneri R, Purrello M, Purrello F. Chronic exposure to free fatty acids or high glucose induces apoptosis in rat pancreatic islets: possible role of oxidative stress. Metabolism 51: 1340–1347, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Richardson H, Campbell SC, Smith SA, Macfarlane WM. Effects of rosiglitazone and metformin on pancreatic beta cell gene expression. Diabetologia 49: 685–696, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem 279: 42351–42354, 2004. [DOI] [PubMed] [Google Scholar]
- 29. Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu CH, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, Spiegelman BM. Targeted elimination of peroxisome proliferator-activated receptor gamma in beta cells leads to abnormalities in islet mass without compromising glucose homeostasis. Mol Cell Biol 23: 7222–7229, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saitoh CpC Y, Noma K, Ueno H, Mizuta M, Nakazato M. Pioglitazone attenuates fatty acid-induced oxidative stress and apoptosis in pancreatic bata-cells. Diabete Obesity Metab 10: 563–573, 2007. [DOI] [PubMed] [Google Scholar]
- 31. Shao J, Iwashita N, Ikeda F, Ogihara T, Uchida T, Shimizu T, Uchino H, Hirose T, Kawamori R, Watada H. Beneficial effects of candesartan, an angiotensin II type 1 receptor blocker, on beta-cell function and morphology in db/db mice. Biochem Biophys Res Commun 344: 1224–1233, 2006. [DOI] [PubMed] [Google Scholar]
- 32. Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA 95: 2498–2502, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taylor SI, Accili D, Imai Y. Insulin resistance or insulin deficiency. Which is the primary cause of NIDDM? Diabetes 43: 735–740, 1994. [DOI] [PubMed] [Google Scholar]
- 34. Uno K, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Imai J, Hasegawa Y, Gao J, Kaneko K, Iwasaki H, Ishihara H, Sasano H, Inukai K, Mizuguchi H, Asano T, Shiota M, Nakazato M, Oka Y. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science 312: 1656–1659, 2006. [DOI] [PubMed] [Google Scholar]
- 35. Wada K, Nakajima A, Katayama K, Kudo C, Shibuya A, Kubota N, Terauchi Y, Tachibana M, Miyoshi H, Kamisaki Y, Mayumi T, Kadowaki T, Blumberg RS. Peroxisome proliferator-activated receptor gamma-mediated regulation of neural stem cell proliferation and differentiation. J Biol Chem 281: 12673–12681, 2006. [DOI] [PubMed] [Google Scholar]
- 36. Wang X, Zhou L, Shao L, Qian L, Fu X, Li G, Luo T, Gu Y, Li F, Li J, Zheng S, Luo M. Troglitazone acutely activates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Life Sci 81: 160–165, 2007. [DOI] [PubMed] [Google Scholar]
- 37. Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 53, Suppl 3: S16–S21, 2004. [DOI] [PubMed] [Google Scholar]
- 38. Welters HJ, McBain SC, Tadayyon M, Scarpello JH, Smith SA, Morgan NG. Expression and functional activity of PPARgamma in pancreatic beta cells. Br J Pharmacol 142: 1162–1170, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zeender E, Maedler K, Bosco D, Berney T, Donath MY, Halban PA. Pioglitazone and sodium salicylate protect human beta-cells against apoptosis and impaired function induced by glucose and interleukin-1beta. J Clin Endocrinol Metab 89: 5059–5066, 2004. [DOI] [PubMed] [Google Scholar]
- 40. Zhang F, Sjoholm K, Zhang Q. Pioglitazone acutely influences glucose-sensitive insulin secretion in normal and diabetic human islets. Biochem Biophys Res Commun 351: 750–755, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.