Abstract
Rodents undergo gestational hepatomegaly to meet the increased metabolic demands on the maternal liver during pregnancy. This is an important physiological process, but the mechanisms and signals driving pregnancy-induced liver growth are not known. Here, we show that liver growth during pregnancy precedes maternal body weight gain, is proportional to fetal number, and is a result of hepatocyte hypertrophy associated with cell-cycle progression, polyploidy, and altered expression of cell-cycle regulators p53, Cyclin-D1, and p27. Because circulating reproductive hormones and bile acids are raised in normal pregnant women and can cause liver growth in rodents, these compounds are candidates for the signal driving gestational liver enlargement in rodents. Administration of pregnancy levels of reproductive hormones was not sufficient to cause liver growth, but mouse pregnancy was associated with increased serum bile acid levels. It is known that the bile acid sensor Fxr is required for normal recovery from partial hepatectomy, and we demonstrate that Fxr−/− mice undergo gestational liver growth by adaptive hepatocyte hyperplasia. This is the first identification of any component that is required to maintain the normal mechanisms of gestational hepatomegaly and also implicates Fxr in a physiologically normal process that involves control of the hepatocyte cell cycle. Understanding pregnancy-induced hepatocyte hypertrophy in mice could suggest mechanisms for safely increasing functional liver capacity in women during increased metabolic demand.
Keywords: cholestasis, bile acids, liver regeneration, cell cycle
during pregnancy, enhanced hepatic metabolism is essential to accommodate the increased demand for energy from the developing fetus and the detoxification of fetal metabolites. The pathological consequences of gestational metabolic stress on the liver are highlighted by pregnancy-specific liver diseases including intrahepatic cholestasis of pregnancy, hemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome, and acute fatty liver of pregnancy (12).
Hepatocytes maintain the ability to enter the cell cycle and proliferate in response to mitogenic signals (24). The increase in hepatic capacity during pregnancy in rodents is considered to be met by marked liver enlargement (20). This gestational hepatomegaly has been incompletely characterized, and the cellular mechanisms and signals driving the growth are not known. Pregnancy is associated with alterations in a number of metabolic parameters that are likely to contribute to gestational liver enlargement; examples of these are hyperphagia, insulin, IGF, growth hormone, placental lactogen, and reproductive hormone signaling (2, 5, 9, 10, 26). These factors play important roles in maintaining other physiological adaptations of pregnancy and are likely to have redundant, additive, and complementary roles on driving liver growth.
Bile acids and their nuclear receptors Fxr (NR1H4), Car (NR1I3), or Pxr (NR1I2) are required for the normal mechanisms of liver regeneration following partial hepatectomy (8, 14). Because bile acids are raised during pregnancy in humans (4) and cause liver growth in rodents (14), they could form a component of the signal driving liver growth during pregnancy. However, long-term exposure to raised serum bile acids results in hepatocellular carcinoma (15, 27, 28) and indicates that tight control of the cell cycle during gestational hepatic enlargement is important. Understanding the mechanisms underlying liver growth during pregnancy is, therefore, of particular interest, as it involves the regulation of the cell cycle and adaptive organ enlargement during a normal physiological event and in the presence of mitogenic signals.
In the present study, pregnancy-induced liver growth is characterized in detail for the first time. In addition, the role of physiological concentrations of reproductive hormones, bile acids, and the nuclear receptors Fxr and Pxr in gestational liver enlargement has been determined. These studies provide novel insights into the temporal control of maternal liver growth and the mechanisms and signals driving gestational liver enlargement.
MATERIALS AND METHODS
Materials.
Diets were made by Special Diet Services (Essex, UK). Wild-type mice were from Harlan Laboratories (Bicester, UK). Pxr−/− mice (23) were from GlaxoSmithKline. Fxr−/− mice were generated and validated (Supplementary Methods and Supplemental Fig. S1; supplemental material for this article is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website) in the laboratory of Prof. Johan Auwerx and Dr. Kristina Schoonjans, provided under a materials transfer agreement with the ICS (GIE-CERBM).
Animal treatments.
Animal treatments were conducted in accordance with The Animals (Scientific Procedures) Act 1986. Procedures were approved by the Imperial College London ethical treatment of animals committee. Age-matched 10–12-wk females were used. Reproductive hormone treatments were conducted as described (6, 16–18). Mice were ovariectomized and allowed to recover for 14 days before subcutaneous implantation of silastic tubing containing 17β-estradiol (E2; 33 μg/ml) and progesterone (P4; 0.5 g/ml) or vehicle (Sigma-Aldrich, Dorset, UK) for 18 days. These concentrations result in serum levels equivalent to those in pregnant mice (16–18). Nonpregnant animals were killed on the day of conception, and pregnant animals were killed on the 18th day of gestation. Nonpregnant and pregnant 0.5% cholic acid-fed animals received dietary modification for 30 ± 2 days. Tissues were collected between 1:00 PM and 2:00 PM, following a 4-h fast.
Serum bile acids.
Serum bile acids were determined enzymatically by the 3α-hydroxysteroid dehydrogenase reaction. Bile acids are converted to 3-keto steroids and NADH, which reacts with nitrotetrazolium blue to form formazan dye that is measured (Sentinel Diagnostics, Milan, Italy).
Immunohistochemistry.
Immunohistochemistry was performed according to manufacturerapos;s instructions for β-catenin (Santa Cruz Biotechnology, Santa Cruz, CA), Ki-67 (Dako, Ely, UK), and phospho-histone-H3 (Santa Cruz Biotechnology). Five images were analyzed per animal at ×40 magnification.
Nuclear DNA content.
Nuclear DNA content was quantified as described (25). Sections were stained with Hoechst 33342 (Invitrogen, Carlsbad, CA) (1 μg/ml) and imaged at ×40 magnification. Cell Profiler software (3) identified hepatocyte nuclei and summed the fluorescence of each pixel within the nucleus. One hundred nuclei per animal were considered. The proportion of binucleate hepatocytes was consistent in all mouse treatments, but these were excluded because of artifacts caused by overlapping nuclei.
Hepatocyte apoptosis.
Hepatocyte apoptosis was assessed by terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) assay on histological sections according the manufacturerapos;s instructions (Promega, Madison, WI).
Western blotting.
Western blotting was performed on 100 μg of protein, pooled from six mice. Rabbit polyclonal Cyclin-D1, p27, p53 (Santa Cruz Biotechnology), p21 (Cell Signaling Technology, Danvers, MA), and mouse monoclonal GAPDH (Chemicon, Billerica, MA) were used. Horseradish peroxidase-conjugated secondary antibodies (Dako, Glostrup, Denmark). Bands were detected by ECL Advance Western Blotting Detection Reagents (GE Healthcare, Little Chalfont, UK).
Statistics.
Data are presented as means ± SE of at least five animals. Statistical significance was calculated using Studentapos;s t-test and one-way or two-way ANOVA, indicated in the figure legends. Significance is defined as P < 0.05.
RESULTS
Characterizing gestational liver growth in normal mice.
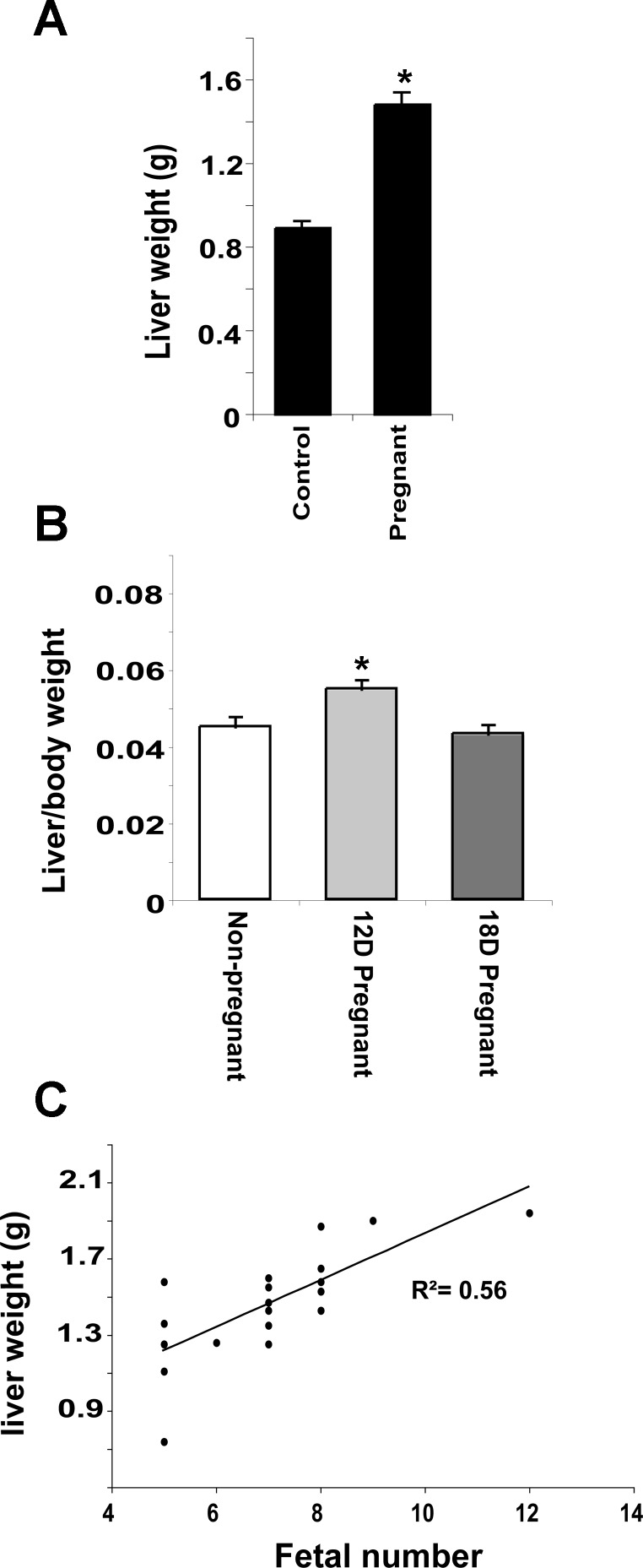
Mean liver weight in 18-day pregnant mice increased 66% (P < 0.01) compared with nonpregnant animals (Fig. 1A). Whereas liver-to-body weight ratio in 18-day pregnant mice is the same as in controls, on the 12th day of gestation, liver weight is disproportional to maternal body weight (Fig. 1B). Liver weight was also determined in pregnant mice with naturally varying numbers of viable implantation sites. Fifty-six percent (r2) of the observed variation in liver weight can be attributed to the number of fetuses (P < 0.001) (Fig. 1C), whereas liver-to-body weight ratio was unaffected by the number of fetuses (data not shown). Together, these data suggest that the liver does not simply sense and respond to changes in maternal body weight and that the signal driving gestational liver enlargement has an effect that is proportional to fetal number or associated placental mass.
Fig. 1.
Gestational liver growth precedes changes in maternal body weight and is proportional to fetal number. A: liver weight in nonpregnant and pregnant mice. B: liver weight as a percentage of body weight at 12 days (12D) and 18 days (18D) postconception. C: liver weight plotted against number of fetuses in utero. Results are shown as means ± SE (n = 6). Studentapos;s t-test, *P < 0.05 compared with nonpregnant animals.
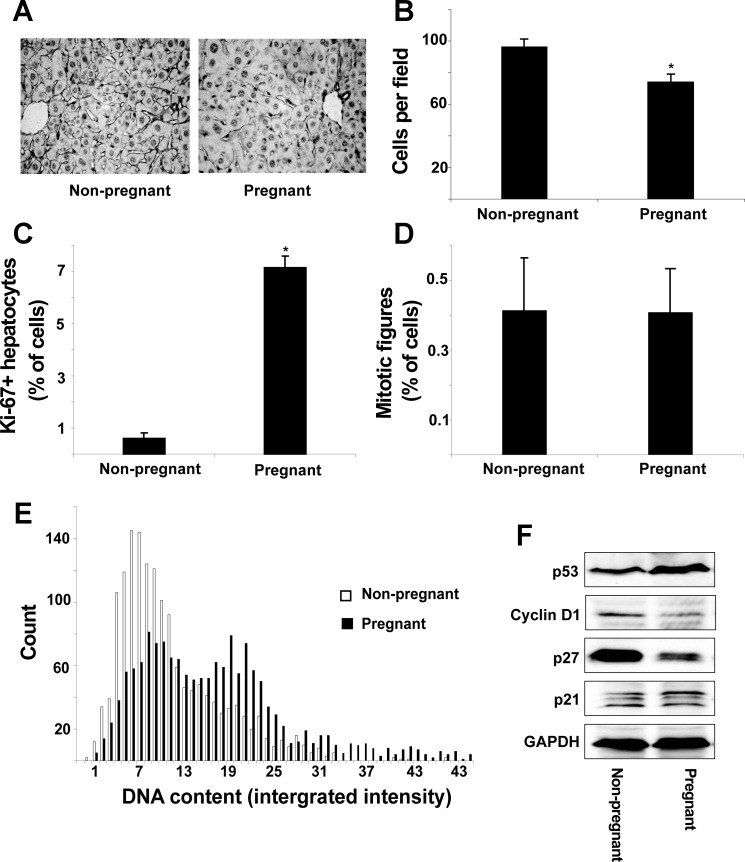
Increased liver size may result from hepatocyte hyperplasia or hypertrophy. Representative liver sections stained with anti-β-catenin antibodies reveal an apparent increase in cell size in pregnant mice compared with nonpregnant controls (Fig. 2A). Indeed, the number of hepatocytes per microscope field was reduced by 19% in pregnant mice, which is consistent with hepatocyte hypertrophy (Fig. 2B). The Ki-67 protein is present during all active phases of the cell cycle (G1, S, G2, and M) but is absent from resting cells (G0). Pregnancy induced hepatocyte cell-cycle progression, as measured by a 10-fold increase in Ki-67-positive hepatocytes (P < 0.01, Fig. 2C), but there was no change in mitotic figures (Fig. 2D), suggesting cell-cycle arrest before mitosis. In addition, TUNEL assays showed no changes in the proportion of apoptotic cells (P = 0.46) (data not shown). Quantitative fluorescent imaging of Hoechst-stained hepatocyte nuclei clearly shows that pregnancy caused the emergence of a cell population containing twice the DNA content of controls (Fig. 2E). This result was confirmed, on biological replicates, by fluorescence-activated cell sorting analysis of propidium iodide-stained crude-isolated primary cells (Supplemental Fig. S2). These results indicate that a large proportion of hepatocytes progress through S-phase before arresting before mitosis.
Fig. 2.
Pregnancy causes liver growth by hepatocyte hypertrophy. A: representative 5-μm liver sections immunostained for β-catenin. B: number of cells per microscope field. Proportion of Ki-67+ (C) and mitotic (phospho-histone-H3 positive) (D) cells is shown. E: hepatocyte nuclear DNA content from 6 nonpregnant and 6 pregnant mice; 1,500 nuclei were considered from each group. F: protein levels of cell-cycle regulators. Results are shown as means ± SE (n = 6). Studentapos;s t-test, *P < 0.05 compared with nonpregnant animals.
The hepatic protein expression of a number of cell-cycle regulators was analyzed (Fig. 2F). The expression of p53 was upregulated, whereas Cyclin-D1 and p27 were considerably downregulated in pregnant mice compared with nonpregnant controls. Hepatic p21 expression was unaffected. These findings demonstrate that pregnancy differentially affects the expression of key cell-cycle regulators during the observed cell-cycle entry and arrest before mitosis.
Therefore, we have demonstrated that gestational liver enlargement is dependent on fetal number, precedes maternal body weight gain, and is achieved by hepatocyte hypertrophy that is associated with altered expression of cell-cycle regulators, cell-cycle entry, progression through DNA replication, and arrest before mitosis.
Gestational liver growth occurs in the presence of raised serum bile acids.
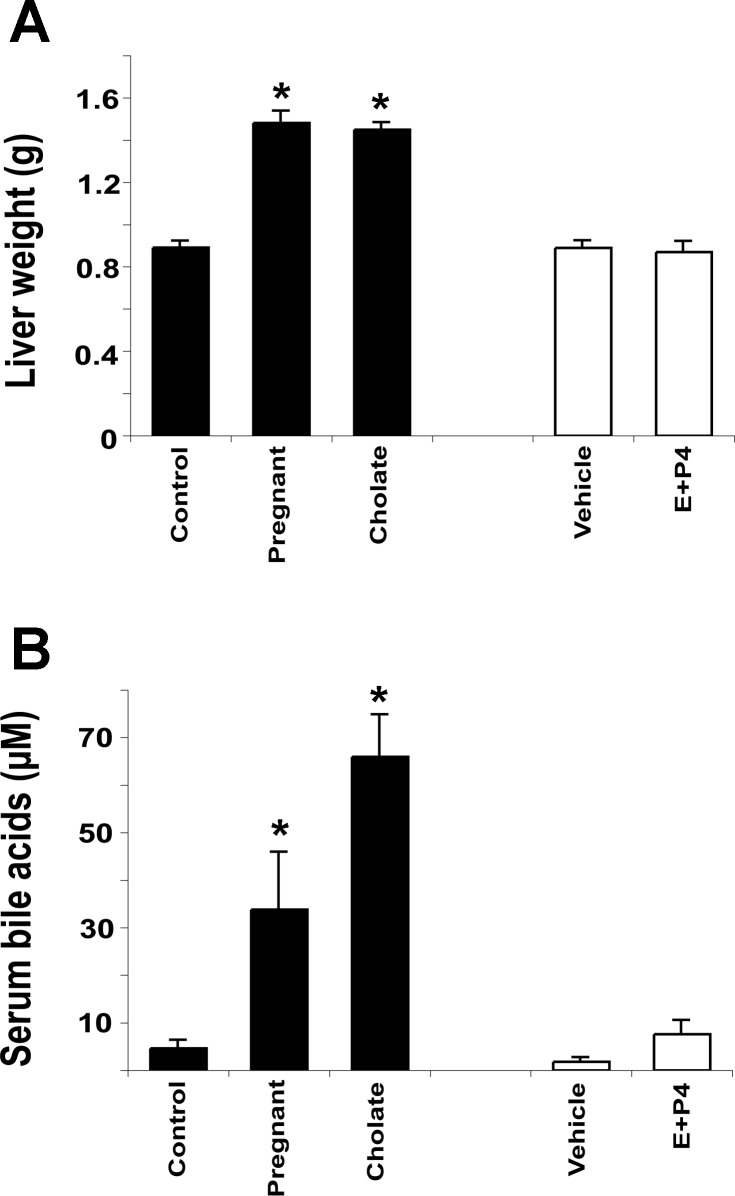
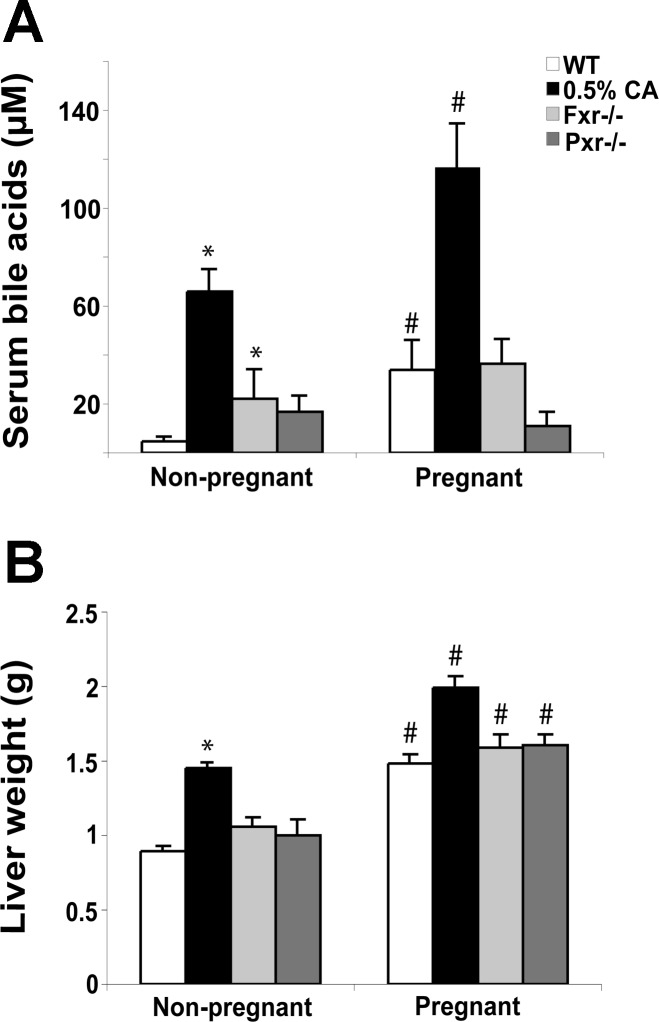
To determine the potential involvement of bile acids and reproductive hormones in gestational liver growth, liver weight and serum bile acid levels were determined in control mice, pregnant mice, mice fed a 0.5% cholate diet for 30 days, and mice implanted with silastic implants containing E2 and P4 for 18 days. Food consumption was not affected by either reproductive hormone or cholate treatment but was increased by 32% in the pregnant mice (data not shown, P < 0.01). Pregnancy and cholate feeding cause a similar increase in liver weight (66% and 63%, respectively; P < 0.01) compared with controls. The E2 and P4 treatments had no significant effect on liver weight (Fig. 3A) but caused uterine swelling in all animals compared with vehicle treatment, demonstrating that the hormone treatment was effective. Serum bile acid levels showed a sevenfold increase in pregnant mice (P < 0.05) and 14-fold increase upon cholate feeding (P < 0.01) compared with control mice and were not significantly affected by the reproductive hormones (Fig. 3B). These data demonstrate that physiological doses of reproductive hormones are not sufficient to drive liver growth. However, bile acids may have a role in mediating gestational liver enlargement.
Fig. 3.
Pregnancy and cholate feeding cause liver growth and increase serum bile acids. A: liver weight. B: total serum bile acid concentration. Results are shown as means ± SE [n = 12 pregnancy and cholate-fed animals and n = 5 17β-estradiol (E) + progesterone (P4)-treated animals]. One-way ANOVA, *P < 0.05 compared with controls.
Cholate feeding causes cell-cycle progression, polyploidy, and hepatomegaly by hyperplasia.
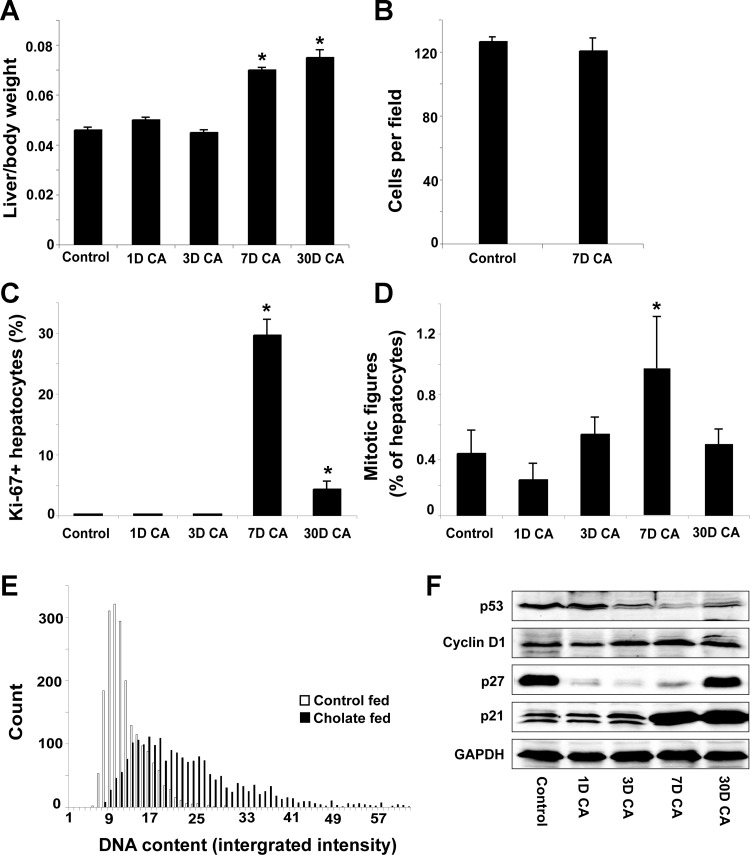
For comparison of pregnancy- induced and cholate feeding-induced liver enlargement, a time course study was conducted to determine the effect of feeding a 0.5% cholic acid-supplemented diet. Cholate feeding caused marked and rapid liver growth between 3 and 7 days (Fig. 4A). There was no effect on hepatocyte size (Fig. 4B), demonstrating that the liver enlargement is not a result of hepatocyte hypertrophy. Ki-67-positive hepatocytes (Fig. 4C) and phospho-histone-H3-positive mitotic figures (Fig. 4D) were increased 290-fold and 2.5-fold, respectively, on the seventh day of feeding, and the cholate diet induced hepatocyte polyploidy, as measured by increased nuclear DNA content (Fig. 4E, Studentapos;s t-test, P < 0.05). These data demonstrate that cholic acid feeding causes liver growth by hepatocyte hyperplasia, which is associated with increased nuclear DNA content.
Fig. 4.
Cholate feeding causes liver growth by hepatocyte proliferation. A: Liver weight as a proportion of body weight. B: hepatocytes per microscope field. Proportion of Ki-67+ (C) and mitotic (phospho-histone-H3 positive) (D) cells is shown. E: hepatocyte nuclear DNA content of 6 control-fed and 6 30-day cholic acid (CA)-fed mice; 2,000 nuclei were considered from each group. F: protein levels of cell-cycle regulators. Results are shown as means ± SE (n = 6). One-way ANOVA, *P < 0.05 compared with control-fed animals.
Cyclin-D1 expression increased after 3 days of cholate feeding and remained higher than controls upon continuation of the feeding regimen (Fig. 4F). p53 protein expression was reduced on the third day of feeding and remained lower than controls despite a small increase in expression between day 7 and day 30. p27 expression was initially downregulated by the cholate diet but increased markedly following long-term cholic acid feeding (Fig. 4F). The expression of p21 was initially unaffected by the cholate diet, but its expression was markedly increased following 7 days of feeding and remained high following long-term treatment. Cholate feeding, therefore, has temporally distinct effects, and upregulation of tumor suppressors following 7 and 30 days of feeding coincides with a stable liver/body weight ratio between these time points (Fig. 4A).
We conclude that, unlike pregnancy-induced hepatomegaly, cholate feeding causes liver growth primarily by hyperplasia. However, both cholate feeding and pregnancy cause cell-cycle entry and polyploidy.
Gestational liver growth is bile acid independent, and Pxr and Fxr are not required.
To further investigate whether bile acids play a role in pregnancy-induced liver growth, we studied gestational liver enlargement in wild-type mice, long-term cholate-fed mice, and mice nullizygous for either Fxr or Pxr. In long-term cholate-fed animals, pregnancy caused a further increase in serum bile acids (76%) and liver weight (37%) (Fig. 5, A and B). These results demonstrate that pregnancy can cause raised serum bile acids and a second wave of liver growth in long-term cholate-fed animals. As expected, nonpregnant Fxr−/− mice had higher bile acids than nonpregnant wild-type mice (Fig. 4A). Despite no significant changes in serum bile acids in pregnancy, we found that both Fxr−/− and Pxr−/− mice undergo gestational liver enlargement that is not significantly different to that observed in wild-type animals (Fig. 5, A and B). These data suggest that bile acid signaling via these receptors is not required to induce or maintain gestational liver growth in mice.
Fig. 5.
Pregnancy induced liver growth and serum bile acid concentrations in long-term cholate-fed, Fxr−/− and Pxr−/− mice. A: total serum bile acids. B: liver weight. Results are shown as means ± SE (n = 6 or more). Two-way ANOVA, *P < 0.05 compared with wild-type (WT), #P < 0.05 compared with nonpregnant animals.
The normal mechanisms of gestational liver growth are not maintained in the absence of Fxr.
Because bile acids, Pxr, and Fxr are implicated in liver regeneration after partial hepatectomy, the mechanism of pregnancy-induced liver growth was analyzed in the absence of Fxr and Pxr and in the presence of cholate-induced maternal cholestasis.
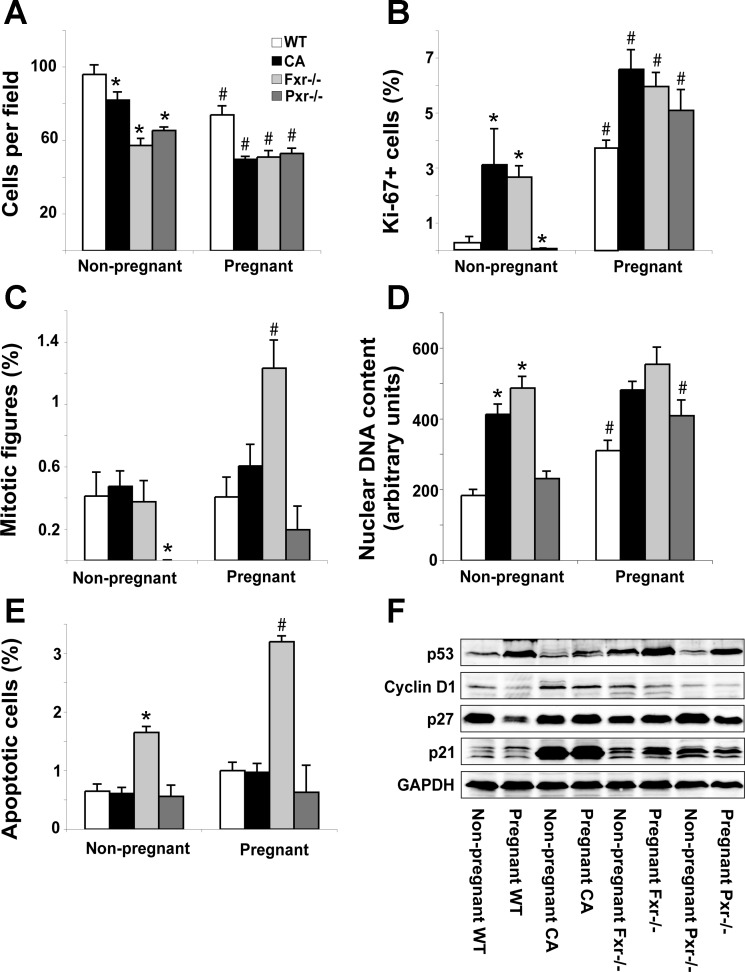
Long-term cholate-fed animals and Pxr−/− mice undergo gestational liver growth by similar mechanisms to wild-type mice. This was shown by a decrease in the number of hepatocytes per microscopic field (Fig. 6A) and increased Ki-67 staining (Fig. 6B) in the absence of mitotic hepatocytes (Fig. 6C). We note, however, that the nonpregnant Pxr−/− animals had a lower proportion of phospho-histone H3-positive mitotic figures than control mice. In contrast to controls and Pxr−/− mice, pregnancy did not result in a further increase in nuclear DNA content in the long-term cholate-fed animals (Fig. 6D), indicating that cell-cycle progression is terminated before S-phase in these mice. In Fxr−/− mice, the pregnancy-associated increase in hepatocyte size was not as marked as in wild-type animals (Fig. 6A). Furthermore, in Fxr−/− mice, the proportion of mitotic figures was significantly increased by pregnancy (Fig. 6C), and there was no significant effect on nuclear DNA content (Fig. 6D). These data indicate that pregnancy-induced liver growth is accomplished by hyperplasia and (limited) hypertrophy in the absence of Fxr. The Fxr−/− mice were the only group to have a raised proportion of apoptotic cells, and this was exacerbated by pregnancy (Fig. 6E).
Fig. 6.
Fxr is required for the normal mechanisms of gestational liver growth. A: number of cells per microscope field. Proportion of Ki-67+ (B) and mitotic (phospho-histone-H3 positive) (C) cells is shown. D: hepatocyte nuclear DNA content. The sum of the DNA content of 100 nuclei per animal was determined as described in materials and methods. E: proportion of apoptotic hepatocytes. F: protein levels of cell-cycle regulators. Results are shown as means ± SE (n = 6). Two-way ANOVA, *P < 0.05 compared with wild-type, #P < 0.05 compared with nonpregnant animals.
Consistent with the phenotypic observations, the effects of pregnancy on cell-cycle regulators were similar in wild-type and Pxr−/− mice. Pregnancy also had similar effects on the expression of p21 and p53 in normally fed and long-term cholate-fed mice, but the expression of Cyclin-D1 and p27 were decreased by pregnancy only in control animals (Fig. 6F). Cyclin-D1 and p53 were similarly affected by pregnancy in wild-type and Fxr−/− mice. However, the expression of the tumor suppressor p27 was induced by pregnancy in Fxr−/− mice, whereas it was markedly repressed by pregnancy in wild-type animals (Fig. 6F). Differences in the observed mechanisms of gestational liver growth in cholate-fed and Fxr−/− mice are, therefore, reflected by the altered expression of cell-cycle regulators.
Although bile acids and pregnancy increased Cyp2b10 in wild-type animals, nonpregnant Fxr−/− mice did not show increased Cyp2b10, and pregnant Fxr−/− mice failed to show an increase in Cyp2b10 (Supplemental Fig. S3). This argues against compensatory activation of Car in mice lacking Fxr. In contrast, Pxr−/− mice showed increases in basal levels of Cyp2b10, suggesting that Car may be activated in Pxr-deficient mice. However, pregnancy-induced increases in Cyp2b10 expression were similar in wild-type and Pxr−/− mice (Supplemental Fig. S3).
From these data, we conclude that the normal mechanism of gestational liver enlargement is broadly maintained in Pxr−/− and cholate-fed mice. In mice lacking Fxr, however, pregnancy-induced hepatocyte hypertrophy is blunted and liver growth is achieved by adaptive hyperplasia.
DISCUSSION
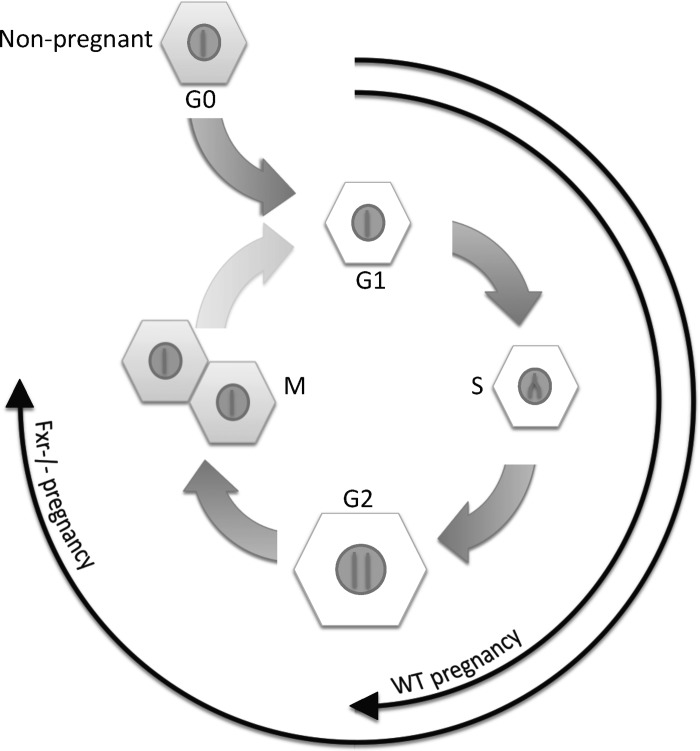
In this study, we have characterized pregnancy-induced liver growth in mice and have provided novel insights into potential signals that drive this process. By demonstrating altered mechanisms of gestational hepatomegaly in the absence of the nuclear receptor Fxr (summarized in Fig. 7), we have shown that adaptive processes exist to maintain gestational liver growth.
Fig. 7.
Schematic representation of the cellular effects of pregnancy in normal and Fxr−/− mice. During pregnancy, the hepatocytes of the liver enter the cell cycle and progress through S-phase. The majority of cells in normal pregnant mice do not progress past G2-phase, resulting in enlarged hepatocytes with increased nuclear DNA content. By contrast, in pregnant Fxr−/− mice, a significant proportion of hepatocytes progress to M-phase, resulting in cells of similar size and nuclear DNA content as those in nonpregnant animals.
Importantly, we show that liver size increases as a linear function of fetal number and is disproportional to maternal body weight during midgestation. These findings demonstrate that the liver is not simply responding to changes in maternal mass and that the increase in size occurs proportionally to the increase in metabolic demand from the fetoplacental unit. Some mechanisms that achieve gestational hepatomegaly have been characterized, in detail, here for the first time. In agreement with Hollister et al. (13), and using independent assays, we report hepatocyte hypertrophy in pregnant mice. Hollister et al. reported that pregnancy in mice did not affect total liver DNA content (13), but, by immunostaining for Ki-67 and performing two independent assays on biological replicates for nuclear DNA content, we have clearly shown that hepatocytes enter the cell cycle and increase their nuclear DNA content during pregnancy. These findings have significant implications in the understanding of the physiological regulation of the hepatocyte cell cycle, in particular its ability to increase functional capacity without risking mitogen-induced proliferation. We propose that the hypertrophic effects of pregnancy are physiologically preferable to potentially harmful hepatocyte proliferation.
The gestational liver growth described here shares some common features with liver growth that occurs during other developmental processes, such as neonatal liver growth (1) and the process of polyploidy that occurs during the suckling to weaning transition (5). However, these processes are distinct because liver growth during pregnancy does not mirror changes in maternal body weight, which is the case in neonatal liver growth. Furthermore, the suckling to weaning transition causes the emergence of binuclear hepatocytes (5), which is a feature not shared with gestational liver growth. Indeed, this is one of the few reports describing a physiological process in adult mice that causes controlled cell-cycle entry and markedly alters the expression of cell-cycle regulators in a normally quiescent organ.
Serum levels of reproductive hormones increase dramatically during pregnancy, and administration of superphysiological levels of E2 (26) or P4 (19) can cause hepatotoxicity and hepatomegaly in rodents. For these reasons, pregnancy levels of E2 and P4 were delivered to mice via silastic implants over a time course similar to that of pregnancy. These treatments had no effect on liver size, suggesting that reproductive hormones alone are unlikely to be the primary liver growth-promoting signal during pregnancy.
Bile acids are raised during pregnancy in humans (4) and can drive liver growth in rodents at relatively low serum concentrations (14). We have shown that total serum bile acids also increase during mouse pregnancy and that the magnitude of liver growth during pregnancy is similar to that caused by feeding a 0.5% cholate diet. However, despite evidence of cell-cycle entry and polyploidy under both conditions, pregnancy and cholate feeding had largely differential effects on the protein expression of the cell-cycle regulators investigated here. Furthermore, cell-cycle progression was uncoupled from mitosis in the pregnant but not cholate-fed mice. Raised serum bile acids in the pregnant mice might contribute to the entry of hepatocytes into the cell cycle, but, if this is the case, other gestational factors prevent the cellular progression to mitosis. Although we have used cholate feeding as a model of bile acid overload, a limitation of this study is that this regimen likely does not mimic the complex changes that occur to the bile acid pool during pregnancy (4). Despite this, the marked differences in cellular events observed in pregnant and cholate-fed mice are unlikely to be explained by subtle alterations in bile acid profiles. Data from Fxr−/− and Pxr−/− mice also demonstrate that raised serum bile acids are not an absolute requirement for gestational liver growth in mice.
Because both cholate feeding and pregnancy cause cell-cycle progression and DNA replication, we assessed the effects of pregnancy in long-term cholate-fed mice and in mice lacking the bile acid receptors Fxr and Pxr. The normal mechanisms of gestational liver enlargement, i.e., hypertrophy, increased DNA replication, and cell-cycle arrest before mitosis, were broadly maintained in Pxr−/− and long-term cholate-fed mice. However, Fxr is required to maintain the normal mechanisms of gestational liver enlargement because, in Fxr−/− mice, hepatocyte proliferation appears to contribute significantly to the liver growth during pregnancy. Although these data indicate redundancy in the mechanisms of gestational liver enlargement, it is possible that the proliferation observed in Fxr−/− mice puts them at even higher risk of developing the hepatocellular carcinoma that is reported in mice lacking Fxr (15, 27). Fxr is required for the normal mechanisms of recovery from partial hepatectomy (14), and this is the first report demonstrating a role for this receptor in maintaining cell-cycle events during a normal developmental process.
Pregnancy is associated with alterations in many factors that could contribute to maternal hepatomegaly. This report has demonstrated that changes in circulating reproductive hormone and bile acid levels are not sufficient to explain pregnancy-induced liver growth. Among others, hyperphagia, insulin, IGF, growth hormone, and placental lactogen signaling are known to affect liver morphology and are also affected during pregnancy (2, 5, 9, 10). Although determining the individual contribution of these factors to gestational liver growth is an important area for future work, this could prove difficult because of their roles in maintaining other physiological adaptations during pregnancy and the fact that these factors are likely to have redundant and overlapping functions. The fact that we observed liver enlargement on the 12th day of pregnancy suggests that lactogens of placental origin may initiate the process because, at this time point, significant placental development has occurred, with placental lactogen release (21). Maternal hepatic growth hormone and prolactin receptor expression increase dramatically during pregnancy (7). In addition, the fact that liver size increases in proportion to fetal number supports the notion that fetal or placental signals contribute to gestational hepatomegaly. Understanding the signals driving pregnancy-induced liver growth, hepatocyte hypertrophy, and cell-cycle arrest at S/G2 in mouse models may suggest strategies to more safely promote increases in liver function in women during periods of increased metabolic demand.
The fact that there are a number of pregnancy-specific liver diseases in humans (e.g., intrahepatic cholestasis of pregnancy, HELLP syndrome, and acute fatty liver of pregnancy) suggests that this organ is under increased metabolic demand during gestation. Our data suggest that, in humans, any effect of pregnancy on liver size will be less marked than in mice because of the reduced fetoplacental mass-to-body weight ratio in pregnant women. Total serum bile acids are increased in pregnant humans (22), and we have also shown this in mice. The biochemical and cell-cycle changes induced in mice by bile acids as observed in the present study may provide useful targets to evaluate during gestational liver disease in women.
In summary, we have characterized the cellular mechanisms that achieve gestational liver enlargement in mice. This is an important physiological process that precedes changes in maternal body weight, causes controlled cell-cycle entry, polyploidy, and cell growth that is uncoupled from potentially harmful mitosis. The bile acid receptor Fxr is the first receptor to be identified as being required for maintaining the normal mechanisms of gestational liver growth. As such, the role of Fxr in controlling functional liver mass is here expanded, beyond liver regeneration, to a normal developmental process.
GRANTS
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC), Institute of Obstetric and Gynaecology (IOG) Trust, Dutch Scientific Organization (NWO), Biomedical Research Centre at Imperial College Healthcare NHS Trust, and GlaxoSmithKline.
DISCLOSURES
The authors disclose no conflict of interest.
Supplementary Material
REFERENCES
- 1. Alfert M, Geschwind The development of polysomaty in rat liver. Exp Cell Res 15: 230–232, 1958. [DOI] [PubMed] [Google Scholar]
- 2. Augustine RA, Ladyman SR, Grattan DR. From feeding one to feeding many: hormone-induced changes in bodyweight homeostasis during pregnancy. J Physiol 586: 387–397, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7: R100, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castano G, Lucangioli S, Sookoian S, Mesquida M, Lemberg A, Di Scala M, Franchi P, Carducci C, Tripodi V. Bile acid profiles by capillary electrophoresis in intrahepatic cholestasis of pregnancy. Clin Sci (Lond) 110: 459–465, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Celton-Morizur S, Merlen G, Couton D, Margall-Ducos G, Desdouets C. The insulin/Akt pathway controls a specific cell division program that leads to generation of binucleated tetraploid liver cells in rodents. J Clin Invest 119: 1880–1887, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen PE, Milligan SR. Silastic implants for delivery of oestradiol to mice. J Reprod Fertil 99: 219–223, 1993. [DOI] [PubMed] [Google Scholar]
- 7. Cramer SD, Wong L, Kensinger RS, Ogren L, Talamantes F. Regulation of the hepatic growth hormone receptor and serum growth hormone-binding protein during pregnancy in the mouse: effects of litter size. Endocrinology 131: 2914–2920, 1992. [DOI] [PubMed] [Google Scholar]
- 8. Dai G, He L, Bu P, Wan YJ. Pregnane X receptor is essential for normal progression of liver regeneration. Hepatology 47: 1277–1287, 2008. [DOI] [PubMed] [Google Scholar]
- 9. El Khattabi I, Remacle C, Reusens B. The regulation of IGFs and IGFBPs by prolactin in primary culture of fetal rat hepatocytes is influenced by maternal malnutrition. Am J Physiol Endocrinol Metab 291: E835–E842, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Freemark M, Kirk K, Pihoker C, Robertson MC, Shiu RP, Driscoll P. Pregnancy lactogens in the rat conceptus and fetus: circulating levels, distribution of binding, and expression of receptor messenger ribonucleic acid. Endocrinology 133: 1830–1842, 1993. [DOI] [PubMed] [Google Scholar]
- 11. Germain AM, Carvajal JA, Glasinovic JC, Kato CS, Williamson C. Intrahepatic cholestasis of pregnancy: an intriguing pregnancy-specific disorder. J Soc Gynecol Investig 9: 10–14, 2002. [DOI] [PubMed] [Google Scholar]
- 12. Hay JE. Liver disease in pregnancy. Hepatology 47: 1067–1076, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Hollister A, Okubara P, Watson JG, Chaykin S. Reproduction in mice: liver enlargement in mice during pregnancy and lactation. Life Sci 40: 11–18, 1987. [DOI] [PubMed] [Google Scholar]
- 14. Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312: 233–236, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28: 940–946, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Milligan SR, Cohen PE. Silastic implants for delivering physiological concentrations of progesterone to mice. Reprod Fertil Dev 6: 235–239, 1994. [DOI] [PubMed] [Google Scholar]
- 17. Milligan SR, Cohen PE, Finn CA. The minimum requirements for oestradiol to induce uterine sensitivity for implantation and decidualization in mice. Hum Reprod 10: 1502–1506, 1995. [DOI] [PubMed] [Google Scholar]
- 18. Milligan SR, Finn CA. Minimal progesterone support required for the maintenance of pregnancy in mice. Hum Reprod 12: 602–607, 1997. [DOI] [PubMed] [Google Scholar]
- 19. Ochs H, Dusterberg B, Schulte-Hermann R. Induction of monooxygenases and growth in rat liver by progesterone. Arch Toxicol 59: 146–149, 1986. [DOI] [PubMed] [Google Scholar]
- 20. Paschkis KE, Cantarow A. Pregnancy, tumor growth, and liver regeneration. Cancer Res 18: 1060–1066, 1958. [PubMed] [Google Scholar]
- 21. Soares MJ. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol 2: 51, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sookoian S, Castano G, Burgueno A, Gianotti TF, Pirola CJ. Association of the multidrug-resistance-associated protein gene (ABCC2) variants with intrahepatic cholestasis of pregnancy. J Hepatol 48: 125–132, 2008. [DOI] [PubMed] [Google Scholar]
- 23. Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98: 3369–3374, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tarla MR, Ramalho FS, Ramalho LN, Silva Tde C, Brandao DF, Ferreira J, Silva Ode C, Zucoloto S. A molecular view of liver regeneration. Acta Cir Bras 21, Suppl 1: 58–62, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Toyoda H, Bregerie O, Vallet A, Nalpas B, Pivert G, Brechot C, Desdouets C. Changes to hepatocyte ploidy and binuclearity profiles during human chronic viral hepatitis. Gut 54: 297–302, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamamoto Y, Moore R, Hess HA, Guo GL, Gonzalez FJ, Korach KS, Maronpot RR, Negishi M. Estrogen receptor alpha mediates 17alpha-ethynylestradiol causing hepatotoxicity. J Biol Chem 281: 16625–16631, 2006. [DOI] [PubMed] [Google Scholar]
- 27. Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res 67: 863–867, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y, Xu P, Park K, Choi Y, Moore DD, Wang L. Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation. Hepatology 48: 289–298, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.