Abstract
The apical membrane Na+-H+ exchanger (NHE)3 is regulated by cAMP-dependent phosphorylation, which inhibits its activity through membrane endocytosis. The clathrin complex adaptor protein synaptotagmin 1 (Syt 1) appears to be essential to this process, but little is known about its expression in intestinal epithelial cells or interaction with NHE3. The intestinal epithelial expression and apical location of Syt 1 were determined by Syt 1 mRNA profiling and immunolocalization. Tandem mass spectrometry was used for protein identification. Bis(sulfosuccinimidyl) suberate (BS3) cross linking suggested that NHE3 and Syt 1 were in a membrane complex following cAMP stimulation of Caco2BBE (Brush Border Expressions) cells. To investigate the regulation of NHE3 appearance in a Syt 1-containing membrane compartment, doxycycline-inducible hemaglutinin (HA)-tagged NHE3 was expressed in Caco2BBE cells. HA-NHE3 correctly targeted to the apical membrane, where, upon cAMP stimulation, it was internalized with a Syt 1-containing compartment. Site-directed mutagenesis of NHE3 showed that serine 605 (S605) was pivotal to NHE3 and Syt 1 association and internalization. Direct Syt 1 interaction with NHE3 was suggested by fluorescence resonance energy transfer (FRET) analysis. The physiological role of S552 was less clear. By FRET, this serine residue appeared to be involved in cAMP-induced Syt 1 binding of NHE3. However, when HA-tagged NHE3 S552A was expressed in Caco2 cells, the mutated construct was not inserted into the apical membrane. We conclude that intestinal epithelial Syt 1 plays an important role in cAMP-stimulated endocytosis of apical NHE3 through cAMP-dependent phosphorylation of S605 that is required for NHE3 and Syt 1 association.
Keywords: protein trafficking, fluorescence resonance energy transfer, Na+ transport, diarrheal disease, clathrin and adaptor protein 2 complex, Na+-H+ exchanger
sodium absorption by the intestine is a tightly regulated bodily function that can accommodate significant alterations in luminal salt load or metabolic demands. The major pathway for mammalian intestinal Na+ absorption involves electroneutral Na+ and Cl− transport mediated by the functional coupling of apical membrane Na+-H+ exchangers (NHE) and Cl−-base exchangers. NHE3 is the predominant NHE apical membrane transporter (34, 40, 41), which is highly regulated by a number of second messenger systems that involve cAMP-dependent protein kinase (PKA), cGMP-dependent protein kinase, intracellular calcium, protein kinase C, phosphatidyl inositol-3-kinase, Akt, and others (8, 42, 43). PKA regulation of NHE3 is best understood and appears to involve phosphorylation of multiple serines of NHE3 that directly affects NHE3 activity or alters its association with key proteins involved in clathrin-mediated NHE3 endocytosis (7, 20–23, 37, 45, 47). Internalization of NHE3 is believed to be a major mechanism for inhibiting intestinal Na+ absorption by regulatory factors. What is unclear are the intermediate events that take place before clathrin-mediated endocytosis. Recently, the role of synaptotagmin 1 (Syt 1) in regulating NHE3 function and endocytosis was demonstrated in Caco2BBE cells (31). In these studies, Syt 1 was shown to associate with NHE3, directing it to the adaptor protein 2-clathrin complex. When Syt 1 expression was specifically silenced by more than 80%, an associated decline in cAMP-induced interaction between NHE3 and Syt 1 and their membrane endocytosis was observed. Syt 1 silencing by siRNA results in decreased association of NHE3 in the adaptor protein (AP)2-clathrin complex after cAMP stimulation. Thus we surmised that NHE3 association with Syt 1 or a Syt 1-containing complex stimulated by protein kinase A must be upstream and essential for clathrin-mediated endocytosis of NHE3.
The Syts are a family of related proteins that have been studied mostly in neuronal cells. Syts regulate trafficking of a number of different proteins such as vesicle-associated membrane protein, syntaxin, S-nitroso N-acetyl penicillamine (SNAP)25, and AP2 (3, 18, 35). In neuronal cells, Syts are essential for mediating “fast vesicle” fusion for neurotransmitter release (1, 17) but also function in endocytosis. Syt 1 regulates endocytosis of the acetylcholine receptor (25), a process that involves the AP2 complex before membrane internalization (12, 13). Syt 7 appears to have a role in membrane sealing and lysosomal trafficking (9, 26, 27). Syts have also been found in nonneuronal tissues, including Syt 8 in kidney (19), Syt 6 in spermatozoa (29), and Syt 5 and 7 in macrophages (5, 36), but their functions have not been well studied. In the intestine, Syt 1 appears to be expressed in the apical membrane and subapical region of intestinal epithelial cells. Syt 1 is most highly expressed in villus epithelial cells and to a lesser extent in the crypt epithelial cells (31). However, the epithelial expression of Syt 1 requires further corroboration because the anti-Syt 1 polyclonal antibody (SySy Systems, Gottingen, Germany) used to identify Syt 1 could potentially cross react with other closely related members of the Syt family. The present studies, therefore, examine the intestinal epithelial expression of Syt 1 and its potential role and action in regulating apical NHEs.
MATERIALS AND METHODS
Cell culture.
Caco2 cells were grown as described previously (28, 31). Confluent Caco2BBE monolayers were grown on rat tail collagen-coated Transwells for 14 days to achieve optimal differentiation, as determined by expression of villin and brush-border alkaline phosphatase (28, 32).
Identification of intestinal Syt 1.
Syt 1 was immunoprecipitated from lysates of Caco2BBE cells or light mucosal scrapings of murine jejunum using polyclonal anti-Syt 1 (SySy). Immunoprecipitated proteins were resolved on SDS-PAGE. Half of the gel was processed for Western blots using the rabbit polyclonal anti-Syt 1, whereas the other half of the gel was stained with Brilliant Blue R250 and processed for mass spectrometry. Gel bands were washed three times with 40% methanol in 0.5% formic acid to destain and remove impurities. Twenty-five microliters of 6 M urea were added to the samples, which were allowed to denature at 25°C for 60 min. After 5 μl of 10 μM Tris were added, the samples were agitated for 20 min to reduce disulfide bonds. The samples were then pelleted at 14,000 g for 2 min, washed with 100 μl of 25 mM ammonium bicarbonate pH 8.9 and 200 μl of 55 mM iodoacetamide, and placed in the dark for 30 min to alkylate free sulfhydryl groups. At this point, the samples were again pelleted and subsequently rehydrated with 100 μl acetonitrile before drying by SpeedVac. Sixty microliters digestion buffer (250 μM CaCl2) with 12.5 ng TPCK-modified trypsin were added to the samples, which were then incubated at 37°C for 18 h. Samples were diluted with 100 μl 0.1% formic acid in acetonitrile and placed into a centrifugal evaporator at room temperature until dry. They were then reconstituted in 50 μl 5% formic acid and 6 μl injected into a Dionex Ultimate 3000 nano HPLC with a Zorbax C18 trapping column. The solvent was an isocratic buffer of 95% 0.1% formic acid in water and 5% acetonitrile. The trapping column retained the peptides and allowed them to be eluted onto the analytical column (Zorbax analytical C18 column using a gradient of mobile phase starting with 95% 0.1% formic acid in water 5% acetonitrile) and over 7 min increased acetonitrile to 15%, then to 55% acetonitrile in 47 min, and finally to 80% acetonitrile in 50 min. The peptides were eluted from the nanocolumn at a flow rate of 200 nl/min and then sprayed into a Therm Instruments (Waltham, MA) LTQ-FT tandem mass spectral instrument running Xcalibur software [version 2.2 equipped with a nonspray source using a New Objectives (Woburn, MA) picotip nanospray needle with a 8-μm ID tip]. Spectra were acquired using positive ion nano ESI mode with the FT-ICR acquiring precursor spectra from 250–1,800 m/z at a resolution of 50,000 at m/z 400. Tandem mass spectra were acquired in a data-dependent manner using the five most intense ions with charge states of +2 or higher from each FT-ICR MS scan to trigger the LTQ ion to perform collision-induced dissociation on each of the selected precursor ions using activation Q of 0.25, a normalized collision energy of 35, and an activation time of 30 ms. The raw data files from each run were then processed with DTA supercharge (version 1.18) to generate MGF peak list files. The software, ReadW (http://tools.proteomecenter.org/software.php), was used to produce binary mzXML files. Protein identification was performed by submitting the MGF files to a server running Mascot sequence database search software (version 2.2; Matrix Science, London, UK) and by submitting the mzXML file to a server running the Sagen Sorcerer (version 1.0; Sagen Research, San Jose, CA) implementation of the Sequest sequence database search software. Searches on both machines were run against the sequences of the IPI-Human and IPI-Rat database (version 3.33, www.ebi.ac.uk). In each search, a peptide precursor mass tolerance of 5 ppm was used, allowing for modifications of peptide mass attributable to such additions as Gly-Gly (114.04292 Th), methionine oxidation (15.99 Th), asparagine deamidation (+0.984016 Th), and cysteine carbamidomethylation (57.021464), as well up to three missed cleavages and strict adherence to tryptic digestion rules. The results obtained from the two separate searches were then loaded into the Scaffold software package (version 6.09; Proteome Software, Portland, OR), and peptides with scores of 95% confidence or better were used to confirm peptide assignments.
PCR analysis of Syts.
For analysis of Syt mRNA expression, RNA was isolated from Caco2BBE monolayers or from villus or crypt enterocytes of murine jejunum. Using a Leica AS laser microdissection (laser capture) system (Bannockburn, IL), crypt and villus epithelial samples from five 4-μm tissue sections were pooled separately, and RNA was extracted using the PicoPure RNA kit (Arcturus/Applied Biosystems, Palo Alto, CA). The RNA was reverse transcribed using the Superscript II system (Invitrogen, Carlsbad, CA) and PCR amplification performed with primers for mouse Syt 1 and GAPDH. Primers were designed for human and mouse Syts using MacVector version 7.2.2 software (Accelrys, San Diego, CA). Primers were selected from the suggested choices so that PCR products would span exon-intron boundaries. Thus the presence of contaminating DNA could be detected by larger than expected products. Human and mouse Syts were analyzed, and the base positions of the primers are as follows (with Genbank number provided after the name): human Syt 1 (NM_005639, bases 150–430 or 403–758), mouse Syt 1 (NM_009306, bases 150–430 or 403–758); human Syt 2 (NM_177402, bases 974–1144), mouse Syt 2 (NM_009307, bases 705–982); human Syt 3 (NM_032298, bases 2017–2130), mouse Syt 3 (NM_016663, bases 1374–1618); human Syt 4 (NM_020783, bases 420–538), mouse Syt 4 (NM_009308, bases 599–882); human Syt 5 (NM_016908, bases 199–404), mouse Syt 5 (NM_003180, bases 832–1146); human Syt 6 (NM_205848, bases 3019–3136), mouse Syt 6 (NM_018800, bases 799–982); human Syt 7 (NM_004200, bases 305–478), mouse Syt 7 (NM_173067, bases 705–982); human Syt 8 (NM_138567, bases 747–849), mouse Syt 8 (NM_018802, bases 350–489); human Syt 9 (NM_175733, bases 521–649), mouse Syt 9 (NM_021889, bases 684–1072); human Syt 10 (NM_198992, bases 1615–1825), mouse Syt 10 (NM_018803, bases 286–576); human Syt 11 (NM_152280, bases 401–499), mouse Syt 11 (NM_018804, bases 138–244); human Syt 12 (NM_177963, bases 698–840), mouse Syt 12 (NM_AB062804, no specific primers could be designed); human Syt 13 (NM_020826, bases 367–446), mouse Syt 13 (NM_030725, bases 584–857); human Syt 14 (NM_153262, bases 582–649), mouse Syt 14 (NM_181546, bases 989–1246); human Syt 15 (NM_031912, bases 871–977); mouse Syt 15 (NM_181529, bases 1797–2137); human Syt 16 (NM_031914, bases 328–479), mouse Syt 16 (NM_172804, bases, 140–392); human SLP-2 variant 2b (NM_032379, bases 496–720), mouse SLP-2b (NM_031394, bases 1289–1414); human GAPDH (NM_002046, bases 430–645), mouse GAPDH (NM_008084, bases 530–747).
For PCR reactions, Takara ExTaq was used according the manufacturer's directions. To optimize the potential to find PCR products, the annealing temperatures were decreased. For all primers, the initial annealing temperature was set to 55°C, and this was decreased by 2°C until a PCR product was obtained for sequencing or when no PCR product was observed at 42°C. For those cases where no product was observed even at 42°C, reactions were set up with varying MgCl2 concentrations from 0.2 to 5 mM. Controls were run for all primer pairs without addition of reverse transcription reaction as a control and to observe formation of primer dimers.
BS3 NHE cross linking.
Caco2BBE cells were treated with 8-chlorophenylthio-cAMP (100 μM, 15 min) and then rapidly chilled in iced saline. Cells were scraped from filters and solubilized in lysis buffer (10 mM HEPES, pH 7.4, 3 mM EDTA with the complete protease inhibitor cocktail; Roche, Indianapolis, IN). An aliquot was removed for protein analysis, and 500 μg protein reacted with bis(sulfosuccinimidyl) suberate (BS3) (5 mM). Tris was added to 10 mM to stop the reaction, and Syt 1 immunoprecipitated using anti-Syt 1 (SySy rabbit polyclonal) or rabbit anti-NHE3. Immunoprecipitated proteins were resolved by SDS-PAGE and subsequently probed for NHE3 and Syt 1 by Western blot analysis.
Association of Syt 1 and NHE3.
To determine the role of serines 505 (S505), S552, and S605 in promoting NHE3 association with Syt 1, rat NHE3 cDNA was cloned into pTre2-HA-hyg2 plasmid (Clontech, Palo Alto, CA). By tagging with hemaglutinin (HA), transgene proteins can be distinguished from endogenous human NHE. Caco2BBE cells that already expressed the reverse tetracycline transactivator (rTTA) driven by the cytomegaloviral promoter-rTTA (pTet-On system of Clontech) and maintained on 200 μg/ml G418 were transfected with plasmid containing either normal rat NHE3 or individually mutated constructs where alanine replaced S505, S552, or S605. Caco2BBE cells were transfected using Mirus LT-1 reagent (2 μg cesium-purified plasmid that was made into a complex with 2 μl of LT-1 stock in serum-free Optimem medium per 10-cm2 dish) when cells were 30% confluent. Media were aspirated, 500 μl Optimem was added containing the LT-1/plasmid complex, and solutions were incubated for 90 min followed by the addition of complete media (2 ml/10 cm2). Transfection efficiency ranged from 20–30%. Two days following transfection, cells were subjected to hygromycin selection (200 μg/ml) for two days and then maintained on 30 μg/ml hygromycin. The G418- and hygromycin-resistant cell population was maintained for 6 wk to provide time for all experiments. Hygromycin-resistant cells were plated on Transwells and used 14 days after plating when Syt 1 is optimally expressed. This methodology resulted in greater than 95% of the cells expressing the transgene protein, assessed by dsRed fluorescent protein cloned into pTre2-HA-hyg2.
For apical surface biotinylation, two previously reported protocols were used (15). For total apical surface NHE3, Sulfo-NHS-biotin was used. To assess internalized NHE3, a disulfide-cleavable form, Sulfo-NHS-S-S-biotin was used. After cyclic AMP stimulation, biotinylated proteins were isolated from cellular homogenates using streptavidin-agarose. To immunoprecipitate NHE3, anti-NHE3 rabbit polyclonal antibody coupled to Seize beads was used (31).
FRET analysis of Syt 1-NHE3 interaction.
Fluorescence resonance energy transfer (FRET) was used to assess direct Syt 1 and NHE3 protein binding. Full-length human Syt 1 (cDNA a gift of Mitsunori Fukuda, RIKEN Institute, Sakita, Japan) was cloned into plasmid pR-SET-A (Invitrogen), providing a NH2-terminal 6-histidine (His) tag. His-tagged Syt 1 was expressed using the manufacturer's directions in BL21DE3pLysS bacteria and purified using a His-Bind kit (Novagen, Madison, WI). For the cytoplasmic tail of rat NHE3, primers were made to amino acids 436–831, which was cloned in frame into plasmid pQE30 (Qiagen Sciences, Valencia, CA), also providing a NH2-terminal 6-His tag, and sequenced to confirm the in-frame ligation and correct sequence of the PCR product. The His-tagged NHE3 tail was expressed and purified in BL21DE3 bacteria and purified using the His-Bind kit above. To change specific serine residues of the His-tagged NHE3 carboxy terminal cytoplasmic region, the QuikChange II mutagenesis kit (Stratagene, San Diego, CA) was used. Primers were designed using the primer mutation software of the company website, and mutagenesis was performed according to the manufacturer's directions. For all NHE3 constructs, the ability of the NHE3 tail to bind to Syt 1 was tested with and without prior treatment of the purified His-tagged NHE3 with cAMP-dependent protein kinase. The purified NHE3 cytoplasmic tails were expressed, purified on His resin, and eluted per manufacturer instructions using standard elution buffer (composition in mmol/l: 300 NaCl, 250 imidazole, 50 sodium phosphate, pH 8.0). The eluted His-tagged NHE3 proteins were dialyzed against PBS (composition in mmol/l: 137 NaCl, 1.3 KCl, 0.5 KH2PO4, 3.7 Na2HPO4) and then half reacted with cAMP-dependent protein kinase (Sigma, St. Louis, MO) that had been activated by reaction with DTT (1 mM DTT in a solution of 10 mM HEPES at pH 7.4). For phosphorylation, 2 M MgCl2 was added to the entire mixture, and 1 mM ATP was added to only half. The half without ATP should result in no cAMP-dependent phosphorylation of NHE3. Reactions were at 37°C for 60 min and then dialyzed against PBS overnight and kept at 4°C and used within 5 days.
Purified His-tagged Syt 1 was labeled with (5-(((2-iodoacetyl)amino) ethyl)amino naphthalene-1-sulfonic acid) (1,5 IAEDANS; Molecular Probes, Eugene OR), and purified His-tagged NHE3 was labeled with (4-(4-(dimethylamino) phenyl)azo)benzoic acid, succinimidyl ester (Dabcyl) essentially according to manufacturer's protocol. IAEDANS was chosen as the fluorescent tag for Syt 1 because it reacts with cysteine residues, of which Syt 1 has six. In contrast, the COOH-terminal cytoplasmic tail of rat NHE3 has only one cysteine and was therefore not a good candidate for this label. The NHE3 carboxy terminal cytoplasmic region constructs were labeled with Dabcyl that reacts with amine groups, including ε-amino groups of lysine, of which there are 29. Purified His-tagged Syt 1 or NHE3 at 500 μg/ml was incubated in PBS with added 100 mM NaHCO3. The sample was then reacted with a fourfold molecular excess of freshly prepared IAEDANS or Dabcyl, both dissolved in DMSO at 10 mg/ml. This was then added to the reaction mixtures and incubated at room temperature for 1 h with constant stirring and stopped by addition of 2-mercaptoethanol (to 1 mM). Labeled proteins were dialyzed against PBS for four changes over 16 h at 4°C to remove unreacted IAEDANS or Dabcyl in the dark. The degree of labeling of IAEDANS-labeled Syt 1 and Dabcyl-labeled His-tagged NHE3 were verified per manufacturer's instructions. The protein concentration was measured by absorbance at 280 nm (and corrected for dye absorbance at 280 nm), and the absorbance of the IAEDANS or Dabcyl was measured at absorbance maxima (336 nm and 472 nm, respectively). For analysis, IAEDANS-labeled Syt 1 was placed into unilamellar phospholipid vesicles. Vesicles were first created by mixing dioctanoyl phosphatidylchlonine with dimethyl phosphatidylserine at a ratio of 3:1 (Avanti Polar Lipids, Alabaster, AL) (final concentrations 150 and 50 μg/ml, respectively) in PBS containing 20 μM CaCl2 and 1 mM EDTA to generate a free [Ca++] of 40 nM (32). To create small unilamellar vesicles, the lipid-containing solution was placed in the Mini-Extruder apparatus (Avanti) and pushed through the membrane 20 times to create uniform vesicles. Vesicles were kept at 4°C for up to a week to perform FRET analysis. Before FRET analysis, IAEDANS-labeled Syt 1 was added to PBS at a concentration of 0.75 mg/ml and briefly sonicated (4 10-s pulses at power setting of 3 on Branson Microsonifier) to incorporate the IAEDANS-labeled Syt 1. Vesicles were then kept on ice until use. Clumped vesicles were removed by centrifugation (14,000 g for 30 s), and an aliquot of the subsequent vesicle suspension was added to a 2-ml aliquot of PBS with 20 μM CaCl2 and 1 μM EDTA (free [Ca++] 40 nM). The cuvette was placed in a FluoroMax-3 (Hitachi) fluorometer, and solution was gently stirred. The excitation maximum IAEDANS of 295 nm was used, and emission was measured from 350–595 nm. Fluorescence measurements were taken every 0.5 s and graphed at 5-nm intervals. A baseline reading of the emission spectrum at 295 nm excitation was taken, and then Dabcyl-labeled NHE3 was added (50–2000-μl cuvette incubation volume) to achieve concentrations of 50, 100, or 150 μg/ml. This protocol was followed for all the NHE3 COOH-terminal protein constructs tested, wild-type and mutations S505A, S552A, and S605A. Vehicle controls were performed to determine that no buffer effects occurred.
RESULTS
Syt 1 is expressed in mouse small intestinal and Caco2BBE epithelial cells.
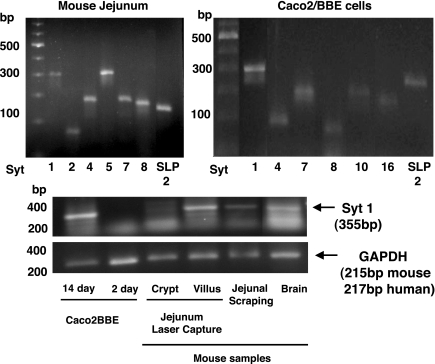
RT-PCR was used to investigate Syt isoform expression in mouse intestine and Caco2BBE cells. A complete listing of the primer positions used for the Syts and Syt-like proteins (SLPs) is provided in the supplemental material, which is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website. A number of Syt isoform mRNAs were detected in Caco2BBE cells as well as mouse jejunal scrapings, including Syt 1, 4, 7, 8, and SLP-2 (Fig. 1, top). Syt 11 and 5 were also detected in mouse jejunum but not in Caco2BBE cells. Syt 10 and 16 were inconsistently detected in mouse jejunum but were expressed by Caco2BBE cells. For Caco2BBE cells, RNA was harvested at 2 and 14 days after plating, corresponding to early and differentiated stages of maturation. At day 2, Syt 1 mRNA abundance was very low but increased by day 14 (Fig. 1, bottom). Although intestinal mucosal scrapings contain predominantly, but not exclusively, epithelial cells, laser capture microdissection was used to harvest villus and crypt epithelial cell RNA. Syt 1 expression was readily detected in crude extracts from jejunal scrapings as well as mature villus epithelial cells; however, Syt 1 expression was minimal in crypt epithelial cells (Fig. 1, bottom). In all cases, the PCR products were sequenced to confirm identity (data not shown). PCR products were cloned into pCR2.1-TOPO, and insert size was confirmed by restriction digest before sequencing. In almost all cases, a lower band was observed using both mouse and human Syt 1 primers (Fig. 1, bottom). This band was likely due to primer-dimer formation, as it was observed when no RNA was added to the PCR reactions (reactions not shown). It should be noted that primers used for Syt 1 were designed to span exon-intron boundaries to detect larger products that would be indicative of DNA contamination. For mouse Syt 1, the forward primer was in exon 4 and reverse primer in exon 6, and, for human, the forward primer was in exon 3 and reverse primer in exon 5.
Fig. 1.
Synaptotagmin (Syt) 1 mRNA is expressed in mouse jejunum and brain and in human Caco2BBE cells. RNA was isolated from mouse jejunum (top, left) and Caco2BBE (top, right) monolayers (after 14 days confluence), reverse transcribed, and amplified using primers for mouse or human Syts. Bottom: RNA was isolated from Caco2BBE cells at 2 days (little differentiation) and 14 days (fully differentiated), mouse jejunum crypt or villus enterocytes (isolated by laser capture), whole mouse jejunal scrapings, and brain as a positive control. Arrows indicate PCR products isolated and sequenced. Images shown are representative of those of 3 separate experiments. SLP, Syt-like protein.
We next determined the expression of Syt 1 protein in Caco2BBE and mouse jejunal cells by tandem mass spectrometry. A number of peptides in each sample were identified that corresponded to mouse or human Syt 1 with high (>95%) probability. These peptides are underlined in the amino acid sequences of mouse and human Syt 1 below (Fig. 2). For the Caco2BBE sample, six tryptic peptides were identified with 20% coverage. For the mouse intestinal Syt 1, seven tryptic peptides were identified with 27%. For all of the peptides from both human and mouse Syt 1, the Scaffold software predicted with greater than 95% probability the presence of Syt 1. These data, along PCR identification of mRNA expression, strongly suggested a villus cell-predominant expression of Syt 1, consistent with the villus-crypt gradient of Syt 1 immunohistochemical localization previously reported (31). Syt 1 is also expressed in ileum, proximal, and distal colon (detected on Western blots using a monoclonal anti-Syt 1, SySy Systems).
Fig. 2.
Tryptic peptides of immunoprecipitated Syt 1 confirm mouse intestinal and Caco2BBE expression. Syt 1 was immunoprecipitated from mouse jejunal brush-border membranes or Caco2BBE cell lysates, run on SDS-PAGE, eluted from the gel, digested with peptides, and analyzed by tandem mass spectrometry as described in materials and methods. Underlined amino acids are the identified peptides, and all had a confidence level of greater than 95% as predicted by Scaffold protein sequencing software.
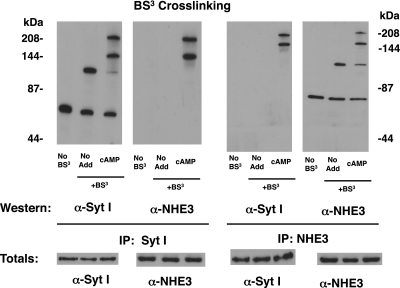
Increased BS3 cross linking of Syt 1 and NHE3 after cAMP stimulation of Caco2BBE cells.
We previously demonstrated that Syt 1 was essential for cAMP-mediated inhibition of NHE3 in Caco2BBE cells but did not have information on the molecular interactions of the two proteins (31). As another approach to assess the interaction of Syt 1 and NHE3, cross-linking studies with the homobifunctional cross linker BS3 were performed. Western blots of cell membrane proteins not treated with BS3 (control) showed only Syt 1 or NHE3 at their predicted molecular mass (Fig. 3, left lane, left and right). Cell membrane proteins from nonstimulated cells subjected to BS3 cross linking exhibited bands of higher molecular mass. For Syt 1 immunoreactivity, a band approximately twice the molecular mass of Syt 1 was seen (Fig. 3, ∼100 kDa, middle lane, left), possibly consistent with a Syt 1 dimer that is known to form (9). However, the possibility that other proteins might be linked to Syt 1 under these conditions could not be excluded. In BS3-cross-linked cell membrane proteins harvested from control cells not treated with cAMP, a NHE3 immunoreactive band near 140 kDa was observed in addition to the NHE3 protein (Fig. 3, middle lane, right). The identity of the protein(s) causing this shift is unknown.
Fig. 3.
Syt 1 and Na+/H+ exchanger (NHE)3 cross linking following cAMP stimulation. Caco2BBE cells were untreated or stimulated with cAMP (100 μM for 15 min) when appropriate. Cells were quickly chilled, and microsomal membranes were isolated and reacted with the 11.4 Angstrom cross linker bis(sulfosuccinimidyl) suberate (BS3) for 30 min at 4°C as designated. Membranes were solubilized and immunoprecipitated with either anti-Syt 1 or anti-NHE3 rabbit polyclonal antisera. Samples were washed and eluted, run on 5% SDS-PAGE for Western blots, and analyzed for Syt 1 or NHE3. An aliquot of each immunoprecipitation reaction was removed before addition of antibodies and analyzed for total Syt 1 or NHE3. Images are representative of those from 3 separate experiments.
After stimulation of cells with cAMP, two bands of greater than 140-kDa molecular mass immunoprecipitated with anti-Syt 1 from BS3-treated membrane proteins that reacted with anti-Syt 1 antibody (Fig. 3, right lane, left). Concurrently, there appeared to be a decrease in the relative intensity of Syt 1 at ∼55-kDa and 100-kDa immunoreactive bands. The two high molecular mass bands that appeared only after cAMP stimulation also exhibited NHE3 immunoreactivity (Fig. 3, right lane, left- middle; right lane, right-middle and right). These findings strongly suggested that cAMP stimulation promotes the association of NHE3 and Syt 1 within a membrane complex. While not conclusive the 140-kDa band that is both NHE3 and Syt 1 immunoreactive suggested that NHE3 (∼85 kDa) and Syt 1 (∼55 kDa) are complexed after cAMP stimulation.
Phosphorylation of S605 of NHE3 is pivotal for Syt 1 interaction.
Although cyclic AMP-dependent protein kinase can phosphorylate NHE3 at a number of sites (7–22, 30, 45), S505, S552, S605, S634, and S690/691 have been identified as potentially the most important for regulated endocytosis. To examine whether some of these sites might be involved in NHE3-Syt 1 association and endocytosis, wild-type and mutagenized HA-tagged rat NHE3 constructs were transfected into human Caco2BBE cells. This approach provides the ability to test the production, targeting, and function of mutagenized constructs in their “native” environment and to compare these properties to those of endogenous NHE3.
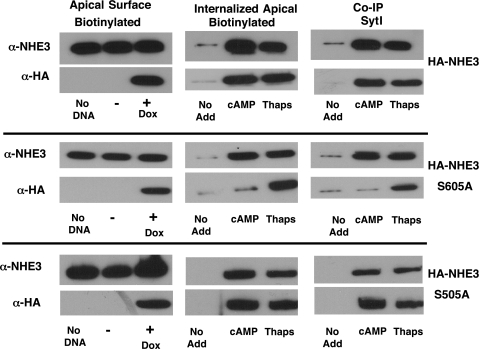
A clone of Caco2BBE cells that stably expressed the reverse tetracycline transactivator was transfected with HA-tagged rat NHE3 or HA-tagged NHEs with serine site-specific mutations. HA-tagged nonmutated rat NHE3 was induced only in cells treated with doxycycline (Fig. 4, second from top, far left). However, normal human NHE3 was expressed in all samples (Fig. 4, top, far left α-NHE3). The HA-tagged rat NHE3 sorted correctly to the brush border as evidenced by analysis of surface biotinylated proteins under nonstimulated conditions (Fig. 4, second from top, far left, α-HA). The HA-tagged doxycycline-induced rat NHE3 was internalized upon cAMP stimulation, as was endogenous (human, non-HA tagged) NHE3 (Fig. 4, top, middle α-NHE3 and α-HA). Little NHE3 endocytosis occurred under basal conditions, but, after cAMP stimulation, endocytosis of endogenous and HA-tagged wild-type NHE3 were observed. Also shown in Fig. 4 are coimmunoprecipitation (Co-IP) studies with anti-Syt 1 antibody followed by Western blots for either HA-tagged or native NHE3. Both wild-type and S505A appeared to co-IP with Syt 1 following cAMP stimulation. In contrast, the S605A NHE3 mutation did not appear to coassociate with Syt 1 pulldowns. Similar studies were performed on the S552A-mutated NHE3. Although the HA-tagged NHE3 S552A appeared to be expressed by Caco2BBE cells, this protein did not appear in the apical membrane of the cells (data not shown). This finding may suggest that S552 is critical for membrane insertion of NHE3. On the other hand, its role in regulated NHE3 endocytosis could not be studied further because the S552A protein failed to insert into the apical membrane.
Fig. 4.
Mutation of serine 605 to alanine (S605A) of NHE3 blocks its interaction with Syt 1 and endocytosis following cAMP stimulation. Histidine (His)-tagged, tet promoter regulated wild-type rat NHE3, and 2 mutant constructs (S505A and S605A) were transfected into Caco2BBE cells stably expressing the reverse tetracycline transactivator. For all 3 NHE3 cDNA, doxycycline (Dox) addition resulted in hemaglutinin (HA)-tagged proteins that could be biotinylated at the apical surface and had the same molecular mass as NHE3. Similar to the endogenous NHE3, the HA-tagged wild-type and S505A mutation were internalized upon cAMP stimulation, demonstrated by detection of the disulfide-cleaved, biotin-labeled NHE3 within the cell (middle row). These constructs could also be co-immunoprecipitated with Syt 1 (Co-IP). In contrast, the S605A NHE3 mutation failed to internalize or associate with Syt 1. Of note, thapsigargin (Thaps) (which increases cytosolic Ca), stimulated membrane endocytosis of all 3 transgene products as well as endogenous NHE3. Thus the S605 mutation is specific to cAMP-regulated NHE3 membrane trafficking. Images shown are representative of 4 separate experiments.
S605 and S552 regulate NHE3-Syt 1 binding.
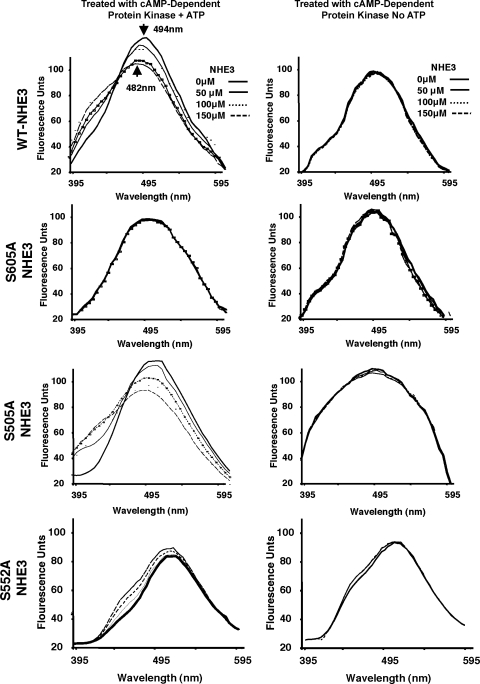
We next determined whether cAMP-dependent protein kinase phosphorylation of NHE3 promotes NHE3-Syt 1 binding, using FRET analysis. Full-length NHE3 has at least 10 transmembrane regions and, therefore, is insoluble when produced in bacteria. Therefore, a construct containing the carboxy terminal cytoplasmic tail (amino acids 436–826) was made. This protein construct contains all the regulatory serine residues required for cAMP regulation. IAEDANS-labeled Syt 1 was excited at 295 nm and demonstrated a predicted emission curve with an emission maximum of 482 nm (Fig. 5). Close proximity of Dabcyl groups within 50 nm of an IAEDANS label decreases the emission and shifts emission maximum to a shorter wavelength, indicative of protein-protein binding. With the use of IAEDANS-Syt 1 and wild-type Dabcyl-His-COOH-terminal NHE3, a dose-related shift (decrease) in fluorescence was observed upon addition of the COOH-terminal NHE3 construct that had been phosphorylated in vitro with protein kinase A. In contrast, no shift was observed with the nonphosphorylated form of COOH-terminal NHE3 (Fig. 5, top, left). When S605 was mutated to alanine, the shift in emission profile was blocked, suggesting that S605A is needed for Syt 1-NHE3 interaction following cAMP stimulation 1 (Fig. 5, top-middle, left). When S505 was mutated to alanine, the same emission shift observed for wild-type COOH-terminal NHE3 was observed (Fig. 5, bottom-middle, left), suggesting that this serine is not involved in cAMP-mediated Syt 1-NHE3 interaction. When S552 was mutated to alanine, the shift in emission profile was altered. The S552A COOH-terminal NHE3 construct caused a shift to the left and increased the IAEDANS-Syt 1 emission. Thus S552 appears to be important in mediating cAMP-induced Syt 1-NHE3 interaction.
Fig. 5.
Syt 1 and NHE3 direct binding enables fluorescent resonance energy transfer. His-tagged full-length Syt 1 was labeled with (5-(((2-iodoacetyl)amino)ethyl)amino naphthalene-1-sulfonic acid) (IAEDANS), and His-tagged rat NHE3 COOH-terminal cytoplasmic tail (amino acids 436–831) was labeled with (4-(4-(dimethylamino) phenyl) azo)benzoic acid, succinimidyl ester (Dabcyl). IAEDANS-Syt 1 was excited at 295 nm, and emission was measured over the range 350–595 nm. NHE3 was phosphorylated in vitro by cAMP-dependent protein kinase with (left) or without ATP (right). Addition of all NHE3 constructs from reactions without ATP did not change the Syt 1 emission spectrum. However, addition of the wild-type (WT) NHE3 that had been phosphorylated by cAMP-dependent protein kinase caused a concentration-related shift in the emission spectral profile and decrease in emission intensity (top, left). Similar results were obtained with the NHE3 S505A mutation. When the PKA-phosphorylated S605A mutation was used, no changes were observed upon addition of the His-Dabcyl-tagged S605A NHE3. Addition of the NHE3 S552A mutation caused a concentration-dependent shift in the emission spectrum that differed from the wild-type or S505A mutation and slightly increased the emission maximum. Spectra shown were obtained on a Hitachi FluoroMax-3 fluorometer and are representative of those of 3 separate experiments with different reagents (IAEDANS-Syt 1 and Dabcyl-NHE3) in each experiment.
DISCUSSION
Syts are a family of proteins that are recognized to participate in the docking and fusion of membrane vesicles in neuronal and nonneuronal cells including macrophages (5, 29), insulin-secreting β-cells (16), osteoblasts, and osteoclasts (46), and airway and gastric mucin-secreting goblet cells (6, 33). SLPs have been identified in many cell types including cytotoxic T lymphocytes (14). The present study demonstrates that Syt 1 is expressed in intestinal epithelial cells and regulates endocytosis of the apical/brush border sodium hydrogen exchanger NHE3. Epithelial mRNA and protein expression of Syt 1 were confirmed in both Caco2BBE colonic epithelial cells as well as native jejunal membranes. Syt 1 protein was also identified by tandem mass spectral analysis in samples isolated from mouse jejunal brush-border membranes as well as Caco2BBE cells, with the use of the Scaffold software analysis of the mass spectral data. Other tryptic peptides that were identified with high (>95%) confidence included α-actin, type II keratin Kb15, keratin type II cytoskeletal 5, CD44, fibrillin-1, and KA22 and KA27 type I keratin. However, these proteins were likely contaminants acquired during the immunoprecipitation process. It is important to note that no other Syts were predicted by the software, confirming the specificity of the anti-Syt 1 polyclonal antiserum. Messenger RNA of other Syts are nevertheless expressed in intestinal cells. By RT-PCR, Syt 4, 7, 8, and SLP-2 appear to be expressed in mouse jejunal and Caco2BBE epithelial cells. However, our prior studies would suggest that Syt 1 is necessary for cAMP-stimulated NHE3 endocytosis because specific RNA silencing of this isoform blocks the endocytic response (31). We cannot, however, exclude a role for other Syts until specific silencing strategies of these other isoforms are performed. We do not know whether Syt multimers (which are known to form) are involved in the process or whether other Syts might regulate a different step to regulate NHE3 endocytosis.
Prior studies demonstrated that, after cAMP stimulation, NHE3 Co-IPs with Syt 1 (31). The present studies, which employed multiple, complementary approaches, suggest that cAMP stimulation promotes direct binding of NHE3 with Syt 1, which is necessary for regulated endocytosis in intestinal epithelial cells. Syt 1-NHE3 association appears to be dependent on protein kinase A-stimulated phosphorylation of S605 (which is conserved in rat, human, and mouse), a site that had been previously identified as being involved in the regulation of cAMP-stimulated inhibition of NHE3 function and endocytosis (7, 20–22, 45). S505, in contrast, does not appear to be involved in cAMP regulation of NHE3. Unfortunately, we could not establish the physiological role of S552 because of the limitations presented by the S552A mutation construct that failed to insert into the apical membrane. A double mutation (S552A, S605A) was not attempted because the effects of S605A mutation alone disrupted NHE3 interaction with Syt 1 nearly completely. Therefore, it would be difficult to interpret the additional effects of the S552 mutation. However, we do not exclude the possibility that S552 has a role in regulation of NHE3, as the FRET studies demonstrated a difference with the S552 mutation. Previous studies of S552 of NHE3 have also not yielded consensus. Some reports (20, 22) have concluded that S552 has no direct role in NHE3 regulation, whereas others have suggested that it is important in regulation of NHE3 (7, 45). The lack of consensus may be a result of the differences in cell systems used for examining S552, ranging from Xenopus A6 cells to fibroblasts. It is notable that our studies were performed in intestinal epithelial cells where conditional requirements for NHE3 regulation are perhaps more physiologically relevant.
We propose that cAMP-dependent phosphorylation of NHE3 is essential for association with Syt 1 and membrane endocytosis. Other studies have demonstrated the pivotal roles of the NHE regulatory factor NHERF-1 to promote cAMP-dependent phosphorylation of NHE3 (23, 37, 47). After phosphorylation, NHE3 has been shown to associate with the AP2 complex (15), which is then directed to clathrin pits for endocytosis (41). Because Syt 1 binds certain proteins of the AP2 complex (12, 13, 24, 44), we propose that it plays a critical intermediary role for NHE3 endocytosis. Syt 1 may interact with a number of proteins including SNARE proteins that are pivotal in assembling complexes of certain membrane proteins (24). Syts have also been demonstrated to act as accessory proteins to stabilize the acceptor complex synaptobrevin, the 1:1 syntaxin/SNAP-25 complex (38). Syts also interact with acidic phospholipids through their C2 domains (10, 39) that may be important in directing NHE3 to specific endocytic compartments.
In conclusion, we demonstrate that Syt 1 is expressed in intestinal epithelial cells, where it plays an important role in cAMP-stimulated endocytosis of apical NHE3 through cAMP-dependent phosphorylation of S605, which is required for direct binding of NHE3 and Syt 1. We cannot rule out the possibility that S552 is also involved in cAMP regulation of NHE3 association with Syt 1 and endocytosis.
GRANTS
This work was supported by NIH grants DK-38510 and DK-47722 (E. Chang), the Digestive Disease Research Center of the University of Chicago (DK-42086), and the Gastrointestinal Research Foundation of Chicago.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Bai F, Wang P, Chapman ER. C2A activates a cryptic Ca(2+)-triggered membrane penetration activity within the C2B domain of synaptotagmin I. Proc Natl Acad Sci USA 99: 1665–1670, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryson A, Fletcher IS. Ligand exchange kinetics in the calcium-EDTA system. Aust J Chem 23: 1095–1110, 1970 [Google Scholar]
- 3.Chapman ER, Desai RC, Davis AF, Tornehl CK. Delineation of the oligomerization, AP-2 binding, and synprint binding region of the C2B domain of synaptotagmin. J Biol Chem 273: 32966–32972, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Chow CW, Khurana S, Woodside M, Grinstein S, Orlowski J. The epithelial Na(+)/H(+) exchanger, NHE3, is internalized through a clathrin-mediated pathway. J Biol Chem 274: 37551–37558, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Czibener C, Sherer NM, Becker SM, Pypaert M, Hui E, Chapman ER, Mothes W, Andrews NW. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J Cell Biol 174: 997–1007, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis CM, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol 70: 487–512, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Di Sole F, Cerull R, Casavola V, Moe OW, Burckhardt G, Helmle-Kolb C. Molecular aspects of acute inhibition of Na(+)-H(+) exchanger NHE3 by A(2)-adenosine receptor agonists. J Physiol 541: 529–543, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donowitz M, Welsh MJ. Ca++ and cyclic AMP in regulation of intestinal Na, K, and Cl transport. Annu Rev Physiol 48: 135–150, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Fukuda M, Mikoshiba K. Distinct self-oligomerization activities of synaptotagmin family. Unique calcium-dependent oligomerization properties of synaptotagmin VII. J Biol Chem 275: 28180–28185, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Fukuda M, Kanno E, Ogata Y, Mikoshiba K. Mechanism of the SDS-resistant synaptotagmin clustering mediated by the cysteine cluster at the interface between the transmembrane and spacer domains. J Biol Chem 276: 40319–40325, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Grass I, Thiel S, Honing S, Haucke V. Recognition of a basic AP-2 binding motif within the C2B domain of synaptotagmin is dependent on multimerization. J Biol Chem 279: 54872–54880, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Haucke V, DeCamilli P. AP-2 recruitment to synaptotagmin stimulated by tyrosine-based endocytic motifs. Science 285: 1268–1271, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Haucke V, Wenk MR, Chapman ER, Farsad K, De Camilli P. Dual interaction of synaptotagmin with mu2- and alpha-adaptin facilitates clathrin-coated pit nucleation. EMBO J 19: 6011–6019, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt O, Kanno E, Bossi G, Booth S, Daniele T, Santoro A, Arico M, Saegusa C, Fukuda M, Griffiths GM. Slp1 and Slp2-a localize to the plasma membrane of CTL and contribute to secretion from the immunological synapse. Traffic 9: 446–457, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem 276: 26906–26915, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Hu ZT, Chen MR, Ping Z, Dong YM, Zhang RY, Xu T, Wu ZX. Synaptotagmin IV regulates dense core vesicle (DCV) release in LbetaT2 cells. Biochem Biophys Res Commun 371 : 781–786, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Hui E, Bai J, Sugimori M, Llinas RR, Chapman ER. Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis. Proc Natl Acad Sci USA 102: 5210–5214, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarousse N, Wilson JD, Arac D, Rizo J, Kelly RB. Endocytosis of synaptotagmin 1 is mediated by a novel, tryptophan-containing motif. Traffic 4: 468–478, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Kishore BK, Wade JB, Schorr K, Inoue T, Mandon B, Knepper MA. Expression of synaptotagmin VIII in rat kidney. Am J Physiol Renal Physiol 275: F131–F142, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol 289: F249–F258, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Kocinksy HS, Dynia DW, Wang T, Aronson PS. NHE3 phosphorylation at serines 552 and 605 does not directly affect NHE3 activity. Am J Physiol Renal Physiol 293: F212–F218, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kurashima K, Yu FH, Cabado AG, Szabo EZ, Grinstein S, Orlowski J. Identification of sites required for down-regulation of Na+/H+ exchanger NHE3 activity by cAMP-dependent protein kinase. Phosphorylation-dependent and -independent mechanisms. J Biol Chem 272: 28672–28679, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Lamprecht G, Weinman EJ, Yun CH. The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J Biol Chem 273: 29972–29978, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Lynch KL, Gerona RR, Larsen EC, Marcia RF, Mitchell JC, Martin TF. Synaptotagmin C2A loop 2 mediates Ca2+-dependent SNARE interactions essential for Ca2+-triggered vesicle exocytosis. Mol Biol Cell 18: 4957–4968, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madziva MT, Bai J, Bhalla A, Chapman ER, Edwardson JM. Effects of synaptotagmin reveal two distinct mechanisms of agonist-stimulated internalization of the M4 muscarinic acetylcholine receptor. Br J Pharmacol 144: 761–771, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol 148: 1141–1150, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeil PL, Terasaki M. Coping with the inevitable: how cells repair a torn surface membrane. Nat Cell Biol 3: E124–E129, 2001 [DOI] [PubMed] [Google Scholar]
- 28.McSwine RL, Musch MW, Bookstein C, Xie Y, Rao MC, Chang EB. Regulation of apical membrane Na/H exchangers NHE2 and NHE3 in intestinal epithelial cell line CACO-2BBE/bbe. Am J Physiol Cell Physiol 275: C693–C701, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Michaut M, De Blas G, Tomes CN, Yunes R, Fukuda M, Mayorga LS. Synaptotagmin VI participates in the acrosome reaction of human spermatozoa. Dev Biol 235: 521–529, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Miller RT. Phospho-specific antibodies: more valuable than originally thought. Am J Physiol Renal Physiol 289: F247–F248, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Musch MW, Arvans DL, Walsh-Reitz MM, Uchiyama K, Fukuda M, Chang EB. Synaptotagmin I binds intestinal epithelial NHE3 and mediates cAMP and Ca++-induced endocytosis by recruitment of AP2 and clathrin. Am J Physiol Gastrointest Liver Physiol 292: G1549–G1588, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Petersen MD, Mooseker MS. An in vitro model for the analysis of intestinal brush border assembly. I. Ultrastructural analysis of cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci 105: 445–460, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Saegusa C, Tanaka T, Tani S, Itohara S, Mikoshiba K, Fukuda M. Decreased basal mucus secretion by Slp2-a-deficient gastric surface mucous cells. Genes Cells 11: 623–631, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Sudhof TC. Synaptotagmins: why so many? J Biol Chem 277: 7629–7632, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Vinet AF, Fukuda M, Descoteaux A. The exocytosis regulator synaptotagmin V controls phagocytosis in macrophages. J Immunol 181: 5289–5295, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Weinman EJ, Steplock D, Donowitz M, Shenolikar S. NHERF associations with sodium-hydrogen exchanger isoform 3 (NHE3) and ezrin are essential for cAMP-mediated phosphorylation and inhibition of NHE3. Biochemistry 39: 6123– 6129, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Weninger K, Bowen ME, Choi UB, Chu S, Brunger AT. Accessory proteins stabilize the acceptor complex for synaptobrevin, the 1:1 syntaxin/SNAP-25 complex. Structure 16: 308–320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, He Y, Bai J, Ji SR, Tucker WC, Chapman ER, Sui SF. Visualization of synaptotagmin I oligomers assembled onto lipid monolayers. Proc Natl Acad Sci USA 100: 2082–2087, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wormmeester L, Sanchez de Medina F, Kokke F, Tse CM, Khurana S, Bowser J, Cohen ME, Donowitz M. Quantitative contribution of NHE2 and NHE3 to rabbit ileal brush-border Na+/H+ exchange. Am J Physiol Cell Physiol 274: C1261–C1272, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Yeo C, Barry K, Gontarek D, Donowitz M. Na+-H+ exchange mediates meal-stimulated ileal absorption. Surgery 116: 338–394, 1994 [PubMed] [Google Scholar]
- 42.Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol Gastrointest Liver Physiol 269: G1–G11, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Zachos NC, Tse CM, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Zhang JZ, Davletov BA, Sudhof TC, Anderson RG. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell 78: 751–760, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Zhao H, Wiederkehr MR, Fan L, Collazo RL, Crowder LA, Moe OW. Acute inhibition of Na/H exchanger NHE-3 by cAMP. Role of protein kinase a and NHE-3 phosphoserines 552 and 605. J Biol Chem 274: 3978–3987, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Zhao H, Ito Y, Chappel J, Andrews NW, Teitelbaum SL, Ross FP. Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteoblast secretion. Dev Cell 14: 914–925, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zizak M, Lamprecht G, Steplock D, Tariq N, Shenolikar S, Donowitz M, Yun CH, Weinman EJ. cAMP-induced phosphorylation and inhibition of Na(+)/H(+) exchanger 3 (NHE3) are dependent on the presence but not the phosphorylation of NHE regulatory factor. J Biol Chem 274: 24753–24758, 1999 [DOI] [PubMed] [Google Scholar]