Abstract
The glutamic acid derivative thalidomide is a transcriptional inhibitor of TNF-α but is also known to affect human blood vessels, which may underlie its teratogenicity. Thalidomide has been used in the treatment of refractory Crohn's disease (CD), but the therapeutic mechanism is not defined. We examined the effect of thalidomide on primary cultures of human intestinal microvascular endothelial cells (HIMEC), the relevant endothelial cell population in inflammatory bowel disease (IBD), to determine its effect on endothelial activation, leukocyte interaction, and VEGF-induced angiogenesis. HIMEC cultures were pretreated with thalidomide before activation with either TNF-α/LPS or VEGF. A low-shear-stress flow adhesion assay with either U-937 or whole blood was used to assess HIMEC activation following TNF-α/LPS, and a Wright's stain identified adherent leukocytes. Expression of cell adhesion molecules (E-selectin, intercellular adhesion molecule-1, vascular cell adhesion molecule-1) was assessed using radioimmunoassay. Effects of thalidomide on NF-κB activation, cyclooxygenase (COX)-2, and inducible nitric oxide synthase (iNOS) expression in TNF-α/LPS-activated HIMEC were determined by RT-PCR and Western blotting. Thalidomide blocked adhesion of both U-937 and whole blood leukocytes by 50% in HIMEC, inhibiting binding of all classes of leukocytes. Thalidomide also blocked NF-κB and cell adhesion molecule expression in HIMEC. In marked contrast, thalidomide did not affect either iNOS or COX-2 expression, two key molecules that play a role in the downregulation of HIMEC activation. VEGF-induced HIMEC transmigration, growth, proliferation, tube formation, and Akt phosphorylation were significantly inhibited by thalidomide. In summary, thalidomide exerted a potent effect on HIMEC growth and activation, suggesting that it may also function via an endothelial mechanism in the treatment of CD.
Keywords: angiogenesis, inflammatory bowel disease, inflammation
thalidomide (N-phthalimidoglutarimide) (2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3 (2H)-dione) is a potent teratogen (7), which has more recently demonstrated anti-inflammatory and antiangiogenic activity with new clinical applications in oncology and the treatment of chronic inflammation, including Crohn's disease (CD). The immunomodulatory activity of thalidomide includes inhibiting the production of TNF-α, IL-1β, IL-6, IL-12, and granulocyte macrophage colony-stimulating factor. In primary human T cells, thalidomide induces an IL-2-mediated proliferation and increases IFN-γ production (2, 19). Thalidomide inhibits NF-κB, a critical regulator of inflammatory processes (22). Moreover, thalidomide inhibits IL-1β-induced IL-8 production, nuclear translocation of NF-κB, degradation of Iκ-Bα (21), and LPS-induced COX-2 (16). In addition to effects on classic immune cell populations, thalidomide also suppresses the induction of cell surface adhesion molecules in human umbilical vein endothelial cells (HUVEC) (17). Despite these findings, the mechanism of the effect of thalidomide on tissue-specific microvascular endothelial populations, specifically the gut microvascular endothelium, during inflammatory and angiogenic activation remains undefined.
Microvascular endothelial cells are a critical cell population in inflammation, wound healing, and the inflammatory response. Angiogenesis, the growth of new microvessels, plays a key physiological role in wound healing while also playing a pathophysiological role in diabetic retinopathy, atherosclerosis, tumor growth, and chronic inflammation (14). Recent investigation has demonstrated that angiogenesis is a common feature in inflammatory bowel disease (IBD) (8). Thus investigation of angiogenic mechanisms in IBD is a promising area for defining the molecular and cellular basis of disease pathophysiology and devising novel forms of therapy that target angiogenesis.
Angiogenesis is regulated through a balance of positive and negative regulatory mediators. VEGF is one of the most important positive angiogenic factors (3, 41). VEGF plays an essential role in endothelial proliferation and angiogenesis during embryogenesis as well as the menstrual cycle, pregnancy, and wound healing (5, 18). Enhanced expression of VEGF may contribute to pathological mechanisms in chronic inflammation (i.e., rheumatoid arthritis, psoriasis, and IBD), diabetic retinopathy, and adenocarcinoma (13). The importance of angiogenesis in the disease processes has been demonstrated by the success of antiangiogenic therapeutic trials, which are approved for the treatment of advanced colorectal adenocarcinoma (33). VEGF plays a key role in tumor neovascularization (12, 32, 39). In the setting of chronic inflammation, antiangiogenic therapy has shown beneficial effects in animal models of IBD (CD, ulcerative colitis) (8), as well as open-label trials of the compound thalidomide in refractory CD.
The ability of endothelial cells to migrate and form capillary-like structures is critical in growth factor-induced angiogenesis. Furthermore, this process is dependent on signaling via phosphatidylinositol 3-kinase (PI3K)-Akt-dependent pathways (6, 26). We therefore set out to determine the antiangiogenic effect of thalidomide on HIMEC in response to VEGF and investigate its potential inhibitory effect on Akt activation. PI3K/Akt is the most targeted pathway in human cancers, as its activation leads to cell proliferation and cell survival via mechanisms that are presently not well defined (38). Akt is an important molecular junction in intracellular cell signaling because multiple growth factor-driven signaling pathways converge through this molecule (28).
In this study, we assess the in vitro effect of thalidomide on human intestinal microvascular endothelial cell (HIMEC) activation by TNF-α/LPS and apoptosis induced by serum deprivation and also investigated the effect of thalidomide on HIMEC angiogenesis mediated by VEGF. Our data demonstrate that thalidomide inhibited HIMEC leukocyte adhesion and intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin expression in TNF-α/LPS-activated HIMEC. Thalidomide also inhibited NF-κB activation but had no effect on either inducible nitric oxide synthase (iNOS) or cyclooxygenase (COX)-2 expression in activated HIMEC. The antiangiogenic effect of thalidomide on VEGF-induced HIMEC activation was evident by its inhibitory affect on cell migration, growth, proliferation, in vitro capillary tube formation, and Akt phosphorylation.
MATERIALS AND METHODS
Antibodies and reagents.
Thalidomide was a generous gift from Celgene (San Diego, CA). VEGF and antibodies to VEGF receptor 2, ICAM-1, VCAM, and E-selectin were from R&D Systems (Minneapolis, MN). Antibodies to iNOS and COX-2 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The selective COX-2 inhibitor NS398 was obtained from Cayman Chemical (Ann Arbor, MI). Antibodies against phosphorylated and nonphosphorylated Akt and NF-κB p65 subunit were from Cell Signaling Technology (Danvers, MA). LY294002 (PI3K/Akt) and SN-50 (NF-κB) inhibitors were obtained from BioMol (Plymouth Meeting, PA). Fluorescein-conjugated phalloidin was from Molecular Probes (Eugene, OR). Fluorescein-conjugated streptavidin was from Pierce (Rockford, IL). Oligonucleotide and primers were from Operon (Alameda, CA) and Integrated DNA Technologies (Coralville, IA). Immun-Star and all other electrophoresis reagents were from Bio-Rad (Hercules, CA). Endothelial cell growth supplement (ECGS) was from Upstate Biotech (Lake Placid, NY). MCDB-131 medium, porcine heparin, penicillin/streptomycin/fungizone, and soybean trypsin inhibitor were from Sigma (St. Louis, MO). FBS was from Bio Whittaker (Walkersville, MD). Collagenase type II was from Worthington Biochemical (Lakewood, NJ), and BSA (Fraction V) was obtained from Fisher Scientific (Fair Lawn, NJ). Human plasma fibronectin was from Chemicon International (Temecula, CA). Unless otherwise indicated, all other chemicals used in this study were purchased from Sigma-Aldrich.
Isolation and culture of HIMEC.
The use of human tissues and all experiments were approved by the Institutional Review Board of the Medical College of Wisconsin. HIMECs were isolated from surgical specimens and maintained as described earlier (4). HIMEC cultures, modified lipoprotein (Dil-ac-LDL) uptake (Biomedical Technology, Stoughton, MA), and expression of Factor VIII-associated antigen were recognized microscopically. All experiments were carried out using passages 8–12 of HIMEC.
Leukocytes.
U-937 cells (monocyte-like) were obtained from American Type Culture Collection (Rockville, MD) and maintained in RPMI 1640 medium with 10% heat-inactivated FBS.
Assessment of CAM surface expression.
CAM surface expression assays were performed as previously described (4, 34). Briefly, HIMEC with or with out thalidomide (1–10 μg/ml) were stimulated with TNF-α (100 U/ml) and LPS (1 μg/ml), for 24 h. E-selectin, ICAM-1, and VCAM-1 surface expressions were detected with 125I streptavidin (80 μCi/ml). The cells were lysed with 1.0% Triton X-100 and quantified in a γ-counter. Data from triplicate wells were expressed as the means of 125I streptavidin bound (cpm/well). Control experiments using a nonspecific monoclonal antibody were performed in parallel at equal concentrations and incubation conditions.
Endothelial-leukocyte adhesion assay.
Adhesion assays were performed as previously described (4, 34). Briefly, endothelial cells were seeded onto fibronectin-coated 24-well tissue culture plates (Corning Costar, Corning, NY) at 0.5 × 105 cells/well using HIMEC medium supplemented with endothelial cell growth factor, and allowed to grow to confluence over 48–72 h. Endothelial cells with or without thalidomide (1–10 μg/ml) were stimulated with a combination of TNF-α (100 U/ml) and LPS (1 μg/ml). After 24–48 h, monolayers were rinsed, and 1 × 106 leukocytes in 1 ml were cocultured on endothelial monolayers and allowed to adhere at 37°C in a 5% CO2 incubator. Following a 1-h incubation, nonadherent cells were removed, and residual cells were rinsed three times with Dulbecco's PBS (Gibco, Grand Island, NY) followed by gentle shaking of the tissue culture plate. Monolayers were fixed and stained using a modified Wright's stain (Diff-Quik Stain; Baxter Scientific, McGraw, IL), and adherent leukocytes were counted in 10 high-power fields (×20) using an ocular grid. Adhesion was expressed as number of adherent leukocytes/mm2.
Endothelial-leukocyte low-shear-stress flow adhesion assay.
Adhesion assays were performed as previously described (4, 34). Briefly, endothelial cells were seeded onto 35-mm fibronectin-coated tissue culture dishes (Corning Costar) at 1 × 105 cells/dish using HIMEC medium and were allowed to grow to confluence over 48–72 h. Endothelial cells were analyzed with or without thalidomide (1–10 μg/ml) directly or following stimulation with a combination of TNF-α (100 U/ml) and LPS (1 μg/ml). After 24 h, monolayers were rinsed and assayed in a low-shear-stress flow chamber (23, 43). Leukocytes (1 × 106 per ml) flowing at a rate of 1 dyne/cm2 were cocultured over the endothelial monolayers and allowed to adhere at 37°C, and adhesion was recorded using a CCD camera attached to an inverted tissue culture microscope. Data were analyzed by counting the number of adherent leukocytes over 10 random high-power fields using an ocular grid, and adhesion was expressed as number of adherent leukocytes/mm2.
Assay of transcription factor NF-κB.
With the use of TransAM NF-κB and Nuclear Protein Extraction Kits, NF-κB activity was measured according to the manufacturer's protocol (Active Motif, Carlsbad, CA). The samples were analyzed in a 96-well plate containing the immobilized NF-κB consensus site (5′-GGG ACT TTCC-3′) oligonucleotide. The activated form of NF-κB in nuclear extract binds to this oligonucleotide. Using an antibody against NF-κB p65 subunit and a horseradish peroxidase-conjugated secondary antibody results in a colorimetric readout, which was quantified at 450 nm using a Beckman DU-650 spectrophotometer. Data from triplicate wells were expressed as means ± SD.
Immunofluorescence staining.
Nuclear translocation of NF-κB p65 subunit was determined using a FITC-conjugated secondary antibody as described previously (31).
Western blot analysis.
SDS-PAGE and Western blot analysis were performed using antibodies to iNOS, COX-2, and Akt as described previously (34).
Semi-quantitative RT-PCR for iNOS.
iNOS gene expression was assessed in HIMEC with or without thalidomide pretreatment using iNOS: F 5′-TCT TGG TCA AAG CTG TGC TC-3′ and R 5′-CAT TGC CAA ACG TAC TGG TC -3′ (237 bp) as previously described (34). β-actin primers were included in the reaction as an internal control.
Real-time PCR for COX-2.
RNA was then isolated using Qiagen's RNeasy Plus Mini Kit according to manufacturer's instructions. Reverse transcription was done with 1 μg of RNA using Bio-Rad's iScript cDNA synthesis kit. COX-2 gene expression was analyzed via real-time PCR using Bio-Rad's Sybr Green Master Mix, 2 μl of cDNA, and 250 nM primer in 25-μl reactions. Cycling parameters were 95°C for 3 min, then 45 cycles of 95°C for 10 s and 60°C for 30 s. Generation of a single product was confirmed with a melt cycle. Real-time data were analyzed using Bio-Rad's iQ5 software. Primer sequences were as follows: β-actin, F 5′-CTG GAA CGG TGA AGG TGA CA-3′ and R 5′- AAG GGA CTT CCT GTA ACA ATG CA-3′; COX-2, F 5′- CAG CAC TTC ACG CAT CAG TT-3′ and R 5′- TCT GGT CAA TGG AAG CCT GT-3′; and GAPDH, F 5′- TGC ACC ACC AAC TGC TTA GC-3′ and R 5′- GGC ATG GAC TGT GGT CAT GAG-3′. All primers were obtained from SA Biosciences (Frederick, MD).
Endothelial cell chemotaxis assay.
Chemotaxis assay was carried out as described earlier (20). Briefly, HIMEC (3 × 104 cells/cm2) were cultured on fibronectin-coated polycarbonate filters (8 μm pore size; BD Biosciences, Bedford, MA). After incubation in media containing 2% FBS overnight, HIMEC were incubated with thalidomide (.01–10.0 μg/ml) for 2 h, and buffers containing VEGF (50 ng/ml) were filled into the lower compartment of the 12-well plates. After overnight incubation, cell culture inserts were removed, and the upper surface of the membrane was gently wiped to remove nonmigrated cells. Filters were stained with DiffQuik (Baxter Scientific), air-dried, and mounted onto glass slides. Migrated HIMEC adherent to the lower side of the membrane were counted [at least 15 random high-power fields (×200) per condition], and data were expressed as a means ± SD. Each condition was assessed in triplicate.
Cell proliferation assay.
HIMEC (3 × 104 per well) were seeded onto fibronectin-coated 24-well plates using growth medium without ECGS as described earlier (20). After pretreatment with the thalidomide (1–10 μg) for 60 min at 37°C, cells were stimulated with VEGF (50 ng/ml) for 24, 48, and 72 h, or left untreated. Following complete detachment, cells were resuspended and counted in a Coulter Counter (Coulter, Brea, CA). In parallel experiments, cell viability was assessed by trypan blue exclusion and was greater than 95%. Each condition was assessed in triplicate.
Cellular DNA synthesis was assessed by [3H]thymidine uptake in HIMEC as described earlier (20). HIMEC were pulsed with [3H]thymidine (1 μCi/ml; Amersham, Arlington Heights, IL) and washed twice on ice with 5% (vol/vol) trichloroacetic acid before fixation. DNA was then released from precipitated material by alkaline lysis in 0.5 N NaOH, and supernatants were quantified in a β-counter. Each condition was assessed in triplicate.
Microscopic wounding assay.
To assess HIMEC migration in response to angiogenic stimuli, a microscopic wounding assay was performed as described earlier (10). In brief, a HIMEC-confluent monolayer was scraped along a straight line, and the remaining monolayer was then incubated with growth medium (without ECGS); cells were then pretreated for 30 min at 37°C with or without thalidomide (1–10 μg). Then, cells were stimulated by addition of VEGF or left untreated. The migration of HIMEC across the demarcation line was monitored using an inverted microscope. At each time point (24, 48, and 72 h), 10 random fields using an ocular grid were counted in a blinded fashion. Data were expressed as cells/mm2, and each condition was assessed in triplicate.
Matrigel in vitro tube formation assay.
Endothelial tube formation was assessed using Matrigel, a solubilized extracellular basement membrane matrix extracted from the Engelbreth-Holm-Swarm mouse sarcoma, as described previously (36). HIMEC were suspended in complete growth medium and were seeded at a density of 5 × 104 cells per well with or without thalidomide. Endothelial tube formation on Matrigel after 16 h was assessed by inverted phase contrast microscopy and photographed with an inverted tissue culture microscope. Five high-power fields per condition were examined, and experiments were repeated in two independent HIMEC cultures.
Statistical analysis.
Statistical analysis was performed by ANOVA using StatView for Macintosh (version 4.51; Abacus Concepts, Berkeley, CA). P ≤ 0.05 was considered significant, and data shown are means ± SD.
RESULTS
Thalidomide suppresses CAM expression in TNF-α/LPS-activated HIMEC.
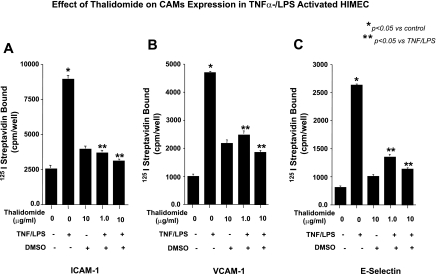
The effect of thalidomide on CAM expression in unstimulated and TNF-α/LPS-activated HIMEC was assessed by radioimmunoassay as described under materials and methods. Resting HIMEC expressed undetectable levels of E-selectin and VCAM-1 and low levels of ICAM-1. TNF-α/LPS activation of HIMEC dramatically increased the expression of all three adhesion molecules, and treatment of HIMEC with thalidomide before TNF-α/LPS activation significantly decreased the level of CAM expression Fig. 1, A, B and C. These findings suggested that anti-inflammatory effect of thalidomide at least in part is through the modulation of CAM surface expression and leukocyte binding in activated HIMEC. Similar results were obtained from fluorescence-activated cell sorting analysis (data not shown).
Fig. 1.
Effect of thalidomide on cell adhesion molecule (CAM) expression in human intestinal microvascular endothelial cells (HIMEC) following TNF-α/LPS activation. A: cell surface immunodetection of CAM with radioimmunoassay demonstrates that TNF-α/LPS activation significantly increased intercellular adhesion molecule (ICAM)-1 surface expression in HIMEC. Thalidomide pretreatment of HIMEC inhibited ICAM-1 expression dose dependently. B: in contrast to the effect on ICAM-1 expression, thalidomide increased vascular cell adhesion molecule (VCAM)-1 surface expression in HIMEC but again appeared to inhibit activation in response to TNF/LPS. C: thalidomide inhibited E-selectin expression in TNF/LPS-activated HIMEC in pattern similar to its effect on ICAM-1. Thalidomide alone slightly increased (but not significantly) CAM expression, which was not beyond the effect of vehicle (DMSO) alone. Thalidomide + DMSO refers to the DMSO used as a vehicle for thalidomide. Data are expressed as means ± SD from triplicate experiments.
Thalidomide suppresses leukocyte adhesion in TNF-α/LPS-activated HIMEC.
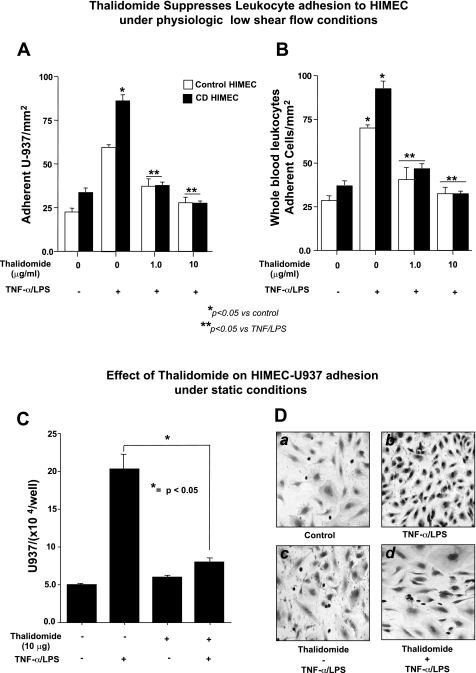
Next we performed a low-shear-stress flow adhesion assay. Monolayers of HIMEC can be used to assess endothelial function in vitro, including their ability to undergo activation and binding of leukocytes through specific interaction of cell adhesion molecules. U-937 cells were used as a target leukocyte population for these assays, as this established monocyte-like cell line is known to express the specific cell adhesion molecules α(4)β(1) integrin and sialyl Lewis X, which mediate binding to their endothelial ligands VCAM-1 and E-selectin, expressed on activated HIMEC. As shown in Fig. 2A, unstimulated HIMEC bound low levels of U-937; in marked contrast, the TNF-α/LPS-activated HIMEC demonstrated a dramatic enhancement in U-937 binding. Thalidomide pretreatment of HIMECs inhibited the leukocyte adhesion to TNF-α/LPS-activated HIMEC. Similar results were obtained with whole blood (Fig. 2B). Quantification of enhanced leukocyte binding by TNF-α/LPS using in vitro static assay demonstrated a significant increase, which was decreased by thalidomide (Fig. 2C). In vitro static assay also demonstrated that unstimulated HIMEC bound low levels of U-937 monocyte-like cells (Fig. 2, D, a) that readily increased with overnight TNF-α/LPS activation by a static endothelial-leukocyte adhesion assay (Fig. 2, D, b). Pretreatment of HIMEC with thalidomide did not affect the adhesion of U-937 leukocytes to unstimulated HIMEC (Fig. 2, D, c). In marked contrast, thalidomide pretreatment of the TNF-α/LPS-activated HIMEC demonstrated a dramatic decrease in U-937 binding (Fig. 2, D, d). These findings suggested that anti-inflammatory effect of thalidomide at least in part is through the modulation of CAM surface expression and leukocyte binding in activated HIMEC.
Fig. 2.
Effect of thalidomide on HIMEC-leukocyte binding under low-shear-stress flow adhesion assay. A: unstimulated control and Crohn's disease (CD) HIMEC bound low levels of U-937 at low-shear-stress physiological flow (1 dyne cm2), which increased significantly following TNF-α/LPS activation. Thalidomide pretreatment of HIMECs inhibited leukocyte adhesion to TNF-α/LPS-activated HIMEC, with a 10 μg/ml dose returning leukocyte binding to baseline levels. B: similar results were obtained with leukocyte binding under physiological low shear stress to control and CD HIMEC from heparinized whole blood. Thalidomide inhibited the effect of TNF/LPS activation of both the control and CD endothelial cells with a 10 μg/ml dose returning levels of adhesion to baseline. Data are expressed as means ± SD from triplicate experiments. C: static adhesion assay was performed, expressing quantification of static adhesion of U-937 cells to HIMEC monolayer in the absence and presence of thalidomide (10 μg/ml). Data from triplicate wells were expressed as the average number of adherent U-937 cells/well ± SD. N = 3 total experiments for each condition; *significant difference between the thalidomide treated and not treated cells (P < 0.05). D: modified Wright's stain of unstimulated HIMEC monolayer after 1 h cocultured with U-937 monocyte-like cells. In a high-power (×200), bright field microscopic view, firmly adherent U-937 nuclei stain dark purple, whereas HIMEC nuclei stain light purple. HIMEC were seeded at 5 × 105 cells per ml and 24 h later were exposed to 1 × 106 U-937 cells for 1 h (a). HIMEC monolayer was activated with TNF-α/LPS and 24 h later were exposed to 1 × 106 U-937 cells for 1 h (b). HIMEC monolayers were treated with thalidomide (10 μg) for 1 h with or without TNF-α/LPS (as above) for 24 h before the 1-h U-937 coculture (c and d).
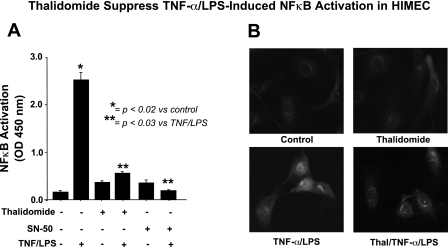
Thalidomide suppresses NF-κB activation in TNF-α/LPS-activated HIMEC.
Previous works from our group have demonstrated the involvement of NF-κB in CAM expression in HIMEC following TNF-α/LPS activation (31). In the present study, we have demonstrated that thalidomide pretreatment of HIMEC before TNF-α/LPS activation completely inhibited NF-κB-DNA binding, similar to the specific inhibitor of NF-κB (SN-50) as determined by a cell-based ELISA-NF-κB assay (Fig. 3A). Moreover, immunofluorescence staining of TNF-α/LPS-activated HIMEC showed nuclear translocation of NF-κB subunit p65 into the nucleus, which was effectively blocked with thalidomide pretreatment (Fig. 3B).
Fig. 3.
Effect of thalidomide on NF-κB in TNF-α/LPS-activated HIMEC. A: NF-κB activation in HIMEC with TNF-α/LPS was determined using a DNA-binding ELISA-based assay. NF-κB-DNA binding activity elevated rapidly following exposure to TNF-α/LPS, and this was significantly inhibited by thalidomide pretreatment before HIMEC activation. The effect of thalidomide 10 μg/ml was similar to the NF-κB inhibitor SN-50. B: immunofluorescence staining demonstrates that TNF-α/LPS activation of HIMEC resulted in NF-κB p65 subunit nuclear translocation, which was inhibited by thalidomide (Thal) pretreatment (10 μg/ml).
Together these results suggest that thalidomide is an effective anti-inflammatory agent in suppressing TNF-α/LPS-induced HIMEC activation.
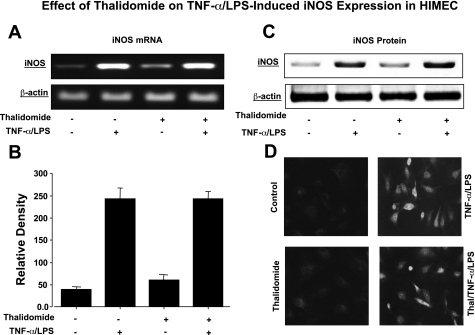
Effect of thalidomide on TNF-α/LPS-induced iNOS expression in HIMEC.
We also examined the effect of thalidomide on iNOS gene and protein expression. As determined by semiquantitative PCR, activation of HIMEC with TNF-α/LPS resulted in increased expression of iNOS mRNA (Fig. 4, A and B). Corresponding with the gene expression, TNF-α/LPS increased the level iNOS protein in HIMEC as determined by Western blotting (Fig. 4C). Pretreatment of HIMEC with thalidomide did not inhibit iNOS gene and protein expression. Immunofluorescence staining of TNF-α/LPS-activated HIMEC showed the cytoplasmic staining for iNOS with no effect from thalidomide beyond the control cells (Fig. 4D).
Fig. 4.
Effect of thalidomide on TNF-α/LPS-induced inducible nitric oxide synthase (iNOS) expression in HIMEC. A: semi-quantitative reverse transcriptase-PCR was used to detect iNOS mRNA expression in HIMEC following activation with TNF-α/LPS for 4 h. HIMEC expressed low levels of iNOS gene product at baseline, which increased following TNF-α/LPS activation. Pretreatment of HIMEC with thalidomide did not alter expression of iNOS mRNA in the TNF-α/LPS-activated HIMEC. B: using ImageJ software, densitometry of iNOS mRNA scanned image was performed. C: effect of thalidomide on expression of iNOS protein in TNF-α/LPS-activated HIMEC was investigated. Similar to the data in A regarding iNOS gene expression, thalidomide did not inhibit expression of iNOS protein in TNF-α/LPS-activated HIMEC. D: immunofluorescence staining of TNF-α/LPS-activated HIMEC demonstrates cytoplasmic staining for iNOS and no inhibitory effect from thalidomide pretreatment. Images are representative of 3 individual experiments.
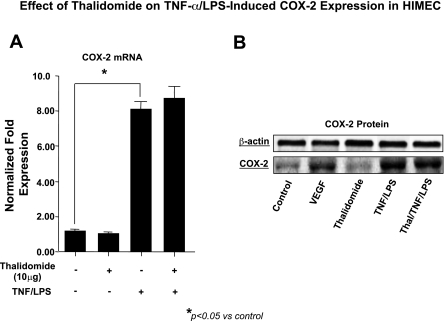
Effect of thalidomide on TNF-α/LPS-induced COX-2 expression in HIMEC.
Next we examined the effect of thalidomide on COX-2 expression. Using real-time PCR we have shown that activation of HIMEC by TNF-α/LPS significantly increased COX-2 mRNA expression, and pretreatment of HIMEC with 10 μg/ml of thalidomide had no inhibitory effect on COX-2 gene expression (Fig. 5A). Corresponding with the effect on COX-2 mRNA expression, COX-2 protein was increased by TNF-α/LPS activation of HIMEC, and, similar to mRNA, thalidomide had no affect on COX-2 protein a (Fig. 5B).
Fig. 5.
Effect of thalidomide on TNF-α/LPS-induced cyclooxygenase (COX)-2 expression in HIMEC. A: real-time PCR shows that activation of HIMEC by TNF-α/LPS significantly increased the expression of COX-2 mRNA, and pretreatment with thalidomide (10 μg/ml) had no inhibitory effect on COX-2 gene expression. B: HIMEC expressed increased levels of COX-2 protein following stimulation with VEGF and TNF-α/LPS. Thalidomide pretreatment of HIMEC had no effect on the expression following TNF-α/LPS activation.
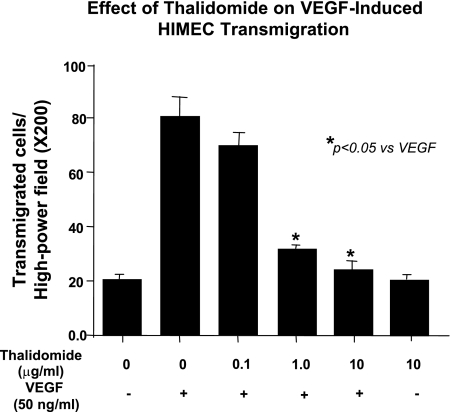
Effect of thalidomide on VEGF-induced HIMEC transmigration.
Endothelial transmigration assay was performed using polycarbonate filters. HIMEC, which transmigrated through the filters to the lower side of the membrane, were counted [at least 15 random high-power fields (×200) per condition], and data were expressed as means ± SD. The number of transmigrated HIMEC was increased by VEGF stimulation, which was significantly inhibited by pretreatment with thalidomide (Fig. 6; representative result from a total of 3 independent experiments; *P < 0.05 compared with VEGF-stimulated HIMEC).
Fig. 6.
Effect of thalidomide on HIMEC transmigration. Angiogenesis can be modeled in vitro by assessing endothelial transmigration across polycarbonate filters. VEGF activation significantly increased the number of transmigrating HIMEC (4-fold increase in transmigrated cells). Thalidomide pretreatment of HIMEC significantly inhibited HIMEC transmigration in a dose-dependent manner. HIMEC, which transmigrated to the lower side of the membrane filter, were quantified [15 random high-power fields (×200) per condition], and data were expressed as a means from triplicate experiments ± SD.
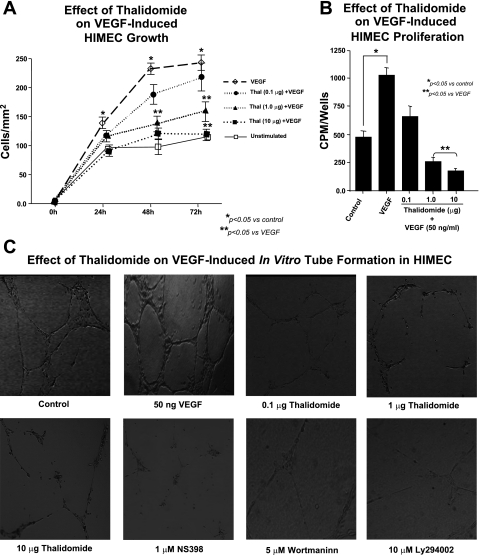
Effect of thalidomide on VEGF-induced growth, proliferation, and tube formation in HIMEC.
To further characterize the antiangiogenic activity of thalidomide in HIMEC, we treated the cells with thalidomide (0.1–10.0 μg/ml) for 30 min before VEGF (50 ng/ml) stimulation. VEGF stimulation of HIMEC increased cell growth significantly, and thalidomide pretreatment inhibited the cell growth (Fig. 7A).
Fig. 7.
Effect of thalidomide on VEGF-induced growth, proliferation, and capillary tube formation in HIMEC. A: potent angiogenic effect of VEGF on HIMEC was quantified by measuring endothelial growth across a leading edge in vitro. Thalidomide pretreatment inhibited HIMEC growth in a dose-dependent manner, lowering overall rates of growth to rates similar to control cells. B: proliferation was assessed by measuring cellular DNA synthesis was assessed by measuring [3H]thymidine uptake in HIMEC activated by VEGF. [3H]thymidine uptake was significantly increased after VEGF stimulation for 15 h, and proliferation was inhibited by thalidomide pretreatment in a dose-dependent manner, ultimately decreasing rates to basal levels. Assays were done in triplicate experiments, and the data are shown as mean cpm ± SD. *P < 0.05 compared with VEGF-stimulated HIMEC cultures. C: third assay to measure angiogenic activity in VEGF-activated HIMEC was tube formation in Matrigel plates. Phase-contrast photomicrographs demonstrate the capacity of HIMEC to form capillary-like tubes on Matrigel. VEGF increased tube formation in HIMEC, and this was inhibited by thalidomide in a dose-dependent fashion, which was similar to the effect of the selective COX-2 antagonist NS-398 and PI3K/Akt inhibitors (wortmannin and LY294002), which completely inhibited formation of capillary-like structures (×40).
Proliferation was determined by measuring both [3H]thymidine uptake and cell number. [3H]thymidine uptake was significantly increased after VEGF stimulation, and VEGF stimulation for 18 h increased the number of cells. Although serum-starved HIMECs increased their DNA synthesis in response to VEGF, treatment with thalidomide abrogated these proliferative effects, decreasing endothelial cell survival dose dependently (Fig. 7B).
Angiogenic ability of HIMECs to spontaneously form branching and thick anastomosing capillaries in vitro, when seeded on a Matrigel surface, was completely abrogated by 10 μg thalidomide. As shown in Fig. 7C, exposure of HIMEC to 50 ng/ml of VEGF increased the numbers of their tube multicentric junctions, which gave rise to a more closely knit network of capillary-like structures. Thalidomide blocked VEGF-promoted angiogenesis, as evidenced by isolated cell clumps with few sprouting capillaries.
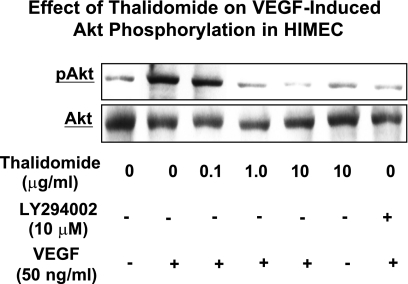
Effect of thalidomide on VEGF-induced Akt phosphorylation in HIMEC.
Given the important role of the PI3K/Akt pathway in endothelial cell survival and proliferation, we assessed the effect of thalidomide on Akt phosphorylation in HIMEC. Previously, using Western blot and phosphorylated Akt (pAkt) antibody, we have shown that VEGF (50 ng/ml) induced increased Akt phosphorylation in HIMEC as early as 15 min. Pretreatment of HIMEC with thalidomide dose dependently resulted in significant inhibition of Akt phosphorylation (Fig. 8). LY294002, a specific PI3K/Akt inhibitor was used as control. These findings suggest that PI3K/Akt may play a role in VEGF-induced cell survival in this endothelial cell population, and inhibition of PI3K/Akt by thalidomide inhibits the angiogenesis in HIMEC.
Fig. 8.
Effect of thalidomide on VEGF-induced Akt phosphorylation in HIMEC. Stimulation of HIMEC with VEGF (50 ng/ml) increased Akt phosphorylation as demonstrated in Western blot analysis. Pretreatment of HIMEC with thalidomide significantly inhibited Akt phosphorylation in a dose-dependent manner, which was similar to the effect of LY294002, a specific PI3K/Akt inhibitor.
DISCUSSION
The present study demonstrates the inhibitory effect of thalidomide on both inflammatory and angiogenic activation of human gut microvascular endothelial cells. Interestingly, the effect of thalidomide did not involve inhibition of NOS-2 or COX-2, which is distinct from the effect of other pharmacological compounds that are known to inhibit vascular inflammation and growth (i.e., curcumin, MAPK inhibitors). These data suggest that the therapeutic effect of thalidomide observed in clinical trials for refractory CD may function through novel vascular mechanisms.
One of the better characterized anti-inflammatory properties of thalidomide has been its capacity to inhibit the production and release of a wide range of proinflammatory cytokines and growth factors (35). Thalidomide inhibits the release and action of TNF-α (37) and is also known to block the synthesis of TNF-α following stimulation of human monocytes by LPS (27). The function of thalidomide as a transcriptional inhibitor of TNF-α in gut endothelial cells may also be a contributing mechanism, as HIMEC were previously shown to produce gene product for this cytokine following activation by IL-1b and TNF-α (29).
The present study demonstrates that thalidomide affects TNF-α/LPS-induced transmigration of leukocytes by a direct action on endothelial cells. The effect of thalidomide on leukocyte-endothelial interaction in the setting of experimental colitis in dextran sulfate sodium rats was explored by Lienenlüke et al. (24). These authors demonstrated that a reduction in VCAM-1 expression was followed by a significant reduction in postcapillary leukocyte adhesion in these animals. We used our human system for modeling gut endothelial-leukocyte interaction to determine specific mechanisms that would underlie the anti-inflammatory effect of thalidomide. Although thalidomide did not alter the expression of E-selectin, ICAM-1, or VCAM-1 on resting HIMEC, it was associated with inhibition of the upregulation of all three of these CAM following induction with TNF-α/LPS. These findings are in agreement with Nogueira and coworkers (30), who demonstrated that thalidomide did not alter CAM expression in resting HUVEC. However, in contrast with the others who have reported increases in ICAM-1, density on TNF-α activated endothelial cells in response to increasing concentrations of thalidomide (17). The results of the present investigation, where pretreatment of HIMEC monolayers with thalidomide did not affect basal leukocyte-endothelial binding, are in agreement with those of Dunzendorfer et al. (11), who demonstrated the lack of effects of thalidomide on resting endothelial cells.
Inflammatory responses in intestinal tissues are mediated by multiple molecular mechanisms, which are distinct for the various involved immune and nonimmune cell populations. Two of the most prominent, general effects following inflammatory activation with TNF-α and LPS will include the production of nitric oxide by NOS-2 and the formation of prostaglandins via the upregulation of COX-2 (25, 40). In general, under normal, resting conditions, neither of these enzymes are constitutively expressed in resting endothelial cells, but following inflammatory stimuli (i.e., LPS, TNF-α) there will be a rapid increase in expression (1). Our results clearly indicate that the TNF-α/LPS treatment of HIMEC resulted in a marked increase in both NOS-2 and COX-2 mRNA and protein expression. However, the pretreatment of HIMEC with thalidomide did not inhibit either of these molecules, while exerting potent inhibitory effects on the functional assessment of both endothelial-leukocyte adhesion as well as endothelial growth, two well established in vitro approaches for the modeling of vascular inflammatory and angiogenic activity. These data suggest that thalidomide will exert its therapeutic effect through novel vascular mechanisms, through selective effect on the PI3K/Akt pathway and activation of the transcription factors activator protein-1 (AP-1) and NF-κB.
On the basis of these results we propose that thalidomide exerts its anti-inflammatory properties in HIMEC through downregulation of the endothelial cell transmigration, decreased CAM expression, and inhibition of HIMEC-leukocyte binding, probably by inhibiting the activation of transcriptional factors such as NF-κB and AP-1. Further investigation should provide additional biochemical data to elucidate the precise anti-inflammatory mechanisms of thalidomide. Taken together, our results suggest that thalidomide promoted a satisfactory inhibition of the inflammatory reaction in HIMEC attributable to the inhibition of NF-κB activation, decreased CAM expression, and leukocyte adhesion. Some chemical properties of thalidomide such as lipophilicity and molecule size, as well as pharmacokinetic factors including movement in the liquids or cell membrane permeability, could facilitate the passage of thalidomide into cells and tissues.
Previous studies from our groups and others have established that growth factor-induced endothelial cell migration and subsequent tube formation are known to be PI3K-Akt dependent (6, 15, 26). In this study, we determine whether the inhibitory effect of thalidomide on VEGF-induced angiogenesis might be attributable to inhibition of signaling via Akt. It is well established that activation of the Akt pathway plays a crucial role in tumor chemoresistance, malignant transformation, and invasiveness by inducing cell survival, growth, migration, and angiogenesis (42). In a number of human cancers overexpression and activation of Akt have been shown, and inhibition of its activity induces apoptosis in a variety of mammalian cells (9), making Akt a very attractive therapeutic target for cancer therapy. In this study we have shown that thalidomide inhibited VEGF-induced Akt phosphorylation to well below its constitutive level. Because activated Akt is required to maintain endothelial cell viability during integrin-mediated interaction with the extracellular matrix (15), this may thus have implications for the survival of endothelial cells during the angiogenic process in vivo. The possibility that other pathways may also be involved in the angiogenic process should not be excluded although thalidomide does not appear to have any effect on VEGF-induced MAPK pathway activation in HIMEC (preliminary studies).
In summary, we provide evidence that thalidomide significantly blocks the vascular inflammatory response including inhibition of NF-κB activation, decreased endothelial-leukocyte binding, and reduced CAM expression in TNF-α/LPS-activated HIMEC. Furthermore, we also report that thalidomide significantly reduced the angiogenic response in human gut endothelial cells, which included inhibition of endothelial transmigration and decreased growth, proliferation, and capillary tube formation in VEGF-activated HIMEC. However, thalidomide did not inhibit COX-2 and NOS-2 mRNA and protein expression in either TNF-α/LPS-activated HIMEC or in VEGF-stimulated HIMEC. Collectively, the present findings suggest that thalidomide may function through novel vascular mechanisms in addition to its known effect on inhibiting TNF-α, in the treatment of patients suffering from refractory CD chronic gut inflammation. Further investigation defining the therapeutic effect of thalidomide in the treatment of chronic inflammatory bowel disease, specifically through novel vascular mechanisms, is warranted.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-065948 and by the Digestive Disease Center of the Medical College of Wisconsin.
DISCLOSURES
The authors have nothing to disclose and declare no conflicts of interest.
ACKNOWLEDGMENTS
Thalidomide was a generous gift from Celgene (San Diego, CA).
REFERENCES
- 1.Appleton I, Tomlinson A, Willoughby DA. Induction of cyclo-oxygenase and nitric oxide synthase in inflammation. Adv Pharmacol 35: 27–78, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer 4: 314–322, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Battegay EJ. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med 73: 333–346, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Binion DG, West GA, Ina K, Ziats NP, Emancipator SN, Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology 112: 1895–1907, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Chavakis E, Dernbach E, Hermann C, Mondorf UF, Zeiher AM, Dimmeler S. Oxidized LDL inhibits vascular endothelial growth factor-induced endothelial cell migration by an inhibitory effect on the Akt/endothelial nitric oxide synthase pathway. Circulation 103: 2102–2107, 2001 [DOI] [PubMed] [Google Scholar]
- 7.D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA 91: 4082–4085, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danese S, Sans M, Spencer DM, Beck I, Donate F, Plunkett ML, DE la Motte C, Redline R, Shaw DE, Levine AD, Mazar AP, Fiocchi C. Angiogenesis blockade as a new therapeutic approach to experimental colitis. Gut 56: 855–862, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 13: 2905–2927, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Denes L, Jednakovits A, Hargitai J, Penzes Z, Balla A, Talosi L, Krajcsi P, Csermely P. Pharmacologically activated migration of aortic endothelial cells is mediated through p38 SAPK. Br J Pharmacol 136: 597–603, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunzendorfer S, Schratzberger P, Reinisch N, Kahler CM, Wiedermann CJ. Effects of thalidomide on neutrophil respiratory burst, chemotaxis, and transmigration of cytokine- and endotoxin-activated endothelium. Naunyn Schmiedebergs Arch Pharmacol 356: 529–535, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med 77: 527–543, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1: 27–31, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 333: 1757–1763, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem 274: 16349–16354, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita J, Mestre JR, Zeldis JB, Subbaramaiah K, Dannenberg AJ. Thalidomide and its analogues inhibit lipopolysaccharide-mediated induction of cyclooxygenase-2. Clin Cancer Res 7: 3349–3355, 2001 [PubMed] [Google Scholar]
- 17.Geitz H, Handt S, Zwingenberger K. Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology 31: 213–221, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development 126: 1149–1159, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Haslett PA, Corral LG, Albert M, Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med 187: 1885–1892, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem 278: 8508–8515, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Jin SH, Kim TI, Han DS, Shin SK, Kim WH. Thalidomide suppresses the interleukin 1beta-induced NFkappaB signaling pathway in colon cancer cells. Ann NY Acad Sci 973: 414–418, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS., Jr Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem 276: 22382–22387, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood 70: 1284–1290, 1987 [PubMed] [Google Scholar]
- 24.Lienenluke B, Stojanovic T, Fiebig T, Fayyazi A, Germann T, Hecker M. Thalidomide impairment of trinitrobenzene sulphonic acid-induced colitis in the rat—role of endothelial cell-leukocyte interaction. Br J Pharmacol 133: 1414–1423, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moncada S. Nitric oxide: discovery and impact on clinical medicine. J R Soc Med 92: 164–169, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res 86: 892–896, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med 177: 1675–1680, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura JL, Karlsson A, Arvold ND, Gottschalk AR, Pieper RO, Stokoe D, Haas-Kogan DA. PKB/Akt mediates radiosensitization by the signaling inhibitor LY294002 in human malignant gliomas. J Neurooncol 71: 215–222, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Nilsen EM, Johansen FE, Jahnsen FL, Lundin KE, Scholz T, Brandtzaeg P, Haraldsen G. Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut 42: 635–642, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nogueira AC, Neubert R, Felies A, Jacob-Muller U, Frankus E, Neubert D. Thalidomide derivatives and the immune system. 6. Effects of two derivatives with no obvious teratogenic potency on the pattern of integrins and other surface receptors on blood cells of marmosets. Life Sci 58: 337–348, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Ogawa H, Binion DG, Heidemann J, Theriot M, Fisher PJ, Johnson NA, Otterson MF, Rafiee P. Mechanisms of MAdCAM-1 gene expression in human intestinal microvascular endothelial cells. Am J Physiol Cell Physiol 288: C272–C281, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 359: 845–848, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Prat A, Casado E, Cortes J. New approaches in angiogenic targeting for colorectal cancer. World J Gastroenterol 13: 5857–5866, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rafiee P, Johnson CP, Li MS, Ogawa H, Heidemann J, Fisher PJ, Lamirand TH, Otterson MF, Wilson KT, Binion DG. Cyclosporine A enhances leukocyte binding by human intestinal microvascular endothelial cells through inhibition of p38 MAPK and iNOS. Paradoxical proinflammatory effect on the microvascular endothelium. J Biol Chem 277: 35605–35615, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Raje N, Anderson KC. Thalidomide and immunomodulatory drugs as cancer therapy. Curr Opin Oncol 14: 635–640, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood 99: 2703–2711, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med 173: 699–703, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441: 424–430, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359: 843–845, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med 53: 35–57, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res 60: 203–212, 2000 [PubMed] [Google Scholar]
- 42.Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, Sebti SM, Cheng JQ. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res 64: 4394–4399, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Zimmerman GA, Prescott SM, McIntyre TM. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today 13: 93–100, 1992. [DOI] [PubMed] [Google Scholar]