Abstract
The previously undescribed heterozygous missense mutation E758K was discovered in the human AE1/SLC4A1/band 3 gene in two unrelated patients with well-compensated hereditary spherostomatocytic anemia (HSt). Oocyte surface expression of AE1 E758K, in contrast to that of wild-type AE1, required coexpressed glycophorin A (GPA). The mutant polypeptide exhibited, in parallel, strong GPA dependence of DIDS-sensitive 36Cl− influx, trans-anion-dependent 36Cl− efflux, and Cl−/HCO3− exchange activities at near wild-type levels. AE1 E758K expression was also associated with GPA-dependent increases of DIDS-sensitive pH-independent SO42− uptake and oxalate uptake with altered pH dependence. In marked contrast, the bumetanide- and ouabain-insensitive 86Rb+ influx associated with AE1 E758K expression was largely GPA-independent in Xenopus oocytes and completely GPA-independent in Ambystoma oocytes. AE1 E758K-associated currents in Xenopus oocytes also exhibited little or no GPA dependence. 86Rb+ influx was higher but inward cation current was lower in oocytes expressing AE1 E758K than previously reported in oocytes expressing the AE1 HSt mutants S731P and H734R. The pharmacological inhibition profile of AE1 E758K-associated 36Cl− influx differed from that of AE1 E758K-associated 86Rb+ influx, as well as from that of wild-type AE1-mediated Cl− transport. Thus AE1 E758K-expressing oocytes displayed GPA-dependent surface polypeptide expression and anion transport, accompanied by substantially GPA-independent, pharmacologically distinct Rb+ flux and by small, GPA-independent currents. The data strongly suggest that most of the increased cation transport associated with the novel HSt mutant AE1 E758K reflects activation of endogenous oocyte cation permeability pathways, rather than cation translocation through the mutant polypeptide.
Keywords: chloride-bicarbonate exchange; Xenopus oocytes; Ambystoma oocytes; erythrocyte band 3, glycophorin A
hereditary stomatocytic anemia (HSt) usually presents as a well-compensated, autosomal-dominant anemia associated with increased red cell cation permeability insensitive to ouabain and bumetanide (15, 45). The disorder has been subdivided into overhydrated and dehydrated categories on the basis of mean corpuscular volume (MCV) and total cation content. The nonspecific monovalent cation leaks in red blood cells of both categories can exhibit a range of temperature sensitivity patterns and can occur in the presence of normal, reduced, or undetectable levels of band 7.2/stomatin.
HSt has been associated with mutations in the genes encoding the red cell Cl−/HCO3− exchanger AE1/SLC4A1/band 3 (6, 27) and with mutations encoding the rhesus antigen-associated glycoprotein (RhAG) (5), but additional families lacking mutations in these two genes have been identified. A dehydrated form of HSt with pseudohyperkalemia has been linked to a locus on chromosome 16q23–24 (20), and a milder form of HSt, in which pseudohyperkalemia is the predominant clinical feature, has been linked to a locus on chromosome 2q25–26 (7).
Both overhydrated and dehydrated categories of HSt have been associated with heterozygous missense mutations of AE1/SLC4A1/band 3, encoding polypeptides that are expressed at normal or near-normal abundance at the red cell surface (3, 16). These unique HSt mutations are distinct from the AE1 mutations associated with autosomal dominant hereditary spherocytic anemias (28) and with distal renal tubular acidosis (dRTA) (31). All HSt mutations reported to date (6, 27) alter amino acid residues located in the central region of the AE1 transmembrane domain believed to flank the first of two putative reentrant loops. Expression of HSt mutations in Xenopus oocytes has been associated with two transport phenotypes to date. AE1 HSt mutations S731P, H734R, D705Y, L687P, and G796R and Southeast Asian Ovalocytosis all exhibit severely reduced or absent 36Cl− influx and Cl−/HCO3− exchange, accompanied by increased nonspecific monovalent cation leak (6, 25, 27). In contrast, the elevated nonspecific cation leak of AE1 HSt mutation R760Q is accompanied by apparently normal Cl−/HCO3− exchange activity (16). The coexistence of normal Cl−/HCO3− exchange activity with lower levels of cation leak has also been reported for AE1 mutations associated with recessive dRTA (50, 51). Xenopus oocytes expressing AE1 HSt mutants exhibited increased cation current as measured by two-electrode voltage clamp in all cases, with the exception of AE1 G796R, for which currents were not reported (27).
The ability of a range of AE1 missense mutations to confer increased cation flux, altered cation content, and increased cation current when expressed in Xenopus oocytes, whether with loss or preservation of Cl−/HCO3− exchange, has been interpreted to represent conductive translocation of cations through the mutant AE1 polypeptide (3, 6, 16, 25, 50, 51). This interpretation has been indirectly supported by the ability of wild-type trout AE1 expression in Xenopus oocytes to confer not only Cl−/HCO3− exchange and 36Cl− flux, but also anion currents and permeability to uncharged and zwitterionic osmolytes. Moreover, engineered mutations in trout AE1 selectively altered magnitude and anion selectivity of anion conductance, with minimal change to electroneutral anion-exchange activity (36, 37).
In this report, we present a novel, heterozygous human AE1 mutation, E758K (Band 3 New Haven), found in two unrelated patients with compensated hemolytic anemia. Patient 1 carried a clinical diagnosis of hereditary overhydrated stomatocytosis, and patient 2 carried a clinical diagnosis of xerocytosis. Expression of AE1 E758K in Xenopus and Ambystoma oocytes was associated with ∼50–80% of wild-type anion exchange activity, increased transport of SO42− and oxalate with altered pH sensitivity, and elevated 86Rb+ influx with a profile of pharmacological inhibitors distinct from that of Cl− influx. Whereas Cl− transport and polypeptide surface expression of AE1 E758K were largely or completely dependent on coexpression of glycophorin A (GPA), 86Rb+ influx was completely GPA-independent in Ambystoma oocytes and largely so in Xenopus oocytes. The small AE1 E758K-associated currents of Xenopus oocytes also exhibited little or no GPA-dependence. The data suggest that, most, if not all, of the AE1 E758K-associated cation transport detected in oocytes transits a permeability pathway outside the confines of the mutant AE1 polypeptide, but stimulated by its expression. The data further suggest that the increased nonspecific cation leak of red blood cells from patients with stomatocytosis associated with some heterozygous AE1 mutations reflects indirect activation of intrinsic cation permeability pathways.
METHODS
Clinical.
Patient 1 was a 47-yr-old male whose history of neonatal jaundice and lifelong anemia, in the setting of maternal anemia and cholelithiasis in his mother and sister, was described by his physicians as “probable hereditary spherocytosis.” The patient presented with a viral respiratory infection and the following laboratory values: whole blood Hb concentration ([Hb]) 10.9 g/dl, MCV 98 fl, reticulocytes 7.2%, serum lactate dehydrogenase 250 U, and serum total bilirubin 0.8 mg/dl. A follow-up visit revealed the values of [Hb] 15 g/dl, MCV 102 fl, reticulocytes 9.6%, total bilirubin 1.8 mg/dl, and haptoglobin 13 U. Peripheral blood smears on both occasions showed polychromasia, mild stomatocytosis, and rare spherostomatocytes and acanthocytes (Fig. 1A). Red cell membranes exhibited mild band 3 deficiency (see supplemental Fig. 1 in the online version of this article), as previously reported for band 3-deficient spherocytic anemias, with spectrin-to-band 3 ratio of 1.24 ± 0.05 (normal 1.00 ± 0.10) and ankyrin-to-band 3 ratio of 0.23 ± 0.04 (normal 0.20 ± 0.04). The membrane protein profile was otherwise qualitatively normal (see supplemental Fig. 1). Tests for glucose-6-phosphate dehydrogenase deficiency and osmotic fragility were normal. Blood urea nitrogen and serum creatinine concentrations were normal, but urine pH was not available.
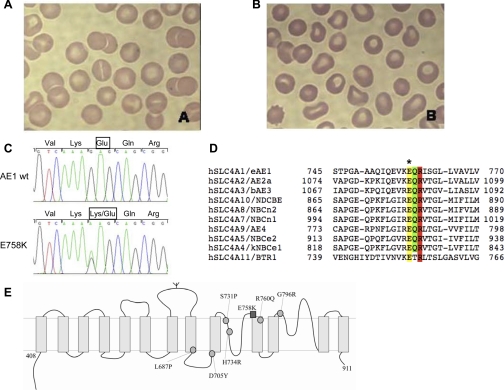
Fig. 1.
The novel AE1 spherostomatocytosis mutation E758K. A and B: peripheral blood Wright-Giemsa smears from patients 1 and 2, respectively. C: partial nucleotide sequence of exon 17 of the SLC4A1 gene from a normal control [wild-type (wt); top] and from patient 2 (bottom). Heterozygosity for the G-to-A mutation at nucleotide 2272 is evident in the sequence from patient 2. Exon 17 from patient 1 DNA exhibited the identical mutation (not shown). D: sequence alignment of part of the human erythroid AE1 (eAE1) transmembrane domain with corresponding regions from other SLC4 polypeptides. Note conservation of AE1 E758 (yellow band with ∗ above) adjacent to Q759 (green band, site of a distal renal tubular acidosis mutation) and R760 (red band, site of a hereditary spherocytosis mutation associated with cation leak). Amino acid residue numbers bracket each sequence. E: schematic diagram of the transmembrane domain of AE1, showing putative location of AE1 E758K (dark square) and previously reported AE1 stomatocytosis mutations (light circles).
Patient 2 was a 51-yr-old male presenting with a history of chronic fatigue, anxiety, and depression, without known history of anemia, jaundice, or cholelithiasis. Laboratory values revealed [Hb] 14–16.2 g/dl, MCV 89.5 fl, mean corpuscular hemoglobin concentration = 38 g/dl, reticulocytes = 7.9%, and total bilirubin = 1.0 mg/dl. Erythrocyte cytosolic cation concentrations, as determined on two occasions by atomic absorption spectrometry, were 55.4 and 39.4 meq/l [Na+] (normal 14.5 ± 5) and 40.0 and 46.7 meq/l [K+] (normal 99.0 ± 6). The peripheral blood smear showed normochromic, normocytic erythrocytes, mild anisocytosis, and polychromasia (Fig. 1B). Erythrocyte osmotic fragility was decreased, as evidenced by incomplete hemolysis in a 0.30% NaCl solution after overnight incubation of whole blood at 37°C. Hb electrophoresis and screens for unstable hemoglobins were normal, as were tests for glucose-6-phosphate dehydrogenase deficiency and pyruvate kinase deficiency. The patient was given a clinical diagnosis of compensated xerocytic anemia. Patient blood samples were processed as described below for preparation of red cell membranes and genomic DNA, after consent was obtained according to protocols approved by the Institutional Review Board of Yale University School of Medicine.
Erythrocyte membrane preparation and quantitation of protein content.
Erythrocyte membrane proteins were prepared from peripheral blood (17, 35), and membrane proteins were analyzed by SDS-PAGE with a 5–15% polyacrylamide gradient (32) or with 3.5% polyacrylamide (17). Spectrin-to-band 3 and ankyrin-to-band 3 ratios were estimated by scanning of Coomassie blue-stained gels at 550 nm (DU8, Beckman Instruments, Fullerton, CA).
Mutation detection.
The 19 coding exons of the SLC4A1 gene were amplified from proband genomic DNA by PCR. The exon-flanking primer pairs (see supplemental Table 1) designed with Primer 3 and WAVEmaker 4.1.40 (Transgenomics, Omaha, NE) were typically ∼40 nucleotides away from the intron-exon boundary to facilitate detection of possible splice junction mutations, and optimized for both PCR and denaturing HPLC (DHPLC). PCR amplification products were subjected to agarose gel electrophoresis and visualized by ethidium bromide staining to ensure robust amplification. For DHPLC, patient and wild-type amplification products mixed in a 1:1 ratio were denatured for 5 min at 94°C, then were reannealed by gradual temperature decrease from 94°C to the target temperature (see supplemental Table 1) on a WAVE DNA fragment analysis system (Transgenomics). Annealed amplification products were separated on a DNASep column (Transgenomics) by ion-pair reverse-phase liquid chromatography through a 2% linear acetonitrile gradient under partially denaturing conditions at the desired temperature. Temperatures for DHPLC were determined by melting domain profile analysis of each amplicon to produce a ≥90% double-helical fraction of wild-type DNA. When two or more distinct melting domains were predicted, DHPLC analysis was performed at two or more different temperatures. Genomic DNA fragments demonstrating a consistently abnormal DHPLC pattern (see supplemental Fig. 2) were reamplified and subjected to nucleotide sequence analysis.
Since variants of HSt have also been associated with two missense mutations in exon 2 of the human RhAG gene (5), RhAG exon 2 was also amplified from patient genomic DNA and sequenced. Oligonucleotides used for amplification and sequencing are listed in supplemental Table 2.
cDNA preparation, mutagenesis, and transcription.
The human SLC4A1/AE1/band 3 cDNA was excised from the pBluescript KS+ plasmid pHB3 (34) and subcloned into the multipurpose expression vector pMAX Maxi N-71 (Invitrogen). The SLC4A1/AE1/band 3 mutation discovered in this report, E758K, and two other SLC4A1 gene mutations associated with defects in erythrocyte cation homeostasis, S731P and H734R (6), were engineered into this human AE1 oocyte expression plasmid as follows. A BbvCI-NotI SLC4A1/AE1/band3 cDNA fragment encoding amino acid residues 643–911 was PCR-amplified with primers G1749/G1750 (see supplemental Table 3) and subcloned into the plasmid TOPO2.1 (Invitrogen). Disease mutants E758K, S731P, and H734R were PCR amplified from this template using mutant oligonucleotide primers listed in supplemental Table 3. Parental methylated and hemimethylated DNA was destroyed by overnight digestion with DpnI. Mutated DNA was retransformed into TOP10 cells. Mutant recombinant clones were selected by direct nucleotide sequencing of the amplified fragments, and absence of unintended nucleotide changes was confirmed. BbvCI-NotI fragments of SLC4A1 were excised from TOPO2.1 and ligated into the wild-type hSLC4A1 backbone plasmid lacking the corresponding BbvCI-NotI fragment. The influenza hemagglutinin (HA) epitope YPYDVPDYA was introduced between amino acids N556 and V557 in the AE1 third extracellular loop by PCR amplification with oligonucleotides listed in supplemental Table 4.
Capped cRNA was transcribed using the T7 MEGAscript kit (Ambion, Austin, TX) from cDNA templates linearized with XbaI (pXT7), HindIII (pBL and pL2AD), or ClaI (pDX) and purified with the Qiagen RNeasy minikit. RNA integrity was confirmed by agarose gel electrophoresis in formaldehyde, and RNA concentration was estimated by absorbance at 260 nm (Nanodrop, Wilmington, DE).
Xenopus oocyte expression of AE1 wild-type and mutant polypeptides.
Ovarian segments were excised from female Xenopus laevis (Department of Systems Biology, Harvard Medical School) anesthetized with 0.17% tricaine according to protocols approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center. Ovarian fragments were incubated overnight at 19°C with 1.3 mg/ml collagenase type A (Boehringer Mannheim) in modified Barth's solution (MBS) containing (in mM) 85 NaCl, 1 KCl, 2.4 NaHCO3, 0.82 MgSO4, 0.41 CaCl2, 0.33 Ca(NO3)2, and 10 Na HEPES (pH 7.40) and supplemented with 50 ng/ml gentamicin. The collagenase-treated oocytes were incubated 20 min in Ca2+-free MBS and then washed in MBS. Defolliculated stage V–VI oocytes were injected on the same day with 50 nl of H2O or 50 nl containing 10 ng of human AE1 cRNA without or with 2.5 ng of human GPA cRNA, unless otherwise indicated. Injected oocytes were maintained for 2–4 days at 19°C in ND-96 with gentamicin and pyruvate.
Ambystoma oocyte expression of AE1 wild-type and mutant polypeptides.
Ambystoma mexicanum oocytes have a much lower basal level of Ca2+-dependent Cl− conductance than is present in Xenopus oocytes. Therefore, ovarian segments were excised from female A. mexicanum (Axolotl Genetic Stock Center, University of Kentucky, Lexington, KY) anesthetized with 0.17% tricaine according to protocols approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center. Manually defolliculated stage V–VI oocytes were injected with capped cRNA and incubated as described elsewhere (1, 43).
Isotopic influx measurements.
Unidirectional 36Cl− influx studies were carried out in 150-μl volumes for 1–2 h in ND-96 containing (in mM) 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 Na HEPES (pH 7.4) supplemented with 10 μM bumetanide, as described elsewhere (10), with minor modifications. 35SO42− influx studies were carried out for 2 h in medium of the indicated pH containing 96 mM Na cyclamate and 5 μCi of carrier-free Na235SO4 (47 nM) in the presence of 100 μM unlabeled Na2SO4. [14C]oxalate influx studies were carried out for 30 min in 96 mM Na cyclamate influx medium lacking Ca2+ and Mg2+, and containing 5 μCi of [14C]oxalate in the presence of 1.0 mM unlabeled Na oxalate. 86Rb+ influx studies were carried out for 2 h in ND-96 containing 1 μCi of 86RbCl, 10 μM bumetanide, and 100 μM ouabain. When indicated, bath Na+ was replaced with N-methyl-d-glucamine (NMDG) and Cl− was replaced with isethionate or SO42−. In some experiments, influx studies were performed in the presence of the AE1 inhibitors DIDS (200 μM) or WW-781 (10 μM) or in the presence of the cation channel inhibitors or modulators Zn2+ (100 μM), Gd3+ (50 μM or 1 mM), Grammostola spatulata mechanotoxin-4 (GsMTx-4, 1 μM), streptomycin (100 μM), hyperforin (50 μM), Li+ (1 mM), La3+ (1 mM), NH4+ (2 mM), or Ba2+ (5 mM).
Isotopic efflux measurements.
36Cl− efflux was assayed as previously described (44). Oocytes in Cl−-free ND-96 were injected with 50 nl of 130 mM Na36Cl (10,000–12,000 cpm). After a 10 min recovery period, the efflux assay was initiated by transfer of individual oocytes to 6-ml borosilicate glass tubes, each containing 1 ml of efflux solution. At intervals of 3 min, 0.95 ml of this efflux solution was removed for scintillation counting and replaced with an equal volume of fresh efflux solution. Following completion of the assay with a final efflux period in Cl−-free isethionate or cyclamate or in the presence of the anion transport inhibitor DIDS (200 μM), each oocyte was lysed in 100 μl of 2% SDS. Samples were counted for 3–5 min, such that the magnitude of 2 SDs was <5% of the sample mean.
Experimental data were plotted as ln (% cpm remaining in the oocyte) vs. time. 36Cl− efflux rate constants were measured from linear fits to data from the last three time points sampled for each experimental condition. Efflux cpm values for H2O-injected oocytes or for AE1 cRNA-injected oocytes in the presence of DIDS were less than threefold higher than machine background (∼20 cpm). Within each experiment, oocytes from the same frog previously injected with H2O or with cRNA encoding wild-type and mutant AE1 were subjected to parallel measurements.
Confocal laser scanning immunofluorescence microscopy.
At 2 days after injection with H2O or with cRNA encoding wild-type or mutant AE1 bearing the HA epitope YPYDVPDYA inserted between amino acids N556 and V557 in the AE1 third extracellular loop, unfixed oocytes (n = 10–12 per group) were blocked in PBS with 1% BSA for 1 h at 4°C, incubated unfixed for 1 h at 4°C with mouse monoclonal anti-HA antibody (Sigma, St. Louis, MO; 1:100 dilution), and washed three times for 10 min at 4°C. Antibody-exposed oocytes were fixed at room temperature for 30 min in PBS containing 3% paraformaldehyde, washed three times in PBS supplemented with 0.002% Na azide, and again blocked in PBS with 1% BSA and 0.05% saponin (PBS-BSA) for 1 h at 4°C. Oocytes were then incubated for 1 h with Cy3-conjugated secondary goat anti-mouse Ig (Jackson Immunochemicals; 1:200 dilution) and again thoroughly washed in PBS-BSA. Oocytes expressing HA-tagged constructs were aligned in uniform orientation along a Plexiglas groove and sequentially imaged through the ×10 objective of a Zeiss LSM510 laser scanning confocal microscope using the 543-nm laser line at 512 × 512 resolution at a uniform setting of 80% intensity, pinhole 180 (2.0 Airy units), detector gain 650, amp gain 1, and 0 amp offset.
Polypeptide abundance at each oocyte surface was estimated by quantitation of specific fluorescence intensity (FI) at the circumference of one quadrant of an equatorial focal plane (Image J version 1.38, National Institutes of Health). Mean background-corrected FI for quadrants of oocytes previously injected with H2O was subtracted from the background-corrected FI for quadrants of individual cRNA-injected oocytes to yield intensity values for surface-associated specific FI for each oocyte.
Fluorescence ratio measurement of oocyte intracellular pH.
Oocyte intracellular pH (pHi) was monitored during bath superfusion using BCECF fluorescence excitation ratio imaging, as previously described. Cl−/HCO3− exchange activities were assayed by measurement of dpHi/dt during bath Cl− substitution with isethionate and during subsequent restoration of Cl− (10). Data acquisition and analysis were carried out with MetaFluor software (Universal Imaging, Chester, PA).
Two-electrode voltage clamp measurements.
Microelectrodes from borosilicate glass made with a Narashige puller were filled with 3 M KCl. Microelectrode resistances were 2–3 MΩ. Oocytes previously injected with H2O or 10 ng of AE1 cRNA and 2 ng of GPA cRNA were placed in a 1-ml chamber (model RC-11, Warner Instruments, Hamden, CT) on the stage of a dissecting microscope and impaled with microelectrodes under direct view. Steady-state currents achieved within 2–5 min following bath change or drug addition were measured with a Geneclamp 500 amplifier (Axon Instruments, Burlingame, CA) interfaced to a Dell computer with a digitizer (Digidata 1322A, Axon Instruments). Standard recording bath solution was ND-96. In anion substitution experiments, NaCl was replaced with Na+ or NMDG+ salts of gluconate.
Data acquisition and analysis utilized pCLAMP 8.0 software (Axon). The voltage pulse protocol generated with the Clampex subroutine consisted of 20-mV steps between −100 and +40 mV, with durations of 738 ms separated by 30 ms at the holding potential of −30 mV. Bath resistance was minimized by the use of agar bridges filled with 3 M KCl, and a virtual ground circuit clamped bath potential to zero during voltage clamp experiments. Unclamped oocyte membrane potential was also recorded with the Geneclamp 500 amplifier. A bath electrode allowed correction for the potentials arising from bath solution changes.
Statistics.
Values are means ± SE. Flux and image intensity data were compared among multiple conditions by Dunnett‘s two-way t-test. Two-electrode voltage clamp data were analyzed by ANOVA followed by Bonferroni’s t-test for multiple samples (Sigmaplot 8.0). Statistical significance was also assessed by Student's paired and unpaired t-tests. P < 0.05 was interpreted as significant.
RESULTS
Mutation detection.
The SLC4A1 gene from the spherostomatocytosis probands was screened by DHPLC of amplified genomic DNA. Both probands revealed an abnormal DHPLC pattern in exon 17 [patient 2's pattern is presented in supplemental Fig. 2; the result for patient 1 was similar (not shown)]. The corresponding DNA sequence from each proband revealed identical heterozygous G-to-A substitutions at nucleotide 2272 encoding a novel missense substitution at codon 758 from glutamic acid to lysine (AE1 E758K, or band 3 New Haven). The lower trace of supplemental Fig. 2C shows mutant genomic DNA from patient 2. Exon 2 of the RhAG gene, previously found to harbor two missense mutations in multiple families with the overhydrated form of stomatocytosis (5), was normal in both patients (not shown). Parental DNA was not available for study.
Human AE1 E758 is completely conserved among all human SLC4 polypeptides, including the most divergent among them, SLC4A11/BTR1 (Fig. 1D,1 yellow). E758 is also conserved in chicken AE1 and trout AE1. E758 is believed to be accessible to the extracellular space (19), at the COOH-terminal end of reentrant loop 1 (44) (Fig. 1E). It is immediately adjacent to the site of the Q759H mutation associated with a compound heterozygous case of autosomal recessive dRTA (12) and one residue removed from R760, the position at which two missense mutations have been associated with autosomal dominant spherostomatocytosis (6, 29).
Cl− transport by AE1 E758K.
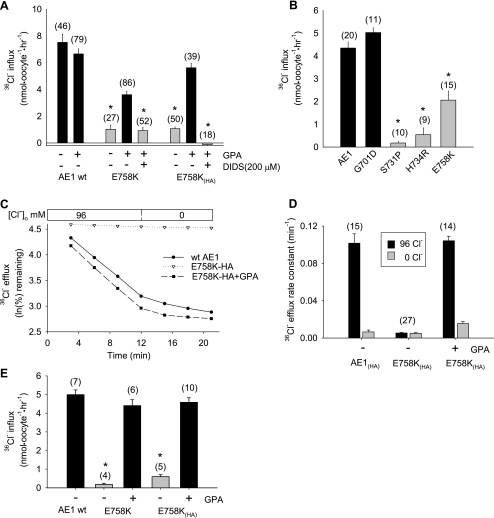
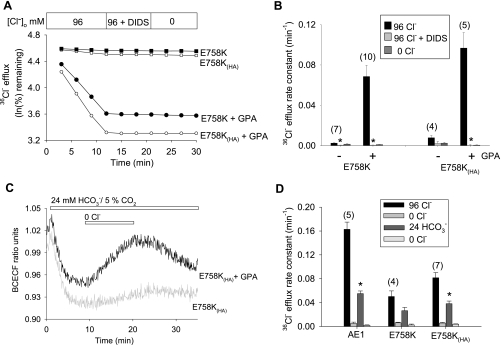
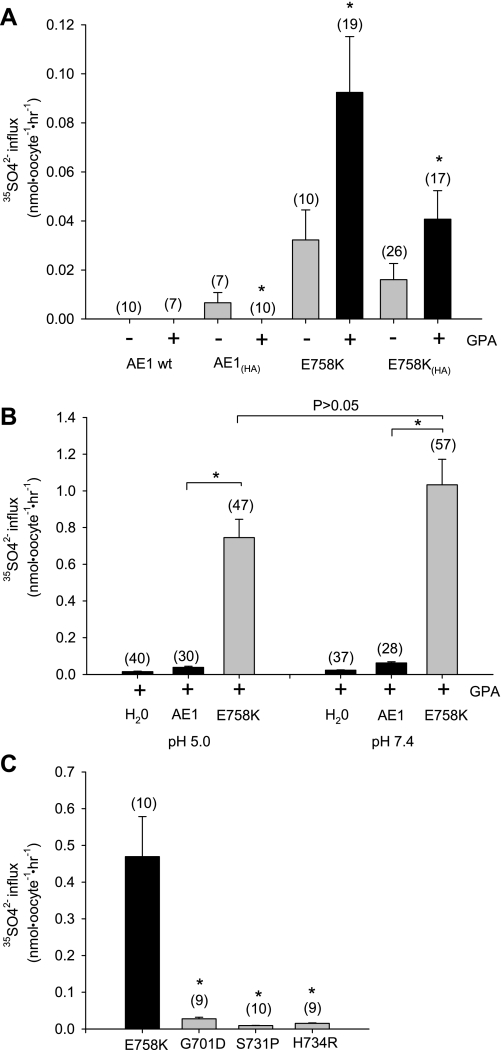
AE1 mutations previously associated with stomatocytosis exhibited severely reduced Cl− transport when coexpressed with GPA (6, 25). Except at very low levels of injected cRNA, wild-type AE1 does not require GPA coexpression for maximal surface expression and activity in oocytes (21, 22, 47). Oocytes expressing AE1 E758K mediated 36Cl− uptake at ∼20% of wild-type rates in the absence of GPA, but at ∼60% of wild-type rates in the presence of coexpressed GPA. With an HA epitope tag inserted into the third extracellular loop of AE1 E758K [E758K(HA)], 36Cl− uptake was indistinguishable from that of wild-type AE1 and completely DIDS-sensitive (Fig. 2A). These rates exceeded 36Cl− uptake rates exhibited by the previously reported stomatocytosis-associated AE1 mutants S731P and H734R (Fig. 2B). AE1 E758K(HA) also mediated wild-type rates of Cl−/Cl− exchange, as measured by 36Cl− efflux, but only in the presence of coexpressed GPA (Fig. 2, C and D). Exchange was DIDS-sensitive and unaffected by the ectofacial HA tag (Fig. 3, A and B). The 36Cl− uptake activity of AE1 E758K was similarly wild-type in magnitude but completely GPA-dependent when expressed and measured in oocytes of the axolotl Ambysoma mexicanum (Fig. 2E), which lack a substantial portion of the background (endogenous) Cl− transport detectable in Xenopus oocytes (43).
Fig. 2.
Human AE1 E758K mediates glycophorin A (GPA)-dependent Cl−/Cl− exchange. A: effect of DIDS (200 μM) on GPA-dependent 36Cl− uptake by Xenopus oocytes expressing wild-type AE1, AE1 mutant E758K, or E758K with a hemagglutinin (HA) tag in the 3rd extracellular loop [E758K(HA)]. H2O-corrected values are means ± SE for (n) oocytes. *and gray bar indicate P < 0.05 vs. E758K or E758K(HA) coexpressed with GPA (Student‘s unpaired t-test). B: 36Cl− uptake by Xenopus oocytes expressing wild-type AE1 and AE1 mutants. *and gray bar indicate P < 0.01 vs. AE1 (Dunnett’s t-test). C: representative traces of 36Cl− efflux from Xenopus oocytes expressing wild-type AE1 or AE1 mutant E758K(HA), without or with coexpressed GPA, into ND-96 or Cl−-free bath. D: summary of data from experiments in C. E: 36Cl− uptake in Ambystoma mexicanum oocytes expressing wild-type AE1 or AE1 mutants E758K or E758K(HA) in the absence and presence of coexpressed GPA. H2O-corrected values are means ± SE for (n) oocytes. *P < 0.01 vs. +GPA (Student's unpaired t-test).
Fig. 3.
AE1 E758K and E758K(HA) mediate GPA-dependent Cl−/HCO3− exchange. A: 36Cl− efflux traces from representative Xenopus oocytes expressing E758K and E758K(HA) in the presence or absence of GPA. DIDS was added at 200 μM. B: summary of data from experiments as in A. *P < 0.01 vs. E758K or E758K(HA) in the absence of DIDS (Student's unpaired t-test). C: BCECF ratio-fluorimetry recordings of intracellular pH in individual Xenopus oocytes expressing E758K(HA) in the presence (representative of 5 oocytes) or absence of GPA (representative of two oocytes, and similar to 3 H2O-injected oocytes). Oocytes were subjected to bath Cl− removal and restoration in the presence of 24 mM HCO3− and 5% CO2. D: summary of 36Cl− efflux rate constants of oocytes expressing AE1 and E758K (in the presence and absence of HA tag) and subjected to sequential solution changes. The 24 mM HCO3− bath contained 5% CO2. *P < 0.05 vs. Cl−/Cl− exchange.
Although the stomatocytosis-associated AE1 mutants S731P and H734R lacked Cl−/HCO3− exchange activity (25), mutant R760Q retained ∼50% of wild-type activity (16). The spherostomatocytosis-associated mutant AE1 E758K also retained considerable Cl−/HCO3− exchange activity (only in the presence of coexpressed GPA), as measured by BCECF fluorometry (Fig. 3C) and by 36Cl− efflux into HCO3− bath (Fig. 3D).
Cation permeability associated with expression of AE1 E758K.
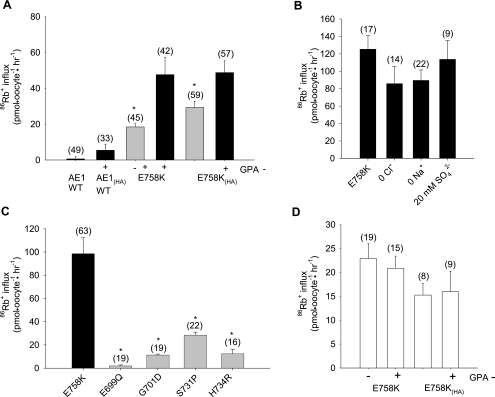
Patient 1 red cell ion contents suggested a cation leak form of spherostomatocytosis. Although wild-type AE1-expressing oocytes did not exhibit bumetanide-insensitive uptake of 86Rb+, 86Rb+ uptake into oocytes expressing AE1 E758K was increased (Fig. 4A) to levels considerably higher than into oocytes expressing stomatocytosis mutants S713P or H734R, or recessive dRTA mutant G701D (Fig. 4C). 86Rb+ uptake was not stimulated by removal of bath Cl− or bath Na+, nor was it modified by replacement of Cl− with 20 mM SO42− (Fig. 4B). In Xenopus oocytes, 86Rb+ uptake in the absence of GPA was as high as 65% of that in the presence of GPA (Fig. 4A). AE1 E758K-mediated 86Rb+ uptake into Ambystoma oocytes was completely GPA-independent (Fig. 4D). Expression of GPA alone in Xenopus oocytes did not elevate 86Rb+ influx.
Fig. 4.
Human AE1 E758K mediates 86Rb+ uptake with reduced or no GPA dependence. A: 86Rb+ uptake by Xenopus oocytes expressing wild-type AE1 or AE1 mutant E758K without or with an HA tag in the 3rd extracellular loop and in the absence or presence of coexpressed GPA. H2O-corrected values are means ± SE for number of oocytes shown in parentheses. *and gray bar indicate P < 0.05 vs. the same construct with coexpressed GPA (Student‘s unpaired t-test). B: 86Rb+ uptake by Xenopus oocytes expressing AE1 E758K was unaltered by gluconate replacement of bath Cl−, by N-methyl-d-glucamine replacement of bath Na+, or with 20 mM SO42− as the only permeant bath anion. C: comparison of 86Rb+ uptake by Xenopus oocytes expressing AE1 E758K with oocytes expressing human AE1 stomatocytosis mutants S731P and H734R, human AE1 distal renal tubular acidosis mutant G701D, and mouse Ae1 mutant E699Q. *and gray bar indicate P < 0.01 vs. E758K (Dunnett’s t-test). D: GPA-independent 86Rb+ uptake by Ambystoma oocytes expressing E758K (10 ng cRNA) without and with an HA tag in the 3rd extracellular loop. Values are means ± SE for (n) oocytes.
Xenopus oocyte surface expression of AE1 E758K.
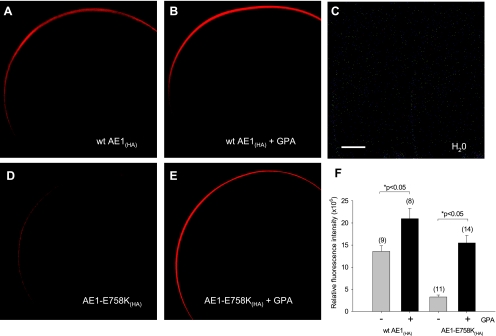
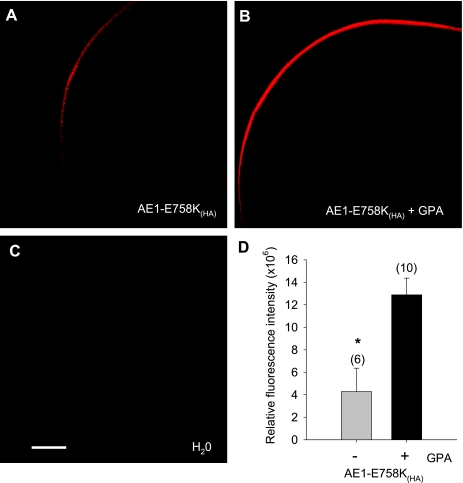
Wild-type AE1(HA) surface expression in Xenopus oocytes was only minimally increased by coexpressed GPA under the conditions tested (Fig. 5, A, B, and F). In contrast, surface expression of AE1 E758K(HA) was increased more than fivefold by coexpression of GPA (Fig. 5, D–F). GPA-dependence of AE1 E758K(HA) surface expression was also evident in Ambystoma oocytes (Fig. 6). In both Xenopus and Ambystoma oocytes, the strong GPA dependence of AE1 E758K(HA) surface expression contrasted with the minimal GPA-dependence of 86Rb+ influx into either type of oocyte expressing AE1 E758K, with or without epitope tag (Fig. 4).
Fig. 5.
Surface expression of AE1 mutant E758K in Xenopus oocytes is increased by coexpression of GPA. A–E: confocal immunofluorescence micrographs of HA antigen surface expression by Xenopus oocytes expressing HA-tagged wild-type AE1 or AE1 mutant E758K (10 ng cRNA) in the presence or absence of coexpressed GPA (2.5 ng cRNA). Scale bar, 100 μm. F: surface expression of wild-type AE1 and AE1 E758K measured as (water-injected oocyte-corrected) mean relative fluorescence intensities ± SE for (n) oocytes. *P < 0.05 compared in the absence (gray bar) and presence (black bar) of GPA (Student's unpaired t-test).
Fig. 6.
Surface expression of AE1 mutant E758K in Ambystoma oocytes is increased by coexpression of GPA. A and B: confocal immunofluorescence micrographs of HA antigen surface expression by Ambystoma oocytes expressing HA-tagged AE1 mutant E758K (10 ng cRNA) in the absence (A) and presence (B) of coexpressed GPA (2.5 ng of cRNA). C: H2O-injected oocyte. Scale bar, 100 μm. D: surface expression of AE1 E758K measured as (water-injected oocyte-corrected) mean relative fluorescence intensities ± SE for (n) oocytes; *P < 0.05 compared in the absence (gray bar) and presence (black bar) of GPA (Student's unpaired t-test).
SO42− and oxalate transport by oocytes expressing AE1 E758K.
The presence of the HA epitope detectably increased wild-type AE1-mediated SO42− uptake at pH 7.4 above the undetectable levels previously noted in Xenopus oocytes (9, 11). However, oocytes expressing AE1 E758K or AE1 E758K(HA) exhibited increased SO42− uptake further enhanced by GPA coexpression (Fig. 7A). AE1 E758K-associated SO42− uptake was not significantly pH-dependent (Fig. 7B) and, at bath pH 7.4, was far greater than in oocytes expressing the AE1 stomatocytosis mutants S731P and H734R or the dRTA mutant G701D (Fig. 7C).
Fig. 7.
AE1 E758K mediates increased GPA-dependent 35SO42− uptake. A: 35SO42− uptake at bath pH 7.4 by Xenopus oocytes expressing AE1 mutant E758K or E758K(HA) (10 ng cRNA) was enhanced by GPA coexpression (2.5 ng). H2O-corrected values are means ± SE for number of oocytes shown in parentheses. *and black bar indicate P < 0.05 compared to values without GPA expression (gray bar). B: pH-independent 35SO42− uptake by oocytes expressing AE1 mutant E758K in the presence of GPA. *and gray bar indicate P < 0.05 compared to wild-type AE1 (Dunnett's t-test). C: 35SO42− uptake by AE1 E758K was compared to that by oocytes expressing the indicated AE1 mutants. Values are mean H2O-subtracted values for (n) oocytes. *and gray bar indicate P < 0.05 compared to AE1 E758K.
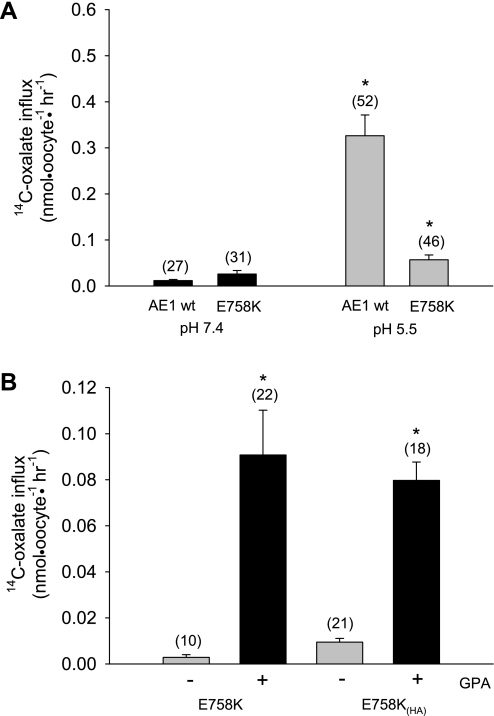
Wild-type AE1 exhibits acid pH-activated [14C]oxalate uptake likely representing H+-oxalate cotransport, as shown in human erythrocytes (30). This acid pH-stimulated oxalate uptake is diminished but still present in oocytes expressing AE1 E758K (Fig. 8A). With or without the exofacial HA tag, oxalate uptake by oocytes expressing AE1 E758K is nearly completely GPA-dependent (Fig. 8B).
Fig. 8.
AE1 E758K mediates GPA-dependent [14C]oxalate uptake. A: [14C]oxalate uptake by Xenopus oocytes expressing wild-type AE1 or AE1 mutant E758K with coexpressed GPA at bath pH 7.4 or 5.5. *and gray bar indicate P < 0.05 compared to pH 7.4 (Student‘s unpaired t-test). B: GPA-dependence of AE1 E758K-mediated [14C]oxalate uptake. *and black bar indicate P < 0.05 vs. absence of GPA (gray bar; Student's unpaired t-test). Values are H2O-corrected means ± SE for (n) oocytes.
Pharmacology of ion transport by AE1 E758K.
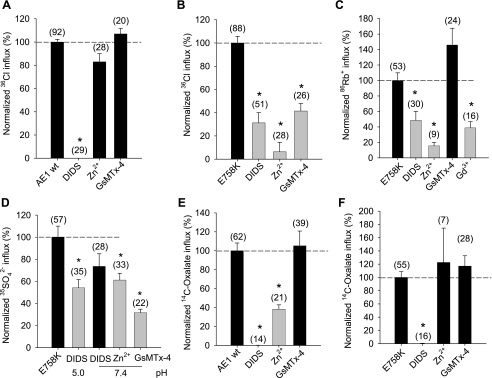
Wild-type AE1-mediated 36Cl− influx is DIDS-sensitive but completely insensitive to Zn2+ and to the toxin inhibitor of mechanosensitive cation channels isolated from the venom of the Chilean rose tarantula Grammastola spatulata, GsMTx-4 (Fig. 9A) (46). In contrast, 36Cl− influx into oocytes expressing AE1 E758K is partially inhibited by DIDS and by GsMTx-4 and is nearly completely inhibited by Zn2+ (Fig. 9B). 86Rb+ influx into AE1 E758K-expressing oocytes is also partially inhibited by DIDS and nearly completely inhibited by Zn2+. In addition, E758K-associated 86Rb+ influx is partially inhibited by the cation channel blocker Gd3+ (50 μM), with no further inhibition by 1 mM Gd3+ (not shown). However, E758K-associated 86Rb+ influx is not inhibited by 1 μM GsMTx-4 (Fig. 9C) or by 100 μM streptomycin, 10 μM WW-781, 1 mM La3+, 1 mM Li+, 2 mM NH4+, or 5 mM Ba2+ (not shown). AE1 E758K-mediated SO42− influx is only partially inhibited by DIDS, Zn2+, and GsMTx-4 (Fig. 9D), whereas oxalate uptake by wild-type AE1 (Fig. 9E) and by AE1 E758K (Fig. 9F) is completely DIDS-sensitive and GsMTx-4-insensitive. Wild-type AE1-mediated oxalate uptake is inhibited 50% by Zn2+ (Fig. 9E), but oxalate uptake by the E758K mutant is Zn2+-insensitive (Fig. 9F). Thus the increased uptakes of Cl−, Rb+, SO42−, and oxalate into oocytes expressing AE1 E758K exhibit ion-specific pharmacological differences.
Fig. 9.
Pharmacological profiles of AE1 E758K-associated ion transport in Xenopus oocytes. Effects of the indicated inhibitors on normalized values of (A) 36Cl− into Xenopus oocytes expressing wild-type AE1; (B) 36Cl− influx into oocytes expressing AE1 E758K; (C) 86R+ influx into oocytes expressing E758K; (D) SO42− influx into oocytes expressing E758K at the indicated bath pH values; (E) 14C-oxalate influx at pH 5.5 into oocytes expressing wild-type AE1; or (F) 14C oxalate influx at pH 5.5 into oocytes expressing E758K. H2O-corrected values are means ± SE for (n) oocytes, and normalized to mean uptake in the absence of inhibitors. Inhibitor concentrations were DIDS (200 μM), Zn2+ (200 μM), GsMTx-4 (1 μM), Gd3+ (50 μM). *and gray bar indicate P < 0.05 compared to absence of inhibitor (Dunnett's t-test).
AE1 E758K-associated currents.
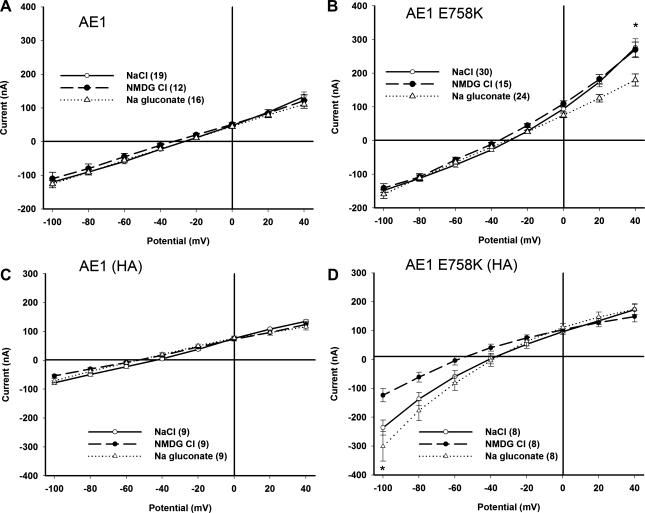
Xenopus oocytes coexpressing GPA with wild-type AE1 or AE1(HA) exhibited background levels of current (Fig. 10, A and C). Oocytes coexpressing GPA with AE1 E758K exhibited a small, outwardly rectifying current at positive holding potentials. The current was smaller than exhibited by oocytes coexpressing GPA with the AE1 HSt mutants H734R and S731P (25). The outwardly rectifying component was insensitive to bath Na+ replacement with NMDG; abolished by bath Cl− replacement with gluconate (Fig. 10B); strongly inhibited by Zn2+ (see supplemental Fig. 3), as was the corresponding 36Cl− influx (Fig. 9B); and partially inhibited by 100 μM SITS (see supplemental Fig. 3) and by 10 μM WW-781 (see supplemental Fig. 4). The outwardly rectifying component of current was of variable magnitude (see supplemental Figs. 3B, 3D, and 4).
Fig. 10.
Xenopus oocytes expressing AE1 E758K exhibit small currents sensitive to the presence of an ectoepitope tag, as recorded by two-electrode voltage clamp. A: Xenopus oocytes expressing wild-type AE1 display basal currents insensitive to bath substitution of Cl− or Na+. B: AE1 E758K-expressing oocytes exhibit a modest, outwardly rectifying current in NaCl bath that is preserved in NMDG Cl but largely abolished in Na gluconate. *P < 0.01, Na gluconate vs. NaCl. C: oocytes expressing HA-tagged wild-type AE1 display basal currents insensitive to bath substitution of Cl− or Na+. D: oocytes expressing HA-tagged AE1 E758K exhibit a modest, inwardly rectifying current in NaCl bath that is preserved, if not enhanced, in Na gluconate but reduced in NMDG Cl. *P < 0.001, NaCl vs. NMDG Cl. All oocytes coexpressed GPA (2 ng cRNA). Values are means ± SE for number of oocytes shown in parentheses. In all panels, NaC1 bath currents are presented as open circles, Na gluconate bath currents as filled circles, and NMDG C1 bath currents as open triangles.
Oocytes coexpressing GPA with AE1 E758K(HA) expressed a small, inwardly rectifying current that was minimally sensitive to bath Cl− replacement, but was reduced by bath Na+ replacement (Fig. 10D). This AE1 E758K(HA)-associated inward current was smaller than reported for oocytes coexpressing GPA with AE1 H734R or with AE1 S731P (25). Thus AE1 E758K coexpression with GPA was characterized by a small nonspecific current with a variable component of outwardly rectifying anion current. In contrast, the small nonspecific current of oocytes coexpressing AE1 E758K(HA) with GPA was characterized by a mildly inwardly rectifying component of cation current. Although 86Rb+ influx was higher in oocytes expressing AE1 E758K than in oocytes expressing the AE1 HSt mutant H734R or S731P, E758K-associated inward currents were of lower magnitude.
The small currents of Xenopus oocytes expressing AE1 E758K were GPA-independent, whether measured in bath solutions containing NaCl, NMDG Cl, or Na gluconate (see supplemental Fig. 5). In contrast, oocytes from the same frog exhibited robust GPA-dependent 36Cl− influx (see supplemental Fig. 5C). These data strongly suggest that AE1 E758K-associated currents are mediated by one or more distinct polypeptide(s) of the oocyte membrane.
DISCUSSION
We have characterized the novel human AE1 missense mutation E758K, encoded by a heterozygous G-to-A transition found in two unrelated patients with well-compensated anemia. Patient 1 presented with spherostomatocytosis and a family history of anemia and cholelithiasis. Patient 2 had a clinical diagnosis of xerocytosis with elevated red cell Na content and reduced K content. The amino acid modified in both patients, E758, is conserved among all human SLC4 polypeptides and resides in a mutational hotspot of the AE1 transmembrane domain at the boundary of a putative reentrant loop (Fig. 1). The nearby heterozygous AE1 mutation R760Q was reported previously in association with cation-leak stomatocytosis (6, 16). The adjacent heterozygous AE1 mutation Q759H, found in trans with the AE1 Southeast Asian ovalocytosis mutation Δ400–408, was reported in association with the syndrome of combined dRTA and severe spherocytic anemia (12).
Preservation of anion exchange activity by AE1 E758K.
AE1 E758K expressed in Xenopus oocytes sustained a substantial fraction of wild-type Cl−/Cl− and Cl−/HCO3− exchange activities. This preservation of anion exchange activity resembles that observed for the AE1 HSt mutation R760Q (16), but contrasts with the loss of Cl− transport and/or Cl−/HCO3− exchange activity by the HSt mutations L687P, D705Y, S731P, H734R (6), and G796R (27) expressed in Xenopus oocytes. Coexpression of GPA with AE1 H734R did not rescue oocyte Cl−/HCO3− exchange activity (25), consistent with anion transport rates in heterozygous H734R red blood cells measured at 50% of wild-type values (6).
AE1 E758K-mediated trans-anion-sensitive 36Cl− efflux and 36Cl− influx were nearly completely dependent on GPA coexpression. Both Cl−/Cl− exchange and Cl−/HCO3− exchange in oocytes expressing AE1 E758K were fully inhibited by 200 μM DIDS (Figs. 2 and 3). In Ambystoma oocytes, AE1 E758K displayed similar Cl− transport properties (Fig. 2E). Wild-type AE1 surface expression and transport function (at 10 ng injected cRNA) was, in contrast, completely independent of GPA coexpression. Introduction of an HA epitope tag into the third extracellular loop of AE1 usually had minor effects on ion fluxes associated with expression of either wild-type or mutant polypeptide. HA epitope detection in unfixed oocytes confirmed that AE1 E758K(HA) surface expression was strongly GPA-dependent (Figs. 5 and 6), consistent with the GPA-dependent transport of Cl− and HCO3− in oocytes of both species.
AE1 E758K exhibited increased SO42− transport that was acid pH-insensitive (Fig. 7), resembling the upregulated SO42− transport by the AE1 mutant E681Q (murine Ae1 E699Q) (11). AE1 E758K-mediated oxalate transport was slightly elevated at neutral pH, but the wild-type stimulation of oxalate uptake by acid pH 5.5 was attenuated for the E758K mutant (Fig. 8). Thus the E758K mutation had pleiform effects on AE1-mediated anion transport, and decreased or abolished acid pH dependence of divalent anion transport. Transport of both monovalent and divalent anions by AE1 E758K displayed strong GPA dependence.
86Rb+ uptake by AE1 E758K.
The elevated Na content and decreased K content of erythrocytes from patient 2 suggested, as with earlier reported stomatocytosis mutations, that AE1 E758K might encode a cation leak. Indeed, oocytes expressing AE1 E758K exhibited greater 86Rb+ uptake than oocytes expressing AE1 HSt mutants S731P and H734R (6) or dRTA mutant G701D (51). Curiously, however, 86Rb+ uptake into oocytes expressing AE1 E758K was not altered by bath Na+ substitution, a maneuver that increased inward current associated with the HSt mutant H734R (25). 86Rb+ uptake by AE1 E758K-expressing oocytes was similarly unaltered by bath Cl− removal or by addition of the substrate SO42−, suggesting functional independence of anion and cation translocation pathways and a lack of involvement of SLC12 cation-chloride cotransport. More remarkably, 86Rb+ uptake into Ambystoma and Xenopus oocytes expressing AE1 E758K or E758K(HA) was completely or nearly GPA-independent (Fig. 4). Thus 86Rb+ flux accompanying AE1 E758K expression is largely or completely independent of AE1 polypeptide surface expression, in remarkable contrast to mutant polypeptide-mediated transport of Cl−, HCO3−, SO42−, and oxalate.
Does AE1 E758K itself transport cations?
The simplest conclusion to be drawn from these data is that AE1 E758K is not itself the plasmalemmal pathway of most, if any, cation transport detected in oocytes overexpressing the mutant polypeptide. A substantial proportion, if not all, of the AE1 E758K-induced cation leak in oocytes may be mediated by endogenous permeability pathway(s) of the oocyte plasma membrane. This endogenous pathway is subject to functional modulation by heterologous expression of AE1 E758K, but not by wild-type AE1 or by AE1 E699Q (Fig. 5C), and is not stimulated by the TRPC6 agonist, hyperforin (50 μM; not shown). This specificity is highlighted by the absence of 86Rb+ flux associated with oocyte overexpression of the recessive dRTA mutant AE1 G701D in the absence of GPA (51), a situation associated with intracellular retention of the mutant protein (47). The lack of induced 86Rb+ influx associated with intra-oocyte retention of mutants of the K+-Cl− cotransporter KCC1 (8) and of the polycystin-1 COOH-terminal tail (48) further attests to the contextual specificity of AE1 E758K overexpression-associated cation transport.
Activation of this apparently endogenous cation pathway could occur within the oocyte plasma membrane (and, potentially, also in the red cell membrane of patients). Increased cation leak in the oocyte might alternatively or additionally reflect endoplasmic reticulum stress specific to overexpression of AE1 E758K, as reported for oocyte overexpression of pathogenic mutants of the voltage-gated Ca2+ channel CaV2.2 associated with episodic ataxia-2 (39), and elicited even by physiological CaV2.2 inhibition by either of the two auxiliary channel subunits γ2 or γ3 (42). Either interpretation is compatible with elevated cation permeability of human erythrocytes subjected to stress conditions such as thalassemia and dyserythropoietic anemia (53) or of normal red blood cells exposed to oxidizing agents or to replacement of extracellular Cl− or Na+ by impermeant ions (13, 16).
Another interpretation would attribute cation transport exclusively to the small GPA-independent fraction of AE1 E758K (Figs. 4–6), perhaps reflecting a low-frequency, GPA-independent folding event during mutant AE1 biosynthesis. Binding of GPA to the mutant would restore plasmalemmal delivery and/or stability and anion-exchange activity, without increasing cation transport (since the majority of GPA-dependent, mutant polypeptides at the surface would be incapable of 86Rb+ transport). A third interpretation would posit a fraction of AE1 E758K(HA) at the oocyte surface, folded in a way that renders the HA epitope inaccessible to extracellular antibody and that accounts for most or all of the E758K-associated cation transport. Either of these two additional interpretations could accommodate the ion-specific pharmacological inhibition profiles of AE1 E758K-associated uptake of Cl−, 86Rb+, 35SO42−, and [14C]oxalate by DIDS, Zn2+, and GsMTx-4 (Fig. 9).
The “band 3 macrocomplex” of the erythroid membrane (4) includes the cation transporter RhAG and its Rh antigen-binding partners, as well as stomatin/band 7.2 [postulated to regulate cation channel(s)]. A conformationally altered mutant AE1, as the most abundant and largest component of this macrocomplex, might upregulate the function of a known (e.g., RhAG) or unidentified component of the macrocomplex to result in increased cation transport. This type of mechanism, although not offering the virtue of simplicity, might apply equally to macrocomplex member GLUT1, in which paroxysmal dyskinesia mutations associated with hemolytic anemia were characterized by increased conductive cation transport in red blood cells and increased cation flux in oocytes (52). Additional examples of activation of endogenous oocyte conductive cation transport have been observed in the settings of heterologous oocyte overexpression of the mosquito electroneutral Na+/H+ exchanger AeNHE8 (41), the plasmodial chloroquine resistance transporter PfCRT (38), and the human heterodimeric amino acid transporter 4F2hc/LAT1 (49).
GPA dependence of previously reported AE1 stomatocytosis mutants.
AE1 E758K is not unique among stomatocytosis mutants in its association with GPA-independent cation uptake. Thus expression in oocytes of AE1 G796R resulted in reduced K content, elevated Na content, and a GPA-independent 20-fold stimulation of Li+ uptake (27). Although the GPA dependence and stilbene sensitivity of the preserved Cl−/HCO3− exchange mediated by the G796R mutant was not reported, the enhanced cation permeability was SITS-insensitive. Thus oocyte expression of the AE1 HSt mutant G796R resembles that of the AE1 mutant E758K in its GPA-independent cation transport and its altered cation transport pharmacology. In contrast, oocyte expression of the AE1 HSt mutants H734R and S731P was associated with currents activated severalfold by GPA coexpression (25). GPA dependence of currents associated with other HSt mutants was not reported, but the cation fluxes associated with oocyte expression of the AE1 recessive dRTA mutant G701D were completely GPA-dependent (51), in concert with GPA dependence of Cl− transport by AE1 G701D (47, 51).
The renal phenotype of the two patients with heterozygous mutations was not characterized. However, the strong GPA-dependence of Cl− transport associated with AE1 E758K suggests that the mutation is not strongly dominant-negative in renal collecting duct intercalated cells. The heterozygous state is likely compatible with normal urinary acidification under unstressed conditions.
Heterozygous AE1 mutations in HSt red blood cells are associated with altered electroneutral cation transport by other transporter polypeptides.
Unlike the situation in oocytes expressing heterologous AE1 H734R, neither cation currents nor anion currents were elevated in AE1 H734R HSt red blood cells (2). However, H734R red blood cells did exhibit elevated, abnormally regulated K+-Cl− cotransport (KCC) activity and increased K+(Na+)/H+ exchange activity. Earlier investigations of HSt cryohydrocytosis in red blood cells from patients later shown to be heterozygous for AE1 H734R also revealed increased Na+ pump activity, as well as increased cation-Cl− cotransport activity (26) [as measured, likely a sum of Na+-K+-2Cl− cotransport (NKCC) and KCC activities (13)]. HSt red blood cells from patients heterozygous for AE1 G796R also exhibited increased activities of KCC, NKCC, and Na+/H+ exchange (27), in addition to increased membrane association of Lyn and Syk kinases and increased tyrosine phosphorylation of multiple membrane proteins. Despite elevated intracellular Na and reduced K contents of AE1 G796R red blood cells, Na+-K+-ATPase activity was reduced, perhaps secondary to pump-mediated ATP depletion (27). The mechanism by which individual AE1 HSt mutations might selectively up- or downregulate red cell Na+-K+-ATPase activity remains unclear.
The modulation of multiple ion transport activities in HSt red blood cells heterozygous for AE1 mutants recalls the pleiform effects of trout AE1 expression in Xenopus oocytes. These include, in addition to increased glyburide- and NPPB-sensitive anion conductance, increased permeability to the cations Na+, K+, and Li+ (24) and to the zwitterion taurine [both AE1-associated and endogenous in origin (18)], as well as secondary activation of endogenous oocyte NKCC activity (23). Similar changes in oocyte transport properties were associated with expression of a mutant human AE1 lacking transmembrane spans 6 and 7 (40). AE1 E758K red blood cells might exhibit comparable secondary modulation of endogenous transporter activities.
AE1 HSt mutant-associated currents in Xenopus oocytes.
Coexpression of AE1 E758K with GPA was associated with small outward currents, and especially for AE1 E758K(HA), with small inward currents (Fig. 10). E758K-associated outward currents were, at low positive potentials, comparable in magnitude to those reported for the AE1 HSt mutants H734R and S731P coexpressed with GPA (25), but were largely carried by anions. The inward currents associated with AE1 E758K(HA) were predominantly carried by cations, as noted with mutants H734R and S731P. However, E758K-associated inward currents were considerably smaller than those associated with H734R and S731P (25), despite the higher rates of 86Rb+ influx associated with E758K expression. In Xenopus oocytes expressing AE1 E758K, 86Rb+ influx was largely GPA-independent (Fig. 4). The small, AE1 E758K-associated currents measured by two-electrode voltage clamp were GPA-independent, whether in baths of NaCl, NMDG Cl, or Na gluconate (see supplemental Fig. 5). Thus the small GPA-dependent component of 86Rb+ flux in Xenopus oocytes (Fig. 5) was not detectably electrogenic. The source of incremental cation current in oocytes expressing AE1 E758K or E758K(HA) remains uncertain.
Neither we nor others (25) included ouabain in our two-electrode voltage clamp bath solutions. As noted above, red blood cells from several HSt patients carrying heterozygous AE1 mutations exhibited increased ouabain-sensitive cation transport. Although ouabain-sensitive outward currents likely contributed ≤40 nA to the measured current in control oocytes and oocytes expressing wild-type AE1 (14, 33), pump current in oocytes expressing AE1 HSt mutants may well have been increased, secondary to elevated intracellular [Na+].
Conclusion.
The newly described, heterozygous AE1 HSt mutation E758K is associated with cation leak in spherostomatocytic red blood cells. Overexpression of AE1 E758K in oocytes reveals retention of most Cl−/anion exchange activity, increased SO42− and oxalate transport, and increased cation fluxes through endogenous oocyte pathways, accompanied by small currents of mixed ion selectivity, likely reflecting small endogenous permeabilities. A smaller component of cation leak mediated by the AE1 polypeptide mutant itself cannot be ruled out. These data, together with multiple reports of secondary increases in defined erythroid cation permeability pathways in red blood cells heterozygous for other AE1 HSt mutations, and with the remarkably pleiotropic effects of trout AE1 expression on oocyte ion and solute transport, support the interpretation that some (if not all) AE1 HSt mutant polypeptides modulate endogenous cation transport pathways in oocytes and erythrocytes. The accumulated data remain consistent with the possibility that some AE1 HSt mutant polypeptides mediate a (mutation-specific) proportion of total cell cation transport.
GRANTS
This work was supported in part by National Institutes of Health Grants DK-62039 (P. G. Gallagher), DK-43495 and HL-077765 (S. L. Alper), and T32 HD-07094 (J. F. Heneghan).
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
Footnotes
REFERENCES
- 1.Barish ME, Baud C. A voltage-gated hydrogen ion current in the oocyte membrane of the axolotl, Ambystoma J Physiol 352: 243–263, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogdanova A, Goede JS, Weiss E, Bogdanov N, Bennekou P, Bernhardt I, Lutz HU. Cryohydrocytosis: increased activity of cation carriers in red cells from a patient with a band 3 mutation. Haematologica In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce LJ. Hereditary stomatocytosis and cation leaky red cells—recent developments. Blood Cells Mol Dis 42: 216–222, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Bruce LJ, Beckmann R, Ribeiro ML, Peters LL, Chasis JA, Delaunay J, Mohandas N, Anstee DJ, Tanner MJ. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 101: 4180–4188, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bruce LJ, Guizouarn H, Burton NM, Gabillat N, Poole J, Flatt JF, Brady RL, Borgese F, Delaunay J, Stewart GW. The monovalent cation leak in overhydrated stomatocytic red blood cells results from amino acid substitutions in the Rh-associated glycoprotein. Blood 113: 1350–1357, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Bruce LJ, Robinson HC, Guizouarn H, Borgese F, Harrison P, King MJ, Goede JS, Coles SE, Gore DM, Lutz HU, Ficarella R, Layton DM, Iolascon A, Ellory JC, Stewart GW. Monovalent cation leaks in human red cells caused by single amino-acid substitutions in the transport domain of the band 3 chloride-bicarbonate exchanger, AE1. Nat Genet 37: 1258–1263, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Carella M, d’Adamo AP, Grootenboer-Mignot S, Vantyghem MC, Esposito L, D’Eustacchio A, Ficarella R, Stewart GW, Gasparini P, Delaunay J, Iolascon A. A second locus mapping to 2q35–36 for familial pseudohyperkalaemia. Eur J Hum Genet 12: 1073–1076, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Casula S, Shmukler BE, Wilhelm S, Stuart-Tilley AK, Su W, Chernova MN, Brugnara C, Alper SL. A dominant negative mutant of the KCC1 K-Cl cotransporter: both N- and C-terminal cytoplasmic domains are required for K-Cl cotransport activity. J Biol Chem 276: 41870–41878, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chernova MN, Jiang L, Crest M, Hand M, Vandorpe DH, Strange K, Alper SL. Electrogenic sulfate/chloride exchange in Xenopus oocytes mediated by murine AE1 E699Q. J Gen Physiol 109: 345–360, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J Biol Chem 280: 8564–8580, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chernova MN, Stewart AK, Barry PN, Jennings ML, Alper SL. Mouse Ae1 E699Q mediates SO42−i/aniono exchange with [SO42−]i-dependent reversal of wild-type pHo sensitivity. Am J Physiol Cell Physiol 295: C302–C312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choo KE, Nicoli TK, Bruce LJ, Tanner MJ, Ruiz-Linares A, Wrong OM. Recessive distal renal tubular acidosis in Sarawak caused by AE1 mutations. Pediatr Nephrol 21: 212–217, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Coles SE, Stewart GW. Temperature effects on cation transport in hereditary stomatocytosis and allied disorders. Int J Exp Pathol 80: 251–258, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, Horisberger JD, Lelievre L, Geering K. Transport and pharmacological properties of nine different human Na,K-ATPase isozymes. J Biol Chem 275: 1976–1986, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Delaunay J. The hereditary stomatocytoses: genetic disorders of the red cell membrane permeability to monovalent cations. Semin Hematol 41: 165–172, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Ellory JC, Guizouarn H, Borgese F, Bruce LJ, Wilkins RJ, Stewart GW. Leaky Cl−-HCO3− exchangers: cation fluxes via modified AE1. Philos Trans R Soc Lond B Biol Sci 364: 189–194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fairbanks G, Steck TL, Wallach DF. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10: 2606–2617, 1971 [DOI] [PubMed] [Google Scholar]
- 18.Fievet B, Gabillat N, Borgese F, Motais R. Expression of band 3 anion exchanger induces chloride current and taurine transport: structure-function analysis. EMBO J 14: 5158–5169, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujinaga J, Tang XB, Casey JR. Topology of the membrane domain of human erythrocyte anion exchange protein, AE1. J Biol Chem 274: 6626–6633, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Grootenboer S, Schischmanoff PO, Laurendeau I, Cynober T, Tchernia G, Dommergues JP, Dhermy D, Bost M, Varet B, Snyder M, Ballas SK, Ducot B, Babron MC, Stewart GW, Gasparini P, Iolascon A, Delaunay J. Pleiotropic syndrome of dehydrated hereditary stomatocytosis, pseudohyperkalemia, and perinatal edema maps to 16q23-q24. Blood 96: 2599–2605, 2000 [PubMed] [Google Scholar]
- 21.Groves JD, Tanner MJ. Glycophorin A facilitates the expression of human band 3-mediated anion transport in Xenopus oocytes. J Biol Chem 267: 22163–22170, 1992 [PubMed] [Google Scholar]
- 22.Groves JD, Tanner MJ. The effects of glycophorin A on the expression of the human red cell anion transporter (band 3) in Xenopus oocytes. J Membr Biol 140: 81–88, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Guizouarn H, Gabillat N, Borgese F. Evidence for up-regulation of the endogenous Na-K-2Cl co-transporter by molecular interactions with the anion exchanger tAE1 expressed in Xenopus oocyte. J Biol Chem 279: 11513–11520, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Guizouarn H, Gabillat N, Motais R, Borgese F. Multiple transport functions of a red blood cell anion exchanger, tAE1: its role in cell volume regulation. J Physiol 535: 497–506, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guizouarn H, Martial S, Gabillat N, Borgese F. Point mutations involved in red cell stomatocytosis convert the electroneutral anion exchanger 1 to a nonselective cation conductance. Blood 110: 2158–2165, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Haines PG, Jarvis HG, King S, Noormohamed FH, Chetty MC, Fisher J, Hill P, Nicolaou A, Stewart GW. Two further British families with the “cryohydrocytosis” form of hereditary stomatocytosis. Br J Haematol 113: 932–937, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Iolascon A, De Falco L, Borgese F, Esposito MR, Avvisati RA, Izzo P, Piscopo C, Guizouarn H, Biondani A, Pantaleo A, De Franceschi L. A novel erythroid anion exchange variant (Gly796Arg) of hereditary stomatocytosis associated with dyserythropoiesis. Haematologica 94: 1049–1059, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarolim P. Disorders of band 3. In: Red Cell Membrane Transport in Health and Disease, edited by Bernhardt IEJ. Berlin: Springer, 2003, p. 603–619 [Google Scholar]
- 29.Jarolim P, Murray JL, Rubin HL, Taylor WM, Prchal JT, Ballas SK, Snyder LM, Chrobak L, Melrose WD, Brabec V, Palek J. Characterization of 13 novel band 3 gene defects in hereditary spherocytosis with band 3 deficiency. Blood 88: 4366–4374, 1996 [PubMed] [Google Scholar]
- 30.Jennings ML, Adame MF. Characterization of oxalate transport by the human erythrocyte band 3 protein. J Gen Physiol 107: 145–159, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurschat CE, Alper SL. Hereditary renal tubular acidosis. In: Molecular and Genetic Basis of Renal Disease, edited by Mount DB, Pollak MR. Philadelphia: Saunders, 2007, p. 269–294 [Google Scholar]
- 32.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 33.Lafaire AV, Schwarz W. Voltage dependence of the rheogenic Na+/K+ ATPase in the membrane of oocytes of Xenopus laevis. J Membr Biol 91: 43–51, 1986 [DOI] [PubMed] [Google Scholar]
- 34.Lux SE, John KM, Kopito RR, Lodish HF. Cloning and characterization of band 3, the human erythrocyte anion-exchange protein (AE1). Proc Natl Acad Sci USA 86: 9089–9093, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchesi SL, Knowles WJ, Morrow JS, Bologna M, Marchesi VT. Abnormal spectrin in hereditary elliptocytosis. Blood 67: 141–151, 1986 [PubMed] [Google Scholar]
- 36.Martial S, Guizouarn H, Gabillat N, Pellissier B, Borgese F. Consequences of point mutations in trout anion exchanger 1 (tAE1) transmembrane domains: evidence that tAE1 can behave as a chloride channel. J Cell Physiol 207: 829–835, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Martial S, Guizouarn H, Gabillat N, Pellissier B, Borgese F. Importance of several cysteine residues for the chloride conductance of trout anion exchanger 1 (tAE1). J Cell Physiol 213: 70–78, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Nessler S, Friedrich O, Bakouh N, Fink RH, Sanchez CP, Planelles G, Lanzer M. Evidence for activation of endogenous transporters in Xenopus laevis oocytes expressing the Plasmodium falciparum chloroquine resistance transporter, PfCRT. J Biol Chem 279: 39438–39446, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Page KM, Heblich F, Davies A, Butcher AJ, Leroy J, Bertaso F, Pratt WS, Dolphin AC. Dominant-negative calcium channel suppression by truncated constructs involves a kinase implicated in the unfolded protein response. J Neurosci 24: 5400–5409, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker MD, Young MT, Daly CM, Meech RW, Boron WF, Tanner MJ. A conductive pathway generated from fragments of the human red cell anion exchanger AE1. J Physiol 581: 33–50, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piermarini PM, Weihrauch D, Meyer H, Huss M, Beyenbach KW. NHE8 is an intracellular cation/H+ exchanger in renal tubules of the yellow fever mosquito Aedes aegypti. Am J Physiol Renal Physiol 296: F730–F750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandoval A, Andrade A, Beedle AM, Campbell KP, Felix R. Inhibition of recombinant N-type CaV channels by the γ2-subunit involves unfolded protein response (UPR)-dependent and UPR-independent mechanisms. J Neurosci 27: 3317–3327, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart AK, Kurschat CE, Vaughan-Jones RD, Alper SL. Putative re-entrant loop 1 of AE2 transmembrane domain has a major role in acute regulation of anion exchange by pH. J Biol Chem 284: 6126–6139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart GW. Hemolytic disease due to membrane ion channel disorders. Curr Opin Hematol 11: 244–250, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol 115: 583–598, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanphaichitr VS, Sumboonnanonda A, Ideguchi H, Shayakul C, Brugnara C, Takao M, Veerakul G, Alper SL. Novel AE1 mutations in recessive distal renal tubular acidosis. Loss-of-function is rescued by glycophorin A. J Clin Invest 102: 2173–2179, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandorpe DH, Wilhelm S, Jiang L, Ibraghimov-Beskrovnaya O, Chernova MN, Stuart-Tilley AK, Alper SL. Cation channel regulation by COOH-terminal cytoplasmic tail of polycystin-1: mutational and functional analysis. Physiol Genomics 8: 87–98, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Wagner CA, Broer A, Albers A, Gamper N, Lang F, Broer S. The heterodimeric amino acid transporter 4F2hc/LAT1 is associated in Xenopus oocytes with a nonselective cation channel that is regulated by the serine/threonine kinase sgk-1. J Physiol 526: 35–46, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh S, Borgese F, Gabillat N, Guizouarn H. Southeast Asian AE1 associated renal tubular acidosis: cation leak is a class effect. Biochem Biophys Res Commun 382: 668–672, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Walsh S, Borgese F, Gabillat N, Unwin R, Guizouarn H. Cation transport activity of anion exchanger 1 mutations found in inherited distal renal tubular acidosis. Am J Physiol Renal Physiol 295: F343–F350, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Weber YG, Storch A, Wuttke TV, Brockmann K, Kempfle J, Maljevic S, Margari L, Kamm C, Schneider SA, Huber SM, Pekrun A, Roebling R, Seebohm G, Koka S, Lang C, Kraft E, Blazevic D, Salvo-Vargas A, Fauler M, Mottaghy FM, Munchau A, Edwards MJ, Presicci A, Margari F, Gasser T, Lang F, Bhatia KP, Lehmann-Horn F, Lerche H. GLUT1 mutations are a cause of paroxysmal exertion-induced dyskinesias and induce hemolytic anemia by a cation leak. J Clin Invest 118: 2157–2168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiley JS. Increased erythrocyte cation permeability in thalassemia and conditions of marrow stress. J Clin Invest 67: 917–922, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.