Abstract
The human sodium-dependent vitamin C transporter-2 (hSVCT2) plays an important role in cellular accumulation of ascorbic acid in liver cells. However, little is known about the molecular determinants that direct hSVCT2 to the cell surface in hepatocytes. We addressed this issue using live cell imaging methods to resolve the distribution and trafficking of truncated or mutated hSVCT2 constructs in a cellular model of human hepatocytes, HepG2 cells. Whereas a full-length hSVCT2-yellow fluorescent protein (YFP) fusion protein was functionally expressed at the cell surface in HepG2 cells, serial truncation and mutation analysis demonstrated an essential role for both NH2- and COOH-terminal sequence(s) for cell surface expression and function. Video-rate confocal imaging showed evidence of dynamic hSVCT2-YFP containing intracellular trafficking vesicles, the motility of which was impaired following disruption of microtubules using nocodazole. However, in a HepG2 cell line stably expressing hSVCT2-YFP at the cell surface, plasma membrane levels of hSVCT2 were unaffected by inhibition of microtubule-associated motor proteins; rather, surface expression of hSVCT2-YFP was increased following treatment with myosin inhibitors. Together, these results show that 1) both NH2- and COOH-terminal sequences are essential for proper localization of hSVCT2, 2) cell surface delivery is dependent on intact microtubules, and 3) peripheral microfilaments regulate insertion and retrieval of hSVCT2 into the plasma membrane.
Keywords: ascorbic acid, uptake, trafficking
vitamin c is an antioxidant that acts as a free radical scavenger and cofactor for several important enzymatic reactions (20). Although many mammals can synthesize vitamin C in the liver, humans have lost the capability for endogenous vitamin C synthesis and must obtain it from dietary sources. Dietary vitamin C deficiency can lead to variety of clinical abnormalities (6, 14, 20), and several recent studies have shown that optimal vitamin C body homeostasis provides protection against hepatic, cardiovascular, and eye disorders as well as cancer (11, 14, 27).
Vitamin C exists in two forms: ascorbic acid (the reduced form), transported via human sodium-dependent vitamin C transporters-1 and 2 (hSVCT1 and hSVCT2, products of the SLC23A1 and SLC23A2 genes) and dehydroascorbic acid (the oxidized form), transported via glucose transporters [such as GLUT1, GLUT3, and GLUT4 (24)]. Both hSVCT1 and hSVCT2 are expressed in human liver, with hSVCT2 expression being higher than hSVCT1 (22, 23, 38). This abundant expression of hSVCT2 in human liver implies a significant physiological role for this particular transporter in liver vitamin C homeostasis. In turn, because the liver plays a crucial role in the distribution, regulation, and maintenance of overall body vitamin C homeostasis, understanding cell biological aspects of hSVCT2 function is important in this context. However, compared with increasing insight into hSVCT function within intestinal absorptive epithelia (5, 18, 30, 35), little is known about the molecular determinants that dictate hSVCT2 expression at the cell surface in hepatic cells.
As with other nutrient transporters, specification of the intracellular trafficking pathways, and hence cell surface targeting profile, likely depends on specific sequence and topological determinants within the hSVCT2 polypeptide. The relevant determinants for hepatocyte cell surface expression could be distributed within the cytoplasmic NH2- and/or COOH-terminal tails of hSVCT2 (10, 19, 21, 29, 30, 34, 36) or throughout the backbone of the polypeptide (16, 31, 33). Alternatively, targeting motifs may be encoded through specific protein conformations resulting from sequences throughout the entire hSVCT2 polypeptide sequence (17, 32, 40). As a first step toward identifying modules within the hSVCT2 sequence important for cell surface expression in hepatocytes, we employed live cell confocal imaging to monitor the trafficking and targeting of several truncated and mutated hSVCT2 proteins fused with the yellow fluorescent protein (YFP) in conjunction with [14C]ascorbic acid uptake measurements performed with the same constructs. These analyses showed that both the cytoplasmic NH2- and COOH-terminal tail regions of hSVCT impact hSVCT2 targeting and functionality, likely by regulating interactions with microtubule and microfilament dependent processes, which we show to be important for regulating hSVCT2 distribution in hepatocytes.
MATERIALS AND METHODS
Materials.
[14C]ascorbic acid (specific activity ∼13 mCi/mmol) was from American Radiolabeled Chemicals (St. Louis, MO). YFP-N1 fluorescent protein was from BD Biosciences (Palo Alto, CA). HepG2 cells were from ATCC (Manassas, VA). DNA oligonucleotide primers were synthesized by Sigma Genosys (Woodlands, TX). Geneticin (G418) was from Invitrogen (Carlsbad, CA). Cytoskeletal disrupting agents and motor protein inhibitors were obtained from Calbiochem (La Jolla, CA).
Generation of hSVCT2 truncated and mutated constructs.
The full-length hSVCT2-YFP and truncated constructs were generated by PCR using the primer combinations shown in Supplemental Table S1 and conditions previously described (30, 31). (The online version of this article contains supplemental data.) The PCR products and the YFP-N1 vectors were digested with HindIII and SacII, and products were gel separated and ligated to generate in-frame fusion proteins with the YFP fused to the COOH terminus of each construct. The Quik change site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used to introduce insertions or deletions of nucleotides into the open reading frame of SLC23A2. Overlapping primers containing the mutated nucleotides to the specified mutation sites (Supplemental Table S2), and full-length hSVCT2-YFP fused plasmid were used as a template for PCR-based site-directed mutagenesis as described (23, 29). The nucleotide sequence of each construct was verified by sequencing (Laragen, Los Angeles, CA).
Cell culture and transient and stable transfections.
The human-derived hepatic epithelial HepG2 cells (from a 15-yr-old male Caucasian) were grown in DMEM supplemented with 10% (vol/vol) FBS, glutamine (0.29 g/l), sodium bicarbonate (2.2 g/l), penicillin (100,000 U/l), and streptomycin (10 mg/l) in 75-cm2 plastic flasks at 37°C in a 5% CO2-95% air atmosphere with medium changes every 2–3 days. For transient transfection, cells were grown on sterile glass-bottomed petri dishes (MatTek, Ashland, MA) and transfected at 90% confluency with 2 μg of plasmid DNA with use of Lipofectamine 2000 (Invitrogen, San Diego, CA). After 24–48 h, live cells were imaged by confocal microscopy. For stable transfection, cells were selected by using G418 (0.5 mg/ml) for 6–8 wk.
Uptake assays.
[14C]ascorbic acid uptake assays were performed on confluent, stably transfected HepG2 cell lines following established procedures (23, 30, 32). Protein contents were estimated on parallel wells by using a protein assay kit (Bio-Rad, Hercules, CA).
Live cell confocal imaging.
Cells cultured on petri dishes were imaged via a Nikon C-1 confocal scanner head attached to a Nikon inverted phase-contrast microscope or a Bio-Rad upright confocal microscope. Fluorophores were excited by use of the 488-nm line from an argon ion laser, and emitted fluorescence was monitored with a 530 ± 20 nm band-pass filter. The motion of individual vesicles was tracked with a custom-built video-rate confocal microscope (4) and data were analyzed by using a frame-to-frame tracking function in Metamorph (Universal Imaging, Downingtown, PA). Videos are provided as Supplemental Movies S1–S3.
Flow cytometry.
Flow cytometry analysis was performed via a FACScalibur bench top cytometer (BD Biosciences, Palo Alto, CA). Stable HepG2 cells were grown within T25 tissue culture flasks. Confluent monolayers were trypsinized, and cells were pelleted and resuspended in 1 ml of Ca2+-free buffer as described previously (16). In all flow cytometry experiments, samples of untransfected and YFP-alone-transfected HepG2 cells were run in parallel with experimental samples, to calibrate optical parameters for identifying the intact, transfected cell population.
Statistical analysis.
Uptake, FACS, and motor protein inhibition data are the result of three separate experiments and are expressed as means ± SE in pmol·mg protein−1·3 min−1. Differences between the means were tested for significance level at P < 0.05 using Student's t-test.
RESULTS
Functional expression of hSVCT2-YFP in HepG2 cells.
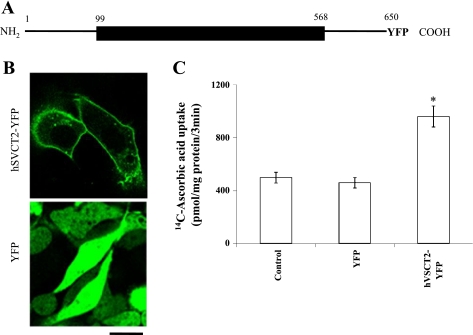
A schematic representation of the full-length hSVCT2-YFP fusion construct is shown in Fig. 1A to illustrate the domain organization of the hSVCT2 protein. This encompasses a long, cytoplasmic NH2-terminal domain (amino acid residues 1–98), followed by a predicted multi-transmembrane-spanning domain (TM) region (TM1–12, amino acid residues 99–568) and a cytoplasmic COOH-terminal tail (amino acids 569–650). The YFP was fused to the COOH terminal tail of the protein to permit visualization of the constructs in live cells. To examine the cell surface expression and functionality of the full-length fused protein, a stable hSVCT2-YFP expressing HepG2 cell line was generated by antibiotic selection with G418. In confluent monolayers of the stable HepG2 cell line, hSVCT2-YFP targeted to the cell surface as well as a population of intracellular vesicles (Fig. 1B). In contrast, cells transfected with YFP showed fluorescence distribution throughout the entire cytoplasmic volume (Fig. 1B). Consistent with these targeting profiles, stable hSVCT2-YFP-expressing cells showed significantly enhanced [14C]ascorbic acid uptake (∼2-fold, P < 0.01) over control or YFP-transfected cells (Fig. 1C).
Fig. 1.
Membrane targeting of human sodium-dependent vitamin C transporter-2 (hSVCT2)-yellow fluorescent protein (YFP) in HepG2 cells. A: schematic representation of the full-length hSVCT2 protein (1–650 amino acids) with YFP fused to the COOH terminus (hSVCT2-YFP). B: lateral (x-y) confocal images of a stable HepG2 cell line expressing hSVCT2-YFP (top) and YFP (bottom). Scale bar = 10 μm. C: [14C]ascorbic acid uptake (32 μM) assays in a stable hSVCT2-YFP expressing HepG2 cell line. Results represent means ± SE (n ≥ 3, *P < 0.01).
Truncation analysis of the hSVCT2 COOH terminus.
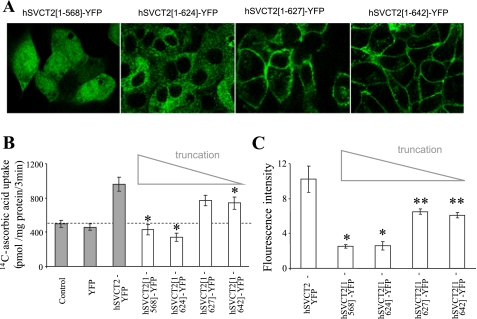
To delimit sequence/molecular determinants within the hSVCT2 polypeptide important for cell surface targeting in HepG2 cells, we first designed a series of four hSVCT2 COOH-terminal truncation constructs (Supplemental Table S1), in which the full-length polypeptide was progressively shortened from the COOH terminus. The cellular expression pattern of each truncated construct was analyzed following stable expression in HepG2 cell lines. Figure 2A shows representative images obtained with these four cytoplasmic COOH-terminal tail truncations (hSVCT2[1-642]-YFP, hSVCT2[1-627]-YFP, hSVCT2[1-624]-YFP and hSVCT2[1-568]-YFP). Complete truncation (82 amino acids) of the COOH-terminal tail of hSVCT2 (hSVCT2[1-568]-YFP) resulted in dim cytoplasmic fluorescence (Fig. 2, A and C), and, in parallel, an ablation of the enhanced [14C]ascorbic acid uptake was observed with the overexpressed full-length hSVCT2 construct (Fig. 2B). Removal of 8 or 23 COOH-terminal amino acids (hSVCT2[1-642]-YFP, hSVCT2[1-627]-YFP) did not affect cell surface expression (Fig. 2A), although an inhibition of [14C]ascorbic acid uptake (P < 0.01, Fig. 2B) and reduced overall population fluorescence intensity was evident (Fig. 2C). However, further deletion of an additional three amino acids (hSVCT2[1-624]-YFP), resulted in the truncated construct being retained within intracellular membranes (Fig. 2A) and enhanced [14C]ascorbic acid uptake was completely abolished (Fig. 2B). Together these results suggest, first, that the region between 625 and 627 within the COOH terminal tail may be important for cell surface expression of hSVCT2 in HepG2 cells and, second, that the COOH-terminal tail is important for regulating optimal cell surface expression of hSVCT2.
Fig. 2.
COOH-terminal truncations disrupt the cell surface targeting of hSVCT2. A: distribution of hSVCT2 truncation constructs in stably expressing HepG2 cells in lateral (x-y) section. B: uptake of [14C]ascorbic acid in control HepG2 cells and cells expressing indicated truncation constructs. Shaded columns are data from Fig. 1C for comparison. C: flow cytometry analysis of the mean fluorescence intensity of populations of HepG2 cells stably expressing indicated constructs different from hSVCT2-YFP. Values are means ± SE (*P < 0.01 and **P < 0.05).
Mutational analysis of the cytoplasmic COOH terminus of hSVCT2.
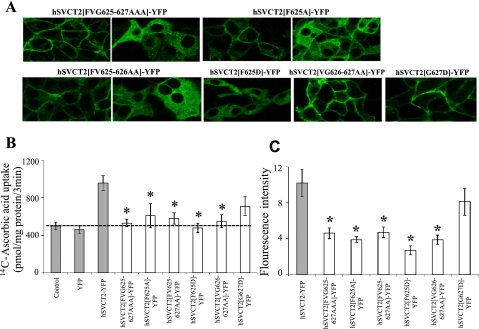
In light of these results, we examined the specific role of the amino acid sequence “FVG” (amino acids 625–627) in regulating the cell surface expression of hSVCT2 in HepG2 cells. To do this, we mutated combinations of these residues within the context of the full-length protein, to validate the importance of this region as implicated by the cruder COOH terminus truncation approach (Fig. 2). First, we substituted each of these three residues with alanine (hSVCT2[FVG/AAA]-YFP). The cellular expression pattern of this construct was pleiotropic, with the majority (∼80%) of cells exhibited retention of the protein within intracellular membranes, whereas a significant proportion of cells exhibited expression at the cell surface (∼20%). Uptake of [14C]ascorbic acid remained at basal levels, likely reflecting the intracellular retention of the mutant transporter, as well as a reduced overall expression level of transporters trafficking to the cell surface (Fig. 3C). Generation of further full-length constructs supported a critical influence of F625 within this triad of residues on cell surface trafficking of hSVCT2; a similar inhibition of expression and uptake profile was observed with both the single-substituted hSVCT2[F/A]-YFP and the double-substituted hSVCT2[FV/AA]-YFP constructs, whereas hSVCT2[VG/AA]-YFP, in which the phenylalanine remained unaltered, trafficked normally to the cell surface. As a further control, we performed a different substitution, comparing the effects of changing F625 or G627 individually to aspartic acid (hSVCT2[F/D]-YFP and hSVCT2[G/D]-YFP). Again, the phenylalanine substitution resulted in a predominantly intracellular expression profile, whereas the same substitution made proximally (hSVCT2[G/D]-YFP) did not affect cell surface targeting. Finally, uptake analysis with all constructs, including those that trafficked normally to the cell surface (hSVCT2[VG/AA]-YFP and hSVCT2[G/D]-YFP), demonstrated that mutations within this sequence impaired accumulation of ascorbic acid, by decreasing transporter functionality and/or expression. These results are consistent with the established theme that individual mutations impairing transporter expression and function are more prevalent than trafficking-deficient alleles.
Fig. 3.
COOH-terminal mutations disrupt the cell surface targeting of hSVCT2 in HepG2 cells. A: distribution of indicated hSVCT2 mutant constructs in stably expressing HepG2 cells in lateral (x-y) section. Images show examples of differing expression profiles observed with indicated constructs. B: uptake of [14C]ascorbic acid in control and stable HepG2 cells. Shaded column data are from Fig. 1C for comparison. C: flow cytometry analysis of the mean fluorescence intensity of population of HepG2 cells stably expressing indicated mutant constructs. Values are means ± SE (*P < 0.01).
In summary, a single amino acid substitution of F625 within the context of the full-length polypeptide resulted in significant, but not complete, retention of hSVCT2 within the endoplasmic reticulum. To investigate other molecular determinants that may function in tandem with this COOH-terminal residue, we proceeded to examine the role of the NH2-terminal cytoplasmic domain of hSVCT2 (36).
Truncation analysis of the hSVCT2 NH2 terminus.
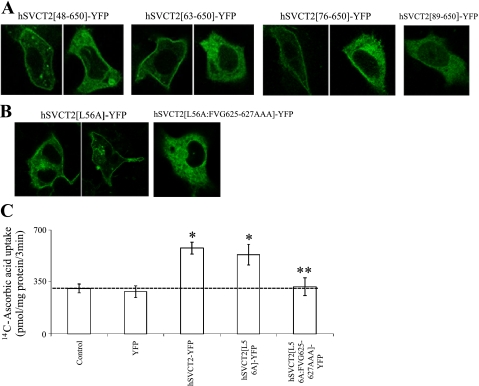
On the basis of the above results, we examined the role of the hSVCT2 NH2-terminal sequence in cell surface expression within HepG2 cells. First, we generated four serial NH2-terminal truncation constructs, ending 10 amino acids upstream of the first transmembrane domain (hSVCT2[89-650]-YFP). These constructs were hSVCT2[89-650]-YFP, hSVCT2[76-650]-YFP, hSVCT2[63-650]-YFP, and hSVCT2[48-650]-YFP. The most severe truncation (hSVCT2[89-650]-YFP) was retained in intracellular membranes, whereas less complete truncations exhibited a progressively greater ability to reach the cell surface (Fig. 4A). Again, for each of these truncations the population expression profile was again pleiotropic: some cells exhibited cell surface expression of hSVCT2, whereas hSVCT2 was retained intracellularly in other cells within the same monolayer. The proportion of cells exhibiting intracellular expression phenotypes was greater with the more extensive truncations, suggesting that the NH2-terminal sequence also plays a role in regulating cell surface expression of hSVCT2 in HepG2 cells.
Fig. 4.
NH2-terminal sequence is important for cell surface targeting of hSVCT2 in HepG2 cells. A and B: distribution of indicated hSVCT2 truncation and mutant constructs transiently (A) and stably expressing HepG2 cell lines (B), in confocal lateral (x-y) section. Images show examples of differing expression profiles observed with indicated constructs. C: [14C]ascorbic acid uptake in control stable HepG2 cell lines. Values are means ± SE; *significantly (P < 0.01) increased compared with control, **significantly (P < 0.01) decreased compared with hSVCT2-YFP.
Consequently, we proceeded to analyze the role of specific residues in the NH2-terminal domain within the context of the full-length hSVCT2 protein. Although truncation results suggested a role of the NH2-terminal sequence upstream of residue 48 in regulating cell surface expression, mutation of several candidate upstream targeting motifs (e.g., hSVCT2[EDE24-26AAA]-YFP, hSVCT2[FF32–33AA]-YFP) did not prevent cell surface targeting (data not shown). In contrast, a recent report (36) discovered the role of a more distal NH2-terminal sequence (LMAI, residues 56–59 in hSVCT2) as a critical regulator of basolateral targeting of hSVCT2 in the polarized Madin-Darby canine kidney (MDCK) cell line. Notably, these authors demonstrated that a single point mutation L56A within the basolateral targeting sequence (albeit within a chimeric hSVCT1/hSVCT2 protein) resulted in increased intracellular retention of the chimeric transporter (36).
In isolation, the single point mutation L56A within the full-length sequence of hSVCT2 (hSVCT2[L56A]-YFP) conferred little functional effect on uptake of [14C]ascorbic acid uptake in HepG2 cells (Fig. 4, B and C), although a subpopulation of cells exhibited an intracellular expression profile in confocal imaging surveys (Fig. 4B). Such intracellular retention was not observed when a trio of upstream residues were mutated (hSVCT2[DTE53–55AAA]-YFP, data not shown). However, when the hSVCT2[L56A]-YFP mutant was made in the context of the COOH terminal FVG/AAA mutant described earlier in which ∼20% of cells retain residual cell surface expression, the fluorescently tagged construct (hSVCT2[L56A][FVG/AAA]-YFP) was retained within the endoplasmic reticulum of stably expressing HepG2 cells, such that little residual cell surface expression was evident. Such an intracellular distribution was consistent with an inhibition of enhanced [14C]ascorbic acid uptake to levels seen in untransfected or mock transfected cells (Fig. 4C). Therefore, these results are consistent with the proposal that both NH2- and COOH-terminal sequences act in concert to regulate the cell surface delivery of hSVCT2 (36).
Intracellular trafficking dynamics of hSVCT2.
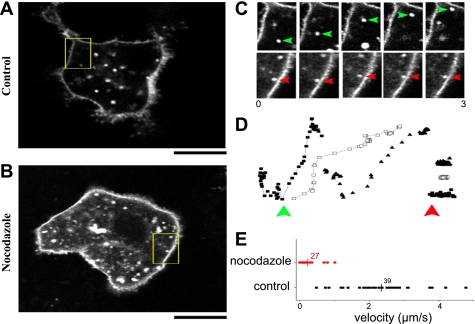
Lateral confocal sections of individual HepG2 cells expressing full-length hSVCT2-YFP revealed numerous vesicular structures within the cytoplasm (Fig. 5A). These structures were resolvable in close juxtaposition to the cell surface, as well as remotely from the plasma membrane within the cytoplasmic volume. These structures appeared heterogeneous in size and dynamics: some were static and resolvable in sequential frames throughout a continuous (≤20 s) record, whereas others were difficult to track from one frame to the next owing to their dynamic behavior (Supplemental Movie S1). Treatment of HepG2 cells with nocodazole (10 μM, 10 min) abolished vesicular motility and resulted in aggregates of immobile vesicles accumulating throughout the cytoplasm (Fig. 5B, Supplemental Movie S2), suggesting an important role for microtubule-dependent events in mediating long-scale vesicular transport. To better quantify this effect and derive insight into the dynamics of these structures in wild-type cells, HepG2 cells expressing hSVCT2-YFP were imaged using a “home-brew,” video-rate confocal microscope (4) permitting x-y imaging at 30 frames/s. A frame-to-frame montage showing representative examples of vesicular motility (e.g., Supplemental Movie S3) in control and nocodazole-treated cells is shown in Fig. 5C. Both examples show that discrete vesicles can move toward, or exist, in close juxtaposition to the cell surface without fusion with the plasma membrane. Point-to-point tracking of individual vesicular dynamics under these conditions (Fig. 5D) reveals that rapid, multidirectional movements of vesicles in intact cells is attenuated by nocodazole, as reflected through collated peak velocity measurements (Fig. 5E).
Fig. 5.
Dynamics of hSVCT2-containing trafficking vesicles. A: lateral (x–y) confocal image of a single hSVCT2-expressing HepG2 cell. B: video still of HepG2 cell treated with nocodazole (10 μM, 15 min). Boxed areas (A and B) are enlarged in C and D. Scale bars = 10 μm. C: image stills exemplifying vesicular dynamics in control (green, top) or nocodazole-treated cells. Tracked vesicles are shown at unequal intervals during a period of 3 s. D: examples of individual tracks representing vesicle motility in control cells (green, left) or cells treated with nocodazole (red, right). E: collated data showing peak velocities (μm/s) of individual vesicles (n > 27 individual puncta) in control (black) or nocodazole-treated (red) cells. Vertical line represents population average.
Effect of pharmacological inhibition of motor proteins.
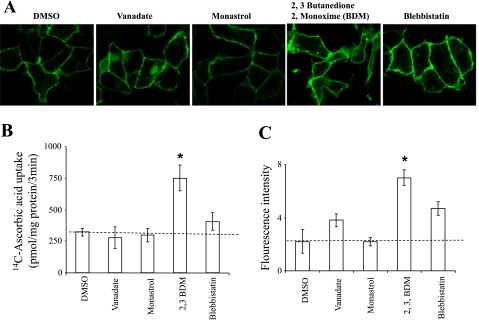
The motor proteins kinesin, dynein, and myosin play important roles in delivering proteins, including vitamin transporters, to the cell surface in many diverse cell types (1, 3, 8, 12, 29, 33). However, the role of these molecular motors in regulating hSVCT2 functional cell surface expression in HepG2 cells, or any other cell type, is unclear. Therefore, we examined the effect of several pharmacological inhibitors, including monastrol (a kinesin inhibitor), sodium orthovanadate (a dynein inhibitor) as well as 2,3-butanedione 2-monoxime (BDM) and blebbistatin (both myosin inhibitors) in the stable hSVCT2-expressing HepG2 cell line. Results showed that neither orthovanadate (100 μM, 24 h) or monastrol (100 μM, 24 h) impacted cell surface expression (Fig. 6A), uptake (Fig. 6B), or overall population fluorescence intensity (Fig. 6C). In contrast, the myosin inhibitors BDM (20 mM, 24 h) and blebbistatin (100 μM, 24 h) markedly increased overall expression of hSVCT2 at the cell surface, as judged by confocal imaging (Fig. 6A) and FACS analysis (Fig. 6C). In parallel with these observations, both treatments resulted in a significant increase in ascorbic acid uptake (Fig. 6B), most notably for BDM (∼3-fold, P > 0.01). Collectively, these data suggest that myosin inhibition regulates the levels of hSVCT present at the cell surface in the stably transfected HepG2 cell line, likely by regulating the final stages of hSVCT2 insertion or by inhibiting endocytosis of hSVCT2.
Fig. 6.
Effect of motor protein inhibitors on hSVCT2 trafficking. A: stable hSVCT2-YFP expressing HepG2 cells were treated with DMSO (control), a cytoplasmic dynein inhibitor (vanadate, 100 μM, 24 h), a kinesin inhibitor (monastrol, 100 μM, 24 h), myosin inhibitors (BDM, 20 mM, 24 h) or blebbistatin (100 μM, 24 h). B: uptake of [14C]ascorbic acid in drug-treated stable hSVCT2-YFP expressing HepG2 cells. C: flow cytometry analysis of the mean fluorescence intensity of populations of hSVCT2-YFP expressing HepG2 cells treated with indicated motor protein inhibitors. Values are means ± SE. (*P < 0.01).
DISCUSSION
Resolving how different cell types absorb, vectorially transport, and recycle vitamin C is crucial for understanding how overall body vitamin C homeostasis is regulated (24, 28). This is especially important in the context of the liver, which is a major organ system for vitamin C utilization. Therefore, in this study, we investigated cell biological aspects of a human sodium-dependent vitamin C transporter (hSVCT2), which is abundantly expressed in liver. We used a human hepatocyte cell line (HepG2) that has been widely used before in such studies (9, 25, 39). In conjunction with hSVCT1, hSVCT2 is presumed responsible for cellular uptake and transport of the reduced form of ascorbic acid (ascorbate) in hepatocytes (22, 23, 26, 28, 38). Both hSVCT1 and hSVCT2 are multi-transmembrane-spanning proteins: topologically, both the NH2 and COOH termini of these transporters reside in the cytoplasm (15, 37), and several pieces of experimental data suggest that these regions play a key role in regulating the cell surface targeting and distribution of transporter expression in polarized cells (10, 19, 21, 29, 30, 34, 36).
First, using the polarized MDCK cell line to express either hSVCT1 (30, 36) or hSVCT2 (36), COOH-terminal regions containing similar sequences [VFKG, amino acids 567–570 in hSVCT1 (30)] and hSVCT2 [SYLPISPTFVGYTWKGLR, amino acids 617–634 in hSVCT2 (36)] were found to be critical for cell surface expression of either transporter isoform. Building on these findings, we confirm that this region also impacts cell surface targeting of hSVCT2 in a human liver cell line and delimit that single amino acid substitutions of F625 in hSVCT2 (Fig. 3A) were sufficient to impair trafficking to the cell surface within the broader 18-residue sequence recently implicated by truncation analysis (36). This phenylalanine residue is conserved in both hSVCT1 (F568) and hSVCT2 (F625), as well as in both mouse and rat SVCT isoforms (15). For other transporters, disruption of specific cytoplasmic phenylalanine residues has previously demonstrated to result in intracellular retention (7, 13). In hSVCT2, F625 occurs within a region that we have previously speculated to be important as a topological motif with high β-turn-forming propensity within the hSVCT1 COOH tail (30). Disruption of this sequence/motif impedes export of either hSVCT isoform to the cell surface. However, the integrity of this region appears more crucial for hSVCT1 than hSVCT2, given that disruption of this region in hSVCT1 completely prevented cell surface expression, resulting in a uniform intracellular expression profile, whereas a small but significant proportion of cells (<20%) expressing hSVCT2[FVG/AAA]-YFP displayed residual cell surface targeting (Fig. 3A) when this COOH-terminal region was mutated in the context of full-length protein. This observation is important in the context of the recent discovery of an additional targeting region needed for correct polarized targeting of hSVCT2 as discussed below.
Second, as regards the role of NH2-terminal sequence, Varma et al. (36) recently identified a basolateral targeting region within the NH2-terminal sequence in hSVCT2, which appears necessary, but not sufficient, for basolateral targeting of hSVCT2 in MDCK cells. By using chimeric analyses, it was demonstrated that swapping only the NH2-terminal domain of hSVCT2 onto hSVCT1 was sufficient to reroute hSVCT1 from the apical to the basolateral domain. Further removal of residues 55–59 (E55LMAI59 of hSVCT2) switched the polarity of hSVCT2 construct expression from basolateral to apical with the caveat that these analyses were predominantly analyzed not in full-length hSVCT2 but within a chimeric template of hSVCT2 and 1, which may be problematic if the cytoplasmic NH2 and COOH termini interact to generate targeting signals. No comparable apical targeting signal was shown to exist in hSVCT1, nor did insertion of this motif into hSVCT1 redirect this transporter to the basolateral surface. Therefore, this important study suggested that, for hSVCT2, both NH2- and COOH-terminal regions are important for appropriate physiological targeting. Our data also are consistent with a role of NH2-terminal sequence in hSVCT2 cell surface expression in hepatocytes: 1) hSVCT2 was retained intracellularly when the NH2 sequence was removed (even upstream of E55LMAI59, Fig. 4A), 2) a single amino acid point mutation (hSVCT2[L56A]) resulted in a shift to intracellular retention of the transporter, and 3) the same mutation, made in the context of the COOH-terminal mutation hSVCT2[L56A][FVG/AAA], completely abolished expression at the hepatocyte plasma membrane. These data collectively imply a role for sequence within both cytoplasmic termini of hSVCT2 for appropriate expression at the cell surface in kidney and liver cell lines. Future work will establish whether similar targeting motifs are also relevant in intestinal epithelia.
Finally, our data implicate roles for both microtubules and microfilaments as regulators of the cell surface expression of hSVCT2. First, the microtubule depolarizing agent nocodazole attenuated the mobility of hSVCT2-containing vesicles when resolved during a period (seconds) of continuous imaging (Fig. 5). Consistent with results with other vitamin transporters (16, 29–31, 33), this result implicates a role of microtubules in dictating transport of hSVCT2-containing vesicles over long (micrometer-scale) distances throughout the cell both to and from the cell surface. Second, results with pharmacological inhibitors demonstrate that incubation (∼24 h) with inhibitors of the myosin-II ATPase, either BDM or blebbistatin, enhanced ascorbic acid uptake into liver cells by increasing the expression of hSVCT2 at the cell surface. This observation may imply a role for microfilament interactions in regulating the insertion and retrieval of hSVCT2 into the cell membrane. Myosin activity may be needed to facilitate endocytic retrieval of hSVCT2 from the cell surface or to regulate cortical actin dynamics that impede insertion of hSVCT2-containing vesicles with the membrane. This result is also consistent with data showing that myosin-II ATPase activity regulates apical lumen morphogenesis in HepG2 cells (12), where inhibition of myosin-II ATPase activity (using either BDM or blebbistatin) enhanced polarization of these cells through apical lumen remodeling. Likely these changes in cellular polarization parallel an increased plasma membrane insertion of hSVCT2 (and possibly endogenous hSVCT1 also). Interestingly, BDM has been shown to decrease apical endocytic uptake of another vitamin, riboflavin, but potentiates vectorial transport of riboflavin across the monolayer (8), a combination of results consistent with upregulation of expression of a basolateral riboflavin transport. However, because this compound has a variety of broad effects beyond inhibition of the myosin ATPase, including effects on membrane protein phosphorylation and membrane characteristics in general (2), these interpretations require further validation.
In conclusion, cell surface targeting of the human SVCT2 transporter in a human hepatocyte cell line is dependent on both NH2- and COOH-terminal sequence within the cytoplasmic domains of these transporters. Cell surface expression of hSVCT2 is also dependent on microtubules for cell surface delivery and modulated by myosin-dependent motor processes.
GRANTS
This study was supported by grants from the Department Veterans Affairs and the National Institute of Health (DK-71538 to V. S. Subramanian, GM-088790 to J. S. Marchant, and DK-58057 to H. M. Said).
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
REFERENCES
- 1.Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2: 149–159, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Artigas P, Al'aref SJ, Hobart EA, Diaz LF, Sakaguchi M, Straw S, Andersen OS. 2,3-Butanedione monoxime affects cystic fibrosis transmembrane conductance regulator channel function through phosphorylation-dependent and phosphorylation-independent mechanisms: the role of bilayer material properties. Mol Pharmacol 70: 2015–2026, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Ashokkumar B, Nabokina SM, Ma TY, Said HM. Identification of dynein light chain road block-1 as a novel interaction partner with the human reduced folate carrier. Am J Physiol Gastrointest Liver Physiol 297: G480–G487, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulware MJ, Marchant JS. Nuclear pore disassembly from endoplasmic reticulum membranes promotes Ca2+ signalling competency. J Physiol 586: 2873–2888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer JC, Campbell CE, Sigurdson WJ, Kuo SM. Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem Biophys Res Commun 334: 150–156, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 69: 1086–1107, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Chen EY, Bartlett MC, Loo TW, Clarke DM. The DeltaF508 mutation disrupts packing of the transmembrane segments of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 279: 39620–39627, 2004 [DOI] [PubMed] [Google Scholar]
- 8.D'Souza VM, Bareford LM, Ray A, Swaan PW. Cytoskeletal scaffolds regulate riboflavin endocytosis and recycling in placental trophoblasts. J Nutr Biochem 17: 821–829, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Dranoff JA, McClure M, Burgstahler AD, Denson LA, Crawford AR, Crawford JM, Karpen SJ, Nathanson MH. Short-term regulation of bile acid uptake by microfilament-dependent translocation of rat ntcp to the plasma membrane. Hepatology 30: 223–229, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Gu HH, Wu X, Giros B, Caron MG, Caplan MJ, Rudnick G. The NH(2) terminus of norepinephrine transporter contains a basolateral localization signal for epithelial cells. Mol Biol Cell 12: 3797–3807, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 98: 2485–2490, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Herrema H, Czajkowska D, Theard D, van der Wouden JM, Kalicharan D, Zolghadr B, Hoekstra D, van Ijzendoorn SC. Rho kinase, myosin-II, and p42/44 MAPK control extracellular matrix-mediated apical bile canalicular lumen morphogenesis in HepG2 cells. Mol Biol Cell 17: 3291–3303, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li HC, Li EY, Neumeier L, Conforti L, Soleimani M. Identification of a novel signal in the cytoplasmic tail of the Na+:HCO3− cotransporter NBC1 that mediates basolateral targeting. Am J Physiol Renal Physiol 292: F1245–F1255, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr 137: 2171–2184, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Liang WJ, Johnson D, Jarvis SM. Vitamin C transport systems of mammalian cells. Mol Membr Biol 18: 87–95, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Marchant JS, Subramanian VS, Parker I, Said HM. Intracellular trafficking and membrane targeting mechanisms of the human reduced folate carrier in Mammalian epithelial cells. J Biol Chem 277: 33325–33333, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Maza R, Poyatos I, Lopez-Corcuera B, Nu E, Gimenez C, Zafra F, Aragon C. The role of N-glycosylation in transport to the plasma membrane and sorting of the neuronal glycine transporter GLYT2. J Biol Chem 276: 2168–2173, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Maulen NP, Henriquez EA, Kempe S, Carcamo JG, Schmid-Kotsas A, Bachem M, Grunert A, Bustamante ME, Nualart F, Vera JC. Up-regulation and polarized expression of the sodium-ascorbic acid transporter SVCT1 in post-confluent differentiated CaCo-2 cells. J Biol Chem 278: 9035–9041, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Muth TR, Ahn J, Caplan MJ. Identification of sorting determinants in the C-terminal cytoplasmic tails of the gamma-aminobutyric acid transporters GAT-2 and GAT-3. J Biol Chem 273: 25616–25627, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Packer L, Fuchs J. Vitamin C in Health and Disease. New York: Dekker, 1997 [Google Scholar]
- 21.Rai T, Sasaki S, Uchida S. Polarized trafficking of the aquaporin-3 water channel is mediated by an NH2-terminal sorting signal. Am J Physiol Cell Physiol 290: C298–C304, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Rajan DP, Huang W, Dutta B, Devoe LD, Leibach FH, Ganapathy V, Prasad PD. Human placental sodium-dependent vitamin C transporter (SVCT2): molecular cloning and transport function. Biochem Biophys Res Commun 262: 762–768, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Reidling JC, Subramanian VS, Dahhan T, Sadat M, Said HM. Mechanisms and regulation of vitamin C uptake: studies of the hSVCT systems in human liver epithelial cells. Am J Physiol Gastrointest Liver Physiol 295: G1217–G1227, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivas CI, Zuniga FA, Salas-Burgos A, Mardones L, Ormazabal V, Vera JC. Vitamin C transporters. J Physiol Biochem 64: 357–375, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Roelofsen H, Wolters H, Van Luyn MJ, Miura N, Kuipers F, Vonk RJ. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology 119: 782–793, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 34: 347–355, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Simon JA, Hudes ES. Serum ascorbic acid and gallbladder disease prevalence among US adults: the Third National Health and Nutrition Examination Survey (NHANES III). Arch Intern Med 160: 931–936, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Sotiriou S, Gispert S, Cheng J, Wang Y, Chen A, Hoogstraten-Miller S, Miller GF, Kwon O, Levine M, Guttentag SH, Nussbaum RL. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med 8: 514–517, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Subramanian VS, Marchant JS, Boulware MJ, Ma TY, Said HM. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am J Physiol Cell Physiol 296: C663–C671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian VS, Marchant JS, Boulware MJ, Said HM. A C-terminal region dictates the apical plasma membrane targeting of the human sodium-dependent vitamin C transporter-1 in polarized epithelia. J Biol Chem 279: 27719–27728, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Subramanian VS, Marchant JS, Parker I, Said HM. Cell biology of the human thiamine transporter-1 (hTHTR1). Intracellular trafficking and membrane targeting mechanisms. J Biol Chem 278: 3976–3984, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Subramanian VS, Marchant JS, Reidling JC, Said HM. N-Glycosylation is required for Na+-dependent vitamin C transporter functionality. Biochem Biophys Res Commun 374: 123–127, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian VS, Marchant JS, Said HM. Targeting and trafficking of the human thiamine transporter-2 in epithelial cells. J Biol Chem 281: 5233–5245, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Sun AQ, Salkar R, Sachchidanand, Xu S, Zeng L, Zhou MM, Suchy FJ. A 14-amino acid sequence with a beta-turn structure is required for apical membrane sorting of the rat ileal bile acid transporter. J Biol Chem 278: 4000–4009, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Takanaga H, Mackenzie B, Hediger MA. Sodium-dependent ascorbic acid transporter family SLC23. Pflügers Arch 447: 677–682, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Varma S, Sobey K, Campbell C, Kuo SM. Hierarchal contribution of N- and C-terminal sequences to the differential localization of homologous sodium-dependent vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochemistry 48: 2969–2980, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Velho AM, Jarvis SM. Topological studies of hSVCT1, the human sodium-dependent vitamin C transporter and the influence of N-glycosylation on its intracellular targeting. Exp Cell Res 315: 2312–2321, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Dutta B, Huang W, Devoe LD, Leibach FH, Ganapathy V, Prasad PD. Human Na(+)-dependent vitamin C transporter 1 (hSVCT1): primary structure, functional characteristics and evidence for a non-functional splice variant. Biochim Biophys Acta 1461: 1–9, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Wojtal KA, de Vries E, Hoekstra D, van Ijzendoorn SC. Efficient trafficking of MDR1/P-glycoprotein to apical canalicular plasma membranes in HepG2 cells requires PKA-RIIalpha anchoring and glucosylceramide. Mol Biol Cell 17: 3638–3650, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou F, Xu W, Hong M, Pan Z, Sinko PJ, Ma J, You G. The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol Pharmacol 67: 868–876, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.