Abstract
Lysophosphatidic acid (LPA), a potent bioactive phospholipid, is a natural component of food products like soy and egg yolk. LPA modulates a number of epithelial functions and has been shown to inhibit cholera toxin-induced diarrhea. Antidiarrheal effects of LPA are known to be mediated by inhibiting chloride secretion. However, the effects of LPA on chloride absorption in the mammalian intestine are not known. The present studies examined the effects of LPA on apical Cl−/OH− exchangers known to be involved in chloride absorption in intestinal epithelial cells. Caco-2 cells were treated with LPA, and Cl−/OH− exchange activity was measured as DIDS-sensitive 36Cl− uptake. Cell surface biotinylation studies were performed to evaluate the effect of LPA on cell surface levels of apical Cl−/OH− exchangers, downregulated in adenoma (DRA) (SLC26A3), and putative anion transporter-1 (SLC26A6). Treatment of Caco-2 cells with LPA (100 μM) significantly stimulated Cl−/OH− exchange activity. Specific agonist for LPA2 receptor mimicked the effects of LPA. LPA-mediated stimulation of Cl−/OH− exchange activity was dependent on activation of phosphatidylinositol 3-kinase/Akt signaling pathway. Consistent with the functional activity, LPA treatment resulted in increased levels of DRA on the apical membrane. Our results demonstrate that LPA stimulates apical Cl−/OH− exchange activity and surface levels of DRA in intestinal epithelial cells. This increase in Cl−/OH− exchange may contribute to the antidiarrheal effects of LPA.
Keywords: downregulated in adenoma, chloride absorption, human intestine, LPA receptor 2, phosphatidylinositol 3-kinase/Akt
lysophosphatidic acid (LPA) is a naturally occurring bioactive glycerophospholipid involved in a broad range of biological processes including platelet aggregation, smooth muscle cell contraction, cell differentiation, and cytoskeletal rearrangements (10, 24, 26). LPA is highly abundant in foods such as soybean and egg yolk (29, 41). Previous studies have suggested that LPA generation is affected by the composition of the diet because high dietary α-linolenic acid has been shown to suppress the formation of LPA in activated platelets (25). LPA is also produced from the plasma membrane lipids in cells and biological fluids by the action of phospholipase A1 or A2 as well as lysophospholipase D (5, 24). Blood platelets are the major source of LPA; however, epithelial cells, macrophages, neuronal cells, and some tumor cells also produce LPA (6, 13, 36). The normal concentration of LPA in the blood ranges from 0.5–50 μM, which increases to high concentrations ranging from 80–100 μM in pathological conditions such as ovarian cancer and inflammation (6, 13, 36, 42).
LPA mediates its effects predominantly by its interaction with specific G protein-coupled receptors (GPCRs) via activation of intracellular signal transduction mechanisms (2–4, 7, 17, 27, 45). To date, seven LPA receptor subtypes have been identified and designated as LPA1–7 receptors (2–4, 7, 17, 21, 30–31, 40). In the gastrointestinal tract, LPA1, 2, and 3 receptor subtypes have been shown to be expressed in colon and in various colonic epithelial cell lines derived from human and rabbit (22, 45). Also, LPA 5 receptor has been shown to be expressed in the mouse small intestine and colon with a moderate level of its expression in stomach (19, 21).
In the gastrointestinal tract, LPA has been implicated to enhance intestinal restitution and wound healing (16, 38). Previous studies have suggested that LPA protects intestinal epithelial cells against radiation- and chemotherapy-induced apoptosis (11). Studies utilizing a rat model of colitis showed that LPA can reduce the degree of inflammation and necrosis in the distal colon compared with control rats (39). Additionally, LPA has been implicated as a potential therapeutic agent in cholera toxin-induced secretory diarrhea in mice by inhibiting CFTR-dependent Cl− channel activity via LPA2 receptor-mediated pathway (22). Diarrhea could result from increased secretion and/or decreased absorption. Previous studies from our laboratories and others have shown NaCl absorption to occur predominantly via an electroneutral process involving the operation of Cl−/OH− exchangers coupled to Na+/H+ exchangers (NHEs) (12, 23, 32, 37). Interestingly, a recent study indicated that LPA stimulates NHE isoform 3 (NHE3) activity in opossum kidney cell line by increasing exocytic trafficking of NHE3 protein on the plasma membrane by activation of phosphatidylinositol 3 (PI3) kinase (20). However, the effects of LPA on intestinal Cl− absorption have not yet been investigated. Recent studies have characterized two members of SLC26 gene family, SLC26A3 or downregulated in adenoma (DRA) and SLC26A6 or putative anion transporter- 1 (PAT-1), as the potential apical membrane Cl−/OH− exchangers involved in electroneutral Cl− absorption along the length of the human intestine (28). Because DRA has been shown to be functionally coupled to NHE3, it was critical to examine the effects of LPA on intestinal apical membrane anion exchangers.
The present studies were undertaken to examine the effects of LPA on apical Cl−/OH− exchangers and to elucidate the signaling pathways and the membrane events involved. Our studies demonstrate that LPA stimulates apical Cl−/OH− exchange activity by increasing the surface levels of DRA on the apical membrane via a LPA2 receptor-mediated process and activation of PI3 kinase/Akt pathways in human intestinal Caco-2 monolayers. These results indicate that an increase in Cl−/OH− exchange activity via increased surface DRA levels may contribute to the antidiarrheal effects of LPA.
MATERIALS AND METHODS
Materials.
Caco-2 cells and MEM were obtained from American Type Culture Collection (ATCC, Manassas, VA). Radionuclide 36Cl was obtained from American Radiolabeled Chemicals (ARC, St. Louis, MO). DIDS and niflumic acid (NFA) were obtained from Sigma Aldrich (St. Louis, MO). 1-Oleoyl-sn-glycerol 3-phosphate sodium salt (LPA) was purchased from Sigma-Aldrich or Avanti Polar Lipids (Alabaster, AL). LPA was prepared in PBS containing 0.1% BSA (vol/vol) and was sonicated before use. Pharmacological inhibitor LY294002 was purchased from Biomol (Plymouth Meeting, PA), and triciribine was procured from Calbiochem (San Diego, CA). Sulfo-NH-SS-biotin was obtained from Pierce Biotechnology (Rockford, IL). All other chemicals were of at least reagent grade and were purchased from Sigma or Fisher Scientific (Pittsburgh, PA).
Cell culture.
Caco-2 cells were grown at 37°C in a 5% CO2 environment in T-75-cm2 plastic flasks. Cells were cultured in MEM medium with high glucose, 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mg/l gentamycin, and 20% fetal bovine serum. For the uptake studies, cells between passages 25 and 45 were plated on 24-well plates at a density of 2 × 104 cells/well. To study the effect of basolateral LPA on Cl−/OH− (HCO3−) exchange activity, Caco-2 cells were plated on Transwell inserts (Costar, Corning, NY) at a density of 1 × 104 cells/Transwell. Uptake studies were performed using fully differentiated cells at day 10–14 postplating. To study the effect of LPA on apical Cl−/OH−(HCO3−) exchange activity, cells were exposed to 100 μM LPA in cell culture medium for 30 min from either the luminal or basolateral side. In a separate set of experiments, cells were pretreated with PI3 kinase inhibitor, LY294002 (50 μM) or Akt inhibitor, triciribine (1 μM), for 1 h before the addition of 100 μM LPA for 30 min. These inhibitors were also coincubated with LPA for another 30 min.
Assessment of Cl−/OH− exchange activity.
Cl−/OH− exchange activity was measured as described previously by our laboratory (35). Caco-2 cells were incubated with loading buffer, pH 8.5, for 30 min at room temperature. The medium was removed, and the cells were rapidly washed with 1 ml tracer-free uptake mannitol buffer containing 260 mM mannitol, 20 mM Tris/2-(N-Morpholino)ethanesulfonic acid, pH 7.0. The cells were then incubated with the uptake buffer containing 1.4 μCi of 36Cl− (2.9 mM) of hydrochloric acid (specific activity: 17.12 mCi/g) for 5 min in the absence or presence of 600 μM DIDS. The 5-min time period was chosen because it falls within the linear range of Cl− uptake in this system. The uptake was terminated by removing the buffer and washing the cells rapidly two times with 1 ml of ice-cold PBS, pH 7.2. Finally, the cells were solubilized by incubation with 0.5 N NaOH for 4 h. The protein concentration was measured by the method of Bradford (9), and the radioactivity was counted by a Packard Liquid Scintillation Analyzer, TRI-CARB 1600-TR (Packard Instruments; PerkinElmer, Boston, MA). The Cl−/OH− exchange activity was assessed as DIDS-sensitive 36Cl− uptake, and the values were expressed as nanomoles per milligram protein per 5 min.
Western blotting.
Differentiated Caco-2 cells were treated with 100 μM LPA for indicated times. After treatment, cells were washed with ice-cold 1× PBS two times and lysed in 20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, and 1× protease cocktail inhibitor mixture. The cells were lysed by sonication, and the lysate was centrifuged at 7,000 revolution/min for 7 min at 4°C. Protein concentration was determined by the Bradford assay (9). To monitor Akt phosphorylation in treated Caco-2 cells, equal amounts (75 μg) of cell lysates were solubilized in gel loading buffer and boiled for 5 min. Samples were loaded on 10% SDS-polyacrylamide gels and transblotted to nitrocellulose membranes. The membranes were incubated in blocking buffer containing 1× TBS and 5% nonfat dry milk for 1 h followed by incubation with rabbit anti-phospho-Akt antibody (1:100 dilution) in 1× TBS and 3% BSA overnight at 4°C. The membranes were washed four times with the wash buffer containing 1× TBS and 0.1% Tween-20 for 5 min. Finally, the membranes were probed with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:2,000 dilution), and the bands were visualized with enhanced chemiluminescence detection reagents.
Cell surface biotinylation and immunoblotting.
Cell surface biotinylation studies were performed in Caco-2 monolayers utilizing Sulfo-NH-SS-Biotin (1.5 mg/ml; Pierce) in borate buffer (in mM: 154 NaCl, 7.2 KCl, 1.8 CaCl2, 10 H3BO3, pH 9.0) as described previously (8). Labeling was allowed to proceed at 4°C to prevent endocytosis and internalization of antigens for 60 min. The biotinylated antigens were immunoprecipitated utilizing streptavidin agarose beads, and the biotinylated proteins were released by boiling in Laemmli buffer containing 100 μM dithiothreitol. Proteins were subjected to SDS-PAGE and transferred to nitrocellulose membranes. Membranes were then labeled with either anti-DRA (custom synthesized against peptide corresponding to COOH-terminal sequence) or anti-PAT-1-purified antibody. The surface DRA or PAT-1 levels were compared with total cell antigen as determined by immunoblotting in solubilized cell extract and with the amount of DRA or PAT-1 not removed by the avidin-precipitation method (intracellular pool).
Statistical analysis.
Results are expressed as means ± SE. Each independent set represents the mean ± SE of data from four to five independent experiments. One-way ANOVA with Dunnett's multiple comparison test was used for statistical analysis. Differences between control and treated groups were considered significant at P < 0.05.
RESULTS
LPA treatment stimulates Cl−/OH− exchange activity.
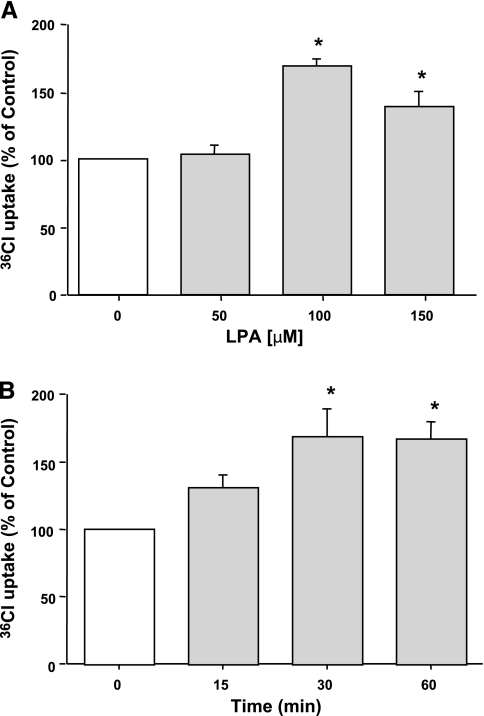
Previous studies have shown that LPA decreases Cl− secretion via inhibition of CFTR chloride channels in intestinal epithelial cells (22). To determine the effects of LPA on Cl− absorption, we examined the effects of LPA on apical Cl−/OH− exchange activity in human intestinal Caco-2 monolayers. Caco-2 cells were serum starved overnight and were treated with different doses of LPA ranging from 50 μM to 150 μM in serum-free media for 30 min, and DIDS-sensitive 36Cl− uptake was assessed in base-loaded differentiated Caco-2 cells. LPA treatment for 30 min resulted in a dose-dependent increase (∼60% at 100 μM) in Cl−/OH− exchange activity (Fig. 1A). A time course for LPA treatment (100 μM) was then assessed in Caco-2 cells for different time periods ranging from 15–60 min. LPA-mediated stimulation of Cl−/OH− exchange activity occurred at 30–60 min with maximal stimulation at 30 min (Fig. 1B). Therefore, for subsequent experiments, 100 μM dose of LPA was used for 30 min.
Fig. 1.
Lysophosphatidic acid (LPA) stimulates Cl−/OH− exchange activity in Caco-2 cells. Serum-starved Caco-2 cells were treated with different doses of LPA (50–150 μM) for 30 min (A) or 100 μM LPA for 15–60 min (B). Cl−/OH− exchange activity was measured as DIDS-sensitive (600 μM) 36Cl− uptake for 5 min in base-loaded cells. Results are expressed as percentages of control and represent means ± SE of 6 separate experiments performed in triplicate. Control values in nmol/mg protein/5 min are as follows: 11.13 ± 1.19 (A) and 8.6 ± 1.62 (B). *P < 0.05 or less compared with control.
LPA 2 receptor is involved in LPA-mediated effects on Cl−/OH− exchange activity.
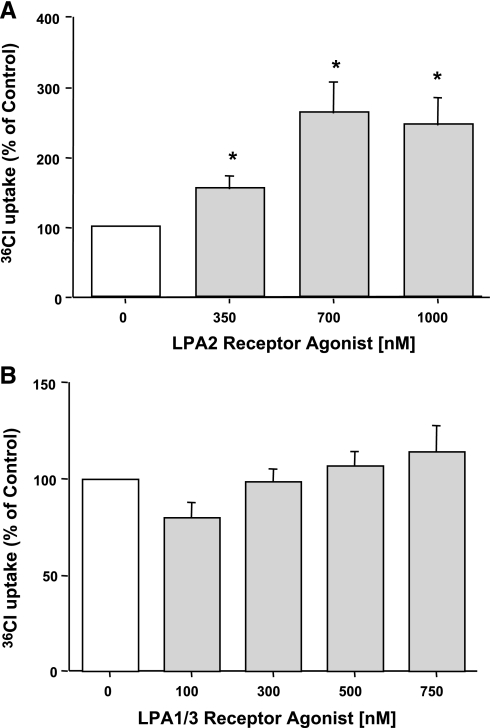
LPA is known to mediate its effects via seven LPA receptors (2–4, 7, 17, 21, 30). To identify the receptor subtype(s) involved in mediating the stimulatory effects of LPA on Cl−/OH− exchange activity, we used specific LPA2 receptor and LPA1/3 receptor agonists. Serum-starved Caco-2 monolayers were treated with different doses of dodecyl phosphate (LPA2 receptor agonist) and VPC 31143 (LPA1/3 receptor agonist) for 30 min. Incubation with LPA2 receptor agonist (350–1,000 nM) significantly increased DIDS-sensitive 36Cl− uptake in Caco-2 cells (Fig. 2A), whereas LPA1/3 receptor agonist (100–700 nM) did not show any effect (Fig. 2B). These results were further confirmed by pretreating the Caco-2 cells with LPA1/3 receptor antagonist VPC 32183 for 1 h followed by treatment with LPA for 30 min. VPC 32183 at 300 nM failed to block the LPA-mediated stimulation of Cl−/OH− exchange activity in Caco-2 cells (data not shown). These studies suggest that LPA2 but not LPA1 and LPA3 receptors mediate the effects of LPA on Cl−/OH− exchange activity.
Fig. 2.
LPA2 receptor is involved. Caco-2 cells were treated with LPA2 receptor agonist dodecyl phosphate (350–1,000 nM) for 30 min (A) or LPA1/3 receptor agonist VPC31143 (100–750 nM) for 30 min (B). Cl−/OH− exchange activity was measured as DIDS-sensitive (600 μM) 36Cl− uptake for 5 min in base-loaded cells. Results are expressed as percentages of control and represent means ± SE of 4 separate experiments performed in triplicate. Control values in nmol/mg protein/5 min are: 5.66 ± 1.3 (A) and 5.8 ± 0.56 (B). *P < 0.05 or less compared with control.
Luminal and basolateral LPA stimulate Cl−/OH− exchange activity on the brush-border membrane of Caco-2 cells.
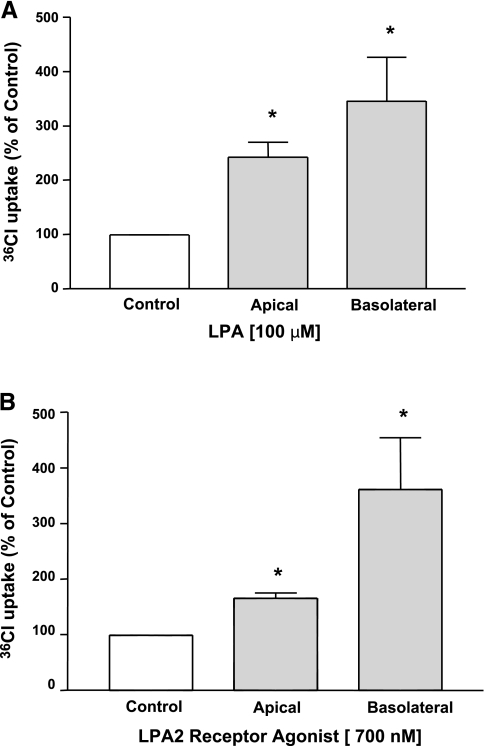
We next examined whether exposure to LPA from basolateral compartment also affected the apical Cl− absorption. For these studies, Caco-2 cells grown on Transwell inserts were exposed to LPA either from the apical or basolateral side, and the effect of another conventional anion exchange inhibitor niflumic acid (NFA) on 36Cl− uptake was assessed in base-loaded differentiated cells. As shown in Fig. 3A, exposure to LPA from both apical and basolateral side (100 μM) for 30 min significantly increased the apical Cl−/OH− exchange activity. Similarly, incubation with LPA2 receptor agonist from either the apical or basolateral side (700 nM) for 30 min significantly increased the NFA-sensitive Cl− uptake across the apical membrane of Caco-2 cells (Fig. 3B). In contrast, treatment with 750 nM of LPA1/3 receptor agonist from either side did not show any effect (data not shown). These data suggest that exposure to LPA from either apical or basolateral side increases the luminal Cl−/OH− exchange activity via LPA2 receptor.
Fig. 3.
Both luminal and serosal LPA increased Cl−/OH− exchange activity. Caco-2 cells grown on Transwells were treated with 100 μM LPA (A) or 700 nM LPA2 receptor agonist (B) from both the apical and basolateral sides. Apical Cl−/OH− exchange activity was measured as niflumic acid-sensitive (100 μM) 36Cl− uptake for 5 min in base-loaded cells. Results are expressed as percentages of control and represent means ± SE of 6 independent wells. Control values in nmol/mg protein/5 min are as follows: apical treatment 1.5 ± 0.29 and basolateral treatment 3.8 ± 0.73 (A); apical treatment 4.7 ± 0.96 and basolateral treatment 3.4 ± 0.46 (B). *P < 0.05 or less compared with control.
Role of PI3 kinase/Akt in LPA-mediated stimulation on Cl−/OH− exchange activity.
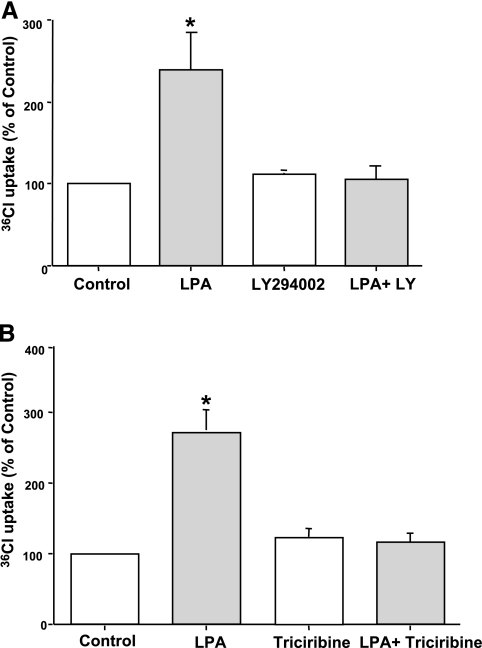
LPA2 receptor has been shown previously to couple to Gi proteins and stimulate PI3-Akt cascade in Caco-2 cells (45). Also, studies have demonstrated the involvement of PI3 kinase in the stimulation of NHE3 by LPA in opossum kidney cell line (20). We thus examined the role of PI3 kinase and Akt pathway in effects of LPA on Cl−/OH− exchange activity utilizing LY294002, a specific inhibitor for PI3 kinase, and triciribine (Akt inhibitor). For these studies, serum-starved Caco-2 cells were pretreated with LY294002 (50 μM) or triciribine (1 μM) for 1 h followed by coincubation in the presence of LPA (100 μM) for 30 min. The stimulation of Cl−/OH− exchange activity in response to LPA treatment was completely abrogated in the presence of LY294002 or triciribine as shown in Fig. 4, A and B. These results indicate that PI3 kinase and Akt are involved in the stimulation of Cl−/OH− exchange activity by LPA.
Fig. 4.
LPA-mediated stimulation of Cl−/OH− exchange activity is phosphatidylinositol 3 kinase (PI3K)-Akt dependent. Caco-2 cells were preincubated with specific PI3K inhibitor, LY294002 (50 μM) (A), or Akt inhibitor, triciribine (1 μM) (B), in the cell culture medium for 60 min. Cells were then coincubated in the presence of LPA (100 μM) for 30 min. Cl−/OH− exchange activity was measured as DIDS-sensitive (600 μM) 36Cl− uptake for 5 min. Results are expressed as percentages of control and represent means ± SE of 4 separate experiments performed in triplicate. Control values in nmol/mg protein/5 min are as follows: 7.19 ± 1.8 (A) and 6.95 ± 1.69 (B). *P < 0.05 or less compared with control.
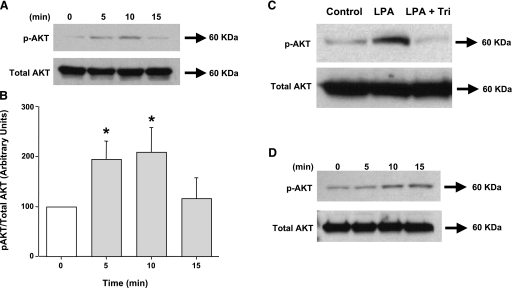
Akt phosphorylation by LPA.
As a direct measure of Akt activation, we determined the phosphorylation of Akt in response to LPA (100 μM) by treating Caco-2 cells with LPA for different time points. Western blot analysis of the total lysates showed that LPA stimulated Akt phosphorylation in Caco-2 cells as early as 5 min, which persisted for at least 10 min (Fig. 5, A and B). This increase in Akt phosphorylation was completely blocked in the presence of Akt inhibitor triciribine (Fig. 5C). Because LPA2 receptors are involved in mediating the effects of LPA on Cl−/OH− exchange activity, we further determined the phospho-Akt levels in response to LPA2 receptor agonist dodecyl phosphate at 700 nM. LPA2 receptor agonist also stimulated Akt phosphorylation in Caco-2 cells at 10 min (Fig. 5D). Also, Akt phosphorylation was attenuated in the presence of PI3 kinase inhibitor LY294002 (data not shown), indicating that Akt is downstream of PI3K.
Fig. 5.
LPA induces Akt phosphorylation in Caco-2 cells. Overnight serum-deprived Caco-2 cells were treated with 100 μM LPA for 5–15 min (A and B), Akt inhibitor, triciribine (Tri) (1 μM) for 60 min and then coincubated with 100 μM LPA for 10 min (C), or LPA2 receptor agonist dodecyl phosphate (700 nM) for 5–15 min (D). Cells were lysed, and extracted proteins were subjected to 10% SDS-polyacrylamide gel for Western blot analysis utilizing phospho-specific Akt antibody. The blots were stripped and reprobed with the anti-Akt antibody to normalize for equal loading of protein in each lane. Quantification of phospho-Akt was performed by densitometric analysis and expressed as arbitrary units and represents means ± SE of 3 different experiments. *P < 0.05 or less compared with control (0 min).
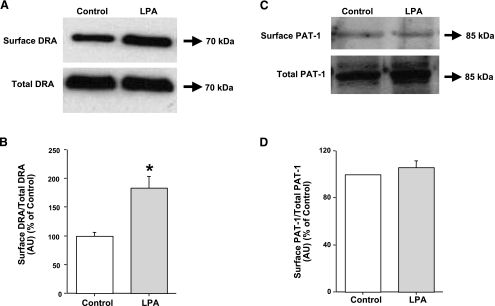
LPA increases surface DRA expression.
Two members of SLC26 gene family DRA and PAT-1 have been implicated in apical Cl−/OH− exchange activity in the human intestine (28). Recently, we have shown that acute modulation of DRA in Caco-2 cells involves alterations in DRA surface levels (8, 14). Therefore, to examine the effects of LPA on surface levels of Cl−/OH− exchangers, cell surface biotinylation studies were performed. Our results showed that LPA treatment significantly increased the surface levels of DRA, whereas the total cellular DRA levels did not change (Fig. 6, A and B). These data are in parallel with an increase in Cl−/OH− exchange activity. Densitometric analysis of the protein bands suggested that LPA treatment increased surface DRA levels by 70–80% compared with control. In contrast, as shown in Fig. 6, C and D, the surface levels of PAT-1 in response to LPA treatment were not significantly altered compared with control. Densitometric analysis showed no quantitative difference in surface PAT-1 levels in LPA-treated cells compared with control.
Fig. 6.
LPA increases surface expression of downregulated in adenoma (DRA) but not putative anion transporter-1 (PAT-1) in Caco-2 cells. Cells were treated with LPA (100 μM) for 30 min in a cell culture medium. Cells were washed with 1× PBS and were subjected to biotinylation at 4°C utilizing sulfo-NH-SS-biotin. Biotinylated proteins were extracted with streptavidin-agarose, and surface and total fractions were run on 10% SDS-polyacrylamide gel. The blot was immunostained with rabbit anti-DRA antibody (A and B) or rabbit anti-PAT-1 antibody (C and D). Representative blots of 3 separate experiments are shown. Results of densitometric analysis are expressed as surface DRA/total DRA or surface PAT-1/total PAT-1. Values represent means ± SE of 3 different experiments. *P < 0.05 or less compared with control.
DISCUSSION
Diarrhea is the most common symptom associated with various gastrointestinal disorders such as inflammatory bowel diseases and bacterial infections and causes high morbidity and mortality. Diarrhea occurs either because of decreased absorption or increased secretion or both of water and electrolytes such as sodium and chloride. Earlier studies have shown that LPA inhibits CFTR-dependent Cl− secretion and stimulates NHE3 activity, which is well known to play a central role in the diarrheal process (22). Therefore, it was of interest to examine the effects of LPA on Cl−/OH− exchange activity in the mammalian intestine. Cl−/OH− exchangers or anion exchangers are known to play a pivotal role in the vectorial transport of Cl− across the plasma membrane of polarized epithelial cells. Previously, we have shown that apical Cl−/OH− exchange activity is inhibited by various inflammatory mediators, such as nitric oxide, serotonin, and phorbol esters, and also by infection with enteropathogenic Escherichia coli in Caco-2 cells and is stimulated by probiotic bacterium, Lactobacillus acidophilus (8, 14, 33–35). In the present studies, our data for the first time demonstrate that LPA significantly stimulates Cl−/OH− exchange activity in Caco-2 cells via activation of PI3 kinase/Akt pathways, suggesting that this effect of LPA could contribute to potential antidiarrheal effects of LPA.
LPA is a biologically active phospholipid that is mainly generated in blood platelets but is also highly abundant in foods such as soybean and egg yolk (13, 29, 41). The effects of LPA are mediated via its interaction with LPA receptors designated as LPA 1–7 (2–4, 7, 17, 21, 30). The LPA1, LPA2, and LPA3 receptors belong to the endothelial differentiation gene family of GPCRs and show about 50% sequence homology to one another (27, 43), whereas, LPA4, LPA5, GPR87 (LPA6), and P2Y5 (LPA7) belong to purinergic receptors and share 35% sequence homology (21, 30–31, 40). LPA receptors differ with respect to their distribution in normal tissues. Earlier studies have shown that LPA1, LPA2, and LPA3 receptors are expressed in colonic epithelial cells and colonic tissues (22, 45). These studies also showed that the expression level of LPA2 is more in colon cancer cell lines (Caco-2, T84, and HT-29) compared with normal colon and noncancerous cells (IEC6) (22, 45). LPA5 is also shown to be expressed at higher levels in small intestine and colon (19, 21, 30). With regard to LPA effects on intestinal Cl−/OH− exchange activity, our studies clearly showed the involvement of LPA2 and ruled out the role of LPA1 and 3 receptors utilizing LPA2 receptor agonists and LPA1/3 receptor agonists and antagonists, respectively. These findings are similar to previous studies showing the involvement of LPA2 receptors in inhibiting the cholera toxin-induced chloride secretion (22). Our results also showed that both luminal and serosal LPA stimulate apical anion exchange activity via LPA2 receptor. Consistent with previous studies (22), these data indicate the expression of LPA2 receptors at both the apical and basolateral membranes of the intestinal epithelial cells. These data suggest that both the luminal (dietary) and serosal (platelets) pools of LPA exert stimulatory effects on apical Cl−/OH− exchange activity. Further studies need to be done to determine the signaling mechanisms involved in the stimulation of Cl−/OH− exchange activity by LPA from the basolateral side.
LPA receptors are known to signal through multiple G proteins, which include G12/13, Gq, Gi, and Gs and trigger distinct intracellular signal transduction mechanisms in mediating the effects (15, 26). Studies have shown that LPA stimulates the PI3 kinase-Akt pathway via Gi in differentiated Caco-2 cells, which predominantly express LPA2 receptor (45). Our data showed that the effects of LPA on Cl−/OH− exchange activity were blocked in the presence of PI3 kinase and Akt inhibitors, indicating the involvement of PI3 kinase-Akt pathway. The involvement of PI3 kinase-dependent pathway is in agreement with other previous studies from our laboratory that have shown the involvement of PI3 kinase in the regulation of Cl−/OH− exchange activity by Lactobacillus acidophilus, taurodeoxycholic acid, and PMA (1, 8, 33). Furthermore, PI3 kinase has been shown to be involved in mediating the effects of LPA on NHE3 in opossum kidney cell line (20). Our data also show the activation of Akt (Akt phosphorylation) by 100 μM LPA and by LPA2 receptor agonist (700 nM) in Caco-2 cells, further confirming the fact that PI3 kinase-Akt activation is downstream of LPA2 receptor.
Previous studies have suggested that PI3 kinase is involved in regulating the amount of transporter on the plasma membrane by translocating the transporters from the intracellular storage sites to the plasma membrane or retrieval of the transporters from the plasma membrane (18). Studies have shown that the stimulation of glucose uptake by interleukin-8 was a PI3 kinase-dependent process and that it involved the membrane trafficking of glucose transporter-1 to the membrane surface mediated via PI3 kinase/Akt pathway (44). In the same manner, our present data clearly demonstrate that LPA treatment significantly increased the surface levels of DRA with no change in the total cellular DRA level. These results are in agreement with our previous studies showing the involvement of PI3 kinase in regulating the surface levels of DRA by Lactobacillus acidophilus (8).
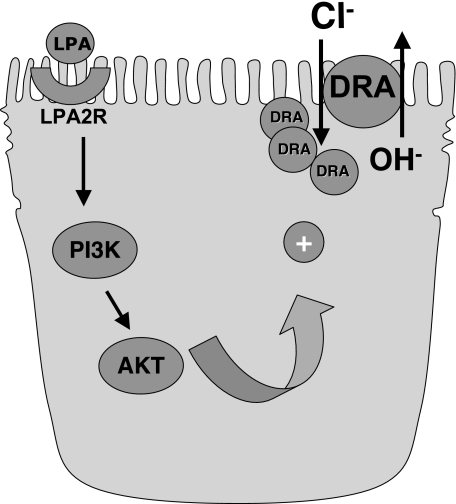
In summary, our results for the first time showed that LPA stimulates Cl−/OH− exchange activity in Caco-2 cells. On the basis of our results, we propose a model (Fig. 7) that LPA through LPA2 receptor and activation of PI3 kinase/Akt pathway increases Cl−/OH− exchange activity. In parallel to increased Cl−/OH− exchange activity, LPA increased the surface levels of DRA but not PAT-1. The results from these studies further enhance our understanding of the mechanisms regulating chloride absorption in the human intestine and also define the mechanisms underlying the beneficial effects of LPA as a potential antidiarrheal and therapeutic agent.
Fig. 7.
Proposed model of LPA-mediated effects on Cl−/OH− exchange activity.
GRANTS
These studies were supported by the department of Veteran Affairs and the NIDDK grants DK 54016 and DK 81858 (P. Dudeja), DK 33349 (K. Ramaswamy), DK 71596 (W. Alrefai), PO1 DK 067887 (P. K. Dudeja and K. Ramaswamy), and CCFA grant reference no. 1942 (S. Saksena).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Alrefai WA, Saksena S, Tyagi S, Gill RK, Ramaswamy K, Dudeja PK. Taurodeoxycholate modulates apical Cl-/OH- exchange activity in Caco2 cells. Dig Dis Sci 52: 1270–1278, 2007 [DOI] [PubMed] [Google Scholar]
- 2.An S, Bleu T, Hallmark OG, Goetzl EJ. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J Biol Chem 273: 7906–7910, 1998 [DOI] [PubMed] [Google Scholar]
- 3.An S, Dickens MA, Bleu T, Hallmark OG, Goetzl EJ. Molecular cloning of the human Edg2 protein and its identification as a functional cellular receptor for lysophosphatidic acid. Biochem Biophys Res Commun 231: 619–622, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Aoki J, Bandoh K, Inoue K. A novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid with unsaturated fatty-acid moiety. Ann NY Acad Sci 905: 263–266, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, Mizuno K, Saku K, Taguchi R, Arai H. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem 277: 48737–48744, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Baker DL, Desiderio DM, Miller DD, Tolley B, Tigyi GJ. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal Biochem 292: 287–295, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, Murakami-Murofushi K, Tsujimoto M, Arai H, Inoue K. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J Biol Chem 274: 27776–27785, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138: 1355–1359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 10.Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol 58: 1188–1196, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Deng W, Wang DA, Gosmanova E, Johnson LR, Tigyi G. LPA protects intestinal epithelial cells from apoptosis by inhibiting the mitochondrial pathway. Am J Physiol Gastrointest Liver Physiol 284: G821–G829, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Dudeja PK, Harig JM, Baldwin ML, Cragoe EJ, Jr, Ramaswamy K, Brasitus TA. Na+ transport in human proximal colonic apical membrane vesicles. Gastroenterology 106: 125–133, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J 291: 677–680, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428–437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetzl EJ, An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J 12: 1589–1598, 1998 [PubMed] [Google Scholar]
- 16.Hines OJ, Ryder N, Chu J, McFadden D. Lysophosphatidic acid stimulates intestinal restitution via cytoskeletal activation and remodeling. J Surg Res 92: 23–28, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Ishii I, Contos JJ, Fukushima N, Chun J. Functional comparisons of the lysophosphatidic acid receptors, LP(A1)/VZG-1/EDG-2, LP(A2)/EDG-4, and LP(A3)/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol Pharmacol 58: 895–902, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Janecki AJ, Janecki M, Akhter S, Donowitz M. Basic fibroblast growth factor stimulates surface expression and activity of Na(+)/H(+) exchanger NHE3 via mechanism involving phosphatidylinositol 3-kinase. J Biol Chem 275: 8133–8142, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther 318: 619–628, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Lee-Kwon W, Kawano K, Choi JW, Kim JH, Donowitz M. Lysophosphatidic acid stimulates brush border Na+/H+ exchanger 3 (NHE3) activity by increasing its exocytosis by an NHE3 kinase A regulatory protein-dependent mechanism. J Biol Chem 278: 16494–16501, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem 281: 23589–23597, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, Nelson DJ, Tigyi GJ, Naren AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med 202: 975–986, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahajan RJ, Baldwin ML, Harig JM, Ramaswamy K, Dudeja PK. Chloride transport in human proximal colonic apical membrane vesicles. Biochim Biophys Acta 1280: 12–18, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev 3: 582–591, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Miyazawa D, Ikemoto A, Fujii Y, Okuyama H. Dietary alpha-linolenic acid suppresses the formation of lysophosphatidic acid, a lipid mediator, in rat platelets compared with linoleic acid. Life Sci 73: 2083–2090, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Moolenaar WH. Bioactive lysophospholipids and their G protein-coupled receptors. Exp Cell Res 253: 230–238, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Moolenaar WH, Kranenburg O, Postma FR, Zondag GC. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr Opin Cell Biol 9: 168–173, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflügers Arch 447: 710–721, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Nakane S, Tokumura A, Waku K, Sugiura T. Hen egg yolk and white contain high amounts of lysophosphatidic acids, growth factor-like lipids: distinct molecular species compositions. Lipids 36: 413–419, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem 278: 25600–25606, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A, Nurnberg P, Nothen MM, Betz RC. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet 40: 329–334, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Ramaswamy K, Harig JM, Kleinman JG, Harris MS, Barry JA. Sodium-proton exchange in human ileal brush-border membrane vesicles. Biochim Biophys Acta 981: 193–199, 1989 [DOI] [PubMed] [Google Scholar]
- 33.Saksena S, Gill RK, Syed IA, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Inhibition of apical Cl-/OH- exchange activity in Caco-2 cells by phorbol esters is mediated by PKCε. Am J Physiol Cell Physiol 283: C1492–C1500, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Saksena S, Gill RK, Syed IA, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Modulation of Cl-/OH- exchange activity in Caco-2 cells by nitric oxide. Am J Physiol Gastrointest Liver Physiol 283: G626–G633, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Saksena S, Gill RK, Tyagi S, Alrefai WA, Sarwar Z, Ramaswamy K, Dudeja PK. Involvement of c-Src and protein kinase C delta in the inhibition of Cl(-)/OH- exchange activity in Caco-2 cells by serotonin. J Biol Chem 280: 11859–11868, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, Igarashi Y, Tigyi G. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem 277: 21197–21206, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Schiller LR, Santa Ana CA, Morawski SG, Fordtran JS. Effect of amiloride on sodium transport in the proximal, distal, and entire human colon in vivo. Dig Dis Sci 33: 969–976, 1988 [DOI] [PubMed] [Google Scholar]
- 38.Sturm A, Sudermann T, Schulte KM, Goebell H, Dignass AU. Modulation of intestinal epithelial wound healing in vitro and in vivo by lysophosphatidic acid. Gastroenterology 117: 368–377, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Sturm A, Zeeh J, Sudermann T, Rath H, Gerken G, Dignass AU. Lisofylline and lysophospholipids ameliorate experimental colitis in rats. Digestion 66: 23–29, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun 363: 861–866, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Tokumura A, Fukuzawa K, Akamatsu Y, Yamada S, Suzuki T, Tsukatani H. Identification of vasopressor phospholipid in crude soybean lecithin. Lipids 13: 468–472, 1978 [DOI] [PubMed] [Google Scholar]
- 42.Tokumura A, Harada K, Fukuzawa K, Tsukatani H. Involvement of lysophospholipase D in the production of lysophosphatidic acid in rat plasma. Biochim Biophys Acta 875: 31–38, 1986 [PubMed] [Google Scholar]
- 43.Toman RE, Spiegel S. Lysophospholipid receptors in the nervous system. Neurochem Res 27: 619–627, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell 18: 1437–1446, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yun CC, Sun H, Wang D, Rusovici R, Castleberry A, Hall RA, Shim H. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol Cell Physiol 289: C2–C11, 2005 [DOI] [PubMed] [Google Scholar]