Abstract
Gastric pacing has been investigated as a potential treatment for gastroparesis. New pacing protocols are required to improve symptom and motility outcomes; however, research progress has been constrained by a limited understanding of the effects of electrical stimulation on slow-wave activity. This study introduces high-resolution (HR) “entrainment mapping” for the analysis of gastric pacing and presents four demonstrations. Gastric pacing was initiated in a porcine model (typical amplitude 4 mA, pulse width 400 ms, period 17 s). Entrainment mapping was performed using flexible multielectrode arrays (≤192 electrodes; 92 cm2) and was analyzed using novel software methods. In the first demonstration, entrainment onset was quantified over successive waves in spatiotemporal detail. In the second demonstration, slow-wave velocity was accurately determined with HR field analysis, and paced propagation was found to be anisotropic (longitudinal 2.6 ± 1.7 vs. circumferential 4.5 ± 0.6 mm/s; P < 0.001). In the third demonstration, a dysrhythmic episode that occurred during pacing was mapped in HR, revealing an ectopic slow-wave focus and uncoupled propagations. In the fourth demonstration, differences were observed between paced and native slow-wave amplitudes (0.24 ± 0.08 vs. 0.38 ± 0.14 mV; P < 0.001), velocities (6.2 ± 2.8 vs. 11.5 ± 4.7 mm/s; P < 0.001), and activated areas (20.6 ± 1.9 vs. 32.8 ± 2.6 cm2; P < 0.001). Entrainment mapping enables an accurate quantification of the effects of gastric pacing on slow-wave activity, offering an improved method to assess whether pacing protocols are likely to achieve physiologically and clinically useful outcomes.
Keywords: gastric electrical stimulation, gastrointestinal, electrophysiology, slow wave
gastric peristalsis is controlled by a propagating electrical activity termed slow waves. Gastric pacing involves electrically stimulating the stomach to entrain the propagation of slow waves for therapeutic purposes. Gastric pacing is a different therapy to high-frequency, short-pulse gastric electrical stimulation, which is not thought to alter slow-wave behavior (18, 24).
Gastric pacing has primarily been investigated for the treatment of gastroparesis. In gastroparesis, researchers have attempted to use pacing to normalize motility and emptying, to control dysrhythmias, and to improve symptoms (15, 16). High-energy stimulation protocols have also been used in obesity to disrupt slow-wave activity in a controlled manner, with the aim of restricting eating and inducing satiety (25). The feasibility of gastric pacing has been evaluated in small trials; however, a clinical role has not been established, and pacing remains experimental. It has been suggested that a new generation of pacing devices will be required to achieve effective entrainment and motility outcomes, while also optimizing energy efficiency to facilitate long-term implantation (9, 26).
Present research methods employed in the study of gastric pacing have not changed significantly since first described almost 40 years ago (12, 19) and have lagged behind major advances in cardiac pacing research (19). Up to now, all gastric pacing studies have employed few recording electrodes (typically ≤ 4) sutured to the gastric serosa. These sparse measurements have enabled a relatively crude analysis of pacing outcomes, such as determining the onset, frequency, and timing of slow-wave entrainment (19, 24). However, it has now been shown that sparse measurements cannot accurately define slow-wave propagation sequences because of the limited field of spatial sampling (13).
New research tools that provide an improved understanding of gastric electrical activity will aid in the development of the new generation of pacing devices. High-resolution (HR) mapping of the gastrointestinal tract involves the placement of spatially dense electrode arrays (typically > 100 electrodes) over the electrically active tissue surface, enabling a precise quantification of the spatiotemporal patterns of slow-wave propagation. HR mapping has recently been successfully applied to improve the understanding of both normal and dysrhythmic gastric slow-wave propagation (13, 14) but has not previously been applied to evaluate outcomes in gastric pacing.
The aim of this study was to demonstrate, evaluate, and validate HR entrainment mapping for gastric pacing research. The technical and analytical tools that enable this methodology are presented, together with the necessary new terminologies. Example studies are presented to evaluate the following four theoretical advantages of HR entrainment mapping over sparse-electrode measurements for investigating gastric pacing outcomes: 1) the capability to track the onset and propagation of entrained slow-wave activity in superior spatial detail and, therefore, to also quantify the interactions between the entrained and native slow-wave activities; 2) improved accuracy in calculating slow-wave propagation velocities; 3) the capability to accurately describe gastric dysrhythmic events during pacing; and 4) the capability to accurately quantify differences in tissue activation that may occur during different pacing protocols.
MATERIALS AND METHODS
Animal preparation.
All experiments were performed in vivo. Pigs of either sex and of mean body weight of 36.1 ± 2.6 kg were used in this study. Ethical approval was granted by the University of Auckland Animal Ethics Committee. The pigs were fasted overnight, before the induction of anesthesia using Zoletil (Tiletamine HCl, 50 mg/ml, and Zolazepam HCl 50 mg/ml), followed by intubation and maintenance anesthesia with isoflurane (2.5–5%, with an oxygen flow of 400 ml within a closed-circuit anesthetic system). The pigs were placed supine on a heating pad, and laparotomy was performed, with continuous monitoring of vital parameters including blood pressure and heart rate throughout the experiments. Rectal and intra-abdominal temperatures were monitored, and a heat lamp was employed when necessary to ensure that both temperatures were maintained within the normal range.
Gastric pacing and HR entrainment mapping.
Pacing was performed using a DS8000 stimulator (World Precision Instruments, Sarasota, FL) attached to two stainless steel 23-gauge pacing needles (8-mm separation). All pacing protocols employed in this study were bipolar, involving constant current pulses of periods 17–19 s, amplitudes of 2 to 4 mA, and a pulse width of 400 ms. These protocols were chosen on the basis of entrainment protocols that were previously found to be successful in canines and humans (1, 24). Baseline recordings were taken before stimulation, and each protocol was evaluated for a duration of 5–20 min.
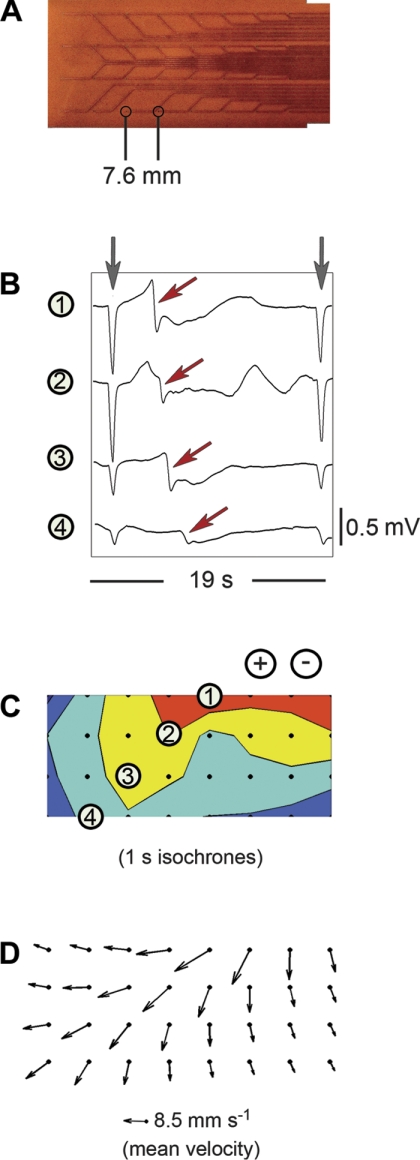
HR mapping was performed using flexible printed circuit board (PCB) electrode arrays, which were recently developed and validated for the HR mapping of gastrointestinal slow-wave activity (Fig. 1A) (5). These arrays are constructed using copper wires and gold contacts in a polyimide ribbon base. Each array has 32 electrodes (4 × 8 configuration), and up to six PCBs (192 electrodes over an area of 93 cm2) were tessellated at any one time to achieve mapping of large areas of tissue. Sample slow-wave sequences recorded using the PCB electrodes are presented in Fig. 1B.
Fig. 1.
Methods for entrainment mapping. A: flexible printed circuit board (PCB) electrodes, each containing 32 electrodes (4 × 8 configuration; interelectrode distance 7.6 mm). B: sample gastric electrograms recorded using the PCBs during pacing. The gray arrows indicate stimulus artifacts, and the red arrows indicate slow waves. C: an isochronal activation map of a single entrained slow wave. The black dots represent the high-resolution (HR) electrode configuration, and the four numbered electrodes correspond to the electrograms shown in B. Each color band between the isochrone lines indicates the area of slow-wave propagation over each 1-s interval. The stimulus location was above the recorded area (+/−). D: velocity field map of the same event. The arrows indicate the direction and velocity of the slow wave at each electrode. The mean velocity is 8.5 ± 3.1 mm/s over this mapped field.
Unipolar recordings were acquired from the PCBs via the ActiveTwo System (Biosemi, Amsterdam, The Netherlands) at a recording frequency of 512 Hz. The common-mode sense electrode was placed on the lower abdomen, and the right leg drive electrode on the hind leg. Each PCB was connected to the ActiveTwo via a 1.5-m 68-way ribbon cable, which was in turn fiber optically connected to a notebook computer. The acquisition software was written in Labview 8.2 (National Instruments, Austin, TX).
Signal analysis.
Signals from all channels were filtered using a second-order Bessel low-pass filter of 10 Hz. Following each experiment, the timing of the slow-wave events were hand marked in SmoothMap v.3.05 at the point of maximum negative slope (22). Unless otherwise specified, all other data analysis and presentation was performed in Matlab v.2006b (The Mathworks, Natick, MA). Isochronal activation maps of selected propagation sequences were computed according to our previously described methods (Fig. 1C) (5), and velocity field maps for selected sequences were computed using an algorithm that was recently adapted for this purpose (Fig. 1D) (7). Slow-wave amplitudes were calculated in SmoothMap v 3.05 (22). Where appropriate, slow-wave parameters were averaged over multiple successive waves and expressed as means ± SD. Students’ t-test was used to compare slow-wave parameters, with a P value <0.05 considered significant.
Methods for analyzing slow-wave interactions.
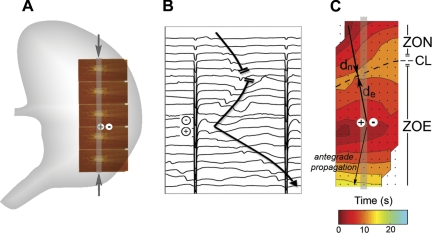
During gastric pacing, it is known that the entrained and native slow-wave activities will often interact within the same field of recordings (17). New analytical methods and terminologies were developed to enable the quantification of these interactions in HR spatiotemporal detail. The “zone of entrainment” (ZOE), “zone of normal” activity (ZON), distance that the native slow wave propagated (dn), and distance that the entrained slow wave propagated in the retrograde direction (de) were defined and calculated for mapped events (see Fig. 2).
Fig. 2.
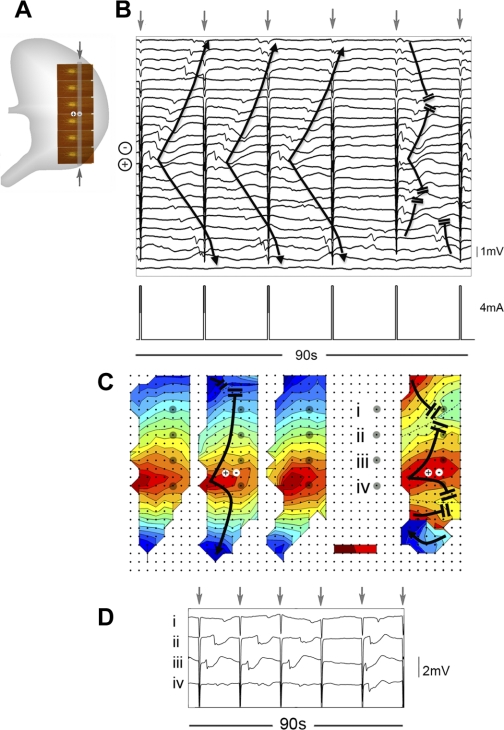
Methods for describing entrainment efficacy and interactions. A: pacing was conducted at a midcorpus site (+/−) (period 17 s, pulse width 400 ms, amplitude 4 mA). HR mapping was performed using 5 tessellated PCBs (160 electrodes; 77 cm2). B: representative electrograms recorded from the column of electrodes indicated by the gray bands and arrows in A. Induced slow waves propagated away from the stimulus site (+/−) and collided with the antegrade native activity (upper 5 channels). C: entrainment map for this same sequence; each color band indicates the area of slow-wave propagation over each 2-s interval. The zone of native activity (ZON) indicates the area where the native slow wave propagated within the mapped field (21.5 cm2). The zone of entrainment (ZOE) indicates the area that was entrained by pacing within the mapped field (51.7 cm2). The “collision line” (CL) denotes where the ZON and ZOE meet. Another line is drawn from the pacing site to the first electrode activated by native activity, and dn indicates the distance that the native slow wave propagated along this line (4.6 cm), whereas de indicates the distance that the entrained slow wave propagated in the retrograde direction along this line (4.3 cm).
Example Study 1 methods: the origin, propagation, and interactions of paced slow waves.
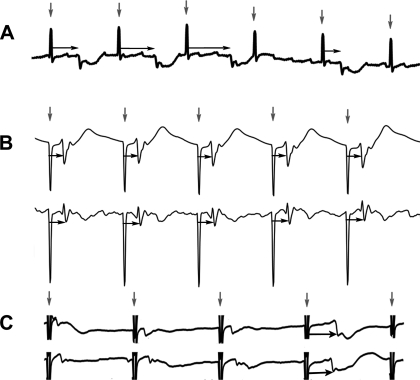
In sparse-electrode studies of gastric pacing, the onset of entrainment is usually determined by the appearance of a constant time lag (phase-locking) between the pacing artifacts and slow waves over multiple waves (Fig. 3). Typically, pacing has, therefore, usually been performed at a frequency slightly higher than the intrinsic slow-wave frequency to permit the verification of pacing onset (1, 24). By contrast, an HR analysis allows pacing outcomes to be evaluated at any pacing frequency because the density of the electrodes allows the slow-wave propagation sequences to be tracked at superior spatial resolutions, allowing the origin of pacing onset to be located precisely as shown in Fig. 2. In the first example study, the baseline gastric slow-wave frequency was 3.5 ± 0.1 counts/min, and stimulation was continuously applied at a similar frequency (period 17 s, amplitude 4 mA, pulse width 400 ms). HR mapping was conducted according to the same electrode configuration as detailed in Fig. 2, and the pacing needles were positioned in the midcorpus to enable the study of entrained slow-wave propagation in all directions from the stimulation site. The onset and propagation of entrained slow-wave activity was quantified in spatiotemporal detail over a 10-min recording period. To quantify the interaction between the native and entrained slow-wave activities, the ZON, ZOE, de, and dn were calculated and compared for every slow-wave sequence that occurred after the onset of pacing.
Fig. 3.
Determining entrainment onset with sparse electrodes. In sparse-electrode studies, the temporal relationship between the pacing artifacts (vertical arrows) and the slow waves is often used to determine the onset of entrainment. A: an irregular period between artifact and slow wave (horizontal arrows) indicates no slow-wave entrainment. B: regular period between the artifacts and slow waves (horizontal arrows) indicates entrainment (phase-locking). C: if the native slow-wave and pacing frequencies were similar, then it would be unclear whether entrainment was achieved or not, as in the first 3 waves shown here (all occurring at 3.5 counts/min). In this case, the last wave in each electrogram is the true time of entrainment onset (horizontal arrows).
Example Study 2 methods: accuracy of slow-wave velocity calculations.
The same pacing and HR mapping configuration used in the first example study was employed again for the second study, in which the accuracy of calculating slow-wave velocities was compared between an HR analysis vs. a standard sparse-electrode analysis. The HR velocity calculation was performed using the recently described velocity field method (7). To compare the velocity calculation method from a typical sparse-electrode study (16, 19), four individual electrodes were selected from the 160 electrodes of the HR array from the greater curvature side at 25-mm spacing intervals. The slow-wave velocity between these electrodes was calculated by determining the time lag between the slow waves recorded by each adjacent electrode pair, then dividing this time by the distance between the electrodes (25 mm) before averaging the velocities for all three adjacent electrode pairs.
Example Study 3 methods: entrainment mapping of gastric dysrhythmia.
A previous study by Lammers et al. (14) has shown that only HR recordings can accurately define the slow-wave behaviors that occur during gastric dysrhythmias, which may include reentrant loops and complex focal activities. In the third example study, pacing was initiated at period of 17 s, amplitude of 4 mA, and pulse width of 400 ms. Periodic instances of gastric dysrhythmias were observed during the resulting entrainment period, and these were mapped in HR using six PCBs (192 electrodes total; 93 cm2). Four individual electrodes were then selected from the HR array in the same manner as described for Example Study 2, and the recordings from these electrodes was presented to compare the level of detail achieved by a sparse analysis vs. an HR analysis.
Example Study 4 methods: entrainment mapping to compare active tissue areas.
In the fourth example study, the pacing electrodes were placed near the greater curvature of the distal antrum in the manner that has been employed for studies investigating gastric stimulation for obesity (25). The baseline slow-wave frequency was 2.7 ± 0.2 counts/min, and stimulation was initiated at a period of 19 s (3.16 counts/min), amplitude of 4 mA, and pulse width of 400 ms. HR mapping was undertaken using three PCB arrays (92 electrodes total; 47 cm2) placed proximal to the stimulus site on the antrum and corpus to map entrained wave propagation in the aboral direction. After 300 s of continuous entrainment, the amplitude of stimulation was halved from 4 mA to 2 mA. HR entrainment mapping was employed to compare the slow-wave velocities, amplitudes, and the area of tissue that was active before and after the change in the stimulation protocol.
RESULTS
Entrainment was successfully established in five of the six evaluated pigs. The following HR entrainment mapping examples were selected to demonstrate the advantages of HR entrainment mapping in the study of gastric pacing for each of the four specified aims.
Example Study 1: the origin, propagation, and interactions of paced slow waves.
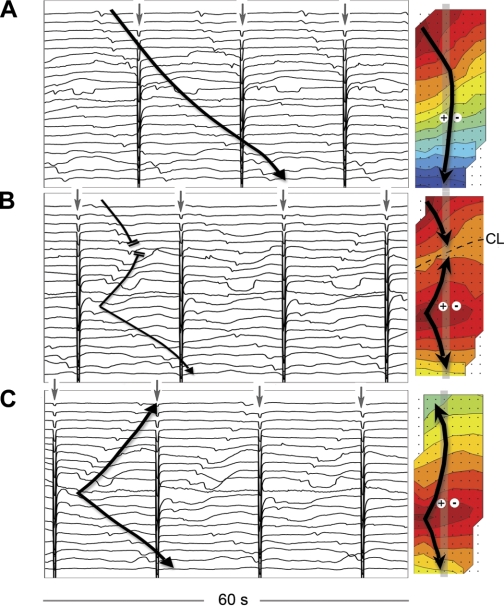
Following the onset of stimulation, baseline slow-wave activity continued unchanged for the first 402 s (6.7 min), with continued propagation of the native slow-wave activity over the entire mapped field (Fig. 4A). Entrainment onset was thereafter shown to be a gradual process, during which the mapped field transitioned from one zone containing the native slow-wave events into two zones (one native and one entrained), each containing independently propagating slow-wave events, and finally into one zone containing only entrained waves (Figure 4). An HR quantification of the onset of entrainment over this period is presented in Fig. 5.
Fig. 4.
Entrainment mapping after the onset of gastric pacing. Gastric pacing was conducted at a period of 17 s (3.5 counts/min), amplitude of 4 mA, and pulse width of 400 ms using electrodes positioned as shown in Fig. 2A. Three 60-s electrogram sequences are presented from recordings taken after pacing onset, and activation maps are shown for each cycle that is designated by the black arrows. The displayed electrograms correspond to the vertical columns of channels indicated by the vertical gray bands on each map. Stimulus artifacts are signaled with gray arrows. The isochronal intervals are 2 s. A: t = 0–60 s after pacing onset. Normal antegrade slow-wave propagation (3.5 ± 0.1 counts/min) initially continued unchanged. B: t = 405–465 s. The entrained area gradually expanded to overtake the native activity. For the mapped event, the ZON = 21.5 cm2, ZOE = 51.7 cm2, dn = 4.6 cm, and de = 4.3 cm. C: t = 575–635 s. Total entrainment of the mapped field was achieved over multiple successive waves.
Fig. 5.
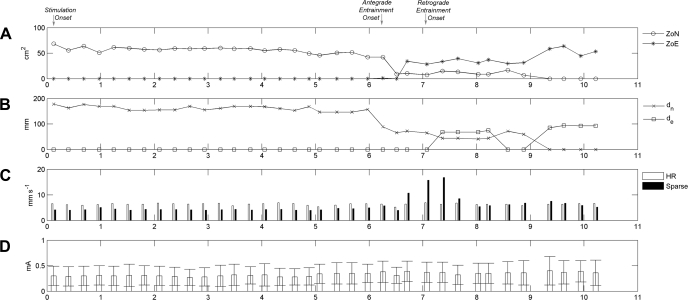
Quantification of slow-wave entrainment. A: ZON and ZOE are quantified during the onset of entrainment for the same experiment detailed in Fig. 4. Entrainment onset is indicated by a fall in the ZON and expansion of the ZOE. B: plot of dn and de measured for every cycle over the same period. Entrainment onset is indicated by a fall in dn. C: propagation velocities as determined with the sparse electrodes and the HR mapping. Before the onset of entrainment, the velocity calculated by a sparse-electrode method was consistently slower than the velocity calculated by the HR velocity method (6.3 ± 2.8 mm/s vs. 4.3 ± 1.8 mm/s; P < 0.001). During the onset of entrainment (6.8–7.5 min) the sparse-electrode method was found to markedly overestimate the slow-wave velocity. D: slow-wave amplitude after entrainment was slightly higher than the baseline amplitude (0.31 ± 0.19 vs. 0.36 ± 0.22 mA; P < 0.001).
The onset of entrainment was signaled by a sharp fall in dn and ZON and a sharp rise in the ZOE. However, the de did not increase immediately at the onset of entrainment because, whereas the entrained activity dominated the mapped field aboral (antegrade) to the pacing electrodes, the native slow-wave activity continued to dominate the mapped field oral (retrograde) to the pacing electrodes. Paced activity began to encroach over the retrograde field at 441 s, with inconsistent gains achieved until 561 s, when complete retrograde entrainment of the whole field was consistently achieved (dn = 0 mm). The average de during complete retrograde entrainment was 91 ± 4 mm.
Example Study 2: accuracy of slow-wave velocity calculations.
Before the onset of entrainment, the velocities calculated using the sparse-electrode method underestimated the propagation velocities compared with the HR calculation method (4.4 ± 1.8 vs. 6.3 ± 2.8 mm/s; P < 0.001). After the onset of entrainment, the sparse-electrode method was markedly inaccurate for the measurement of slow-wave velocities (Fig. 5).
Using the HR methods, entrained gastric slow-wave propagation was found to be anisotropic (Fig. 4, B and C), with a faster velocity in the circumferential axis of the organ than in the longitudinal axis (4.5 ± 0.6 mm/s vs. 2.6 ± 1.7 mm/s; P < 0.001). The average slow-wave velocity did not significantly change after the onset of entrainment (6.3 ± 2.8 vs. 6.4 ± 2.8 mm/s; P = 0.39). The mean slow-wave amplitude increased after the onset of entrainment; however, this difference was minimal (0.31 ± 0.19 vs. 0.36 ± 0.22 mA; P < 0.001).
Example Study 3: entrainment mapping of gastric dysrhythmias.
The baseline slow-wave frequency was 3.1 ± 0.1 counts/min, and pacing at 3.52 counts/min successfully induced slow-wave entrainment with a 1:1 relationship between each stimulus and entrained wave (Fig. 6, A and B). After a period of stable entrainment, a skipped cycle occurred, which was followed by a dysrhythmic episode that was revealed by HR entrainment mapping to be characterized by ectopic events and uncoupled slow-wave propagation (Fig. 6C). By comparison, a sparse-electrode analysis would have detected some irregularity in the slow-wave activity in terms of the rhythm but could not have accurately discerned the simultaneous uncoupled slow-wave events and their complex propagation patterns (Fig. 6D).
Fig. 6.
Dysrhythmia during gastric pacing. A: entrainment mapping was performed with 192 electrodes (area ∼93 cm2). B: gastric pacing (period 17 s, amplitude 4 mA, pulse width 400 ms) achieved regular entrainment. The displayed electrograms were recorded from the column of electrode channels indicated by the vertical gray band and arrows in A. Three entrained waves are presented, followed by a skipped cycle (no wave was entrained), followed by a dysrhythmic event. C: HR maps of the 3 entrained cycles, one skipped cycle, and a dysrhythmic episode are presented in sequence. The dysrhythmic event is shown to feature, from top of mapped field to bottom: a native wave, an entrained wave (retrograde and antegrade propagation), an ectopic pacemaker, and an uncoupled wavefront propagating medially. D: sparse selection of 4 electrodes is shown over the same recording period (interelectrode spacing 30 mm), from the electrodes (i–iv) highlighted with gray circles in C. A sparse electrode analysis would have detected an irregularity in the gastric rhythm but would not have detected the ectopic events and uncoupled propagation.
Example Study 4: entrainment mapping to compare active tissue areas.
The baseline slow-wave frequency was 2.7 ± 0.2 counts/min, and 1:1 entrainment of slow waves was readily achieved in the retrograde direction over the mapped field at 3.16 counts/min. Following the change in the stimulation amplitude, the return of propagation in the antegrade direction indicated a loss of entrainment (Fig. 7), and the slow-wave frequency was observed to drop from 3.5 counts/min (entrained frequency) to 1.7 ± 0.1 counts/min (a native frequency that was lower than the baseline). The velocity of slow-wave propagation during entrainment was significantly slower than that of the subsequent native activity (6.2 ± 2.8 mm/s vs. 11.5 ± 4.7 mm/s; P < 0.001), and the amplitude of the slow-wave events was also significantly lower during entrainment compared with when the native activity returned (0.24 ± 0.08 mV vs. 0.38 ± 0.14 mV; P < 0.001). Entrainment mapping showed that a smaller area of tissue had been activated during the entrainment period, compared with the tissue area that was activated after the return of the native activity (20.6 ± 1.9 cm2 vs. 32.8 ± 2.6 cm2; P < 0.001).
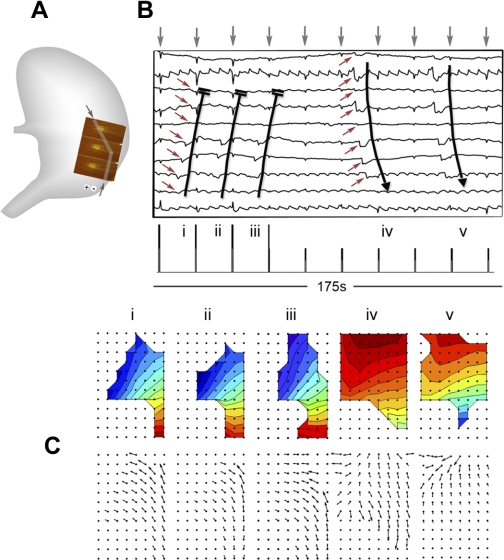
Fig. 7.
Comparison of pacing protocols at HR. A: entrainment mapping was performed with 3 PCBs (92 electrodes; area ∼47 cm2). B: pacing was performed at an antral site (period 19 s, amplitude 4 mA, pulse width 400 ms). The presented electrograms are from the representative channels indicated by the gray bars and arrows in A. Following successful entrainment of the mapped field, the amplitude was halved to 2 mA, resulting in loss of entrainment, indicated by the return of native antegrade propagation, which showed a lower frequency, faster velocity, and higher amplitude than the entrained activity. The mapped events in C correspond to the slow-wave cycles (i–v) that are marked on the electrograms. Activation and velocity field maps are shown for each cycle. The retrograde entrained activity activated less of the mapped tissue area than the native activity (20.6 ± 1.9 cm2 vs. 32.8 ± 2.6 cm2; P < 0.001).
DISCUSSION
This study introduces and validates the methodology for entrainment mapping, in which HR mapping has been adapted for evaluating gastric pacing outcomes. Multielectrode arrays, novel analytical software tools, and new terminologies have enabled this methodology, and their combined utility has been demonstrated in a series of example studies.
The example studies demonstrate that entrainment mapping has a number of potential advantages over previous sparse-electrode methods for researching gastric pacing outcomes. Most significantly, entrainment mapping enables gastric pacing outcomes to be quantified in accurate spatiotemporal detail. Unlike in sparse-electrode studies, entrainment mapping allows the propagation of the entrained activity to be mapped, the interaction between the native and entrained activities to be defined, slow-wave velocities to be accurately calculated, dysrhythmias to be accurately described, and the area and local characteristics of tissue activation to be quantified for a given stimulation protocol.
The inaccuracy of sparse-electrode configurations for calculating slow-wave velocities has previously been predicted and discussed (7, 11). This inaccuracy arises because slow-wave velocities can only be accurately quantified if velocity components are evaluated orthogonally to the wavefront (7). The orientation of the wave front must therefore be known before velocity calculations, which is less likely when using sparse-electrode configurations. The velocity vector field estimation method recently introduced by Du et al. (7) overcomes this problem by employing an algorithm that calculates the slow-wave velocity at each electrode in a manner that is dependent on a spatially averaged analysis of each propagating event. The efficacy of the velocity vector field method has been validated in this study, and its advantages were especially evident after the onset of entrainment, when sparse-electrode calculations were found to be inadequate.
This study aimed to validate a new methodology and was therefore not powered to analyze specific pacing protocol outcomes. However, a number of interesting findings from the example studies warrant further comment. In the first experiment, it was observed that the onset of entrainment did not occur until several minutes after the onset of stimulation. In a previous study by Xing et al. (24), entrainment in canines was usually induced within 1 min of beginning stimulation. A likely explanation for the delayed onset of entrainment in this study is that initially the stimulation impulses fell on the refractory tail of the native slow waves, which were occurring at a similar frequency. After a period of minutes, it is likely that the native activity and the stimulation impulses gradually desynchronized, until the impulses no longer occurred during the refractory period, allowing entrainment to began.
In the first experiment, it was also shown that entrainment of the whole tissue field aboral to the pacing electrodes (i.e., antegrade entrainment) can be achieved immediately, provided that the tissue is in an excitable state. By contrast, successfully achieving entrainment in the retrograde direction is strongly dependent on the interactions between the native and the entrained activities. In this study, it took many cycles until the retrograde activity successfully encroached on, and ultimately dominated, the area that was under the control of the native antegrade activity. Investigators should therefore carefully consider the placement of gastric pacing electrodes and the phase relationships of the native and paced events to minimize or optimize slow-wave interactions according to the physiological and clinical motives of their research. A software tool has recently been developed and validated to assist with this task (6).
In the second experiment, slow-wave conduction velocities in the corpus were found to be anisotropic, with circumferential propagation being faster than longitudinal propagation. Anisotropic gastric slow-wave conduction has previously been demonstrated in vitro (10); however, this study shows that an in vivo evaluation can be accurately performed. Anisotropic propagation in the gastric corpus has not been documented in previous HR gastric mapping studies because, under normal circumstances, the slow-wave front rapidly organizes to propagate as a transverse band in the organoaxial direction, and therefore a circumferentially propagating component is not observed (11, 13). In this study, anisotropic propagation could be calculated at HR only because a slow-wave event was evoked in the middle of the mapped field.
In the third experiment, it was shown that slow-wave dysrhythmias may occur during gastric pacing. Multiple aberrant wave fronts were found to propagate simultaneously during dysrhythmia, as previously demonstrated in a detailed study by Lammers et al. (14). It is not clear why periodic blocks to slow-wave conduction had occurred during pacing in this study or why parts of the stomach became dysrhythmic or uncoupled during these events. Future research may address these questions, which previous research tools have been unable to evaluate.
A new finding from the fourth example study was that a different area of the stomach could be active during pacing compared with during native activity. A difference in the active tissue area of canine stomachs was previously described by Sarna et al. (20) after acetylcholine administration, but it is unclear why the differences had occurred in this study. The fourth experiment also showed that different slow-wave amplitudes and velocities could occur during pacing compared with during native activity. This finding is in contrast to a previous sparse-electrode study that showed that slow-wave amplitudes and velocities are similar during pacing compared with during native activity (24). These inconsistent and poorly understood results underscore the present lack of understanding regarding the effects of gastric stimulation on slow-wave activity, and future research employing entrainment mapping will be useful to improve this understanding.
There are a number of research areas of present interest in gastric pacing where entrainment mapping offers greatest potential. It has previously been suggested that gastric pacing may revert or reduce the dysrhythmias that contribute to dysmotility in gastroparesis (15). As discussed above, the complex slow-wave events that occur during dysrhythmias, such as reentrant circuits, can only reliably be described by using HR mapping (14). Therefore, to accurately define the effects of gastric stimulation on gastric dysrhythmias, entrainment mapping should be employed. It would be particularly interesting to evaluate the effects of pacing in gastroparesis patients using this technique.
Some investigators are now applying gastric pacing via multiple coordinated electrode sites (multichannel stimulation) in an attempt to improve the efficacy and energy efficiency of gastric pacing (2). Entrainment mapping would be a valuable tool for investigating slow-wave behaviors during multichannel stimulation because it would enable an accurate and detailed analysis of the local slow-wave events surrounding each stimulus point and their subsequent interactions.
Researchers investigating gastric pacing as a treatment for obesity have previously attempted to apply stimulation at the distal antrum to entrain slow waves in the retrograde direction or disrupt normal slow-wave propagation in an attempt to inhibit gastric emptying and induce satiety and weight loss (25). The retrograde entrained activity must interfere with the native antegrade activity originating in the upper corpus. Entrainment mapping is shown here to allow an accurate quantification of the phase interactions between native and entrained activities, and the technique could therefore assist researchers interested in optimizing the de and ZOE achieved during antral stimulation for obesity (6).
In addition, entrainment mapping is already being adopted by researchers interested in the mathematical modeling of gastric stimulation outcomes (3, 6). Gastric stimulation research is experimentally demanding because there is a vast range of potential stimulation protocols available for investigation, involving different combinations of numerous variables such as pulse frequency, amplitude, width, electrode positions, and pulse on-off timing. Biophysically based modeling is being used to perform in silico trials that reduce this vast range of protocols to a substantially smaller number of potentially valuable protocols, before more targeted experimental trials (4). However, as the mathematical models become more sophisticated and resemble the physiological complexity of gastrointestinal tissues, the requirement for comprehensive experimental validation of the models also becomes more demanding to prove that the model has worthwhile predictive capabilities. The high quantitative level of spatial detail achieved by entrainment mapping makes it an ideal tool for model validation (6).
Importantly, HR entrainment mapping requires open surgical access and therefore cannot presently be employed in recovery studies on conscious animals. In future, implantable miniarrays of electrodes may enable an entrainment mapping analysis of slow-wave propagation in conscious animals and humans (23). As demonstrated in a recent study describing a new laparoscopic device for recording slow waves, useful spatial detail regarding the direction and velocity of slow-wave propagation can be determined from as few as four electrodes (18). Future intraoperative studies may also employ different electrode configurations to achieve mapping of both the anterior and posterior gastric surfaces simultaneously. The efficiency of future entrainment mapping studies is also likely to improve with the recent advent of an automated algorithm for the rapid identification of the activation times of serosal slow-wave events (8).
In summary, HR entrainment mapping offers a number of important advantages compared with traditional sparse-electrode methods for evaluating gastric pacing outcomes. Entrainment mapping is a major advance for improving the understanding of the effects of gastric electrical stimulation on slow-wave activity and will therefore be valuable in research efforts to improve the clinical efficacy of gastric pacing.
GRANTS
This work is partially supported by grants from the New Zealand Health Research Council, the NIH (R01 DK64775), the New Zealand Society of Gastroenterology, and the Auckland Medical Research Foundation.
DISCLOSURES
No author has any conflicts of interests to declare in relation to this work.
ACKNOWLEDGMENTS
We thank Linley Nisbett for technical assistance.
REFERENCES
- 1.Bortolotti M. The “electrical way” to cure gastroparesis. Am J Gastroenterol 97: 1874–1883, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Chen JD, Xu X, Zhang J, Abo M, Lin X, McCallum RW, Ross B. Efficiency and efficacy of multi-channel gastric electrical stimulation. Neurogastroenterol Motil 17: 878–882, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Cheng LK, O'Grady G, Du P, Egbuji J, Windsor JA, Pullan AJ. Gastrointestinal system. Wiley Interdiscip Rev Syst Biol Med In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du P, Li S, O'Grady G, Cheng LK, Pullan AJ, Chen JDZ. Effects of electrical stimulation on isolated rodent gastric smooth muscle cells evaluated via a joint computational simulation and experimental approach. Am J Physiol Gastrointest Liver Physiol 297: G672–G680, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du P, O'Grady G, Egbuji JU, Lammers WJ, Budgett D, Nielsen P, Windsor JA, Pullan AJ, Cheng LK. High-resolution mapping of in vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: methodology and validation. Ann Biomed Eng 37: 839–846, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du P, O'Grady G, Windso JA, Cheng LK, Pullan AJ. A tissue framework for simulating the effects of gastric electrical stimulation and in-vivo validation. IEEE Trans Biomed Eng 56: 2755–2761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du P, Qiao W, O'Grady G, Egbuji J, Lammers WJ, Cheng LK, Pullan AJ. Automated detection of gastric slow wave events and estimation of propagation velocity vector fields from serosal high-resolution mapping. Conf Proc IEEE Eng Med Biol Soc: 2527–2530, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson JC, O'Grady G, Du P, Obioha C, Qiao W, Richards WO, Bradshaw LA, Pullan AJ, Cheng LK. Falling-edge, variable threshold (FEVT) method for the automated detection of gastric slow wave events in serosal high-resolution electrical recordings. Ann Biomed Eng. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasler WL. Methods of gastric electrical stimulation and pacing: a review of their benefits and mechanisms of action in gastroparesis and obesity. Neurogastroenterol Motil 21: 229–243, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Hirst GD, Garcia-Londono AP, Edwards FR. Propagation of slow waves in the guinea-pig gastric antrum. J Physiol 571: 165–177, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huizinga JD, Lammers WJEP. Gut peristalsis is coordinated by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol 296: G1–G8, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Kelly KA, La Force RC. Pacing the canine stomach with electric stimulation. Am J Physiol 222: 588–594, 1972 [DOI] [PubMed] [Google Scholar]
- 13.Lammers WJ, Ver Donck L, Stephen B, Smets D, Schuurkes JA. Origin and propagation of the slow wave in the canine stomach: the outlines of a gastric conduction system. Am J Physiol Gastrointest Liver Physiol 296: G1200–G1210, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Lammers WJEP, Ver Donck L, Stephen B, Smets D, Schuurkes JAJ. Focal activities and re-entrant propagations as mechanisms of gastric tachyarrhythmias. Gastroenterology 135: 1601–1611, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol Gastrointest Liver Physiol 274: G186–G191, 1998 [DOI] [PubMed] [Google Scholar]
- 16.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology 114: 456–461, 1998 [DOI] [PubMed] [Google Scholar]
- 17.O'Grady G, Du P, Egbuji JU, Lammers WJ, Wahab A, Pullan AJ, Windsor JA. A novel laparoscopic device for measuring gastrointestinal slow-wave activity Surg Endosc 23: 2842–2848, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Grady G, Egbuji JU, Du P, Cheng LK, Pullan AJ, Windsor JA. High-frequency gastric electrical stimulation for the treatment of gastroparesis: a meta-analysis. World J Surg 33: 1693–1701, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarna SK, Daniel EE. Electrical stimulation of gastric electrical control activity. Am J Physiol 225: 125–131, 1973 [DOI] [PubMed] [Google Scholar]
- 20.Sarna SK, Daniel EE, Kingma YJ. Premature control potentials in the dog stomach and in the gastric computer model. Am J Physiol 222: 1518–1523, 1972 [DOI] [PubMed] [Google Scholar]
- 21.Shenasa M, Hindricks G, Borggrefe M, Breithardt G. Cardiac Mapping, 3rd edition.Oxford, UK: Blackwell, 2009 [Google Scholar]
- 22.SmoothMap. SmoothMap. Version 3.05 (Online) Wim Lammers, Mapping Laboratory, Dept. of Physiology, Al Ain, United Arab Emirates: http://www.smoothmap.org [ September 2009]. [Google Scholar]
- 23.Ver Donck L, Lammers WJ, Moreaux B, Smets D, Voeten J, Vekemans J, Schuurkes JA, Coulie B. Mapping slow waves and spikes in chronically instrumented conscious dogs: implantation techniques and recordings. Med Biol Eng Comput 44: 170–178, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Xing J, Brody F, Rosen M, Chen JD, Soffer E. The effect of gastric electrical stimulation on canine gastric slow waves. Am J Physiol Gastrointest Liver Physiol 284: G956–G962, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Yao S, Ke M, Wang Z, Xu D, Zhang Y, Chen JD. Retrograde gastric pacing reduces food intake and delays gastric emptying in humans: a potential therapy for obesity? Dig Dis Sci 50: 1569–1575, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Yin J, Chen JD. Implantable gastric electrical stimulation: ready for prime time? Gastroenterology 134: 665–667, 2008 [DOI] [PubMed] [Google Scholar]