Abstract
Oxidative stress is one of the causative factors in progression and etiology of age-related cataract. Peroxiredoxin 6 (Prdx6), a savior for cells from internal or external environmental stresses, plays a role in cellular signaling by detoxifying reactive oxygen species (ROS) and thereby controlling gene regulation. Using targeted inactivation of the Prdx6 gene, we show that Prdx6-deficient lens epithelial cells (LECs) are more vulnerable to UV-triggered cell death, a major cause of skin disorders including cataractogenesis, and these cells display abnormal protein profiles. PRDX6-depleted LECs showed phenotypic changes and formed lentoid body, a characteristic of terminal cell differentiation and epithelial-mesenchymal transition. Prdx6−/− LECs exposed to UV-B showed higher ROS expression and were prone to apoptosis compared with wild-type LECs, underscoring a protective role for Prdx6. Comparative proteomic analysis using fluorescence-based difference gel electrophoresis along with mass spectrometry and database searching revealed a total of 13 proteins that were differentially expressed in Prdx6−/− cells. Six proteins were upregulated, whereas expression of seven proteins was decreased compared with Prdx6+/+ LECs. Among the cytoskeleton-associated proteins that were highly expressed in Prdx6-deficient LECs was tropomyosin (Tm)2β. Protein blot and real-time PCR validated dramatic increase of Tm2β and Tm1α expression in these cells. Importantly, Prdx6+/+ LECs showed a similar pattern of Tm2β protein expression after transforming growth factor (TGF)-β or H2O2 treatment. An extrinsic supply of PRDX6 could restore Tm2β expression, demonstrating that PRDX6 may attenuate adverse signaling in cells and thereby maintain cellular homeostasis. Exploring redox-proteomics (Prdx6−/−) and characterization and identification of abnormally expressed proteins and their attenuation by PRDX6 delivery should provide a basis for development of novel therapeutic interventions to postpone ROS-mediated abnormal signaling deleterious to cells or tissues.
Keywords: peroxiredoxin, oxidative stress, proteomics, tropomyosin
oxidative stress has been identified as one of the major causes of age-related diseases, including cataracts (1, 45, 62, 74–76, 82). Oxidative stress-induced etiology and progression of diseases may result either from diminished natural antioxidants such as catalase, glutathione peroxidase, and peroxiredoxins (PRDXs) due to aging or from increased generation of reactive oxygen species (ROS) by exposure to environmental factors, X-rays, chemicals, toxins, and ultraviolet (UV) radiation. Environmental stressors such as these have been heavily implicated in the etiology and progression of several diseases by inducing ROS-mediated oxidative stress.
UV radiation exposure induces several complex sets of acute or chronic responses that can lead to initiation of diseases through overproduction of ROS. ROS modify cellular signaling. Modulation of signal transduction pathways includes changes in gene expression (18). However, investigation into how the depletion or reduced expression of antioxidants influences cellular signaling is needed. Several recent reports have emphasized the role of antioxidants in maintaining cellular physiology by optimizing ROS levels (6, 21, 41, 88). The skin and eyes are the organs most exposed to environmental stress, and UV radiation has been proven to generate ROS such as hydrogen peroxide and superoxide ions in the eye lens (83–85, 89, 100–102). UV radiation-modulated production of ROS results in degradation, cross-linking, and aggregation of lens proteins and DNA damage and is regarded as an important factor in cataractogenesis (51, 84, 88). PRDX6 is a relatively newly discovered protective protein (21, 22, 41, 58). We previously (22) cloned PRDX6 from a human lens epithelial cell (LEC) cDNA library. PRDX6 has both glutathione peroxidase and acidic Ca2+-independent phospholipase A2 activity (56, 58) and can protect cells from membrane, DNA, and protein damage mediated by ROS-driven oxidative stress or lipid peroxidation (15, 21, 22, 40, 41, 44, 49, 97, 98).
PRDXs represent a superfamily of selenium-independent peroxidases that are widely distributed in many major organs, including the lens (22, 41). The mammalian PRDX family contains six members, PRDX 1–6 (21, 22, 55, 97, 98). All PRDXs have two catalytically active cysteines, except for PRDX6 (also known as antioxidant protein 2, Aop2), a cytosolic antioxidant protein, which contains only one. In our previous studies, we demonstrated (21, 44) the presence of all six known PRDXs in the lens and, more importantly, that PRDX6 was present in significantly higher concentrations than other family members. PRDX6 is classified as a peroxiredoxin based on homology of structure, but its properties clearly differentiate it from other members of the mammalian PRDX family, and the sequence associated with activity of PRDX6 is not present in other peroxiredoxins (56). Our other previous studies (21, 41) revealed that Prdx6-depleted (Prdx6−/−) mouse LECs exhibited elevated expression of ROS and were vulnerable to oxidative stress-induced apoptosis. We recently reported (21, 24) that Prdx6-depleted mouse lenses develop cataracts after exposure to oxidative stress. Of interest, cells cultured from these mice had elevated levels of ROS and bioactive transforming growth factor (TGF)-β (21) and displayed phenotypic alterations showing differentiation of LECs. Furthermore, the cells had elevated levels of α-smooth muscle actin (α-SMA) and TGF-β-inducible genes (βig-h3), which are factors known to be indicative of cataractogenesis and posterior capsular opacifications after cataract surgery (25, 53, 60, 61) and are indistinguishable from TGF-β-induced changes.
TGF-β, a known physiological effecter of various extracellular matrix genes and their products, is present in the aqueous and vitreous humors (90) and has been shown to induce cataracts (28, 29, 52). Of its three isoforms, TGF-β1 has been shown to promote tissue fibrosis, formation of stress fiber, epithelial-mesenchymal transition (EMT), myofibroblast formation, fibrosis, and apoptosis by overstimulating genes such as extracellular matrix proteins, α-SMA, and βig-h3, which are implicated in several pathogenic conditions (11, 79, 86, 92). Our previous reports and several other reports have demonstrated that elevated levels of ROS activate TGF-β (3, 8, 9), which subsequently induces oxidative stress (7). Transcription factors provide a significant target molecule underlying age-dependent modulation in gene expression and during oxidative stress or oxidative stress-induced modulation of growth factors. We previously demonstrated (21, 80) that TGF-β1 downregulated transcriptional factor lens epithelium-derived growth factor (LEDGF) expression and diminished its affinity for DNA during TGF-β1-induced phenotypic changes and apoptosis observed in human LECs. However, details regarding the wide-spectrum activity of TGF-β and its contributions to oxidative stress in cataract formation remain to be clarified.
No study has yet clarified the consequences of deleting Prdx6 at the cellular and molecular levels in the lens with attention to changes in protein expression or conducted proteomic analyses of Prdx6−/− LECs. However, with the recent development of proteome analysis, examination of protein profiles in LECs has become feasible. To identify proteins involved in the direct and indirect response to Prdx6 depletion in LECs (redox state) or the contributions of oxidative stress, we used the relatively new proteomic differential display method fluorescence-based difference gel electrophoresis (DIGE) (91) coupled with matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS).
Here we demonstrate for the first time the differences in the protein profiles of Prdx6−/− and wild-type (Prdx6+/+) LECs and their response to UV-B-induced ROS-mediated cellular insults. These findings may support an important role of PRDX6 in regulating gene protein expression and thereby function as a cell savior.
MATERIALS AND METHODS
Generation, characterization, and breeding of Prdx6-targeted mutant mice.
The generation, characterization, mating, and analysis of the offspring of Prdx6-targeted mutant mice has been described in detail in previous studies (34, 71, 94). Briefly, a genomic clone with the 129/SvJ (129) Prdx6 gene was isolated as described previously (71). Prdx6−/−129/SvJ mice were then generated at Harvard Medical School under the supervision of Dr. David Beier. These mice are maintained in a fully inbred 129/SvJ background, which minimizes variation due to genetic background. Then to generate Prdx6-targeted mutant mice of 129/SvJ (129) background, chimeric Prdx6-targeted mutants were crossed with 129 mice, and the offspring heterozygous for the Prdx6-targeted mutation were intercrossed to generate mice homozygous for the targeted Prdx6 gene mutation (Prdx6−/−). This study used Prdx6−/− mutant mice of pure 129 background. Wild-type 129/SvJ inbred mice of the same sex and age were used as controls (Prdx6+/+).
Mutant genotypes of Prdx6−/− were confirmed by polymerase chain reaction (PCR) of genomic DNA obtained from the tail (94). All animals were maintained under specific pathogen-free conditions in the animal house at Harvard Medical School. Mice lacking Prdx6 developed normally. No differences were noted in the body weights of age- and sex-matched adult homo- and heterozygous mutant and wild-type control animals. Microscopic examination revealed no abnormalities in any of the organs and tissues of mutant or control animals (94).
Culture.
Lenses were isolated from seven mice that were 8 wk old, and Prdx6−/− and Prdx6+/+ cell lines were generated and maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS; Sigma, St. Louis, MO) as described previously (21, 84). For each assay, Prdx6+/+ and Prdx6−/− mouse LECs were first cultured in DMEM containing 10% FBS for 24 h. The medium was then replaced with DMEM containing 10 μg/ml bovine serum albumin (BSA) + 2% FBS and incubated for another 24 h, after which the assay was performed.
All animal experiments were approved by the Committee of Animal Research at the University of Fukui and conducted in accordance with the regulations of the University of Fukui.
UV-B exposure.
UV-B light was generated from a 15-W UV-B light source (UVP, Upland, CA). The light intensity was standardized with a UV light meter (UVP). Prdx6+/+ and Prdx6−/− mouse LECs were cultured in DMEM containing 10% FBS for 24 h, and then the medium was replaced with DMEM containing 10 μg/ml BSA and incubated for a further 24 h. For UV-B exposure, the medium was replaced with 1× phosphate-buffered saline (PBS) and irradiated with UV-B radiation at 0, 400, or 800 J/m2. After 24–48 h of exposure, experimental assays were performed.
Assay for intracellular redox state.
Intracellular redox state levels were measured with the fluorescent dye dichlorofluorescin diacetate (DCFH-DA) as described previously (19, 21, 67). For the assay, LECs were cultured for 24 h with DMEM-0.1% BSA medium in a 96-well chamber slide after UV-B irradiation at 0, 400, and 800 J/m2. The medium was then replaced with Hanks’ solution (Sigma) containing 10 μM DCFH-DA and incubated for 10 min at room temperature. Fluorescein intensity was measured with a fluorescent photometer.
Cell viability assays and TdT-mediated dUTP-biotin nick end-labeling.
To assess apoptotic cell death, a TdT-mediated dUTP-biotin nick end-labeling (TUNEL) assay was performed with an ApoAlert DNA fragmentation assay (Clontech, Mountain View, CA). The percentage of TUNEL-positive cells per 100 cells was assessed from the number of Hoechst-stained (Molecular Probes, Eugene, OR) nuclei identified in six different fields of each slide in each group. The number of surviving cells in each group was assessed via a cell viability assay using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS; Promega, Madison, WI) as described previously (19, 21, 41, 47).
Protein extraction and fluorescence labeling.
The cell pellets were incubated for 15 min in a lysis buffer containing 20 mM Tris·HCl, 7 M urea, 2 M thiourea, and 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) (pH 8.6). Protein extracts from LECs obtained from Prdx6+/+ and Prdx6−/− mice were labeled with Cy3 or Cy5. An internal pool was generated by combining equal amounts of extracts from each cell type; this pool was labeled with Cy2 dye and was included in all gel runs.
Differential two-dimensional electrophoresis and gel image analysis.
Samples were run with commercial immobilized pH gradient strips (pH 4–7; 7 cm and 13 cm long) for isoelectrofocusing and a ready-made 10–20% gradient sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) for the second dimension. A preparative gel containing 800 μg of each protein was run and stained with Coomassie brilliant blue (SeePico CBB; Benebiosis, Seoul, South Korea).
Proteins were visualized with a fluorescence scanner (Typhoon Trio; GE Healthcare, Fairfield, CT), and images were processed with Progenesis PG240 software v2006 (Nonlinear Dynamics, Newcastle, UK).
Protein identification by MS.
Forty-two differentially expressed protein spots of interest were excised from the gel and subjected to tryptic digestion in accordance with a previous protocol. Briefly, proteins were first destained (1 mM ethylenediaminetetraacetic acid, 25 mM ammonium bicarbonate, 50% acetonitrile) and then reduced (10 mM dithiothreitol) and alkylated (55 mM idoacetamide). After drying, the gel pieces were incubated with modified bovine trypsin (Roche, Basel, Switzerland) at a final concentration of 20 ng/ml in 25 mM ammonium bicarbonate for 16 h at 37°C. After incubation, tryptic peptides were collected and treated with ZipTip (Millipore, Billerica, MA) according to the manufacturer's instructions. For the MALDI study, the extracted peptides were concentrated by SpeedVac, avoiding complete drying, and then spotted on a MALDI target plate (Prespotted AnchorChip, Bruker Daltonics, Billerica, MA). MALDI-TOF MS analysis of the samples was carried on a mass spectrometer (Autoflex, Bruker Daltonics) in positive ion reflector mode. For peptide mass fingerprinting identification, the tryptic mass maps were transferred with MS Bio Tools (Bruker Daltonics) as input to search the National Center for Biotechnology Information (NCBI) database with Mascot software (Matrix Science, Boston, MA).
Western blot analysis.
Protein lysates of Prdx6+/+ and Prdx6−/− mouse LECs were prepared in ice-cold radioimmune precipitation buffer, and Western blot analysis was performed as described previously (41, 43, 44, 48). The membranes were probed with anti-Prdx6 monoclonal antibody (Ab) (LabFrontier, Seoul, South Korea), anti-tropomyosin (Tm) monoclonal Ab (TM311) (Acris Antibodies, Hiddenhausen, Germany), and anti-α-SMA monoclonal Ab (Sigma). Anti-β-actin monoclonal Ab (Sigma) was used to show that equal amounts of protein were loaded in each lane.
Real-time PCR.
To monitor the levels of Prdx6 and Tm in Prdx6+/+ and Prdx6−/− mouse LECs, total RNA was isolated with the RNeasy Mini Kit (Qiagen, Valencia, CA) and real-time reverse-transcribed PCR was performed as described previously (41). We used 20× predeveloped murine Prdx6 probe mix and murine Tm1α, Tm2β, vimentin, ubiquinol-cytochrome c (Uqcrcp1), mitochondrial ribosomal protein S22 (MrpS22), thioredoxin domain containing 5 (Txndc5), serine protease inhibitor, clade B, member 6a (Serpinb6a), and TGF-β1 probe mixes (Applied Biosystems, Foster City, CA) for real-time PCR. The relative quantity of each mRNA was obtained with the comparative threshold cycle (Ct) method and normalized with predeveloped TaqMan assay reagent ribosomal RNA as an endogenous control (Applied Biosystems). In each group, the Ct values of the target genes were normalized to the levels of ribosomal RNA used as an endogenous control. The ΔCt for each gene was calculated as described previously (46). One-factor ANOVA was used for statistical analyses.
For treatment with TGF-β1 (R&D Systems, Minneapolis, MN), control (Prdx6+/+) mouse LECs were treated with DMEM containing 0–10 ng/ml TGF-β1 for 24 h.
Prokaryotic expression of recombinant Prdx6 protein.
The human immunodeficiency virus (HIV)-trans-activating transduction (TAT) domain has 11 amino acids (YGRKKRRQRRR) and 100% potential for intracellular delivery of proteins across the plasma membrane and the blood-brain barrier (10, 41, 59, 66, 73). In the present study, we took advantage of the ability of the TAT domain to infiltrate cells and used the recombinant TAT-linked Prdx6 protein.
The cDNA encoding the open reading frame of Prdx6 was isolated from the human LEC cDNA library as described previously (22). To prepare TAT-HA-PRDX6 constructs, we followed the method described by Dowdy and coworkers (78, 93), and expression and purification of TAT-HA-PRDX6 fusion proteins were performed as described in our previous study (41).
Immunohistochemistry and F-actin staining.
Prdx6+/+ and Prdx6−/− mouse LECs were cultured in DMEM containing 10% FBS for 24 h. After culturing, the medium was replaced with DMEM containing 10 μg/ml BSA in four-well chamber slides (Nalge Nunc) for 24 h and then fixed in 4% paraformaldehyde in PBS. Immunostaining was conducted with an Alexa Fluor 488 Signal-Amplification Kit for mouse Abs (Molecular Probes) as described previously (44) Tm1α and -2β were visualized with anti-Tm monoclonal Ab (TM311, Acris; dilution 1:200). F-actin staining was conducted with fluorescent phallotoxin (Texas Red-X Phalloidin, Molecular Probes) after immunostaining. Fluorescence images were captured with a confocal laser scanning microscope (TCS SP II; Leica Microsystems, Bannockburn, IL).
Statistical methods.
Data are presented as means ± SD of the indicated number of experiments. Data were analyzed by one-way ANOVA, which was followed by Dunnett's multiple comparison test or Student's t-test when appropriate. A P value of <0.05 was defined as indicating a statistically significant difference.
RESULTS
Phenotypic changes and spontaneous cell death of Prdx6−/− LECs.
LECs isolated from Prdx6-targeted mutant (Prdx6−/−) and wild-type (Prdx6+/+) mice were cultured with DMEM containing 0.2% BSA. The cells were observed at different time intervals (at 24, 48, 72, and 96 h) and photomicrographed. Of particular note, we observed significant alterations in phenotype in the Prdx6-depleted cells. Under serum-depleted conditions, Prdx6−/− LECs became elongated and fiberlike, formed cellular aggregates, and packed irregularly (Fig. 1B). Furthermore, these cells frequently detached and underwent spontaneous death. Surprisingly, lengthy incubation of these cells in culture (≥48 h) resulted in greater cell aggregation and adoption of a lentoid shape (4, 5). Figure 1B is a photomicrograph taken after 96 h. However, although these differences were obvious in cells cultured in complete medium, the changes were not as distinguishable in cells cultured under serum depletion conditions (data not shown). This discrepancy may be attributed to the presence of catalase in serum. Interestingly, these morphological changes in Prdx6−/− LECs were quite similar to those observed in transdifferentiated and fibroblastic LECs in anterior subcapsular cataracts and posterior capsule opacifications after cataract surgery.
Fig. 1.
Photomicrograph of Prdx6−/− lens epithelial cells (LECs) cultured in vitro showing phenotypic changes. Prdx6+/+ (A) and Prdx6−/− (B) LECs were cultured in complete Dulbecco's modified Eagle's medium DMEM containing 10% fetal bovine serum and washed, and the medium was changed to 0.1% bovine serum albumin (BSA) in DMEM. Cells were photomicrographed after 48 h of serum depletion. Under serum-depleted conditions, Prdx6−/− cells became elongated and fiberlike, formed cellular aggregates, packed irregularly, and finally aggregated into lentoid formations and underwent spontaneous apoptosis.
Several previous studies have emphasized that ROS-induced TGF-β expression is a culprit that induces abnormal changes in LECs, in turn leading to cataractogenesis (21, 41, 63, 90). We therefore believe that the cells derived from Prdx6−/− mice are a suitable model for studying the mechanism involved in the etiology and progression of cataractogenesis.
Increased vulnerability to UV-B radiation, increased ROS expression, and apoptosis in Prdx6−/− LECs.
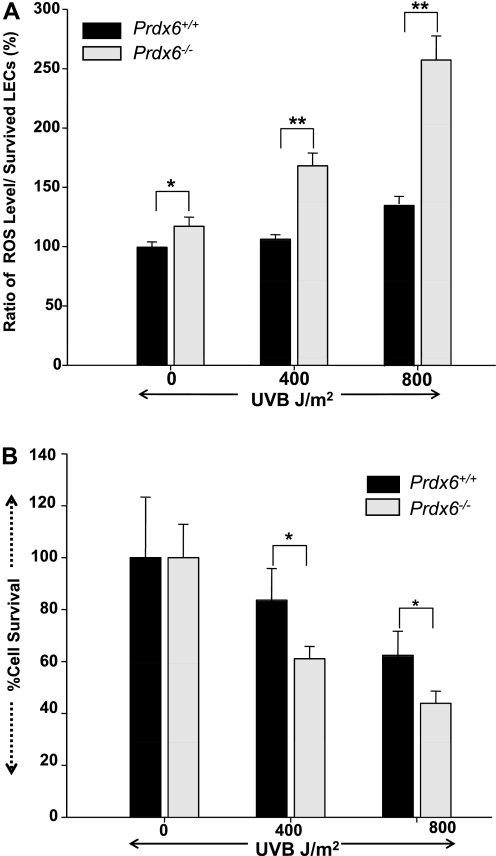
We initially measured ROS level to determine whether UV-induced cell death in LECs is associated with elevated ROS level. Prdx6-depleted and wild-type LECs were exposed to UV-B radiation at 0, 400, and 800 J/m2. After a 24-h recovery period, we conducted quantization by the H2DCFH-DA method and confirmed an elevated level of ROS in Prdx6−/− cells (Fig. 2A) compared with wild-type LECs. Furthermore, we noticed a significant decrease in cell survival for Prdx6-depleted cells (Fig. 2), indicating that these cells are more susceptible to UV-B stress. These results reveal a direct correlation between cell survival and ROS level and suggest that UV-B exposure induces cell death through elevated ROS levels. However, it should be mentioned that DCF fluorescence is not specific for H2O2, and other oxidants such as peroxynitrite, O2−, and NO may also oxidize DCH2 to DCF. DCF fluorescence may thus reflect overall oxidative stress in cells (21).
Fig. 2.
Prdx6−/− cells showed increased reactive oxygen species (ROS) expression after exposure to UV-B radiation in vitro. Prdx6+/+ and Prdx6−/− cells were cultured for 24 h as described in materials and methods. After culturing, the cells were subjected to UV-B radiation at 0, 400, and 800 J/m2. The medium was replaced 24 h later with Hanks’ solution containing 5–10 μM H2-dichlorofluorescin diacetate (DCFH-DA). Fluorescence intensity (ROS expression) was measured. Histogram in A represents the results, showing elevated expression of intracellular ROS in Prdx6−/− cells compared with Prdx6+/+ cells. *P < 0.006, **P < 0.00001. B: cell survival assay (MTS assay) demonstrating that Prdx6−/− LECs cells are highly vulnerable to UV-B-radiation-induced insults. Cells were cultured as stated above and exposed to UV-B radiation, and an MTS assay was conducted as described in text 24 h later. A significant decrease in cell survival of Prdx6−/− LECs was observed compared with Prdx6+/+ LECs (*P < 0.00015), suggesting that PRDX6 is essential to protect cells from UV-B-radiation-induced damage. Results are means ± SD of 3 independent experiments, and A and B are representative of the experiments.
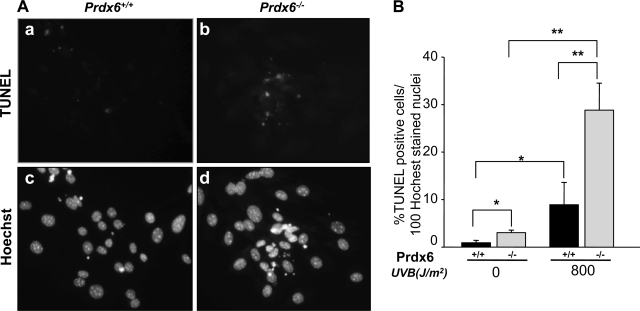
To determine whether UV-B exposure induces apoptotic cell death, we performed Hoechst staining and a TUNEL assay. Cells were first photomicrographed and analyzed. Typical apoptotic nuclei (Fig. 3A) were observed in Prdx6−/− LECs (Fig. 3A, b and d). On the basis of percentage of TUNEL-positive cells/100 Hoechst-stained nuclei, we observed more apoptotic cell deaths in Prdx6−/− LECs (Fig. 3B) than in Prdx6+/+ LECs. These data indicate that cells lacking Prdx6 undergo apoptosis on exposure to UV-B radiation. In contrast, wild-type Prdx6+/+ cells were resistant to UV-B radiation, demonstrating the protective role of PRDX6. However, in light of the finding that PRDX6-depleted LECs contain elevated levels of ROS and are thereby highly susceptible to UV-B-induced ROS-mediated oxidative stress, several reports have noted that elevated expression of ROS is a common signal in different cell pathways, indicating the complex relationship between ROS levels and cell death (21, 30, 31, 87).
Fig. 3.
Prdx6−/−-deficient cells, which are highly vulnerable to UV-B radiation exposure, undergo apoptosis. Cells were cultured in DMEM + 10% fetal bovine serum (FBS) in 60-mm petri dishes. The day after culturing, cells were exposed to UV-B radiation (800 J/m2). After a 24-h recovery period, cells were stained with either TdT-mediated dUTP-biotin nick end-labeling (TUNEL; A, top) or Hoechst (A, bottom) and photomicrographed. A significant number of apoptotic cell deaths were seen in Prdx6−/− cells (A, b and d). B: % of apoptotic cells/100 Hoechst-stained nuclei. *P < 0.04, **P < 0.001.
Differential distribution of proteins detected with DIGE.
Prdx6-depleted cells lose their bona fide phenotypes and are more susceptible to damage induced by environmental stress. Several recent reports have documented the pivotal role of Prdx6 in controlling cellular signaling by optimizing ROS expression, enabling the enzyme to function in cellular differentiation, proliferation, and cytoprotection. To examine the abnormal changes that occur in cells lacking Prdx6 (21, 23, 41, 94), we examined the pattern of protein expression in Prdx6−/− LECs with a proteomics approach.
Prdx6+/+ and Prdx6−/− LEC lysates were prepared and labeled with fluorescent dyes. Image analysis of fluorescently labeled lysates loaded together on two-dimensional gels showed differing distribution patterns between protein expressions on comparison of Prdx6−/− and Prdx6+/+ LECs (Fig. 4). Protein spots that were decreased or increased >1.5-fold were selected for MALDI-TOF identification. Approximately 920 spots were detected on the large gel (13 cm) (Fig. 4A) and 320 on the small gel (7 cm) (Fig. 4B). Forty-two spots were differentially expressed (>1.5-fold change; P < 0.05 on ANOVA analysis) and analyzed with MALDI-TOF MS. Thirteen proteins were identified from the NCBI database search with Mascot software (Matrix Science). Among the 13 altered proteins, expression was increased for 6 proteins and decreased for 7 proteins in Prdx6−/− LECs (Table 1).
Fig. 4.
Representative difference gel electrophoresis (DIGE) images of protein extracts of Prdx6−/− and Prdx6+/+ LECs. Labeled samples (Cy3 for Prdx6−/− LECs in green and Cy5 for Prdx6+/+ LECs in red) were subjected to 2-dimensional (2D) gel electrophoresis. Fluorescence was scanned, and the derived images were superimposed with pseudocolors in the dyes. Proteins that are up- or downregulated in Prdx6−/− cells compared with Prdx+/+ LECs are represented by red (up) and green (down) spots, whereas proteins that are equally abundant in both samples appear yellow. The full range of the horizontal axis is from 4 (left) to 7 (right) pH units, and the full range of the vertical axis is from ∼10 (bottom) to ∼100 (top) kDa. A: superimposed images from Cy3- and Cy5-labeled samples in the large gel (13 × 13 cm). Arrows and spot numbers (see Table 1) indicate significant upregulated (red) or downregulated (green) proteins present in Prdx6−/− LECs vs. Prdx6+/+ LECs. B: enlarged area from a small (7 × 7 cm) 2D-SDS gel showing Prdx6−/− (left) and Prdx6+/+ (right) LECs. Protein spots that were decreased or increased >1.5-fold were selected for matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) identification. Mr, molecular weight ratio.
Table 1.
Identified up- or downregulated proteins in Prdx6−/− LECs
| 2D-DIGE Sequence |
||||||
|---|---|---|---|---|---|---|
| Spot No. | Name | Average Ratio | Accession No. | Mr, kDa | Coverage % | Molecular Function |
| 910* | Tropomyosin (Tm)2β | 6.354↑ | gi:11875203 | 32.931 | 19.0 | Cytoskeleton |
| 1374 | Vimentin | 6.085↑ | gi:2078001 | 53.69 | 31.2 | Cytoskeleton |
| 1479 | Vimentin | 6.194↑ | gi:2078001 | 53.69 | 29.4 | Cytoskeleton |
| 1429 | Ubiquinol-cytochrome c (Uqcrcl) | 1.660↑ | 53.42 | 27.5 | Metalloprotease | |
| Reductase complex core protein | gi:14548301 | |||||
| 1708 | Mitochondrial ribosomal protein S22 (MrpsS22) | 1.563↑ | gi:12963591 | 38.475 | 29.7 | Unclassified |
| 1778 | Hypothetical protein | 1.958↑ | gi:83010429 | 16.539 | ||
| 422 | ALG-1 interacting protein | 0.625↓ | gi:4633515 | 96.661 | 16.0 | Select calcium binding protein |
| 933 | Lamin C | 0.583↓ | gi:1794160 | 65.464 | 19.0 | Intermediate filament |
| 1126 | Tubulin-specific chaperone e | 0.416↓ | gi:31543843 | 59.790 | 16.6 | Chaperone |
| 1499 | Thioredoxin domain containing 5 (Txndc5) | 0.597↓ | gi:83921612 | 36.631. | 49.8 | Isomerase |
| 1511 | Serine (cysteine) protease inhibitor, clade B, member 6a (Seprinb6m) | 0.523↓ | gi:6678097 | 42.913 | 19.6 | Protease inhibitor |
| 1557 | Gelsolin-like capping protein | 0.564↓ | gi:110227377 | 39.030 | 28.7 | Cytoskeleton |
| 2266 | Peroxiredoxin6 (PRDX6) | 0.088↓ | gi:3219774 | 24.969 | 61.6 | Oxidoreductase |
LEC, lens epithelial cell; 2D-DIGE, 2-dimensional difference gel electrophoresis; Mr, molecular weight ratio.
Identified on small gel (7 × 7 cm).
PRDX6 protein expression provides an internal control for comparative proteomic analysis (Table 1). Proteins with dramatically elevated expression included cytoskeleton proteins, Tm2β, and vimentin (Table 1). The increased abundance of these proteins may be related to the phenotypic abnormalities of Prdx6−/− LECs. Protein PRDX6 is involved in detoxifying (21–23, 37, 38, 41) ROS and is the most sensitive marker of a redox cellular state (14, 35) as well as being involved in defense against ROS-generated lipid peroxidation-induced damage (23, 41).
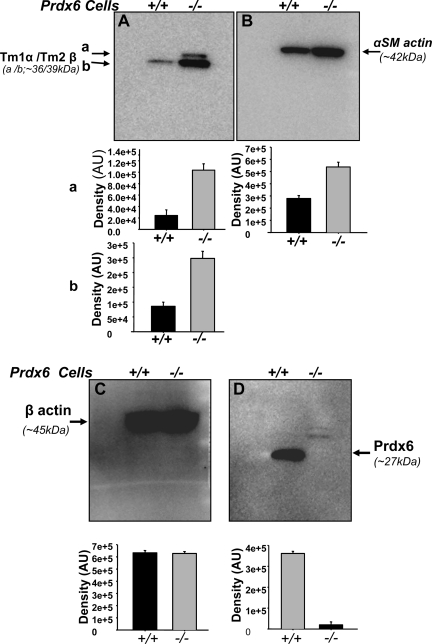
Elevated expression of Tm1α and -2β protein in Prdx6−/− LECs validated by one-dimensional Western blot analysis.
To confirm the results obtained in the proteomic study, we selected Tm1α and Tm2β proteins, which were increased in Prdx6−/− LECs (Fig. 5A), as well as α-SMA (Fig. 5B) as a positive control for EMT of LECs. Proteins corresponding to isoforms from Tm1α and Tm2β genes and α-SMA protein were overproduced in the Prdx6−/− LECs (Fig. 5, A and B).
Fig. 5.
Western blot analysis of protein extracts from Prdx6−/− and Prdx6+/+ LECs showing upregulation of Tm1α, Tm2β, and α-SMA proteins in Prdx6−/− LECs. The mouse monoclonal Tm Ab (TM311) detects the amino terminal exon 1a of the Tm1α and Tm2β genes and is therefore able to detect recombinant Tm1, -2, -3, and Br-1 (27, 77). Although Tm1 and the β-isoform (36 kDa) from Tm2β gene (A, a) protein were not detected in Prdx6+/+ LECs, they were detected in Prdx6−/− LECs. Tm1 or β (A, a = 36 kDa), and Tm2 or α (A, b = 39 kDa) isoforms from Tm2β and Tm1α genes, respectively, and α-SMA proteins (B) were increased in Prdx6−/− LECs. No change was observed in β-actin expression (C, compare black vs. gray bars), confirming equal protein loading and suggesting that expression modulation for other proteins is specific. D confirms that LECs Prdx6−/− LECs do not express PRDX6 protein. Histograms represent densities of protein bands. Results are derived from 3 different cell preparations. AU, arbitrary units.
We then determined whether cells isolated from the lenses were indeed LECs. A protein blot using the αA-crystalline Ab confirmed the presence of αA-crystalline, a specific marker of LECs, in both Prdx6−/− and Prdx6+/+ LECs (data not shown). β-Actin Ab was used to confirm equal sample loads (Fig. 5C). A protein blot using Prdx6-specific Ab revealed the absence of PRDX6 protein in Prdx6−/− LECs (Fig. 5D).
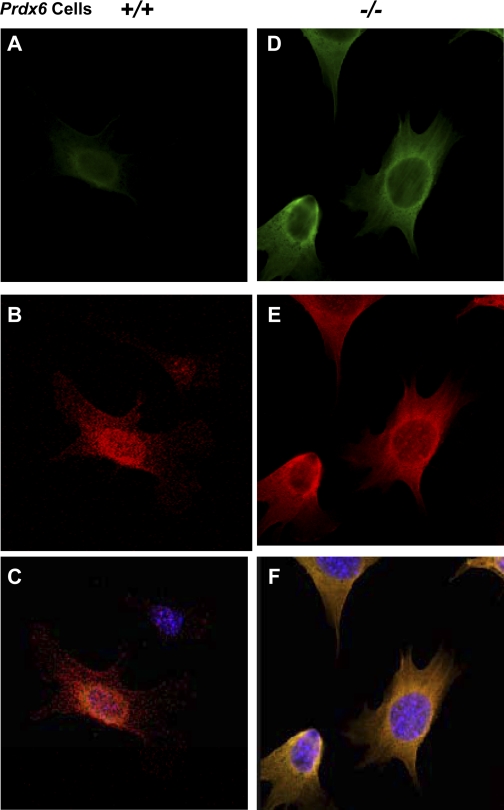
We then performed immunohistochemistry tests using anti-Tm monoclonal Ab (TM311), which recognizes isoforms from Tm1α and Tm2β genes in Prdx6+/+ and Prdx6−/− LECs (Fig. 6, A and B, respectively). It is known that Tm binds to actin, including F-actin. F-actin was double-stained with fluorescein phallotoxin. We observed induction of Tm1α/2β protein and actin stress fibers in Prdx6−/− LECs (Fig. 6, D–F), suggesting that depletion of Prdx6 is involved in the formation of stress fibers, which stabilize actin filaments and thus reduce cell motility and growth (12, 26, 92). These results are suggestive of Prdx6's role in maintaining cellular integrity. Further investigation is required to clarify whether Prdx6 depletion alters protein integrity or induces posttranslational modification.
Fig. 6.
Confocal laser scanning images of Prdx6+/+ and Prdx6−/− LECs showing Tm1α/2β immunolocalization and F-actin. F-actin was labeled with Texas Red in both Prdx6+/+ (B) and Prdx6−/− (E) LECs. Tm1α/2β protein was strongly stained in Prdx6−/− LECs (D) compared with Prdx6+/+ LECs (A) after administration of anti-Tm1α/2β Ab and use of an Alexa Fluor 488 Signal-Amplification Kit. Tm1α/2β proteins can be seen to be localized to stress fiber formed in Prdx6−/− LECs (B). C and F: merged images of Tm immunostaining and F-actin staining.
Increased expressions of Tm2β and other related gene mRNA in Prdx6−/− LECs at transcriptional level.
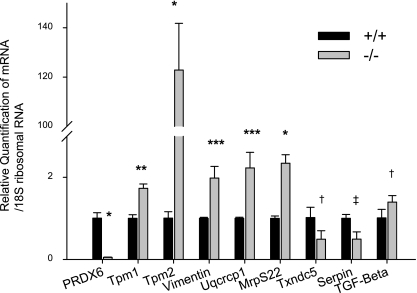
To estimate the transcriptional levels of Tm1α, Tm2β, Vimentin, Uqcrcp1, Mrps22, Txndc5, and Serpinb6, which are up- or downregulated in proteomic analysis, we measured the mRNA expression of Tm1α, Tm2β, Vimentin, Uqcrcp1, MrpS22, Txndc5, and Serpinb6a transcripts in Prdx6−/− LECs by real-time PCR analysis (Fig. 7). The results showed that the level of Tm2β mRNA was extremely elevated in Prdx6−/− LECs compared with wild-type cells (>120-fold). However, we also noted a significant increase in expression of Tm1α, Vimentin, Uqcrcp1, and MrpS22 and a significant decrease in expression of Txndc5 and Serpinb6a (Fig. 7). Tm isoforms are known to be responsive to TGF-βs (7, 92). Both we (21, 46) and others (63, 90) have previously reported that TGF-βs are factors in the development of cataracts. Furthermore, we have reported (21) that ROS induce TGF-β activation and expression. To determine whether TGF-β1 mRNA expression is increased in Prdx6−/− LECs (redox state), real-time PCR analysis using a specific primer revealed that the level of TGF-β1 mRNA was significantly increased in Prdx6−/− LECs compared with Prdx6+/+ LECs (Fig. 7), suggesting that the increase in Tm isoforms in Prdx6−/− may be ROS mediated.
Fig. 7.
Quantitative real-time PCR analysis of Prdx6−/− and Prdx6+/+ LEC mRNA showing expression of Tm1α, Tm2β, Vimentin, Uqcrcp1, MrpS22, Txndc5, Serpinb6a, and TGF-β1. Total RNA was isolated and transcribed into cDNA. Real-time PCR was conducted with specific primers corresponding to genes (see materials and methods). mRNA expression of each gene was adjusted to the 18S ribosomal RNA. Results showed that Tm1α, Tm2β, Vimentin, Uqcrcp1, MrpS22, and TGF-β1 mRNA were upregulated and Txndc5 and Serpinb6a mRNA were downregulated in Prdx6−/− LEC. *P < 0.000, **P < 0.0008, ***P < 0.005, †P < 0.05, ‡P < 0.001.
Induction of Tm isoform transcripts in Prdx6-depleted LECs associated with TGF-β.
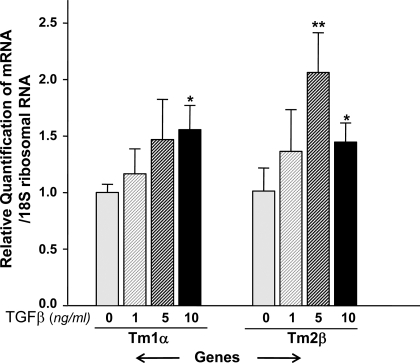
TGF-β induces phenotype alteration, expression of α-SMA, and apoptosis in many cell types that in turn leads to pathophysiology of tissues, including the lenses. To test whether TGF-β is indeed involved in induction of Tm isoform transcripts, we utilized wild-type LECs and treated them with variable concentrations of TGF-β1 (0, 1, 5, and 10 ng/ml) for a predefined duration. We found that Tm1α and Tm2β mRNA were significantly modulated by treatment with 5.0 or 10.0 ng/ml of TGF-β1 in Prdx6+/+ LECs (Fig. 8), suggesting the role of TGF-β in regulation of Tm isoforms. These observations suggest that TGF-β-induced stress fiber formation and EMT of cells may be associated with induction of Tm1α and Tm2β in LECs.
Fig. 8.
Real-time PCR analysis of Prdx6+/+ LEC mRNA with or without addition of transforming growth factor (TGF)-β1, showing TGF-β1-mediated regulation of Tm1α and Tm2β. Cells were cultured in petri dishes in DMEM containing 10% FBS. After 24 h, cells were washed and treated with varying concentrations of TGF-β1 containing 0.2% BSA for 48 h or more. After incubation, RNA was isolated and real-time PCR was conducted. Treatment of Prdx6+/+ LECs with 5.0 or 10.0 ng/ml of TGF-β1 resulted in induction of Tm1α and Tm2β (*P < 0.05, **P < 0.015), suggesting that upregulation of these genes is associated with activation and expression of TGF-β1 in Prdx6−/− cells, as reported elsewhere (21).
Restoration of Tm2β transcripts with TAT-HA-Prdx6.
We investigated whether addition of PRDX6 could attenuate the abnormal expression of proteins in Prdx6−/− LECs with Tm2β (overstimulated). RNA was isolated from TAT-HA-PRDX6-treated or untreated Prdx6−/− LECs as described previously (41). Results of real-time PCR are shown in Fig. 9. After incubation with TAT-HA-PRDX6 for 96 h, the overstimulated expression of Tm2β mRNA was significantly reduced in Prdx6−/− LECs (Fig. 9). These results support our hypothesis that reduced expression of PRDX6 in cells due to aging or environmental stress is a cause of tissue pathophysiology and support the role of PRDX6 in controlling gene expression by optimizing ROS.
Fig. 9.
Real-time PCR analysis of Prdx6−/− LECs mRNA supplied with PRDX6 (TAT-HA-PRDX6) protein revealing attenuation of overactivation of Tm2β genes. Cells were cultured in the presence or absence of 10 μg/ml of recombinant TAT-HA-linked Prdx6 protein, as described previously (41). Addition of TAT-HA-Prdx6 protein significantly suppressed overproduction of Tm2β mRNA in Prdx6−/− LECs. *P < 0.05.
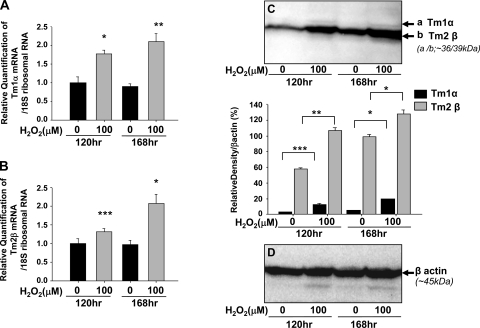
Induction of Tm isoform expression in Prdx6+/+ LECs associated with H2O2.
We previously reported (21, 41) that ROS levels were increased in Prdx6−/− LECs. H2O2 regulates various signaling pathways and induces apoptosis based on ROS levels. Here, we determined the effects of H2O2 on expression levels of Tm in Prdx6+/+ LECs (Fig. 10). We measured mRNA expression of Tm1α and Tm2β transcripts by real-time PCR analysis (Fig. 10, A and B, respectively). Levels of Tm1α and Tm2β transcripts were significantly elevated in Prdx+/+ LECs after incubation in 0 and 100 μM H2O2 for 120 and 168 h compared with untreated controls. Furthermore, Tm1α and Tm2β protein expression was also significantly elevated in Prdx+/+ LECs incubated in 0 and 100 μM H2O2 for 120 and 168 h compared with untreated controls (Fig. 10, C and D).
Fig. 10.
Real-time PCR and protein blot analysis of Prdx6+/+ LECs mRNA with or without addition of H2O2 showing ROS regulation of Tm1α and -2β. Prdx+/+ LECs were cultured in the presence or absence of 100 μM H2O2 for 120 and 168 h. Quantitative real-time PCR (A, B) and protein blotting (C, D) were performed to analyze the expression level of Tm1α and Tm2β transcripts and proteins. Expression of Tm1α and Tm2β transcripts (A) and proteins (B) were significantly increased in Prdx+/+ LECs treated with H2O2. *P < 0.002, **P < 0.0001, ***P < 0.028.
DISCUSSION
In the present study, we investigated the cytoprotective role of PRDX6 against UV-induced LEC insults and the effect of deficiency of this molecule on protein expression profiles. Our experimental data using Prdx6−/− and Prdx6+/+ LECs as a model showed that UV-B radiation exposure enhanced ROS levels in Prdx6-depleted LECs, rendering these cells more susceptible to death and inducing apoptosis (Fig. 2). The skin and eyes are the organs most exposed to UV radiation from the sun, toxic chemicals, and pollutants. Environmental factors such as these have been heavily implicated in the etiology and progression of several cellular and tissue-related diseases due to malfunctions of cellular organelles and damage to biomolecules by ROS-driven oxidative stress (21, 41, 94). ROS generated by cells are tightly regulated by antioxidants to maintain their expression at physiologically tolerable levels (36, 38, 39). However, reduced expression or activity of natural antioxidants such as catalase, glutathione peroxidase, superoxide dismutase, and thioredoxins due to aging or environmental stress may alter optimal expressions of genes and proteins involved in maintaining cellular homeostasis. UV irradiation is generally acknowledged to generate ROS, including hydrogen peroxide and superoxide ions (2, 64). Furthermore, several studies have already reported UV irradiation-induced cataractogenesis (2, 6, 64, 83–85, 89, 100–102). Thus Prdx6 may protect LECs against UV-B-induced cell damage by reducing ROS.
In our previous study (21), we found that lenses from Prdx6-depleted mice were more susceptible to oxidative stress and developed opacity in the superficial cortex after exposure to hydrogen peroxide or paraquat. Several recent reports have shown that Prdx6-depleted cells are more susceptible to death in the presence of oxidative stress and internal stress, (21, 41, 94) suggesting that Prdx6 is crucial for cell survival. In the above scenario, we hypothesize that reduced expression of Prdx6 is a cause of cellular damage and impaired homeostasis against UV-B radiation in the lens, potentially leading to cataractogenesis. The crucial role of Prdx6 in cellular stress response is further evidenced by the morphological changes seen in Prdx6-depleted LECs and their increased vulnerability to UV-B radiation-induced cell death. However, humans often develop cataracts upon aging; a recent report has shown that Prdx6 expression decreases with age and abundance is significantly reduced in cataractous lenses (68). Furthermore, as seen in Fig. 1, deficiency of Prdx6 in LECs induces phenotypic changes, including proliferation of fibroblastic, elongated, lentoid-shaped cells, characteristic of cellular differentiation. Most impressive is that changes similar to those in Prdx6−/− LECs have also been observed in wild-type LECs undergoing TGF-β-induced changes (54, 63), and these cellular abnormalities were able to be reversed after administration of Prdx6 (21, 41).
Given the findings of this and previous studies, we hypothesize that Prdx6 expression is crucial to preventing the development of ROS-mediated abnormalities in the eye lens. Furthermore, Prdx6 deficiency leads to abnormal expression of genes and phenotypic changes in LECs; this inability of other cellular enzymes to maintain cellular homeostasis in its absence underscores the importance of Prdx6.
Prdx6 differs from other antioxidant enzymes because of its unique antioxidant defense function. Prdx6 protects cells by removing ROS (GSH peroxidase activity), thereby controlling survival signaling and maintaining cellular membrane integrity by optimizing phospholipid turnover (phospholipase A2, aiPLA2 activity) (23, 57). The results of the present study are consistent with recent studies conducted with Prdx6-deficient keratinocytes, endothelial cells, and LECs, revealing that PRDX6 is essential to maintaining cellular function (21, 41, 50, 94).
ROS-induced damage to cells is related to ROS-driven abnormal signaling, which overstimulates TGF-β-mediated signaling (21, 41, 67) and in turn leads to overmodulation of gene expression of genes such as α-SMA and βig-h3, implicated in cataractogenesis as well as other pathophysiological disorders of cells and tissues (21, 63, 90). We hypothesize that this mechanism is likely behind the development of cataractogenesis specifically, and other diseases in general. This hypothesis was supported by proteomic analysis of Prdx6−/− cells, which revealed elevated expression of cytoskeleton proteins such as Tm1α and Tm2β (Figs. 4–6). Using DIGE coupled with MALDI-TOF, we identified several LEC proteins that were modulated in response to the depletion of Prdx6 (Fig. 4, Table 1). Levels of cytoskeletal proteins, Tm2β, and vimentin were increased more than sixfold in Prdx6−/− LECs over Prdx6+/+ LECs. Surprisingly, while expression of Tm2β mRNA and proteins was extremely low in Prdx6+/+ LECs, expression was abnormally high (>120 times) in Prdx6−/− LECs and localized in stress fibers (Fig. 6). Furthermore, using anti-Tm monoclonal Ab (TM311), we determined that isoforms from the Tm1α gene (36 kDa) were also increased in Prdx6−/− LECs (Fig. 7).
Tm is composed of nearly 40 closely related proteins generated from 4 different genes through alternative splicing (70). The balance between Tm isoforms present in a given cell determines the specificity of Tm functions (13). In nonmuscle cells, Tm is involved in the formation and stabilization of stress fibers and protection of existing fibers (17). The optimum expression of Tm has been suggested as extremely important for cellular integrity, and decreased expression of Tm has been shown to contribute to tumorigenesis (92). In this respect, Tm appears to function as a tumor suppressor and is tightly regulated. However, Tm regulation in cells under oxidative stress may be disrupted because of overactivation of TGF-β, as in Prdx6−/− cells. Tm isoforms belong to a multigene family of actin-binding proteins. Oxidative stress-induced remodeling of the actin cytoskeleton increases actin polymerization and reorganization into stress fibers (32). In the present study, we demonstrated for the first time overstimulated expression of Tm2β and Tm1α in cells with elevated levels of ROS in Prdx6−/− LECs and Prdx6+/+ LECs after addition of H2O2. This overexpression may lead to abnormal reorganization and intense membrane blebbing in LECs that may alter dramatically the cellular integrity, as seen in Fig. 1.
Focal adhesions are sites of adhesion where the membrane and extracellular matrix and the membrane and cytoskeleton meet. These organizations provide a bridge allowing stress fibers to anchor to the membrane and integrins (24). Previous studies have shown abnormal activation and deactivation of the signaling pathway in response to increased levels of ROS; focal adhesions do not assemble and polymerized actin generated through the SAPK2/p38 pathway is unable to bundle into stress fibers (33). We therefore surmised that abnormalities in LECs are associated with abnormal expression and improper organization of these cytoskeleton proteins, ultimately leading to impaired focal adhesion and resulting in abnormal cellular phenotypes and apoptosis (Figs. 1 and 3).
Our proteomic experiment coupled with Western blot analysis using Prdx6−/− LECs extracts revealed ROS-mediated overshooting of cellular signaling, which resulted in severe defects in normal cellular physiology, particularly in expression of cytoskeleton and extracellular matrix proteins. (Figs. 1–4 and 6). The actin cytoskeleton plays a fundamental role, including maintaining cell morphology, division, motility, and organelle and vesicle trafficking. Dysregulation of proteins such as Tm isoforms, which are involved in regulating actin dynamics, should be a major indicator of altered cell behavior and likely disease (17, 96). The various nonmuscle Tm isoforms are recognized and grouped into high (∼34–40 kDa, such as Tm1, Tm2, Tm3, and Tm6)- and low (∼28–32 kDa, such as Tm5)-molecular-mass forms (95). Using Prdx6-deleted cells, we demonstrated in the present study for the first time that Tm is involved in differentiation or EMT of LEC. Altered expression of Tm isoforms derived from both the Tmα and Tmβ genes (Figs. 4 and 5, Table 1) in mouse LECs suggests that these isoforms are involved in the phenotypic alteration of Prdx6-depleted LECs. Most importantly, a supply of TAT-HA-PRDX6 should be able to reduce Tm2β (Fig. 9).
Prdx6−/− LECs exhibit increased sensitivity to oxidative stress, and changes observed in PRDX6-depleted LECs involved increased ROS levels (Fig. 2). ROS activate TGF-β, which is an inducer of oxidative stress and cataract development (21, 28, 52). LECs from Prdx6-depleted mice have elevated levels of ROS and bioactive TGF-β (21). An extrinsic supply of Prdx6 or addition of a superoxide dismutase mimetic such as Mn(III) tetrakis(4-benzoic acid) porphyrin (MnTBAP), a known ROS inhibitor, hampers TGF-β-induced ROS generation or morphological changes in Prdx6−/− LECs (21), findings that suggest the potential involvement of TGF-β in phenotypic changes in Prdx6−/− LECs (21). Several TGF-β target genes including α- and β-Tms, α-actinin1, and calponin2-encoding actin-binding proteins have been implicated in the assembly of stress fibers (7, 99). Of these, Tms in particular have been shown to play a crucial role in stabilizing actin filaments (69). TGF-β specifically upregulates expression of α- and β-Tm genes while not regulating Tm3 and Tm4 genes, which encode low-molecular-mass Tms (7, 99).
Induction of Tm isoforms and stress fibers has been suggested to play an essential role in TGF-β control of cell motility and is necessary for TGF-β induction of stress fibers (7). Of note, EMT and stress fiber formation of LECs were observed in anterior subcapsular fibrosis of human cataracts and posterior capsule opacity after cataract surgery (16, 65, 72). Our findings regarding ROS-induced TGF-β-mediated induction of Tm may thus be associated with cataractogenesis and posterior-capsular opacification after cataract surgery.
We previously demonstrated (41) that biologically active recombinant PRDX6 proteins bearing the protein transduction domain TAT can enter cells and offer protection from TGF-β- or hydrogen peroxide-induced cell apoptosis, thereby enhancing cell survival. Furthermore, the applicability of this new approach has been demonstrated for inter- and intramolecular targeting of TAT-fusion proteins capable of modulating mitochondrial function and cell survival (41, 81). Further study is required, however, before drawing hard conclusions.
In the present study, we used the proteomics approach with Prdx6−/−-deficient cells as a model for aging or redox-active cells to show that Prdx6 is crucial for maintaining cellular integrity and regulating cell differentiation and proliferation. Prdx6 accomplishes this by stabilizing ROS levels and thereby normalizing gene expression, and its deficiency can attenuate normal physiological signaling, resulting in the failure of cell homeostasis due to overmodulated expression of genes or proteins. We also demonstrated that ROS-induced expression of TGF-β in LECs lacking Prdx6 is related to the upregulation of cytoskeletal proteins, Tms, and EMT of LECs. Regardless of the pathophysiological significance of Tm isoforms, the distinct pattern of Tm2β expression may prove useful as a clinical marker of LEC differentiation or of posterior capsule opacification or lens injury. Together, these observations clarifying the role of Prdx6 in regulating cytoskeleton proteins suggest the possibility of using Prdx6 delivery to prevent or delay the progression of cataractogenesis or posterior capsule opacifications.
GRANTS
This work was supported by National Eye Institute Grant EY-017613 (to D. P. Singh).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. David R. Beier (Div. of Genetics, Brigham and Women's Hospital, Harvard Medical School, Boston, MA) for supplying the Prdx6-targeted mutant mice.
REFERENCES
- 1.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 90: 7915–7922, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andley UP, Clark BA. The effects of near-UV radiation on human lens beta-crystallins: protein structural changes and the production of O2- and H2O2. Photochem Photobiol 50: 97–105, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 116: 217–224, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Arita T, Lin LR, Reddy VN. Differentiation of human lens epithelial cells in tissue culture. Exp Eye Res 47: 905–910, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Arita T, Lin LR, Susan SR, Reddy VN. Enhancement of differentiation of human lens epithelium in tissue culture by changes in cell-substrate adhesion. Invest Ophthalmol Vis Sci 31: 2395–2404, 1990 [PubMed] [Google Scholar]
- 6.Ayala MN, Soderberg PG. Vitamin E can protect against ultraviolet radiation-induced cataract in albino rats. Ophthalmic Res 36: 264–269, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bakin AV, Safina A, Rinehart C, Daroqui C, Darbary H, Helfman DM. A critical role of tropomyosins in TGF-beta regulation of the actin cytoskeleton and cell motility in epithelial cells. Mol Biol Cell 15: 4682–4694, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barcellos-Hoff MH. Latency and activation in the control of TGF-beta. J Mammary Gland Biol Neoplasia 1: 353–363, 1996 [PubMed] [Google Scholar]
- 9.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 10: 1077–1083, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods 24: 247–256, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 360: 361–364, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Braverman RH, Cooper HL, Lee HS, Prasad GL. Anti-oncogenic effects of tropomyosin: isoform specificity and importance of protein coding sequences. Oncogene 13: 537–545, 1996 [PubMed] [Google Scholar]
- 13.Bryce NS, Schevzov G, Ferguson V, Percival JM, Lin JJ, Matsumura F, Bamburg JR, Jeffrey PL, Hardeman EC, Gunning P, Weinberger RP. Specification of actin filament function and molecular composition by tropomyosin isoforms. Mol Biol Cell 14: 1002–1016, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesaratto L, Vascotto C, D'Ambrosio C, Scaloni A, Baccarani U, Paron I, Damante G, Calligaris S, Quadrifoglio F, Tiribelli C, Tell G. Overoxidation of peroxiredoxins as an immediate and sensitive marker of oxidative stress in HepG2 cells and its application to the redox effects induced by ischemia/reperfusion in human liver. Free Radic Res 39: 255–268, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Chae HZ, Uhm TB, Rhee SG. Dimerization of thiol-specific antioxidant and the essential role of cysteine 47. Proc Natl Acad Sci USA 91: 7022–7026, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobo LM, Ohsawa E, Chandler D, Arguello R, George G. Pathogenesis of capsular opacification after extracapsular cataract extraction. An animal model. Ophthalmology 91: 857–863, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Cooper JA. Actin dynamics: tropomyosin provides stability. Curr Biol 12: R523–R525, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Dudek EJ, Shang F, Taylor A. H2O2-mediated oxidative stress activates NF-kappaB in lens epithelial cells. Free Radic Biol Med 31: 651–658, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Fatma N, Kubo E, Sen M, Agarwal N, Thoreson WB, Camras CB, Singh DP. Peroxiredoxin 6 delivery attenuates TNF-alpha- and glutamate-induced retinal ganglion cell death by limiting ROS levels and maintaining Ca2+ homeostasis. Brain Res 1233: 63–78, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fatma N, Kubo E, Sharma P, Beier DR, Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6-/- mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFbeta. Cell Death Differ 12: 734–750, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Fatma N, Singh DP, Shinohara T, Chylack LT., Jr Transcriptional regulation of the antioxidant protein 2 gene, a thiol-specific antioxidant, by lens epithelium-derived growth factor to protect cells from oxidative stress. J Biol Chem 276: 48899–48907, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Fisher AB, Dodia C, Yu K, Manevich Y, Feinstein SI. Lung phospholipid metabolism in transgenic mice overexpressing peroxiredoxin 6. Biochim Biophys Acta 1761: 785–792, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2: 793–805, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Gerardi JG, Colitz CM, Dubielzig RR, Davidson MG. Immunohistochemical analysis of lens epithelial-derived membranes following cataract extraction in the dog. Vet Ophthalmol 2: 163–168, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Gimona M, Kazzaz JA, Helfman DM. Forced expression of tropomyosin 2 or 3 in v-Ki-ras-transformed fibroblasts results in distinct phenotypic effects. Proc Natl Acad Sci USA 93: 9618–9623, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunning PW, Schevzov G, Kee AJ, Hardeman EC. Tropomyosin isoforms: divining rods for actin cytoskeleton function. Trends Cell Biol 15: 333–341, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Hales AM, Chamberlain CG, McAvoy JW. Cataract induction in lenses cultured with transforming growth factor-beta. Invest Ophthalmol Vis Sci 36: 1709–1713, 1995 [PubMed] [Google Scholar]
- 29.Hales AM, Schulz MW, Chamberlain CG, McAvoy JW. TGF-beta1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr Eye Res 13: 885–890, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Herrera B, Alvarez AM, Sanchez A, Fernandez M, Roncero C, Benito M, Fabregat I. Reactive oxygen species (ROS) mediates the mitochondrial-dependent apoptosis induced by transforming growth factor beta in fetal hepatocytes. FASEB J 15: 741–751, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Herrera B, Murillo MM, Alvarez-Barrientos A, Beltran J, Fernandez M, Fabregat I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-beta in fetal rat hepatocytes. Free Radic Biol Med 36: 16–26, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res 80: 383–392, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Huot J, Houle F, Rousseau S, Deschesnes RG, Shah GM, Landry J. SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis. J Cell Biol 143: 1361–1373, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iakoubova OA, Pacella LA, Her H, Beier DR. LTW4 protein on mouse chromosome 1 is a member of a family of antioxidant proteins. Genomics 42: 474–478, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Immenschuh S, Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signal 7: 768–777, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Kim HK, Lee TH, Park ES, Suh JM, Park SJ, Chung HK, Kwon OY, Kim YK, Ro HK, Shong M. Role of peroxiredoxins in regulating intracellular hydrogen peroxide and hydrogen peroxide-induced apoptosis in thyroid cells. J Biol Chem 275: 18266–18270, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Kim HS, Manevich Y, Feinstein SI, Pak JH, Ho YS, Fisher AB. Induction of 1-cys peroxiredoxin expression by oxidative stress in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 285: L363–L369, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Kim HS, Pak JH, Gonzales LW, Feinstein SI, Fisher AB. Regulation of 1-cys peroxiredoxin expression in lung epithelial cells. Am J Respir Cell Mol Biol 27: 227–233, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Kim JR, Yoon HW, Kwon KS, Lee SR, Rhee SG. Identification of proteins containing cysteine residues that are sensitive to oxidation by hydrogen peroxide at neutral pH. Anal Biochem 283: 214–221, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Kim TS, Dodia C, Chen X, Hennigan BB, Jain M, Feinstein SI, Fisher AB. Cloning and expression of rat lung acidic Ca2+-independent PLA2 and its organ distribution. Am J Physiol Lung Cell Mol Physiol 274: L750–L761, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Kubo E, Fatma N, Akagi Y, Beier DR, Singh SP, Singh DP. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am J Physiol Cell Physiol 294: C842–C855, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Kubo E, Fatma N, Sharma P, Shinohara T, Chylack LT, Jr, Singh DP. Transactivation of involucrin, a marker of differentiation in keratinocyte, by lens epithelium derived growth factor (LEDGF). J Mol Biol 320: 1053–1063, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Kubo E, Miyazawa T, Fatma N, Akagi Y, Singh DP. Development- and age-associated expression pattern of peroxiredoxin 6, and its regulation in murine ocular lens. Mech Ageing Dev 127: 249–256, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Kubo E, Miyoshi N, Fukuda M, Akagi Y. Cataract formation through polyol pathway is associated with free radical production. Exp Eye Res 68: 457–464, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Kubo E, Singh DP, Akagi Y. Gene expression profiling of diabetic and galactosaemic cataractous rat lens by microarray analysis. Diabetologia 48: 790–798, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Kubo E, Singh DP, Fatma N, Akagi Y. TAT-mediated peroxiredoxin 5 and 6 protein transduction protects against high-glucose-induced cytotoxicity in retinal pericytes. Life Sci 84: 857–864, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Kubo E, Singh DP, Fatma N, Shinohara T, Zelenka P, Reddy VN, Chylack LT. Cellular distribution of lens epithelium-derived growth factor (LEDGF) in the rat eye: loss of LEDGF from nuclei of differentiating cells. Histochem Cell Biol 119: 289–299, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Kubo E, Urakami T, Fatma N, Akagi Y, Singh DP. Polyol pathway-dependent osmotic and oxidative stresses in aldose reductase-mediated apoptosis in human lens epithelial cells: role of AOP2. Biochem Biophys Res Commun 314: 1050–1056, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Kumin A, Schafer M, Epp N, Bugnon P, Born-Berclaz C, Oxenius A, Klippel A, Bloch W, Werner S. Peroxiredoxin 6 is required for blood vessel integrity in wounded skin. J Cell Biol 179: 747–760, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li WC, Spector A. Lens epithelial cell apoptosis is an early event in the development of UVB-induced cataract. Free Radic Biol Med 20: 301–311, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Hales AM, Chamberlain CG, McAvoy JW. Induction of cataract-like changes in rat lens epithelial explants by transforming growth factor beta. Invest Ophthalmol Vis Sci 35: 388–401, 1994 [PubMed] [Google Scholar]
- 53.Lois N, Taylor J, McKinnon AD, Smith GC, van't Hof R, Forrester JV. Effect of TGF-beta2 and anti-TGF-beta2 antibody in a new in vivo rodent model of posterior capsule opacification. Invest Ophthalmol Vis Sci 46: 4260–4266, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG, McAvoy JW. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br J Ophthalmol 86: 220–226, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lyu MS, Rhee SG, Chae HZ, Lee TH, Adamson MC, Kang SW, Jin DY, Jeang KT, Kozak CA. Genetic mapping of six mouse peroxiredoxin genes and fourteen peroxiredoxin related sequences. Mamm Genome 10: 1017–1019, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med 38: 1422–1432, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Manevich Y, Reddy KS, Shuvaeva T, Feinstein SI, Fisher AB. Structure and phospholipase function of peroxiredoxin 6: identification of the catalytic triad and its role in phospholipid substrate binding. J Lipid Res 48: 2306–2318, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci USA 99: 11599–11604, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mann DA, Frankel AD. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J 10: 1733–1739, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marcantonio JM, Rakic JM, Vrensen GF, Duncan G. Lens cell populations studied in human donor capsular bags with implanted intraocular lenses. Invest Ophthalmol Vis Sci 41: 1130–1141, 2000 [PubMed] [Google Scholar]
- 61.Marcantonio JM, Vrensen GF. Cell biology of posterior capsular opacification. Eye 13: 484–488, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Marsili S, Salganik RI, Albright CD, Freel CD, Johnsen S, Peiffer RL, Costello MJ. Cataract formation in a strain of rats selected for high oxidative stress. Exp Eye Res 79: 595–612, 2004 [DOI] [PubMed] [Google Scholar]
- 63.McAvoy JW, Chamberlain CG, de Iongh RU, Hales AM, Lovicu FJ. Peter Bishop Lecture: growth factors in lens development and cataract: key roles for fibroblast growth factor and TGF-beta. Clin Exp Ophthalmol 28: 133–139, 2000 [DOI] [PubMed] [Google Scholar]
- 64.McCormick JP, Fischer JR, Pachlatko JP, Eisenstark A. Characterization of a cell-lethal product from the photooxidation of tryptophan: hydrogen peroxide. Science 191: 468–469, 1976 [DOI] [PubMed] [Google Scholar]
- 65.McDonnell PJ, Zarbin MA, Green WR. Posterior capsule opacification in pseudophakic eyes. Ophthalmology 90: 1548–1553, 1983 [DOI] [PubMed] [Google Scholar]
- 66.Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med 4: 1449–1452, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Ohba M, Shibanuma M, Kuroki T, Nose K. Production of hydrogen peroxide by transforming growth factor-beta1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J Cell Biol 126: 1079–1088, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pak JH, Kim TI, Joon Kim M, Yong Kim J, Choi HJ, Kim SA, Tchah H. Reduced expression of 1-cys peroxiredoxin in oxidative stress-induced cataracts. Exp Eye Res 82: 899–906, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Pawlak G, Helfman DM. Cytoskeletal changes in cell transformation and tumorigenesis. Curr Opin Genet Dev 11: 41–47, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Perry SV. Vertebrate tropomyosin: distribution, properties and function. J Muscle Res Cell Motil 22: 5–49, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Phelan SA, Johnson KA, Beier DR, Paigen B. Characterization of the murine gene encoding Aop2 (antioxidant protein 2) and identification of two highly related genes. Genomics 54: 132–139, 1998 [DOI] [PubMed] [Google Scholar]
- 72.Rungger-Brandle E, Conti A, Leuenberger PM, Rungger D. Expression of alphasmooth muscle actin in lens epithelia from human donors and cataract patients. Exp Eye Res 81: 539–550, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Rusnati M, Coltrini D, Oreste P, Zoppetti G, Albini A, Noonan D, D'Adda di Fagagna F, Giacca M, Presta M. Interaction of HIV-1 Tat protein with heparin. Role of the backbone structure, sulfation, and size. J Biol Chem 272: 11313–11320, 1997 [DOI] [PubMed] [Google Scholar]
- 74.Salganik RI. The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr 20: 464S–475S, 2001 [DOI] [PubMed] [Google Scholar]
- 75.Salganik RI, Shabalina IG, Solovyova NA, Kolosova NG, Solovyov VN, Kolpakov AR. Impairment of respiratory functions in mitochondria of rats with an inherited hyperproduction of free radicals. Biochem Biophys Res Commun 205: 180–185, 1994 [DOI] [PubMed] [Google Scholar]
- 76.Salganik RI, Solovyova NA, Dikalov SI, Grishaeva ON, Semenova LA, Popovsky AV. Inherited enhancement of hydroxyl radical generation and lipid peroxidation in the S strain rats results in DNA rearrangements, degenerative diseases, and premature aging. Biochem Biophys Res Commun 199: 726–733, 1994 [DOI] [PubMed] [Google Scholar]
- 77.Schevzov G, Vrhovski B, Bryce NS, Elmir S, Qiu MR, O'Neill GM, Yang N, Verrills NM, Kavallaris M, Gunning PW. Tissue-specific tropomyosin isoform composition. J Histochem Cytochem 53: 557–570, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Schwarze SR, Dowdy SF. In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol Sci 21: 45–48, 2000 [DOI] [PubMed] [Google Scholar]
- 79.Seomun Y, Kim J, Lee EH, Joo CK. Overexpression of matrix metalloproteinase-2 mediates phenotypic transformation of lens epithelial cells. Biochem J 358: 41–48, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharma P, Fatma N, Kubo E, Shinohara T, Chylack LT, Jr, Singh DP. Lens epithelium-derived growth factor relieves transforming growth factor-beta1-induced transcription repression of heat shock proteins in human lens epithelial cells. J Biol Chem 278: 20037–20046, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Shokolenko IN, Alexeyev MF, LeDoux SP, Wilson GL. TAT-mediated protein transduction and targeted delivery of fusion proteins into mitochondria of breast cancer cells. DNA Repair (Amst) 4: 511–518, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J 9: 1173–1182, 1995 [PubMed] [Google Scholar]
- 83.Spector A. Oxidative stress and disease. J Ocul Pharmacol Ther 16: 193–201, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Spector A, Kuszak JR, Ma W, Wang RR, Ho Y, Yang Y. The effect of photochemical stress upon the lenses of normal and glutathione peroxidase-1 knockout mice. Exp Eye Res 67: 457–471, 1998 [DOI] [PubMed] [Google Scholar]
- 85.Spector A, Wang GM, Wang RR, Li WC, Kuszak JR. A brief photochemically induced oxidative insult causes irreversible lens damage and cataract. I. Transparency and epithelial cell layer. Exp Eye Res 60: 471–481, 1995 [DOI] [PubMed] [Google Scholar]
- 86.Srinivasan Y, Lovicu FJ, Overbeek PA. Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataracts. J Clin Invest 101: 625–634, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tan S, Wood M, Maher P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J Neurochem 71: 95–105, 1998 [DOI] [PubMed] [Google Scholar]
- 88.Taylor A, Nowell T. Oxidative stress and antioxidant function in relation to risk for cataract. Adv Pharmacol 38: 515–536, 1997 [DOI] [PubMed] [Google Scholar]
- 89.Taylor HR, West SK, Rosenthal FS, Munoz B, Newland HS, Abbey H, Emmett EA. Effect of ultraviolet radiation on cataract formation. N Engl J Med 319: 1429–1433, 1988 [DOI] [PubMed] [Google Scholar]
- 90.Tripathi BJ, Tripathi RC, Livingston AM, Borisuth NS. The role of growth factors in the embryogenesis and differentiation of the eye. Am J Anat 192: 442–471, 1991 [DOI] [PubMed] [Google Scholar]
- 91.Unlu M, Morgan ME, Minden JS. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis 18: 2071–2077, 1997 [DOI] [PubMed] [Google Scholar]
- 92.Varga AE, Stourman NV, Zheng Q, Safina AF, Quan L, Li X, Sossey-Alaoui K, Bakin AV. Silencing of the Tropomyosin-1 gene by DNA methylation alters tumor suppressor function of TGF-beta. Oncogene 24: 5043–5052, 2005 [DOI] [PubMed] [Google Scholar]
- 93.Vocero-Akbani A, Lissy NA, Dowdy SF. Transduction of full-length Tat fusion proteins directly into mammalian cells: analysis of T cell receptor activation-induced cell death. Methods Enzymol 322: 508–521, 2000 [DOI] [PubMed] [Google Scholar]
- 94.Wang X, Phelan SA, Forsman-Semb K, Taylor EF, Petros C, Brown A, Lerner CP, Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem 278: 25179–25190, 2003 [DOI] [PubMed] [Google Scholar]
- 95.Warren KS, Lin JL, McDermott JP, Lin JJ. Forced expression of chimeric human fibroblast tropomyosin mutants affects cytokinesis. J Cell Biol 129: 697–708, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wawro B, Greenfield NJ, Wear MA, Cooper JA, Higgs HN, Hitchcock-DeGregori SE. Tropomyosin regulates elongation by formin at the fast-growing end of the actin filament. Biochemistry 46: 8146–8155, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300: 650–653, 2003 [DOI] [PubMed] [Google Scholar]
- 98.Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci 28: 32–40, 2003 [DOI] [PubMed] [Google Scholar]
- 99.Zheng Q, Safina A, Bakin AV. Role of high-molecular weight tropomyosins in TGF-beta-mediated control of cell motility. Int J Cancer 122: 78–90, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Zigman S. Ocular light damage. Photochem Photobiol 57: 1060–1068, 1993 [DOI] [PubMed] [Google Scholar]
- 101.Zigman S. The role of sunlight in human cataract formation. Surv Ophthalmol 27: 317–325, 1983 [DOI] [PubMed] [Google Scholar]
- 102.Zigman S, Vaughan T. Near-ultraviolet light effects on the lenses and retinas of mice. Invest Ophthalmol 13: 462–465, 1974 [PubMed] [Google Scholar]