Abstract
Colonic migrating motor complexes (CMMCs) propel fecal contents and are altered in diseased states, including slow-transit constipation. However, the mechanisms underlying the CMMCs are controversial because it has been proposed that disinhibition (turning off of inhibitory neurotransmission) or excitatory nerve activity generate the CMMC. Therefore, our aims were to reexamine the mechanisms underlying the CMMC in the colon of wild-type and neuronal nitric oxide synthase (nNOS)−/− mice. CMMCs were recorded from the isolated murine large bowel using intracellular recordings of electrical activity from circular muscle (CM) combined with tension recording. Spontaneous CMMCs occurred in both wild-type (frequency: 0.3 cycles/min) and nNOS−/− mice (frequency: 0.4 cycles/min). CMMCs consisted of a hyperpolarization, followed by fast oscillations (slow waves) with action potentials superimposed on a slow depolarization (wild-type: 14.0 ± 0.6 mV; nNOS−/−: 11.2 ± 1.5 mV). Both atropine (1 μM) and MEN 10,376 [neurokinin 2 (NK2) antagonist; 0.5 μM] added successively reduced the slow depolarization and the number of action potentials but did not abolish the fast oscillations. The further addition of RP 67580 (NK1 antagonist; 0.5 μM) blocked the fast oscillations and the CMMC. Importantly, none of the antagonists affected the resting membrane potential, suggesting that ongoing tonic inhibition of the CM was maintained. Fecal pellet propulsion, which was blocked by the NK2 or the NK1 antagonist, was slower down the longer, more constricted nNOS−/− mouse colon (wild-type: 47.9 ± 2.4 mm; nNOS−/−: 57.8 ± 1.4 mm). These observations suggest that excitatory neurotransmission enhances pacemaker activity during the CMMC. Therefore, the CMMC is likely generated by a synergistic interaction between neural and interstitial cells of Cajal networks.
Keywords: cholinergic transmission, NK2 receptors, NK1 receptors, tachykinins, neuronal nitric oxide synthase, circular muscle, longitudinal muscle, smooth muscle, interstitial cells of Cajal, slow waves
a major motor pattern involved in regulating the movement of fecal matter along the large bowel is the colonic migrating motor complex (CMMC) that consists of a rhythmically occurring sequence of electrical activity and/or contractions over varying lengths of bowel (4–6, 9, 13, 24, 25, 28, 30).
It has been shown that the large bowel is under tonic neural inhibition because TTX increases spiking activity in the muscle (4, 5, 6, 35). The tonic inhibitory drive appears to result from the release of nitric oxide and purines from inhibitory motor neurons to the circular muscle (CM) (4, 15, 18, 23, 28, 30, 31). The mechanisms underlying the CMMC are unclear. A withdrawal of this tonic inhibitory nerve drive (disinhibition) has been proposed to largely underlie the CMMC (2, 4, 18, 28, 30, 31), whereas others, using tension recordings, have suggested that, rather than disinhibition, the CMMC is generated entirely by the release of both acetylcholine and tachykinins from excitatory motor nerves (3). The differences in these two mechanisms have been suggested to stem from the use of two different mice strains or that the tachykinin antagonists do not affect the contractile responses directly but interrupt neuronal transmission underlying the CMMC (28).
It is important to determine whether tachykinins are involved in directly generating the CMMC because a reduction in neurokinin (NK)2 receptors on the muscle has been shown to be associated with inflammatory bowel disease and slow-transit constipation in both adults and children (22, 33).
In this study, we have used simultaneous intracellular microelectrode recordings of electrical activity together with tension recordings from the CM of wild-type and neuronal nitric oxide synthase (nNOS) knockout mice to determine the mechanisms underlying the CMMC, without the complicating effects of L-type calcium channel blockers to paralyze the muscle.
We show that CMMCs still occur in nNOS−/− mice, albeit at a faster frequency. Our data suggest that the major component of the CMMC is mediated by the release of acetylcholine and tachykinins that likely leads to enhanced slow-wave activity in both types of mice, while the role of nitric oxide is to maintain the colon in a hyperpolarized state and to regulate the frequency of CMMCs.
MATERIALS AND METHODS
Forty-two male C57BL/6 wild-type and 12 nNOS−/− mice (28–42 days old) were humanely killed by inhalation of anesthetic (Isofluorane; Baxter, Deerfield, IL) followed by cervical dislocation. The protocol for animal use was reviewed and approved by the animal ethics committee of the University of Nevada School of Medicine. The entire colon was removed from the terminal end of the caecum down to the end of the distal colon (13).
Preparations
Several different preparations were utilized in this study to examine the mechanisms underlying the CMMC in the murine colon and are detailed below.
Simultaneous intracellular microelectrode and tension recordings from whole colon preparations (intact mucosa).
Microelectrode impalements were made into longitudinal muscle (LM) and CM cells from the middle of the colon, as described previously (7, 8, 13). To facilitate these impalements, micropins (∼500 μm) were used to stabilize a small region (3 mm × 4 mm) at the antimesenteric border. At the same time, CM tension was recorded directly opposite the pinned site using a frog heart clip attached to the mesenteric border. The initial resting tension was set to 8 mN before impalements were made into CM.
Intracellular microelectrode recordings from preparations without the mucosa.
In other experiments, the mucosa was sharp dissected away from the entire length of colon in 4°C Krebs solution to prevent uptake of serotonin from the mucosa into descending interneurons (20, 21). To do this, the mucosa was cut in the middle at the anal or oral end and gently peeled outward along its length. This procedure removes the mucosa and submucosa without damaging the underlying CM and myenteric plexus. The colon was then pinned circumferentially with the serosa uppermost and impalements made from the CM. Platinum transmural stimulating wires (diameter 0.2 mm), which were connected to a Grass SD stimulator (Grass Medical Instruments, Quincy, MA), were placed above and below the preparation (1 mm apart and 2–4 mm oral of the recording site).
Mechanical activity of the CM during the CMMC.
The isolated large bowel was attached to the floor of the organ bath by pinning the mesentery. Suture silk was used to connect two force transducers (model TST125C; Biopac Systems, Santa Barbara, CA) at the oral and anal cut ends of the colon. Resting tension was initially set at 8 mN and monitored using an MP100 interface and recorded on a PC running Acqknowledge software 3.2.6 (Biopac Systems).
Video recording of fecal pellet movements and spatiotemporal map analysis.
Fecal pellet velocity was measured in the wild-type mice, and nNOS−/− mice monitored with a video camera (WV-BP330, Panasonic CCTV, iMac; Apple Computers, Cupertino, CA) (7, 8, 13). Video frames were converted into spatiotemporal diameter or D maps, from which the velocity could be calculated (8, 13). Each preparation was continuously perfused with oxygenated Krebs solution at 37.0 ± 0.5°C.
RT-PCR
Total RNA was isolated from either wild-type or nNOS−/− brain and colon (without the mucosa) using TRIzol reagent (Invitrogen, Carlsbad, CA) and DNase treated with 1 U/μl DNase I (Promega, Madison, WI). First-strand cDNA was amplified from 1 μg RNA using SuperScript II reverse transcriptase (Invitrogen). PCR was performed using region-specific primers for nNOS (NM_008712; S:ATCTGTCTCGCCAGCCATCAGCCA; AS: GGAGCTTTGTGCAGTTTGCCGTCG), endothelial NOS (eNOS; NM_008713; S:CCCCAGGAAATGGCCGCTTTGA; AS: AGGGGCAGCAGGAAACTCCAGGC), and inducible nitric oxide synthase (iNOS; NM_010927; S:CAAGCTGCATGTGACATCGACCCG; AS: TTGGACCACTGGATCCTGCCGATG) using AmpliTaq Gold (Applied Biosystems, Foster City, CA). All primers were designed against Mus musculus GenBank sequences. Amplification of hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1) was used as a control for cDNA integrity. Nontemplate PCR reactions served as controls for primer contamination. PCR reactions were performed in a GeneAmp 2700 thermal cycler (Applied Biosystems). The amplification profile was 95°C for 10 min, 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s, followed by a final step of 72°C for 5 min. RT-PCR amplification fragments were analyzed by size analysis on 2% agarose gels alongside a 100-base pair (bp) marker.
Analysis of Data and Statistical Methods
Microelectrode electrophysiology and tension recordings were analyzed as described previously (7, 8, 13). Data analysis of changes in electrical activity, following the addition of antagonists, was only performed on continuous impalements. Frequency, duration, and amplitude of contractile complexes were measured using Acqknowledge 3.2.6 (Biopac Systems), and tests for statistical significance were made using Sigma Plot 5.0 (Jandel Scientific, San Rafael, CA). Statistical comparisons of data were performed using Student's (paired or unpaired) t-tests, ANOVA, or Wilcoxon rank sum test, and a minimum level of significance was reached at P < 0.05. P < 0.05 confers a statistically significant effect in the text, whereas P > 0.05 is a nonstatistically significant effect. In results, n refers to the number of animals from which colons were taken. All data are presented as means ± SE.
Drugs and Solutions
Apamin, atropine, Nω-nitro-l-arginine (l-NA) and nifedipine were purchased from Sigma-Aldrich (St. Louis, MO). MEN 10,376 and RP 67580 were purchased from Tocris Bioscience (Ellisville, MO). The Krebs solution was (in mM) 120.35 NaCl, 5.9 KCl, 15.5 NaHCO3, 1.2 NaH2PO4, 1.2 MgSO4, 2.5 CaCl2, and 11.5 glucose and was gassed continuously with a mixture of 3% CO2-97% O2 (vol/vol) to give a final pH of 7.3–7.4.
RESULTS
Most previous recordings of the CMMC have been conducted in the presence of L-type calcium channel antagonists (nifedipine or nicardipine) to paralyze the smooth muscle, facilitating easier impalement into the CM (4, 18, 30, 31, 32). In this present study, we decided against using these calcium channel antagonists, because 1) slow waves are blocked following application of these drugs (36), and 2) in recordings made without these drugs, submucosal slow waves are synchronized during the CMMC (13).
Spontaneous CMMCs in Wild-type Mice
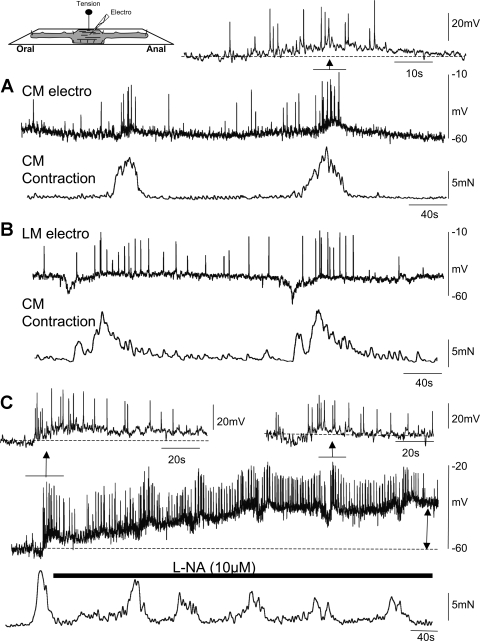
In wild-type mice, colonic CMMCs occurred in the CM at a frequency of 0.3 ± 0.1 cycles/min (n = 10). CMMCs consisted of fast electrical oscillations (frequency: 1.6 ± 0.1 Hz; n = 10) that gave rise to action potentials (21.8 ± 3.6 per complex; n = 10) superimposed on a slow depolarization (amplitude 14.0 ± 0.6 mV; duration 39.5 ± 1.7 s; n = 10; Fig. 1A). Each spontaneous CMMC was associated with a robust increase in tension of the CM (14.3 ± 1.0 mN; n = 10). In four out of ten (40%) preparations, a hyperpolarization (7.2 ± 0.4 mV; duration 8.1 ± 1.0 s; n = 10) occurred before the slow-depolarization phase of the CMMC (Fig. 1A, first CMMC).
Fig. 1.
Simultaneous electrical and tension recordings during the colonic migrating motor complex (CMMC) in wild-type colon. A: intracellular microelectrode recordings (electro) were made from circular muscle (CM) cells in the midcolon. Electrical activity, which consisted of fast electrical oscillations and action potentials (Inset) during the CMMC, was tightly coupled to contraction of the CM. B: CMMCs were recorded from longitudinal muscle (LM) cells in the midcolon that occurred at the same time as a CMMC contraction in the CM. Note the fact that the longitudinal muscle is more depolarized than the CM. C: effects of blocking nitric oxide synthesis with Nω-nitro-l-arginine (l-NA) on electrical and mechanical activity.
In several experiments, we impaled cells in the LM, which exhibited continuous action potential firing and a lower resting membrane potential (46.0 ± 1.0 mV; n = 6) than the CM (61.6 ± 2.0 mV; n = 10) (Fig. 1B). The CMMC in the LM also consisted of a brief hyperpolarization followed by increased action potential firing during the slow-depolarizing phase of the CMMC (Fig. 1B; n = 6). The CMMC occurred at the same time as a CMMC contraction in the CM, suggesting that both muscle layers were synchronously activated during the CMMC, as we have previously shown using calcium imaging of the muscle (29).
We found that antagonizing nitric oxide synthesis with l-NA (10 μM) depolarized the CM (control: 61.6 ± 2.0 mV; after l-NA: 46.2 ± 0.8 mV) and increased the firing frequency of action potentials in the muscle. l-NA did not, however, abolish the spontaneous CMMCs, which increased in frequency (0.5 ± 0.1 cycles/min; n = 3), eliciting contractions of the CM (Fig. 1C).
Spontaneously Occurring CMMCs in nNOS−/− Mice
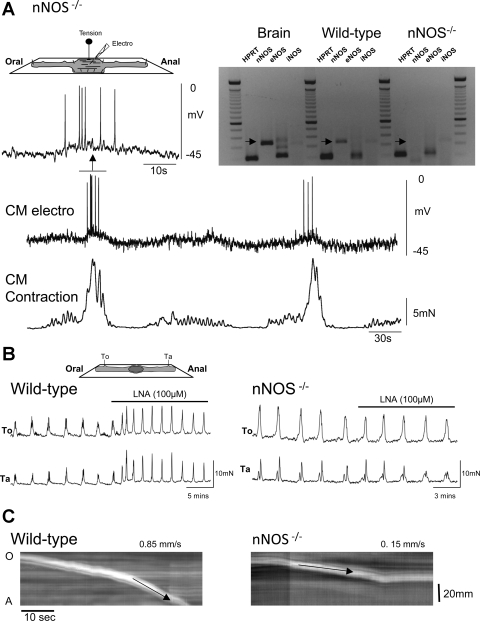
Although nNOS−/− mice had a significantly more depolarized resting membrane potential (wild-type CM: 61.6 ± 2.0 mV; nNOS−/− CM: 47.7 ± 1.8 mV; P < 0.05; n = 10), they still exhibited spontaneously occurring CMMCs (Fig. 2A). The CMMCs occurred at a rate of 0.39 ± 0.03 (n = 10), which was significantly more frequent than in the wild-type mice.
Fig. 2.
CMMCs in neuronal nitric oxide synthase (nNOS)−/− mouse colon. A: simultaneous electrical and mechanical activity of the CM. Note the underdeveloped middle complex. Inset: nNOS is expressed only in the brain and colon of wild-type mice but not in the nNOS−/− mouse. HPRT, hypoxanthine guanine phosphoribosyl transferase; eNOS, endothelial NOS; iNOS, inducible NOS. B: tension recordings at 2 sites along the colon 4 cm apart. l-NA increased the frequency of contractions in the wild-type colon but had little effect in the nNOS−/− mouse. To, oral tension transducer; Ta, anal tension transducer. C: spatiotemporal maps show that pellet propulsion was faster in the wild-type colon (left) compared with that in the nNOS−/− mouse (right).
The duration (7.4 ± 0.9 s; n = 10) of the preceding hyperpolarization was similar to that in the wild-type colon, but its amplitude (5.7 ± 0.3 mV; P < 0.05; n = 10) was significantly reduced.
The duration (46.35 ± 5.08 s), amplitude (11.2 ± 1.5 mV), and area (131.0 ± 13.7 mV/s) under the slow depolarization were significantly smaller in the nNOS−/− mouse. Although the number of action potentials (14.3 ± 1.9; n = 8) upon the fast oscillations was similar to those in the wild-type mice, their amplitude was significantly reduced (wild-type: 47.9 ± 3.29 mV; nNOS−/−: 27.6 ± 0.4 mV; P < 0.01; n = 9). Despite this reduced action potential amplitude, the associated increase in tension generated by the CMMC was similar in both mice (wild-type: 13.4 ± 1.5 mN; nNOS−/−: 11.2 ± 1.5 mV: P > 0.05; n = 8).
l-NA (10 μM) transiently increased the frequency and amplitude of contractions of CMMCs in wild-type mice but had no significant effect on nNOS−/− mice (nNOS−/− amplitude: control: 10.9 ± 0.6 mN; l-NA: 10.6 ± 0.4 mN; n = 5, Fig. 2B). RT-PCR analysis confirmed that the nNOS gene expression was absent, as was iNOS; however, some eNOS expression was observed in these mice (Fig. 2A; n = 3).
nNOS−/− mice exhibited other phenotypical differences: 1) an increased length (wild-type: 47.9 ± 2.4 mm; nNOS−/−: 57.8 ± 1.0 mm; P < 0.05; n = 8), 2) a more constricted colon, both when empty (diameter: wild-type: 1.7 ± 0.1 mm; nNOS−/−: 1.6 ± 0.1 mm; P < 0.05; n = 8) or full of fecal pellets (diameter: wild-type: 3.0 ± 0.2 mm; nNOS−/−: 2.2 ± 0.1 mm; P < 0.05; n = 8), and 3) a reduced velocity of pellet propulsion (velocity: wild-type: 0.9 ± 0.1 mm/s; nNOS−/−: 0.4 ± 0.1 mm/s; P < 0.05; n = 6; Fig. 2C).
Naturally occurring fecal pellets in these mice were of smaller diameter, usually longer, and loosely formed (data not shown). Also, the contractions recorded at two different sites along the colon usually occurred synchronously.
Effects of Cholinergic and Tachykinin Antagonists on Spontaneous CMMCs in Wild-type Mice
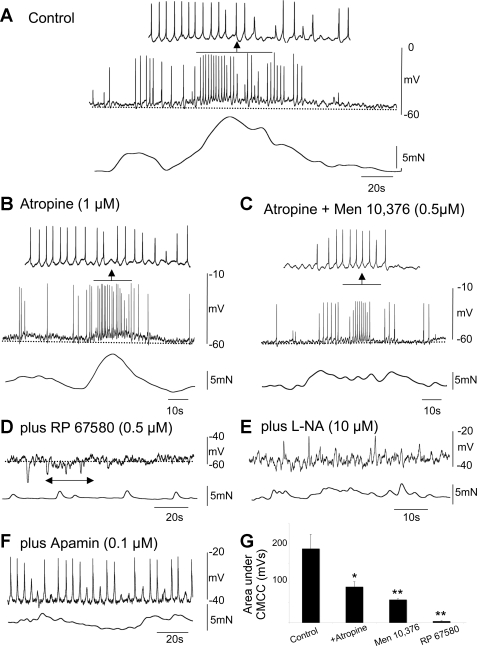
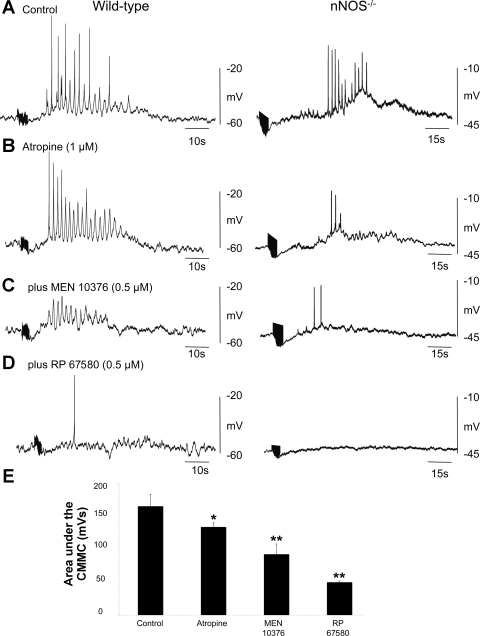
The muscarinic antagonist atropine (1 μM) significantly reduced 1) the area (control: 189.0 ± 35.7 mV/s; atropine: 90.5 ± 12.7 mV/s; P < 0.05; n = 10) under the complex, 2) the number of action potentials (control: 21.8 ± 3.6; atropine: 11.0 ± 1.6; P < 0.05; n = 10), and 3) the strength of contraction (control: 14.3 ± 1.0 mN; atropine: 107.0 ± 0.5 mN; P < 0.05; n = 10) associated with the CMMC (compare Fig. 3, A, B, and G). Atropine failed, however, to abolish the fast oscillations superimposed on top of the slow-depolarizing phase of the complex, which persisted at a frequency similar to those in control (control: 1.6 ± 0.13 Hz; atropine: 1.8 ± 0.2 Hz; P > 0.05; n = 10; Fig. 3B).
Fig. 3.
Effect of cholinergic and tachykinin antagonists on the CMMC in wild-type mice. A: control CMMC with associated contraction. B: effect of atropine on the CMMC. Note that the fast oscillations and action potentials were present; however, atropine reduced the contraction. C: atropine plus the addition of MEN 10,376 further reduced the area under the CMMC and its associated contraction although the fast oscillations and action potentials were still present. D: following the further addition of RP 67580, the fast oscillations, action potentials, and contraction were abolished. However, following the addition of the neurokinin (NK)1 antagonist, periodic bursts of inhibitory junction potentials were observed (see arrow). E: further addition of l-NA revealed fast oscillations and fast inhibitory junction potentials in the CM near the myenteric border. F: further addition of apamin revealed robust slow-wave activity. G: summary of the effects of atropine and tachykinin antagonists on the area under the slow depolarization during the CMMC (n = 10); *P < 0.05, **P < 0.01.
After atropine, the NK2 receptor antagonist MEN 10,376 (0.5 μM) caused a significant reduction in 1) the area under the complex (atropine: 90.5 ± 12.7 mV/s; atropine + MEN 10,376: 58.1 ± 2.1 mV; P < 0.05; n = 10), 2) the number of action potentials (atropine: 11.0 ± 1.6; atropine plus MEN 10,376: 6.0 ± 1.5; P < 0.05), and 3) the strength of the contractions (atropine: 8.1 ± 0.5 mN; atropine + MEN 10,376: 4.2 ± 0.6 mN) associated with the complex. MEN 10,376 had no significant affect on the frequency of the fast oscillations (atropine: 1.8 ± 0.2 Hz; atropine + MEN 10,376: 1.2 ± 0.2 Hz; P > 0.05; n = 10; Fig. 3C). Similar results were obtained if atropine and the NK2 antagonist were administered in the reverse order.
Adding the NK1 antagonist RP 67580 (0.5 μM) concurrently with atropine and MEN 10,376 completely abolished the depolarizing phase of the CMMC, along with the action potentials and the electrical oscillations that were superimposed on it. The area under the complex was also reduced (atropine + MEN 10,736: 58.1 ± 2.1 mV/s; atropine + MEN 10,736 + RP 67580: 4.1 ± 1.2 mV/s; P < 0.05; n = 10; compare Fig. 3, A–D and G). Following the addition of the NK1 antagonist, we did observe periodic bursts of inhibitory junction potentials occurring at the same frequency as the CMMC (see Fig. 3D, arrow), suggesting that this was the recurrence of the CMMC without its excitatory components.
None of the above antagonists had a significant effect on the resting membrane potential (control: 61.6 ± 2.0 mV; atropine: 59.7 ± 2.8 mV; atropine + MEN 10,736: 62.4 ± 1.6 mV; atropine + MEN 10,736 + RP 67580: 61.9 ± 2.8 mV; P > 0.05; n = 10), suggesting that the release of excitatory transmitters rather than the removal of inhibitory neural activity (disinhibition) was responsible for the generation of the CMMCs.
Following the further addition of l-NA (10 μM) and apamin (0.1 μM), which blocks small conductance calcium-activated K+ channels (1, 18, 24), we recorded robust slow waves in the CM that elicited action potentials (Fig. 3, E and F) (13).
In addition, propulsion of an artificial fecal pellet appeared to be completely blocked because the pellet remained stationary for over 2 h after the addition of either the NK1 antagonist (0.3 μM; RP 67580; n = 3) or the NK2 antagonist (0.25 μM; MEN 10,376; n = 3).
Effects of Cholinergic and Tachykinin Antagonists on Spontaneous CMMCs in nNOS−/− Mice
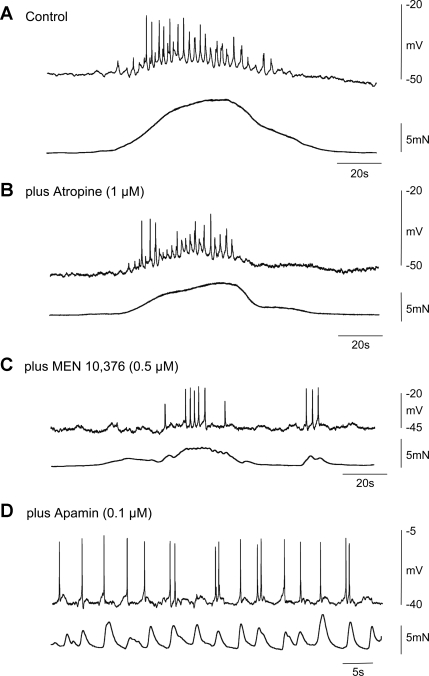
In one out of six experiments, application of atropine (1 μM) completely blocked the CMMC and its associated contraction. In the remaining experiments, atropine did not abolish the CMMC but did cause 1) a significant reduction in the amplitude of the slow depolarization (control: 11.2 ± 1.5 mV; atropine: 8.5 ± 1.1 mV; P < 0.05; n = 5), 2) a reduction in the area under the slow depolarization (control: 131.0 ± 13.7 mV; atropine: 72.4 ± 6.7 mV; P < 0.05; n = 5), 3) a reduction in the number of action potentials (control: 14.3 ± 1.9; atropine: 8.2 ± 1.5; P < 0.05; n = 5) on top of the slow depolarization, and 4) a reduction in contractile amplitude (control: 13.4 ± 1.5 mN; atropine: 7.3 ± 0.9 mN; P < 0.05; n = 5). Atropine did not affect the duration of the slow depolarization (control: 46.4 ± 5.08 s; atropine: 39.6 ± 1.8 s; P < 0.05; n = 5) or frequency of the fast oscillations (control: 1.1 ± 0.2 Hz; atropine: 1.2 ± 0.1 Hz; n = 5; compare Fig. 4, A and B).
Fig. 4.
Effect of cholinergic and tachykinin antagonists on the CMMC in nNOS−/− mice. A: control CMMC with associated contraction. Note that the muscle was more depolarized than in the wild-type mice. B: atropine reduced the amplitude of the fast oscillations and spiking activity during the CMMC and its associated contraction. C: atropine plus the addition of MEN 10,376 further reduced the area under the CMMC, the fast oscillations, and its associated contraction although the fast oscillations and action potentials were still present. D: following the further addition of apamin, slow waves with action potentials occurred, giving rise to phasic contractions of the muscle.
Following the subsequent addition of MEN 10,376 (0.5 μM), there was a further reduction in 1) the area under the slow depolarization (atropine: 72.4 ± 6.7 mV/s; MEN 10,376: 55.4 ± 7.1 mV/s; P < 0.05; n = 5), 2) the amplitude of the slow depolarization (atropine: 8.5 ± 1.1 mV; MEN 10,376: 4.2 ± 1.2 mV; P < 0.05; n = 5), and 3) the contractile amplitude (atropine: 7.3 ± 0.9 mN; MEN 10,376: 4.1 ± 1.0 mN; P < 0.05; n = 5) of the CMMC. However, the duration (atropine: 39.6 ± 1.8 s; MEN 10,376: 32.8 ± 3.1 s; P < 0.05; n = 5), frequency of the fast oscillations (atropine: 1.2 ± 0.1 cycles/min; MEN 10,376: 0.8 ± 0.3 cycles/min; P > 0.05; n = 5), and the number of action potentials (atropine: 8.2 ± 1.5; MEN 10,376: 11.3 ± 2.6) on the slow-depolarization phase of the CMMC remained unchanged. Following the further addition of apamin (0.1 μM), underlying slow-wave activity became apparent (Fig. 4D).
Preparations Without the Mucosa
We have recently shown that the generation of spontaneous CMMCs require 5-HT release from the mucosa (13). To eliminate the possibility that we might be blocking tachykinin receptors on the mucosa epithelium and in neural pathways in the myenteric plexus (14, 26), we conducted a series of experiments in preparations devoid of the mucosa (13). No spontaneous CMMCs were observed in either wild-type (n = 20) or nNOS−/− mice (n = 3) following removal of the mucosa. The lack of spontaneous CMMCs was not due to damage of the myenteric plexus because the resting membrane potential was unaltered (mucosa present: wild-type CM: 59.6 ± 3.1 mV; nNOS−/− CM: 48.7 ± 1.2 mV; mucosa absent: wild-type: 57.3 ± 3.9 mV; nNOS−/− CM: 49.5 ± 2.6 mV; P > 0.05; n = 3). CMMCs were evoked by transmural nerve stimulation (20 Hz for 1 s, 0.5-ms duration pulses) in both the wild-type and nNOS mice (Fig. 5). The effects of the cholinergic and tachykinin antagonists on the evoked CMMCs in both wild-type (Fig. 5, A–E) and nNOS−/− mice were similar to their effects on spontaneous CMMCs with the mucosa intact (compare Figs. 3–5).
Fig. 5.
Effect of cholinergic and tachykinin antagonists on the evoked CMMC in colons without the mucosa. A: transmural nerve stimulation (0.5-ms pulses at 20 Hz for 1 s, 50 V) evoked robust CMMCs in both wild-type (left) and nNOS−/− (right) mice. B–D: atropin (B), atropine plus NK2 (C), and atropine plus NK2 and NK1 (D) receptor antagonists had similar effects to these drugs on spontaneous CMMCs. E: summary of the effects of atropine and tachykinin antagonists on the area under the slow depolarization during the CMMC in wild-type mice (n = 10); *P < 0.05, **P < 0.01.
Slow Waves and the CMMC
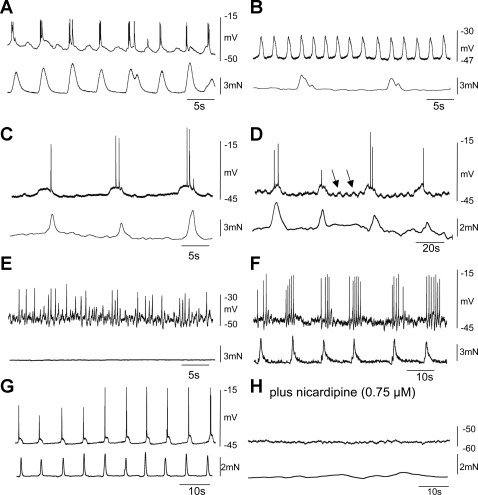
In the presence of the muscarinic and tachykinin antagonists, the addition of l-NA (10 μM) followed by apamin (0.1 μM) depolarized the CM (depolarization wild-type: l-NA: 13.3 ± 1.1 mV, apamin: 7.6 ± 1.0 mV; n = 10; depolarization nNOS−/−: l-NA: 0.5 ± 0.0 mV; apamin: 6.9 ± 0.8 mV; n = 6) and revealed three different types of slow waves in the CM and LM of wild-type and nNOS−/− mice (Fig. 6, A–F; see Ref. 36). The most common electrical rhythm was slow waves eliciting action potentials, which occurred at a frequency of 16.3 ± 2.0 cycles/min (duration: 2.3 ± 0.3 s); these were observed more readily when we penetrated CM cells nearer the submucosal border (Fig. 6, A and B). Another slow-wave rhythm also occurred in LM and in CM cells near the submucosal border; these had a frequency of 5.6 ± 1.95 cycles/min (duration 7.2 ± 1.2 s; n = 3; Fig. 6, C, D, and F). On several occasions both these slow-wave rhythms appeared to be superimposed on one another (Fig. 6D). Fast oscillations were also observed in myenteric CM cells at a frequency of 36.7 ± 3.7 cycles/min (duration 0.9 ± 0.2 s; see Fig. 6E). Nicardipine (0.75 μM; n = 5) blocked all three types of slow-wave activity (Fig. 6, G and H).
Fig. 6.
Types of slow-wave activity observed following blockade of inhibitory nerves. Following the addition of l-NA (10 μM) and apamin (0.1 μM), 3 types of electrical oscillations were observed in the CM (atropine and the tachykinin antagonists were present). A and B: slow waves, which had a frequency of ∼16 cycles/min, were recorded in CM cells near the submucosal border. When they generated action potentials, they gave rise to phasic contractions. C: A less frequent slow-wave activity (∼6 cycles/min) was also observed in deeper CM cells. D: sometimes both these activities were superimposed on one another. E: in CM near the myenteric border, we observed fast electrical oscillations (∼35 cycles/min). F: slow-wave activity recorded from the longitudinal muscle occurs at the same frequency as contractions in the CM. G and H: following nicardipine, slow-wave activity was blocked.
DISCUSSION
Previously it has been proposed that the slow depolarization underlying the CMMC is produced by the removal of inhibitory neural activity (disinhibition) (4, 6, 18, 28, 30, 31). The fast oscillations, superimposed on the slow-depolarizing phase of the CMMC, were thought to be mediated entirely by the release of acetylcholine from cholinergic motor neurons that either caused myogenic oscillations in the muscle (4, 18) or the repetitive firing of excitatory junction potentials in the muscle (32). These previous experiments were performed in the presence of L-type Ca2+ channel antagonists (4, 30–32) that block slow-wave activity (36).
We still recorded robust spontaneous CMMCs in the depolarized nNOS−/− mouse colon despite the fact that neuronal nitric oxide, a major component of inhibitory neurotransmission to the muscle, had been knocked out. This initially suggested to us that a reduction in the release of NO from inhibitory motor neurons (disinhibition) was unlikely to play a major role in generating the CMMC. Also, previous studies have shown that spontaneous CMMCs can occur in nitro-l-arginine (blocker of NO synthesis) and apamin (blocker of small-conductance calcium-activated potassium channels; opened by the inhibitory neurotransmitter ATP) (23, 31). In addition, only the preceding hyperpolarization phase of spontaneous CMMCs invades the anal end of the colon, but the fast oscillations and the slow depolarization are not observed (32). This suggests that, although descending inhibitory neural pathways are intact, the ascending excitatory nerve pathways at the cut anal end have been interrupted and are responsible for not only the generation of the fast oscillations but also for the slow depolarization.
Specifically, we show that the slow-depolarizing phase of the CMMC was gradually reduced in amplitude following the sequential addition of muscarinic and tachykinin (NK2 and NK1) antagonists in both the wild-type or nNOS−/− mice although, in one nNOS mouse preparation, atropine blocked both excitatory components of the CMMC. Usually neither atropine nor the NK2 antagonist had a significant effect on the frequency of the fast oscillations. However, in most preparations, both atropine and the NK2 antagonist reduced the number of action potentials occurring during the complex, suggesting that not all the fast oscillations were reaching threshold for the generation of an action potential. In contrast, the further addition of the NK1 antagonist blocked the remaining slow depolarization and the fast oscillations. Most importantly, in the present study, we found that the sequential addition of these antagonists produced no discernable change in resting membrane potential, suggesting that the inhibitory drive to the CM was unaffected by these drugs.
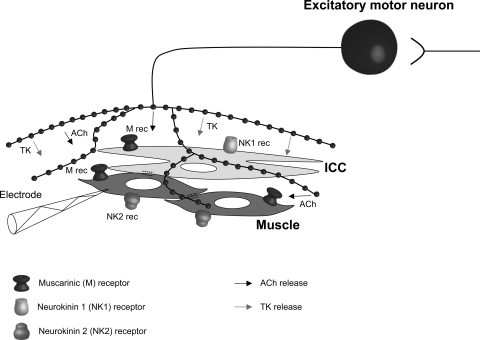
Therefore, we believe that a reevaluation of the mechanisms underlying the generation of the CMMC is warranted. Our results suggest that pacemaker activity is suppressed between CMMCs by ongoing tonic inhibition but activated by excitatory nerves during CMMCs. Presumably the repetitive firing of excitatory motor neurons, which are activated by ascending interneurons, release both acetylcholine and tachykinins onto CM cells and interstitial cells of Cajal (ICC) (see ref. 11; Fig. 7), leading to the generation of the underlying slow depolarization and the fast oscillations. This hypothesis is supported by our recent calcium imaging experiments where we have shown that myenteric ICC (ICC-MY) in the colon, which normally have low and uncoordinated activity between complexes (2, 16), suddenly coordinate their firing rates and oscillate at a similar frequency to the fast oscillations we observed in the muscle (2). The fast oscillations, which were recorded in the CM near the myenteric border, are likely to be the same events that have been referred to as myenteric potential oscillations that originate in ICC-MY and pace both the LM and CM (16, 27). Therefore, the fast oscillations in the muscle appear to result from the neural activation of pacemaker activity that spreads electronically into the muscle (27). This excitation is most likely attributable to the neural activation of ICC-MY pacemakers, mainly by ACh acting on muscarinic receptors and tachykinins acting on NK1 receptors on ICC-MY (11, 15, 16, 26, 34). Much of the slow-depolarization phase of the CMMC seems to result from the activation of muscarinic and NK2 receptors on the muscle (3, 10, 11, 19, 26, 34). Furthermore, submucosal ICC, which generate slow-wave activity (27, 36), appear to also be activated by excitatory motor neurons because slow waves increase in amplitude and frequency during the CMMC (13).
Fig. 7.
Excitatory control of smooth muscle and interstitial cells of Cajal (ICC) during the CMMC. Ascending interneurons activate excitatory motor neurons that release ACh and tachykinins (TK) to stimulate myenteric ICC and submucosal ICC. ACh and tachykinins activate muscarinic (M) receptors and NK2 receptors on muscle cells and muscarinic and NK1 receptors on ICC (adapted from Ref. 11).
In electrophysiological experiments where L-type calcium channel antagonists (which block slow waves and muscle action potentials) are used to paralyze the muscle, atropine has been shown to block the fast oscillations (4) occurring during the slow-depolarization phase of the CMMC, suggesting that they are excitatory junction potentials (EJPs) (32). In a normal physiological environment (i.e., no L-type calcium channel antagonists), it is expected that excitatory motor neurons activate both the muscle and ICC at the same time (Fig. 7; Ref 11, 16). Presumably, EJPs and much of the slow depolarization are excited by the direct stimulation of muscarinic and NK2 receptors on the muscle, respectively, whereas the fast oscillations and some of the slow depolarization are activated by acetylcholine and tachykinins acting on muscarinic and NK1 receptors on ICC. Under normal circumstances, the fast EJPs and slow depolarization in the muscle likely summate with ICC activity, leading to muscle action potentials and contraction.
We observed phenotypical changes in the nNOS−/− mouse. The colon of the nNOS−/− mouse was more depolarized and electrically similar to the wild-type mouse after l-NA. The amplitude of the slow depolarization and its associated action potentials was smaller in the nNOS−/− mouse, suggesting that this was attributable to a decreased driving force for these excitatory events associated with the CMMC. The colon was also longer and more constricted and displayed slower transit than the colon of the wild type mouse, suggesting that it may be an interesting model for constipation. Our studies with the nNOS−/− mouse suggest that the ongoing nitric oxide component of tonic inhibition is important for 1) helping to maintain the membrane potential in a hyperpolarized state, 2) regulating the frequency of CMMCs, 3) suppressing pacemaker activity between CMMCs, 4) normal stool formation, 5) normal transit, and 6) perhaps accommodation (7, 8).
We have recently shown that the CMMC initiates pellet propulsion along the murine colon (13). It appears, from this present investigation, that the CMMC is most likely generated by the release of acetylcholine and tachykinins from excitatory motor neurons that activate both myenteric and submucosal pacemaker cells. The importance of the role of tachykinins in the CMMC can be appreciated by the fact that lower doses of NK1 or NK2 receptor antagonists appear to completely block fecal pellet propulsion along the murine and guinea pig colons (10). Clinically, this study is important because it points to an essential role for tachykinins in generating the CMMC and might explain why a reduction in NK2 receptors has been observed in slow-transit constipation (22, 33). Our study also suggests that deficits in pacemaker ICC, as been observed in slow-transit constipation (12, 17), may compromise neuronal coupling to the muscle during the CMMC (16). Therefore, CMMC generation cannot be considered as just a neural event but as a synergistic interaction between neural and ICC networks.
GRANTS
This study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases: RO1 DK45713.
DISCLOSURES
There are no conflicts of interest to disclose.
REFERENCES
- 1.Albertí E, Mikkelsen HB, Wang XY, Díaz M, Larsen JO, Huizinga JD, Jiménez M. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol 292: G1499–G1510, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bayguinov PO, Hennig GW, Smith TK. Calcium imaging of myenteric neurons and ICC during local mucosal reflexes and the colonic migrating motor complex in the murine colon (Abstract). Neurogastroenterol Motil 21: 319, 2009 [Google Scholar]
- 3.Brierley SM, Nichols K, Grasby DJ, Waterman SA. Neural mechanisms underlying migrating motor complex formation in the mouse isolated colon. Br J Pharmacol 132: 507–517, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bywater RA, Small RC, Taylor GS. Neurogenic slow depolarisations and rapid oscillations in circular muscle of mouse colon. J Physiol 413: 505–519, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen J, Anuras S, Arthur C. Influence of intrinsic nerves on electromyogram of cat colon in vitro. Am J Physiol 234: E641–E647, 1978 [DOI] [PubMed] [Google Scholar]
- 6.Christensen J, Anuras S, Hauser RL. Migrating spike bursts and electrical slow waves in the cat colon: effect of sectioning. Gastroenterology 66: 240–247, 1974 [PubMed] [Google Scholar]
- 7.Dickson EJ, Hennig GW, Heredia DJ, Lee HT, Bayguinov PO, Spencer NJ, Smith TK. Polarized intrinsic neural reflexes in response to colonic elongation. J Physiol 86: 4225–4240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson EJ, Spencer NJ, Hennig GW, Bayguinov PO, Ren J, Heredia DJ, Smith TK. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology 132: 1912–1924, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dinning PG, Szczesniak M, Cook IJ. Removal of tonic nitrergic inhibition is a potent stimulus for human proximal colonic propagating sequences. Neurogastroenterol Motil 18: 37–44, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Foxx-Orenstein AE, Grider JR. Regulation of colonic propulsion by enteric excitatory and inhibitory neurotransmission. Am J Physiol Gastrointest Liver Physiol 271: G433–G437, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Furness JB. Pharmacology of transmission and sites of drug action in the enteric nervous system. In: The Enteric Nervous System Malden, MA: Blackwell, 2006, p. 103–131 [Google Scholar]
- 12.He CL, Burgart L, Wang L, Pemberton J, Young-Fadok T, Szurszewski J, Farrugia G. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology 118: 14–21, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136: 1328–1338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holzer P, Holzer-Petsche U. Tachykinin receptors in the gut: physiological and pathological implications. Curr Opin Pharmacol 1: 583–590, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Lavin ST, Southwell BR, Murphy R, Jenkinson KM, Furness JB. Activation of neurokinin 1 on interstitial cells of Cajal of the guinea-pig small intestine by substance P. Histochem Cell Biol 110: 263–271, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Lee HT, Hennig GW, Park KJ, Bayguinov PO, Ward SM, Sanders KM, Smith TK. Heterogeneities in ICC Ca2+ activity within canine large intestine. Gastroenterology 136: 2226–2236, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JI, Park H, Kamm MA, Talbot IC. Decreased density of interstitial cells of Cajal and neuronal cells in patients with slow-transit constipation and acquired megacolon. J Gastroenterol Hepatol 20: 1292–1298, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Lyster DJ, Bywater RA, Taylor GS. Neurogenic control of myoelectric complexes in the mouse isolated colon. Gastroenterology 108: 1371–1378, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Maggi CA, Giuliani S, Zagorodnyuk V. Sequential activation of the triple excitatory transmission to the circular muscle of guinea-pig colon. Neuroscience 79: 263–274, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Meedeniya ACB, Brookes SJH, Costa M. Sources of 5-hydroxytryptamine immunoreactivity in the myenteric plexus of the guinea-pig small intestine. Proc Aust Neurosci Soc 5: 176, 1994 [Google Scholar]
- 21.Meedeniya ACB, Brookes SJH, Costa M. Identification of 5- hydroxytryptamine handling neurones and their distribution in the myenteric plexus of the guinea-pig small intestine. Proc Aust Physiol Pharmacol Soc 25: 157, 1994 [Google Scholar]
- 22.Menzies JR, McKee R, Corbett AD. Differential alterations in tachykinin NK2 receptors in isolated colonic circular smooth muscle in inflammatory bowel disease and idiopathic chronic constipation. Regul Pept 15: 151–156, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Powell AK, Fida R, Bywater RA. Ongoing nicotinic and non-nicotinic inputs to inhibitory neurons in the mouse colon. Clin Exp Pharmacol Physiol 28: 792–798, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Sarna SK. Physiology and pathophysiology of colonic motor activity (1). Dig Dis Sci 36: 827–862, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Sarna SK. Cyclic motor activity; migrating motor complex. Gastroenterology 89: 894–913, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Shimizu Y, Matsuyama H, Shiina T, Takewaki T, Furness JB. Tachykinins and their functions in the gastrointestinal tract. Cell Mol Life Sci 65: 295–311, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith TK, Reed JB, Sanders KM. Interaction of two electrical pacemakers in muscularis of canine proximal colon. Am J Physiol Cell Physiol 252: C290–C299, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Spencer NJ. Control of migrating motor activity in the colon. Curr Opin Pharmacol 1: 604–610, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Spencer NJ, Bayguinov P, Hennig GW, Park KJ, Lee HT, Sanders KM, Smith TK. Activation of neural circuitry and Ca2+ waves in longitudinal and circular muscle during CMMCs and the consequences of rectal aganglionosis in mice. Am J Physiol Gastrointest Liver Physiol 292: G546–G555, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Spencer NJ, Bywater RA, Holman ME, Taylor GS. Inhibitory neurotransmission in the circular muscle layer of mouse colon. J Auton Nerv Syst 28: 10–14, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Spencer NJ, Bywater RA, Taylor GS. Disinhibition during myoelectric complexes in the mouse colon. J Auton Nerv Syst 71: 37–47, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Spencer NJ, Hennig GW, Dickson E, Smith TK. Synchronization of enteric neuronal firing during the murine colonic MMC. J Physiol 564: 829–847, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanton MP, Hengel PT, Southwell BR, Chow CW, Keck J, Hutson JM, Bornstein JC. Cholinergic transmission to colonic circular muscle of children with slow-transit constipation is unimpaired, but transmission via NK2 receptors is lacking. Neurogastroenterol Motil 15: 669–678, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Southwell BR, Furness JB. Immunohistochemical demonstration of the NK(1) tachykinin receptor on muscle and epithelia in guinea pig intestine. Gastroenterology 120: 1140–1151, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Wood JD. Electrical activity of the intestine of mice with hereditary megacolon and absence of enteric ganglion cells. Dig Dis 18: 477–487, 1973 [DOI] [PubMed] [Google Scholar]
- 36.Yoneda S, Takano H, Takaki M, Suzuki H. Properties of spontaneously active cells distributed in the submucosal layer of mouse proximal colon. J Physiol 542: 887–897, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]