Abstract
TBC1D4 (also known as AS160) regulates glucose transporter 4 (GLUT4) translocation and glucose uptake in adipocytes and skeletal muscle. Its mode of action involves phosphorylation of serine (S)/threonine (T) residues by upstream kinases resulting in inactivation of Rab-GTPase-activating protein (Rab-GAP) activity leading to GLUT4 mobilization. The majority of known phosphorylation sites on TBC1D4 lie within the Akt consensus motif and are phosphorylated by insulin stimulation. However, the 5′-AMP-activated protein kinase (AMPK) and other kinases may also phosphorylate TBC1D4, and therefore we hypothesized the presence of additional phosphorylation sites. Mouse skeletal muscles were contracted or stimulated with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), and muscle lysates were subjected to mass spectrometry analyses resulting in identification of novel putative phosphorylation sites on TBC1D4. The surrounding amino acid sequence predicted that S711 would be recognized by AMPK. Using a phosphospecific antibody against S711, we found that AICAR and contraction increased S711 phosphorylation in mouse skeletal muscle, and this increase was abolished in muscle-specific AMPKα2 kinase-dead transgenic mice. Exercise in human vastus lateralis muscle also increased TBC1D4 S711 phosphorylation. Recombinant AMPK, but not Akt1, Akt2, or PKCζ, phosphorylated purified muscle TBC1D4 on S711 in vitro. Interestingly, S711 was also phosphorylated in response to insulin in an Akt2- and rapamycin-independent, but a wortmannin-sensitive, manner, suggesting this site is regulated by one or more additional upstream kinases. Despite increased S711 phosphorylation with AICAR, contraction, and insulin, mutation of S711 to alanine did not alter glucose uptake in response to these stimuli. S711 is a novel TBC1D4 phosphorylation site regulated by AMPK in skeletal muscle.
Keywords: mass spectrometry, AS160, glucose metabolism
tbc1d4/akt substrate of 160 kDa (AS160) is the fourth member of the TBC1D family of Rab-GTPase-activating proteins (Rab-GAP). TBC1D4 was found to be an Akt substrate in cultured adipocytes (22) and is expressed in insulin-responsive tissues in both rodents and humans (34, 38). TBC1D4 has been proposed to regulate Rab proteins involved in glucose transporter 4 (GLUT4) vesicle mobilization to the plasma membrane (22, 25, 33). TBC1D4 has multiple domains and is regulated by phosphorylation of specific serine (S)/threonine (T) residues. For example, in cultured adipocytes and skeletal muscle, expression of TBC1D4 mutated on S318, S588, T642, and S751 residues to alanine (known as the 4P mutant) reduces GLUT4 translocation and glucose uptake in response to insulin (24, 33). Furthermore, expression of a mutant TBC1D4 construct containing a mutation of the critical arginine residue (R973K) responsible for TBC1D4 GAP activity reverses the inhibitory effect of the 4P mutant (24, 33). These data suggest that phosphorylation of TBC1D4 regulates Rab-GAP activity mediated by R973. TBC1D4 is a multikinase substrate since activation of several kinases mediates TBC1D4 phosphorylation (18, 35). As such, TBC1D4 may be a point of convergence for upstream signaling events, and unraveling how individual kinases influence phosphorylation status and ultimately activity of TBC1D4 may enhance our mechanistic understanding of vesicular translocation.
TBC1D4 phosphorylation is commonly evaluated using the phospho-Akt substrate (PAS) antibody, which recognizes phosphorylated Akt substrates in an (R/K)X(R/K)XXS*/T* recognition motif. Although TBC1D4 contains at least six amino acid residues phosphorylated by Akt, the PAS antibody may not detect more than one or two of these sites (18, 22). The PAS antibody also strongly detects phosphorylated TBC1D1, a TBC1D4 paralog that migrates to a similar molecular weight during SDS-PAGE (6, 30, 34). Therefore, to specifically investigate how different stimuli affect TBC1D4 and TBC1D1 phosphorylation, development of phospho-antibodies against specific S/T residues on TBC1D4 and TBC1D1 is warranted (7, 18, 38).
Most reported TBC1D4 phosphorylation sites have a perfect Akt consensus sequence (i.e., RXRXXS*/T*; Ref. 1). However, muscle contraction, which stimulates GLUT4 translocation, increases the activity of a host of protein kinases in skeletal muscle including the AMP-activated protein kinase (AMPK; Ref. 16). Therefore, we hypothesized the presence of additional contraction-stimulated phosphorylation sites on TBC1D4. Here, we report the identification of several new candidate TBC1D4 phosphorylation sites found in mouse skeletal muscle treated with contraction and/or the AMPK activator, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR). One of these novel sites, S711, was found to be located within a consensus AMPK recognition motif (36). In addition, S711 was found to be located in the splice exon specific for the long version of TBC1D4, which is the major splice isoform in skeletal muscle (34, 38). We developed a phosphospecific antibody and investigated the regulation of S711 phosphorylation in both mouse and human skeletal muscle.
EXPERIMENTAL PROCEDURES
Animals.
Protocols for animal use were in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Joslin Diabetes Center and the National Institutes of Health. Protocols were also approved by the Danish Animal Experimental Inspectorate and complied with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Council of Europe no. 123, Strasbourg, France, 1985). Female ICR mice (8–10 wk of age) were purchased from Charles River Laboratories (Wilmington, MA). Female Akt2 knockout (KO) and wild-type (WT) littermates on a C57BL/6N background (10–12 wk of age; Ref. 8) and female muscle-specific AMPKα2 kinase-dead (KD) mice and WT littermates on a C57BL/6J background (12–14 wk of age; Ref. 26) were also studied. All mice were housed in a 12:12-h light-dark cycle and fed a standard laboratory chow and water ad libitum.
In vivo insulin and AICAR administration and in situ contraction of tibialis anterior muscles.
For insulin, AICAR, and contraction experiments, mice were anesthetized by intraperitoneal injection of sodium pentobarbital (60 mg/kg). To elicit a maximal insulin or AICAR response, mice were injected intraperitoneally with 6 nmol/g of recombinant human insulin (100 Humulin R, cat. no. HI 210; Lilly) or 0.5–1 mg/g AICAR (cat. no. A8129; Sigma-Aldrich, St. Louis, MO) dissolved (50 mg/ml) in saline, respectively. Controls were injected with an equal volume of saline (0.9% NaCl). Thirty minutes following insulin or AICAR treatment, mice were euthanized by cervical dislocation, and soleus muscles were dissected and snap-frozen in liquid nitrogen. For contraction experiments, an electrode was placed on the common peroneal nerve, and tibialis anterior muscles were stimulated to contract in situ (15 min, train rate: 1/s, train duration: 500 ms, pulse rate: 100 Hz, duration: 0.1 ms at 1–10 V). The contralateral leg was sham-operated and served as a rested control.
In vitro incubation of isolated muscles.
Soleus muscles were quickly removed from anesthetized mice and incubated in Krebs-Henseleit buffer (117 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, and 24.6 mM NaHCO3 with addition of 2 mM pyruvate and 0.1% BSA, pH 7.4) at 30°C oxygenated with a gas containing 95% O2-5% CO2. Incubations were carried out in the presence or absence of DMSO (0.01%; Sigma-Aldrich), AICAR (2 mM; Toronto Research Chemicals, Toronto, Canada), wortmannin (0.5 μM; Sigma-Aldrich), rapamycin (600 nM; cat. no. 553210; Calbiochem), and/or insulin (60 nM; Actrapid; Novo Nordisk) as indicated. All muscles were preincubated for 10 min following dissection and subsequently incubated for 40 min in the absence or presence of the chemical indicated in the figure legends. In experiments using wortmannin, muscles were first preincubated for 10 min ± wortmannin and then stimulated for the 40-min period. In experiments with rapamycin, muscles were preincubated and incubated for 30 min ± rapamycin followed by insulin stimulation for 30 min. Wortmannin, insulin, AICAR, and rapamycin were present during the stimulation period. For in vitro contraction experiments, soleus muscles were preincubated for 40 min with a buffer change after 10 min. The contraction protocol consisted of 10-s trains (100 Hz, 0.2 ms, ∼40 V) repeated once every minute for 10 min. After incubation, muscles were quickly frozen in liquid nitrogen and stored at −80°C.
Immunoblot procedure.
Tissues were homogenized using a Polytron (Brinkmann Instruments, Westbury, NY) in the following buffer: 50 mM Tris · HCl, 250 mM mannitol, 50 mM NaCl, 50 mM NaF, 1 mM EDTA, 1 mM EGTA, 5 mM sodium pyrophosphate, 5 mM glycerophosphate, 10% glycerol (vol/vol), 1% Nonidet P-40 substitute (vol/vol; Calbiochem), 1 mM Na3VO4, 1 mM DTT, 0.02 mM leupeptin, 0.5 mM PMSF, pH 7.4, at 4°C. Homogenates were rotated end-over-end for 1 h at 4°C followed by centrifugation at 14,000 g for 15 min. Lysate protein concentrations were determined by the Bradford assay (5). Lysates (30-μg protein) were separated by SDS-PAGE using 6–8% self-cast gels and transferred to nitrocellulose membranes. Antibody-bound proteins were visualized with chemiluminescence reagents (PerkinElmer Life Sciences) using a FluorChem 2.0 detection unit (Alpha Innotech, San Leandro, CA). Commercially available primary antibodies were anti-TBC1D4 (cat. no. 07-741; Millipore, Billerica, MA), anti-PAS (cat. no. 9611), anti-phospho-Akt-T308 (cat. no. 9275), anti-phospho-AMPK-T172 (cat. no. 2535), anti-phospho-GSK-3α/β (S21/9; cat. no. 9331; Cell Signaling Technology, Danvers, MA), and anti-Akt/PKB (cat. no. 05-591; Millipore). Serum-purified anti-phospho-TBC1D4 S711 antibody was generated by immunizing rabbits. The phosphospecificity of this antibody was confirmed (Supplemental Fig. S1, available in the data supplement online at the AJP-Cell Physiology web site).
In vivo gene electrotransfer and glucose uptake in mouse skeletal muscle.
Human WT TBC1D4 DNA and two mutant TBC1D4 DNA constructs were used in this study. One mutant, termed TBC1D4-4P, contains four S/T-to-A point mutations at S318, S588, T642, and S751 (33). The other mutant, TBC1D4-S711A, was generated from the WT TBC1D4 construct using site-directed mutagenesis (cat. no. 200522; Stratagene, La Jolla, CA). TBC1D4 genes were inserted into a pCAGGS vector containing an NH2-terminal Myc-tag (24), and the procedure for gene electrotransfer of plasmid DNA was performed as previously described (24). Seven days following electroporation of TBC1D4 constructs, muscle lysates were prepared and used for signaling studies or used to measure glucose uptake. Glucose uptake was measured in tibialis anterior muscle in response to muscle contraction, AICAR (0.5 g/kg body wt), or glucose injection (1 g/kg body wt) to induce a physiological insulin response as previously described (24). Blood draws were done from the tail vein at time points 0, 5, 10, 15, 25, 35, and 45 min. Immediately following the first blood draw, a bolus containing 3H-labeled 2-deoxyglucose (333 μCi/kg body wt) dissolved in saline (67 μCi/ml for contraction- and AICAR-induced glucose uptake experiments) or ± 20% glucose (for glucose-induced glucose uptake experiments) was delivered retroorbitally. After the last blood sample, animals were euthanized by cervical dislocation, and tibialis anterior muscles were rapidly dissected and snap-frozen in liquid nitrogen. 3H-labeled 2-deoxyglucose uptake was assessed as described previously (15).
Mass spectrometry.
AICAR- and contraction-stimulated gastrocnemius muscle lysates were prepared as described above. Lysates were pooled, and TBC1D4 was immunoprecipitated from 50 mg of total protein using a goat polyclonal TBC1D4 antibody (cat. no. ab5909; Abcam, Cambridge, MA). Protein G-agarose beads (cat. no. 22851; Pierce) were used to bind anti-TBC1D4 antibodies. Bead-antibody-protein complexes were washed 1× with lysis buffer, 2× with lysis buffer + 500 mM NaCl, and 1× with lysis buffer. Pellets were aspirated and spotted with 5–10 μl of 1 μg/μl BSA before elution to maximize the efficiency of protein elution. Proteins were eluted from protein G beads by adding Laemmli buffer and heating for 5 min at 95°C. Eluates were subjected to SDS-PAGE, and gels were stained with GelCode Blue Stain Reagent (cat. no. 24592; Pierce). AICAR-stimulated samples were digested with trypsin and separated by nanoflow reverse phase capillary HPLC. Eluted peptides were ionized by electrospray and analyzed with an LTQ two-dimensional linear quadrupole ion trap mass spectrometer (Thermofinnigan, San Jose, CA; Refs. 14, 17, 20). Contraction-stimulated samples were reduced and alkylated in-gel. Samples were digested with trypsin or chymotrypsin and analyzed by liquid chromatography-tandem mass spectrometry in an LTQ Orbitrap mass spectrometer (Thermofinnigan, San Jose, CA; Refs. 3, 19, 41). In all cases, data were analyzed with the SEQUEST algorithm, and reported phosphopeptides were verified by manual inspection of spectra.
In vitro phosphorylation of TBC1D4 using recombinant AMPK, PKCζ, and Akt.
To phosphorylate S711 using recombinant proteins in vitro, TBC1D4 containing a flag-tag (33) was overexpressed by gene electrotransfer in tibialis anterior muscles as described above and subsequently immunoprecipitated overnight from 300 μg of muscle lysate using an α-flag antibody (F1804; Sigma-Aldrich). The immunoprecipitate was washed 3× with PBS, and reaction buffer (10 mM HEPES, 5 mM MgCl2, 1 mM EGTA, 0.5 mM Na3VO4, 200 μM ATP, 100 μM AMP) was added with or without constitutively active AMPK (cat. no. P47-10H), PKCζ (cat. no. P75-10G), Akt1 (cat. no. A16-10G), and Akt2 (cat. no. A17-10G) from SignalChem (Richmond, British Columbia, Canada). The reaction ran for 30 min at 30°C and was terminated by adding Laemmli buffer and heating the samples to 96°C for 5 min. Samples were then separated by SDS-PAGE. As a positive control for the recombinant PKCζ, assays were run with PKCε pseudosubstrate (cat. no. 77-120; BioSource International) with the addition of [γ-33P]ATP in the reaction buffer. After incubation, 33P-labeled substrate was trapped on P81 filter paper, washed 4 × 10 min in 1% phosphoric acid, and counted in a liquid scintillation counter (Tri-Carb 2000; Packard Instruments).
Human exercise experiment.
The human skeletal muscle samples used have been described previously (4). In brief, 11 healthy young men [age 25 ± 1 yr, body wt 76 ± 1 kg, body mass index (BMI) 24 ± 1 kg/m2, peak oxygen consumption (V̇o2peak) 53 ± 2 ml/kg] gave their written informed consent to participate in this study, which was approved by the Copenhagen Ethics Committee (no. KF01277313) and was in agreement with the Declaration of Helsinki II. The subjects arrived at the laboratory in the morning 3 h after a light breakfast. After 45 min of rest, a needle biopsy from the vastus lateralis muscle was obtained under local anesthesia (2–3 ml of 2% lidocaine). The subjects completed 20 min of bicycling at 77 ± 3% V̇o2peak. Immediately after the exercise was completed, a second biopsy was obtained from the vastus lateralis muscle in the other leg. The biopsies were frozen in liquid nitrogen within 15 s after termination of the exercise and stored at −80°C.
Statistics.
All statistical analyses were performed in SPSS 17.0 (SPSS, Chicago, IL). Data are expressed as means ± SE. Levene test of equality of variance was used to test whether groups had equal variances. Data were log-transformed if Levene test was significant. Statistical differences were assessed using paired or unpaired t-tests or two-way ANOVA ± repeated measures where appropriate. For post hoc testing, Tukey honestly significant difference test was applied. Statistical significance was accepted at P < 0.05.
RESULTS
Mass spectrometry analyses revealed novel contraction- and AICAR-responsive sites on TBC1D4.
Using mass spectrometry analyses, we identified several novel candidate phosphorylation sites on TBC1D4 (Table 1). In contraction-stimulated samples, we found four novel sites (S276, S598, S616, and S789) and two sites that have been previously identified (S595 and T649). In AICAR-stimulated samples, we found one previously characterized site (S764; Ref. 11) and four novel phosphorylation sites (S680, S711, S761, and S1135). We chose to further investigate the regulation of S711 because it falls within the consensus AMPK phosphorylation motif [Φ(X,β)XXS/TXXXΦ, Φ = M, V, L, I, F, β = R, K, H; Ref. 10]. Furthermore, S711 is within a splice exon that is unique to skeletal muscle and heart. These two facts suggested that this site could transduce contraction-related energetic signals arising from stimuli activating AMPK.
Table 1.
Candidate TBC1D4 phosphorylation sites identified by mass spectrometry
| Site | Stimulus | Phosphopeptide | XCorr |
|---|---|---|---|
| S595 (PAS) | Contraction/AICAR | LGS*M#DSFER | 2.1 |
| T649 (PAS) | Contraction | AHT*FSHPPSSSR | 3.2 |
| S276 | Contraction | ADGTVNS*PR | 2.1 |
| S598 | Contraction | LGSM#DS*FER | 2.3 |
| S616 | Contraction | ANSLASEKDFSPGDS*PPGTPPASPLSSAWHAFPEEDSDSPQFR | 3.1 |
| S789 | Contraction | VAS*PVNK | 1.7 |
| S680 | AICAR | QSS*SEQCSIVPSAR | 2.8 |
| S711 | AICAR | ESNSSC∧SLPSLHTSFS*APSFTAPSFLK | 5.7 |
| S761, S764 | AICAR | TSSTC∧S*NES*LNAGGTPVTPR | 5.4 |
| S1135 | AICAR | VALSLLSS*QEALIM#ECENFENIVEFLK | 3.7 |
TBC1D4 was immunoprecipitated from contraction- and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR)-stimulated (1 mg/g) mouse gastrocnemius muscle lysates and analyzed for phosphorylation by liquid chromatography/tandem mass spectrometry (LC-MS/MS). Phosphopeptides were identified by analyzing mass spectral data with the SEQUEST algorithm. Cross-correlation (XCorr) is a measure of confidence for correct identification of a phosphopeptide. Depending on the charge state and the size of the phosphopeptide, scores >1.5 can indicate a correct match pending further verification by manual inspection of spectra. Here, all phosphopeptides reported were verified by manual inspection. AICAR-stimulated samples were analyzed by an LTQ linear ion trap mass spectrometer, and contraction-stimulated samples were analyzed by an LTQ Orbitrap mass spectrometer. Since these mass spectrometers operate by different principles, identification of a phosphopeptide in only contraction- or AICAR-stimulated samples does not indicate that the site is not phosphorylated with the other. S/T* denotes phosphorylation, C∧ denotes carbamidomethylation, and M# denotes methionine oxidation. PAS, phospho-Akt substrate.
Multiple stimuli induce S711 phosphorylation in vivo.
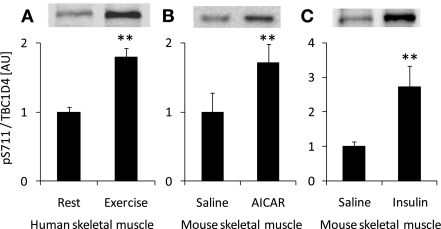
To determine whether S711 was phosphorylated in response to stimuli known to activate AMPK in vivo, we studied exercise in human skeletal muscle and AICAR in mouse skeletal muscle. Exercise at ∼80% of V̇o2peak for 20 min resulted in a pronounced increase in S711 phosphorylation in skeletal muscle from young healthy male subjects (Fig. 1A). AICAR injection increased TBC1D4 S711 phosphorylation in mouse soleus muscle (Fig. 1B). Based on the amino acid sequence around S711, this site appeared to be ideal for AMPK recognition and less so for Akt (32), another well-known TBC1D4 kinase. To rule out that kinases activated by insulin were able to phosphorylate S711, we stimulated ICR mice with insulin in vivo and measured S711 phosphorylation in soleus muscle. Surprisingly, insulin induced a response similar to that observed with AICAR and exercise (Fig. 1C).
Fig. 1.
Different stimuli induce TBC1D4 S711 phosphorylation. A: healthy human male subjects (n = 11) exercised at ∼80% of their peak oxygen consumption (V̇o2peak) for 20 min on a cycle ergometer. Before and immediately after termination of the exercise, biopsies were taken from the vastus lateralis muscle; muscle lysates were prepared followed by Western blot analysis using a TBC1D4 S711-phosphospecific antibody. B: mice (n = 3–6) were injected in vivo with saline or 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR; 1 mg/g body wt ip, 30 min), and soleus muscles were dissected and prepared for Western blot analyses. C: mice (n = 3–6) were injected in vivo with saline or insulin (6 nmol/g ip, 30 min), and soleus muscles were dissected and prepared for Western blot analyses. **Treatment effect (P < 0.01). AU, arbitrary units.
AMPKα2 is required for AICAR- and contraction-stimulated S711 phosphorylation.
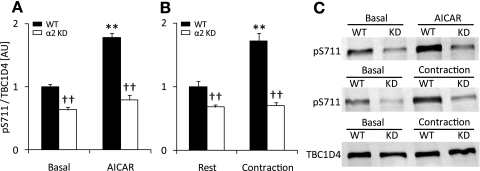
To determine the upstream kinases that mediated the AICAR- and contraction-induced S711 phosphorylation, soleus muscles from mice expressing a muscle-specific AMPKα2 KD subunit were incubated with AICAR or contracted in vitro. Lack of AMPKα2 activity resulted in a significant decrease in basal S711 phosphorylation. We found that both AICAR- and contraction-mediated S711 phosphorylation were completely inhibited in the AMPKα2 KD mice (Fig. 2, A and B). As a control for contraction and AICAR, we tested the effects of these stimuli on AMPK T172 and ACC S227 phosphorylation and found both to be normal in the WT muscles (Supplemental Table S1). Total TBC1D4 expression was not different between genotypes (Fig. 2C), as previously reported (39). These data strongly suggest that AMPK is required for both AICAR and contraction effects on S711 phosphorylation and that AMPK also regulates basal S711 phosphorylation.
Fig. 2.
S711 phosphorylation in response to AICAR and contraction is AMP-activated protein kinase-α2 (AMPKα2)-dependent. A: soleus muscles (n = 8) from wild-type (WT; black bars) and AMPKα2 kinase-dead (α2 KD; white bars) mice were incubated in vitro ± AICAR (2 mM, 40 min). Muscle lysates were prepared followed by Western blot analysis using a TBC1D4 S711-phosphospecific antibody. B: soleus muscles (n = 10–12) from WT (black bars) and AMPKα2 KD (white bars) mice were incubated for 40 min in vitro and stimulated to contract (10 s/60 s, 100 Hz, 0.2 ms) or left at rest during the last 10 min of the incubation period. Muscles were processed and prepared for Western blot analyses. C: representative Western blots for phosphorylation of S711 and total TBC1D4. **Treatment effect (P < 0.01); ††genotype effect (P < 0.01).
Insulin-stimulated S711 phosphorylation is wortmannin-sensitive but independent of Akt2 and AMPKα2 and rapamycin-insensitive.
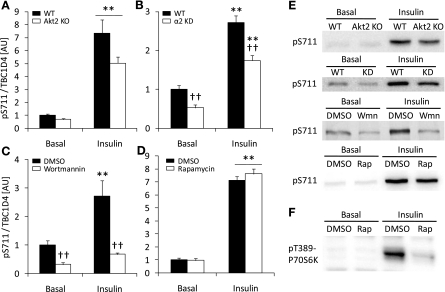
We next tested the hypothesis that insulin-mediated S711 phosphorylation requires expression of Akt2. Therefore, soleus muscles from Akt2 KO mice were incubated with insulin in vitro. Although Akt phosphorylation of T308 was not significantly increased in Akt2 KO mice (Table 2), insulin induced a similar increase in S711 phosphorylation in both Akt2 KO and WT mice (Fig. 3A). We verified that in the Akt2 KO mice, PAS phosphorylation of the 160-kDa band was severely blunted (Supplemental Fig. S2A), as we have previously reported (23). GSK-3 phosphorylation was also diminished in the Akt2 KO mice in response to insulin showing that downstream signaling from Akt was severely affected in this model (Supplemental Fig. S2B). To rule out that the effect of insulin on S711 was dependent of AMPKα2, we incubated soleus muscles from AMPKα2 KD and WT littermates with insulin and measured S711 phosphorylation. As shown in Fig. 2, basal S711 phosphorylation was reduced in the AMPKα2 KD mice. However, the insulin-stimulated increase in S711 phosphorylation above basal was preserved (Fig. 3B). Akt S473 phosphorylation with insulin was normal in the AMPKα2 KD mice (Table 2), indicating an adequate insulin stimulus. Since neither Akt2 nor AMPK were mediating the effects of insulin on S711 phosphorylation, we used the phosphatidylinositol 3 (PI3) kinase inhibitor wortmannin to test whether inhibition of the insulin signaling cascade further upstream would alter insulin-induced S711 phosphorylation. Wortmannin completely inhibited Akt T308 and S473 phosphorylation (Table 2) and abolished the effect of insulin on S711 phosphorylation (Fig. 3C). Insulin is known to stimulate the activation of mammalian target of rapamycin (mTOR) signaling, resulting in the phosphorylation of T389 on the p70S6 kinase, a response that can be inhibited by rapamycin (27). To test the possibility that mTOR/p70S6 kinase could be upstream of insulin-stimulated S711 phosphorylation, we incubated soleus muscles with rapamycin. Although insulin-stimulated p70S6 kinase T389 phosphorylation was severely blunted (Fig. 3F), S711 phosphorylation was not affected (Fig. 3D).
Table 2.
Total protein or phosphorylation level of Akt
| pT308 Akt |
pS473 Akt |
Akt total |
|||||
|---|---|---|---|---|---|---|---|
| Genotype | Condition | Basal | Stimulated | Basal | Stimulated | Basal | Stimulated |
| Akt2 WT | Insulin | 100 ± 7‡ | 2,406 ± 251*‡ | N/A | N/A | 100 ± 5 | 89 ± 3 |
| Akt2 KO | Insulin | 67 ± 2†‡ | 273 ± 58†‡ | N/A | N/A | 31 ± 2† | 31 ± 2† |
| AMPKα2 WT | Insulin | N/A | N/A | 100 ± 11 | 957 ± 32* | N/A | N/A |
| AMPKα2 KD | Insulin | N/A | N/A | 121 ± 9 | 969 ± 43* | N/A | N/A |
| C57BL/6 | Insulin (DMSO) | 100 ± 8‡ | 469 ± 32*‡ | 100 ± 10‡ | 610 ± 43*‡ | N/A | N/A |
| C57BL/6 | Insulin (Wmn) | 47 ± 7†‡ | 51 ± 5†‡ | 6 ± 4†‡ | 17 ± 6†‡ | N/A | N/A |
| ICR | Insulin (DMSO) | 100 ± 24 | 1,327 ± 52* | N/A | N/A | N/A | N/A |
| ICR | Insulin (Rap) | 106 ± 25 | 1,607 ± 132* | N/A | N/A | N/A | N/A |
All values are arbitrary and related to the basal value in either wild-type (WT) or DMSO-treated muscles ± SE.
Different from basal within genotype/condition; P < 0.01.
Different between genotypes/conditions within treatment condition; P < 0.01.
Sets of data containing statistical interactions between genotype and treatment. pT308 Akt, phospho-Akt-Thr308; pS473 Akt, phospho-Akt-Ser473; KO, knockout; KD, kinase-dead; AMPK, AMP-activated protein kinase; Wmn, wortmannin; rap, rapamycin; N/A, not applicable.
Fig. 3.
Insulin-stimulated S711 phosphorylation is wortmannin-sensitive but independent of Akt2 or AMPKα2. A: soleus muscles (n = 10) from WT (black bars) and Akt2 knockout (KO; white bars) mice were incubated in vitro in the absence or presence of insulin (60 nM, 30 min). Muscle lysates were prepared followed by Western blot analysis using a TBC1D4 S711-phosphospecific antibody. B: soleus muscles (n = 10) from WT (black bars) and AMPKα2 KD (white bars) mice were incubated in vitro with insulin (60 nM, 40 min) and then processed and prepared for Western blot analyses. C: soleus muscles (n = 8) from C57BL/6 mice were incubated without (black bars) or with (white bars) wortmannin (500 nM) dissolved in DMSO and stimulated with insulin (60 nM, 40 min). D: soleus muscles (n = 6) from WT ICR mice were incubated without (black bars) or with (white bars) rapamycin (600 nM) dissolved in DMSO and stimulated with insulin. E: representative phospho-S711 Western blots from experiments described in A–D. F: representative phospho-T389-p70S6K Western blot. **Treatment effect (P < 0.01); ††genotype effect (B) or effect of wortmannin (C) (P < 0.01). Wmn, wortmannin; Rap, rapamycin.
Recombinant α1β1γ1-AMPK, but not PKCζ, Akt1, or Akt2, phosphorylates S711 in vitro.
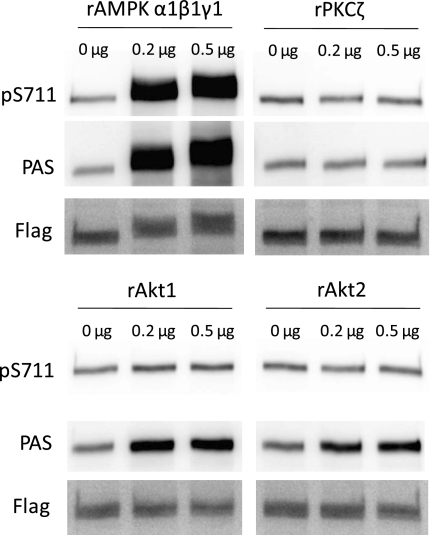
Since AMPKα2 was required for S711 phosphorylation in response to AICAR and contraction, we tested whether AMPK directly phosphorylates S711 in vitro. We also tested the possibility that recombinant constitutively active PKCζ, Akt1, or Akt2 could directly phosphorylate S711 in vitro. As shown in Fig. 4, immunopurified TBC1D4 was heavily phosphorylated at PAS sites by AMPK, Akt1, and Akt2. However, only AMPK was able to phosphorylate S711. Recombinant PKCζ did not phosphorylate either PAS sites or S711. Using a PKCε pseudosubstrate and [γ-33P]ATP in the same reaction conditions as with immunopurified TBC1D4, we confirmed that the PKCζ protein was indeed active (data not shown). It should be noted that the specific activity of the recombinant AMPK was ∼6 times higher than that for Akt1 and Akt2. This may explain the difference in degree of PAS phosphorylation between AMPK and Akt1/2.
Fig. 4.
Recombinant (r) α1β1γ1-AMPK but not PKCζ, Akt1, or Akt2 phosphorylate S711 in vitro. Flag-tagged TBC1D4 was immunopurified from tibialis anterior muscles and incubated in a reaction buffer ± recombinant AMPK, PKCζ, Akt1, or Akt2. The reaction in the absence (0 μg) or presence (0.2 and 0.5 μg) of recombinant protein was run for 30 min at 30°C and stopped by adding Laemmli buffer and heating the samples at 96°C for 5 min. Samples were subjected to SDS-PAGE immunoblotting with antibodies recognizing phosphorylated S711, phospho-Akt substrate (PAS) sites, and total flag-tagged protein. The specific activity of AMPK was ∼6 times higher than that for PKCζ, Akt1, and Akt2, which may explain the difference between the levels of PAS phosphorylation.
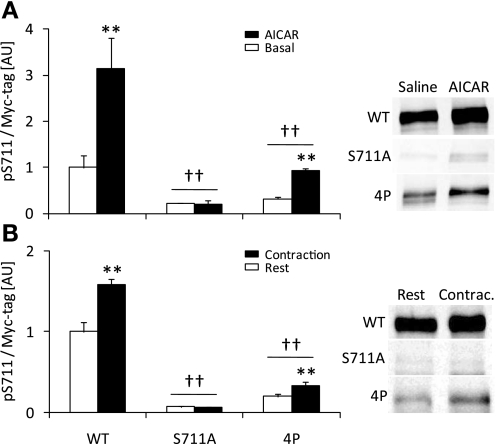
Phosphorylation of S711 by AICAR and contraction is inhibited in skeletal muscles expressing the TBC1D4-4P mutant.
Some interdependency between phosphorylation sites on TBC1D4 has been reported (18). Therefore, to investigate the regulation of S711, we expressed TBC1D4-WT, TBC1D4-S711A, and TBC1D4-4P (containing 4 S-to-A mutations, i.e., S318A, S588A, T642A, and S751A) constructs in tibialis anterior muscles of ICR mice by gene electrotransfer. The level of ectopically expressed proteins reached similar levels (approximately 10- to 15-fold) to that reported previously (24). Mice were subjected to either in situ contraction via stimulation of the peroneal nerve or AICAR stimulation in vivo by a subcutaneous injection. Contraction and AICAR stimulation increased S711 phosphorylation in muscles expressing TBC1D4-WT, and this response was completely ablated in muscles expressing the TBC1D4-S711A mutant (Fig. 5, A and B). Interestingly, S711 phosphorylation was markedly blunted in muscles expressing the TBC1D4-4P mutant indicating that to induce S711 phosphorylation, 1 or more of the 4P sites is required. Although S711 phosphorylation was clearly reduced in the muscles expressing the TBC1D4-4P mutant, AICAR and contraction resulted in a modest but significant increase in phosphorylation at this site.
Fig. 5.
S711 phosphorylation is controlled by 1 or more of the TBC1D4-4P sites. Gene electrotransfer of tibialis anterior muscles from ICR mice in vivo was used to express TBC1D4-WT, TBC1D4-S711A, and TBC1D4-4P and the mice were studied 7 days later. A: mice (n = 4) were stimulated with AICAR (0.5 mg/g body wt subcutaneously, 30 min) or saline (subcutaneous, 30 min). Muscle lysates were prepared followed by Western blot analysis using phosphospecific antibodies as indicated in the figure. B: tibialis anterior muscles (n = 6) were stimulated to contract in situ (15 min; train rate: 1/s; train duration: 500 ms; pulse rate: 100 Hz; duration: 0.1 ms at 1–10 V) via the common peroneal nerve or sham-operated and left in the rested state (15 min). **Treatment effect (P < 0.01); ††effect of the DNA construct expressed compared with WT (P < 0.01).
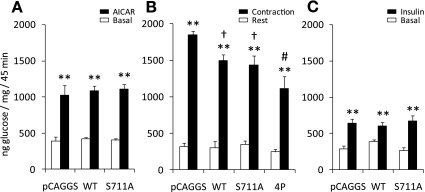
Glucose uptake by AICAR, contraction, and insulin is not affected in muscles expressing the S711A mutant.
AICAR-induced glucose uptake in skeletal muscle has been shown to require AMPKα2 (21), and our current study shows that AICAR-induced S711 phosphorylation requires AMPKα2. Therefore, we hypothesized that S711 would be necessary to increase glucose uptake in skeletal muscle by AICAR. Furthermore, as contraction- and insulin-mediated glucose uptake have been shown to depend in part on the ability of TBC1D4 to be phosphorylated on S318, S588, T642, and S751 (24), and as one or more of these phosphorylation sites appear to be required for phosphorylation of S711 to occur (Fig. 5B), we also tested whether contraction- and insulin-mediated glucose uptake required S711. Although contraction-induced glucose uptake in the TBC1D4-4P mutant was significantly reduced, AICAR-, contraction-, and insulin-stimulated glucose uptake were not affected in muscles expressing the S711A mutant (Fig. 6, A–C). Thus S711 phosphorylation is dispensable for these stimuli to induce glucose uptake.
Fig. 6.
Mutation of S711 does not impair glucose uptake in skeletal muscle in response to AICAR, contraction, or insulin. Tibialis anterior muscles from ICR mice were injected with either pCAGGS empty vector, TBC1D4-WT, TBC1D4-S711A, or TBC1D4-4P plasmid DNA constructs, which were then electroporated into the muscles cells. Seven days later, glucose transport was measured in response to AICAR (n = 6–12), contraction (n = 4–12), and physiological insulin levels induced by a glucose intravenous injection (n = 8–11). A: AICAR (0.5 mg/g body wt) or saline was injected (subcutaneously) 10 min before injecting (intravenously) a bolus of 3H-labeled 2-deoxyglucose (2DG). Blood samples were taken at time points 0, 5, 10, 15, 25, 35, and 45 min, and muscles were dissected after 45 min of stimulation. B: at time 0, a blood sample was taken, and a bolus of 3H-labeled 2DG was injected intravenously. An electrode was placed on the common peroneal nerve, and tibialis anterior muscles were stimulated to contract in situ (15 + 30 min recovery). The contralateral leg was sham-operated and used as rested control. Blood samples were taken as in A, and tibialis anterior muscles were dissected after 45 min. C: glucose or saline containing 3H-labeled 2DG were injected intravenously at time 0, and blood samples were taken as in A. Tibialis anterior muscles were dissected after 45 min of stimulation. Muscles and blood samples were processed as previously described (15). **Treatment effect (P < 0.01); †different from empty vector (P < 0.05); #different from WT and S711A (P < 0.05).
DISCUSSION
TBC1D4 (AS160) is a Rab-GAP with multiple domains and phosphorylation sites, including several sites that have previously been shown to be phosphorylated by Akt. In the current study, we investigated the possibility that novel phosphorylation sites are present on TBC1D4 and identified several putative phosphorylation sites that are regulated by contraction and AICAR in skeletal muscle. Of these, S711 is located in an AMPK recognition motif (10, 36). We found that recombinant AMPK phosphorylates TBC1D4 on S711 and that AICAR and contraction increase S711 phosphorylation in an AMPK-dependent manner. These findings demonstrate that AMPK directly phosphorylates TBC1D4 on S711 and that this occurs in skeletal muscle in vivo. In addition to known AMPK activators increasing phosphorylation of S711 on TBC1D4, we were intrigued to find that insulin also increased S711 phosphorylation and that the magnitude of the insulin effect was comparable with that of AICAR and muscle contraction. Experiments using wortmannin suggest that the upstream kinase that mediates insulin-stimulated S711 phosphorylation is a PI3 kinase-regulated protein, but our experiments with Akt2 KO mice and recombinant Akt1/2 suggest that Akt1 and Akt2 are not the responsible kinases. Atypical PKC isoforms have been reported to be downstream of PI3 kinase (9) and have also been implicated in the regulation of glucose metabolism in skeletal muscle (13). However, using recombinant constitutively active PKCζ, we did not observe phosphorylation of TBC1D4 on either S711 or PAS sites. TBC1D4 PAS phosphorylation has been reported not to be reduced in insulin-stimulated skeletal muscle from PKCλ KO animals (13). Collectively, these data suggest that atypical PKC isoforms may not play a functional role in the regulation of TBC1D4 phosphorylation. We also tested the hypothesis that insulin-mediated S711 phosphorylation was induced by intermediates in the mTOR-p70S6K pathway. However, although rapamycin severely blunted p70S6K T389 phosphorylation, S711 phosphorylation was unaffected. We (31) have previously shown that Akt3 activity is increased with insulin stimulation and by muscle contraction in skeletal muscle, but this protein is considered to be of very low abundance in skeletal muscle (12). However, we cannot rule out a role for Akt3 in mediating S711 phosphorylation with insulin. An alternative mechanism for insulin-stimulated TBC1D4 S711 phosphorylation is that a wortmannin-sensitive kinase could inhibit a S711 phosphatase.
Our finding that expression of TBC1D4 mutated on four Akt substrate motifs (4P mutant) resulted in a marked reduction of S711 phosphorylation suggests that for optimal S711 phosphorylation to occur, a priming phosphorylation of one or more of these Akt sites is required. The concept of interdependency among the different TBC1D4 phosphorylation sites is consistent with previous work where it was reported that a S318A mutation decreased the phosphorylation of S341 (18). For our study, it could be that phosphorylation of T642, the most highly immunoreactive of the 4P sites on TBC1D4 using the PAS antibody, or one or more of the other sites mutated in the TBC1D4 4P mutant would make S711 a much better substrate for AMPK, perhaps through a conformational change of the protein. It is important to point out that although phosphorylation was quite low, AICAR and contraction were still able to increase S711 phosphorylation in the muscles expressing the 4P mutant. Thus one or more of the 4P sites may potentiate phosphorylation of S711 and enhance the signal, but these sites are not mandatory for the ability of upstream kinases to phosphorylate S711.
Exercise in humans is a potent stimulator of AMPK activity in skeletal muscle, and exercise in humans increases TBC1D4-PAS phosphorylation (40). Here, we found that S711 phosphorylation increased in response to cycle ergometer exercise in human skeletal muscle. We (4) have previously reported that of the three different AMPK trimers detected in human skeletal muscle (α1β2γ1, α2β2γ1, and α2β2γ3), only α2β2γ3 was activated in response to this type of exercise. Thus α2β2γ3-containing AMPK complexes could play a central role in regulating S711 on TBC1D4 in skeletal muscle. In this study, we also saw S711 phosphorylation in response to AICAR and contractions in soleus and tibialis anterior muscles from mice. We do not know the AMPK expression profile for the tibialis anterior muscle, but soleus muscles from C57BL/6 mice contain five different AMPK trimers of which α2β2γ3-containing complexes constitutes only a very small fraction (<2%) of the total pool of AMPK trimers (37). In contrast, human vastus lateralis muscle contains ∼20% α2β2γ3-AMPK complexes (4). Future studies will investigate which specific AMPK trimer is responsible for phosphorylating S711, but it is possible that the specific AMPK trimer may be different among species.
The ability of the AMPK activator AICAR to increase glucose uptake in skeletal muscle is dependent on both the α2- and γ3-subunits of AMPK (2, 26), and in the current study we found that S711 of TBC1D4 is regulated by AMPK. However, expression of the S711A mutant did not impair AICAR-stimulated glucose uptake in muscle, raising the possibility that AMPK-stimulated S711 phosphorylation of TBC1D4 may regulate another, yet to be determined function of TBC1D4. Alternatively, S711 phosphorylation may function in the regulation of glucose uptake, but AICAR may phosphorylate other TBC1D4 sites in skeletal muscle (e.g., S588; Ref. 18), and eliminating one phosphorylation site may not be sufficient for regulation of glucose uptake. There could also be redundancy in TBC1D4 and TBC1D1 function. Although we do not know the absolute amount of the TBC1D4 and TBC1D1 proteins in muscle, mRNA analyses suggest lower endogenous TBC1D4 protein expression compared with TBC1D1 in tibialis anterior muscle (34), and overexpression of TBC1D4 does not result in decreased expression of TBC1D1 (D. An and L. J. Goodyear, unpublished observations). Thus the lack of effect on AICAR-induced glucose uptake in the S711A-expressing muscles may be due to redundancy both at the level of phosphorylation and Rab-GAP protein expression.
Recently, we (34) found several new putative phosphorylation sites on TBC1D1. TBC1D1 S660 is located on the splice exon specific for the long isoform of TBC1D1, and based on the ability of AICAR to increase phosphorylation of this site and the surrounding amino acid sequence, this residue may correspond to S711 on TBC1D4 (K. Vichaiwong, D. An, and L. J. Goodyear, unpublished observations). This may further support the idea that TBC1D1 could compensate for the loss of TBC1D4 S711 phosphorylation in the TBC1D4-S711A-expressing muscles. In line with the concept of redundancy, TBC1D1 and TBC1D4 show Rab-GAP activity against the same Rab proteins, at least in vitro (25, 30), and this could also possibly occur in vivo. Similar Rab specificity is likely due to the high homology between TBC1D1 and TBC1D4, especially in the GAP domain of the proteins (30). On the other hand, both TBC1D1 and TBC1D4 have been shown to have multiple binding partners (i.e., IRAP, 14-3-3, and RUVBL2; Refs. 7, 18, 28, 29, 42), and it is possible that the “signature” phosphorylation patterns induced by upstream kinases on TBC1D1 and TBC1D4 result in differential binding of these partners and thereby distinct regulation of Rab-GAP activity. For example, in L6 myotubes, insulin stimulation causes phosphorylation of specific sites and concomitant 14-3-3 binding of TBC1D4, whereas for TBC1D1, insulin also results in site-specific phosphorylation but no 14-3-3 binding (7). Thus regulation of TBC1D1 and TBC1D4 may occur by distinct association of binding partners. The finding that TBC1D1 can be phosphorylated with insulin without resulting 14-3-3 binding may suggest multiple modes of TBC1D1 regulation, which likely also applies for TBC1D4. This could potentially indicate that these Rab-GAPs are not only involved in vesicular traffic related to GLUT4 mobilization, but also that they could control transport of other vesicle structures.
Whether phosphorylation of S711 regulates Rab-GAP activity has yet to be established. However, so far, only truncated, but not full-length, versions of TBC1D1 and TBC1D4, containing just the GAP domain, have been shown to have Rab-GAP activity (25, 30). Thus an open question is whether phosphorylation of TBC1D1 and TBC1D4 regulates Rab-GAP activity per se or whether phosphorylation is required for binding of TBC1D1/4-associated proteins. This issue should be considered in future studies.
In summary, we have identified several new phosphorylation sites on TBC1D4. TBC1D4 S711 is phosphorylated in response to AICAR, contraction, and insulin in mouse skeletal muscle and in response to exercise in human skeletal muscle. AMPK regulates S711 phosphorylation, as both AICAR and contraction effects were completely abolished in muscles from AMPKα2 KD mice, but we are uncertain regarding the identity of the kinase(s) responsible for insulin-induced S711 phosphorylation. Nevertheless, these data strengthen the view of TBC1D4 as a convergence point between insulin- and contraction-mediated signaling pathways in skeletal muscle. TBC1D4 S711 phosphorylation does not regulate glucose uptake, raising the likelihood of redundancy in phosphorylation of the protein or suggesting that TBC1D4 may regulate additional cellular functions in skeletal muscle.
GRANTS
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants R01-AR-42238 and R01-AR-45670 (L. J. Goodyear), funding from the Graetz Challenge Grant of the Joslin Diabetes Center (L. J. Goodyear), and Diabetes Endocrinology Research Centers (DERC) Grant P30-DK-36836 (Joslin Diabetes Center). Additional support was from the Novo Nordisk Foundation (J. F. P. Wojtaszewski), the Danish Medical Research Council (J. F. P. Wojtaszewski), the Danish Diabetes Association (J. F. P. Wojtaszewski), and an integrated project (LSHM-CT-2004-005272) funded by the European Commission (J. F. P. Wojtaszewski). E. B. Taylor was supported by an Individual Ruth L. Kirschstein National Research Service Award (F32-DK-075851), C. A. Witczak by a National Institutes of Health K99/R00 Pathway to Independence Award (AR-056298), D. An from fellowships from the Canadian Institutes of Health Research and the Canadian Diabetes Association, T. Toyoda from an American Diabetes Association Mentor-based Award (to L. J. Goodyear), and H. J. Koh from an American Physiological Society Postdoctoral Fellowship in Physiological Genomics.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Morris J. Birnbaum (Howard Hughes Medical Institute, Philadelphia, PA) for providing founder AMPKα2 KD and Akt2 KO mice.
REFERENCES
- 1.Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett 399: 333–338, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Barnes BR, Marklund S, Steiler TL, Walter M, Hjalm G, Amarger V, Mahlapuu M, Leng Y, Johansson C, Galuska D, Lindgren K, Abrink M, Stapleton D, Zierath JR, Andersson L. The 5′-AMP-activated protein kinase γ3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J Biol Chem 279: 38441–38447, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol 24: 1285–1292, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Birk JB, Wojtaszewski JF. Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J Physiol 577: 1021–1032, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 6.Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J Biol Chem 283: 9187–9195, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, MacKintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409: 449–459, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292: 1728–1731, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, Newton AC, Schaffhausen BS, Toker A. Regulation of protein kinase Cζ by PI 3-kinase and PDK-1. Curr Biol 8: 1069–1078, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Dale S, Wilson WA, Edelman AM, Hardie DG. Similar substrate recognition motifs for mammalian AMP-activated protein kinase, higher plant HMG-CoA reductase kinase-A, yeast SNF1, and mammalian calmodulin-dependent protein kinase I. FEBS Lett 361: 191–195, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA 105: 10762–10767, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol 25: 1869–1878, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WR, Jr, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M. Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 117: 2289–2301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feener EP, Rosario F, Dunn SL, Stancheva Z, Myers MG., Jr Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signaling. Mol Cell Biol 24: 4968–4978, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferre P, Leturque A, Burnol AF, Penicaud L, Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J 228: 103–110, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii N, Jessen N, Goodyear LJ. AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab 291: E867–E877, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gao BB, Hansen H, Chen HC, Feener EP. Angiotensin II stimulates phosphorylation of an ectodomain-truncated platelet-derived growth factor receptor-beta and its binding to class IA PI3K in vascular smooth muscle cells. Biochem J 397: 337–344, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geraghty K, Chen S, Harthill JE, Ibrahim AF, Toth R, Morrice NA, Vandermoere F, Moorhead GB, Hardie DG, MacKintosh C. Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to insulin-like growth factor 1, EGF, PMA and AICAR. Biochem J 407: 231–241, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282: 30107–30119, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Ishida-Takahashi R, Rosario F, Gong Y, Kopp K, Stancheva Z, Chen X, Feener EP, Myers MG., Jr Phosphorylation of Jak2 on Ser(523) inhibits Jak2-dependent leptin receptor signaling. Mol Cell Biol 26: 4063–4073, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jørgensen SB, Viollet B, Andreelli F, Frøsig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the α2 but not α1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279: 1070–1079, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277: 22115–22118, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55: 2067–2076, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281: 31478–31485, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Miinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, Lane WS, Lienhard GE. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase activating protein domain. Biochem J 391: 87–93, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, Thomas G. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J 14: 5279–5287, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peck GR, Ye S, Pham V, Fernando RN, Macaulay SL, Chai SY, Albiston AL. Interaction of the Akt substrate, AS160, with the glucose transporter 4 vesicle marker protein, insulin-regulated aminopeptidase. Mol Endocrinol 20: 2576–2583, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Ramm G, Larance M, Guilhaus M, James DE. A role for 14–3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J Biol Chem 281: 29174–29180, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J 403: 353–358, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakamoto K, Hirshman MF, Aschenbach WG, Goodyear LJ. Contraction regulation of Akt in rat skeletal muscle. J Biol Chem 277: 11910–11917, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab 295: E29–E37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 283: 9787–9796, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thong FS, Bilan PJ, Klip A. The Rab GTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes 56: 414–423, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100: 328–341, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Treebak JT, Birk JB, Hansen BF, Olsen GS, Wojtaszewski JF. A-769662 activates AMPK β1-containing complexes but induces glucose uptake through a PI3-kinase-dependent pathway in mouse skeletal muscle. Am J Physiol Cell Physiol 297: C1041–C1052, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Treebak JT, Frøsig C, Pehmøller C, Chen S, Maarbjerg SJ, Brandt N, MacKintosh C, Zierath JR, Hardie DG, Kiens B, Richter EA, Pilegaard H, Wojtaszewski JF. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia 52: 891–900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 55: 2051–2058, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of α2β2γ1- but not α2β2γ3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab 292: E715–E722, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Villen J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci USA 104: 1488–1493, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie X, Chen Y, Xue P, Fan Y, Deng Y, Peng G, Yang F, Xu T. RUVBL2, a novel AS160-binding protein, regulates insulin-stimulated GLUT4 translocation. Cell Res 19: 1090–1097, 2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.