Abstract
Superoxide (O2−) produced by NADPH oxidase regulates Na absorption and renal hemodynamics. Increased NaCl in the thick ascending limb (TAL) stimulates O2− generation. However, we do not know whether physiological changes in NaCl concentration augment O2− generation, nor do we know the mediator(s) involved. In other cells, Rac1, a regulatory subunit of NADPH oxidase, is activated by elevated NaCl. We hypothesized that increasing luminal NaCl within the physiological range activates Rac1 and NADPH oxidase and, thereby, increases O2− production. We increased NaCl from 10 to 57 mM in medullary TAL suspensions and used lucigenin to measure O2− generation and Western blot to measure Rac1 activity. Increasing NaCl stimulated O2− generation from 1.41 ± 0.16 to 2.71 ± 0.30 nmol O2−·min−1·mg protein−1 (n = 6, P < 0.05). This increase was blocked by the Na-K-2Cl cotransporter inhibitor furosemide and the NADPH oxidase inhibitor apocynin. To examine the role of Rac1 in NaCl-induced O2− production, we measured Rac1 translocation by Western blot. When we added NaCl, Rac1 in the particulate fraction increased from 6.8 ± 0.8 to 11.7 ± 2.4% of total Rac1 (n = 7, P < 0.05). Then we measured O2− generation in the presence and absence of the Rac1 inhibitor. In the absence of the Rac1 inhibitor, NaCl increased O2− generation from 1.07 ± 0.24 to 2.02 ± 0.49 nmol O2−·min−1·mg protein−1, and this increase was completely blocked by the inhibitor. Similarly, in vivo treatment of TALs with adenovirus expressing dominant-negative Rac1 decreased NaCl-induced O2− generation by 60% compared with control (0.33 ± 0.04 vs. 0.81 ± 0.17 nmol O2−·min−1·mg protein−1, n = 6, P < 0.05). We concluded that physiological increases in NaCl stimulate TAL O2− generation by activating Rac1.
Keywords: reactive oxygen species, sodium-potassium-chloride cotransport(er), NaCl transport
superoxide (O2−) is a reactive oxygen species that regulates renal function, acting as an autocrine paracrine factor (20, 23). Increased renal O2− generation causes enhanced Na and water retention, ultimately leading to hypertension (13). O2− increases tubular Na absorption along the nephron, including the thick ascending limb (TAL) (23). This segment reabsorbs 20–30% of total NaCl and creates the corticomedullary gradient necessary for water reabsorption in the collecting duct (5). In addition, increased O2− generation in the TAL has been implicated in several forms of hypertension (20). However, the mechanisms involved in stimulation and regulation of O2− generation by this segment are not fully understood.
The luminal NaCl concentration in the TAL varies over a wide range. At the distal end of the segment, NaCl concentrations from as high as 50 mM (3, 9) to as low as ∼15 mM (3, 19) have been reported. In some nephrons, luminal NaCl may be as low as 10 mM (7) as a result of interruptions of luminal flow resulting from peristalsis of the pelvis (18). Mori and Cowley (15) first reported that raising NaCl concentration increases TAL O2− generation. In their studies, increasing bath and luminal NaCl from 150 to 250 mM enhanced O2− production. Subsequently, it was shown that increasing luminal NaCl delivery stimulates O2− (1) via activation of NADPH oxidase (10). Although a rise in luminal NaCl has been shown to stimulate O2− production in perfused tubules (1, 10), it is not clear whether: 1) increasing NaCl per se without flow will have a similar effect; and 2) physiological changes in luminal NaCl stimulate O2− production. Thus it has not been established exactly how increased NaCl heightens NADPH oxidase activity.
Activation of NADPH oxidase involves trafficking and assembly of cytosolic and membrane subunits. One such subunit, the small GTPase Rac1, translocates from the cytosol to the plasma membrane, where it binds the NADPH oxidase complex, increasing O2− generation (11). Rac1 has been considered an intracellular osmosensor in mammalian cells (25). In cultured cells not exposed to luminal flow, increasing osmolality with NaCl enhanced Rac1 activity (4). However, it is not known whether Rac1 participates in NaCl-induced O2− generation in the TAL. We hypothesized that increasing NaCl within the physiological range activates Rac1 and NADPH oxidase and, thereby, increases O2− production.
METHODS
Animals.
This study was approved by the Henry Ford Hospital Institutional Animal Care and Use Committee. All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats weighing 150–200 g (Charles River Breeding Laboratories) were fed a diet containing 0.2% Na and 1% K (Purina) for 7–12 days before the experiment was started.
Medullary TAL suspensions.
Medullary TAL suspensions were prepared as we previously described (21, 22). Briefly, kidneys were perfused retrograde via the abdominal aorta using 40 ml of 0.1% collagenase type I (Sigma-Aldrich) and 100 U of heparin in HEPES-buffered physiological saline containing (in mM) 10 NaCl, 4 KCl, 2.5 NaH2PO4, 1.2 MgSO4, 2 calcium dilactate, 5.5 glucose, 6 d/l-alanine, 1 trisodium citrate, 120 mannitol, and 10 HEPES. The inner stripe of the outer medulla was dissected from coronal slices of the kidney, minced, and incubated at 37°C for 30 min in 0.1% collagenase type I while the suspension was agitated and gassed with 100% O2 every 5 min. Tissue was centrifuged at 93 g for 2 min, resuspended in cold HEPES-buffered physiological saline, and stirred on ice for 30 min. The resulting tubule suspension was filtered through a 250-μm nylon mesh and spun again for 2 min. The pellet was washed and resuspended in 1 ml of cold HEPES-buffered physiological saline.
Measurement of O2− generation.
Aliquots of TAL suspensions were placed in glass tubes in HEPES-buffered physiological saline containing 10 mM NaCl (290 mosmol/kgH2O), N,N′-dimethyl-9,9′-biacridinium dinitrate (lucigenin; Sigma) was added to a final concentration of 5 μM, and the tubes were incubated for 10 min at 37°C. The tubes were placed in a luminometer (FB12/Sirius, Zylux) and maintained at 37°C. After a 5-min baseline period, NaCl in bath and lumen was increased to 57 mM by addition of an isotonic NaCl solution (290 mosmol/kgH2O), which allowed us to raise NaCl without changing osmolality. Measurements were taken for 5 min; then the O2− scavenger 4,5-dihydroxy-1,3-benzenedisulfonic acid (tiron; Sigma) was added to a final concentration of 10 mM, and the measurements were repeated. In the protocols involving furosemide, Rac1 inhibitor, and apocynin, the inhibitors were present from the beginning of the experiment. The difference in average luminescence between periods with and without tiron was used to calculate the luminescence produced by O2−. Measurements were normalized for protein content.
Western blot measurement of Rac1 translocation.
We measured Rac1 translocation as described previously (20, 23). TAL suspensions were exposed to HEPES-buffered physiological saline containing 10 or 57 mM NaCl. Solutions were similar, except that NaCl was increased in the 57 mM NaCl samples and mannitol concentration was reduced to maintain osmolality. After 2 min of incubation at 37°C, tubules were centrifuged at 96 g and resuspended in 250 μl of homogenization buffer containing 50 mM Tris·HCl, 150 mM NaCl, and a protease inhibitor cocktail containing 5 μg/ml antipain, 10 μg/ml aprotinin, 5 μg/ml leupeptin, 5 μg/ml chymostatin, 5 μg/ml pepstatin A, and 0.105 M [4(2-aminoethyl)]-benzene sulfonyl fluoride plus 4 mM benzamidine (Sigma). The suspensions were homogenized using 20 manual strokes in a glass homogenizer and then sonicated on ice for two 20-s cycles at output 2 of an ultrasonic processor (W-385 sonicator, Ultrasonics). The homogenate was centrifuged at 1,200 g for 10 min at 4°C to pellet cell debris and nuclei, and the supernatant was ultracentrifuged at 100,000 g for 60 min at 4°C. The resulting pellet was washed and resuspended in homogenization buffer containing 0.1% Triton X-100 and the protease inhibitor mixture and ultracentrifuged again at 100,000 g. The resulting supernatant was considered the membrane-enriched fraction. SDS-polyacrylamide gels (14%) were loaded with equal amounts of protein for soluble (7 μg) and particulate (15 μg) fractions. Proteins were separated by electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore) at 90 mA. The membrane was incubated in blocking buffer containing 50 mM Tris, 500 mM NaCl, 5% nonfat dry milk, and 0.1% Tween 20 for 60 min. Then a 1:2,000 dilution of a chicken polyclonal antibody against Rac1 (Abcam) was added in blocking buffer for 60 min at room temperature. The membrane was washed in a buffer containing 50 mM Tris, 500 mM NaCl, and 0.1% Tween 20 and incubated with a 1:2,000 dilution of a secondary antibody conjugated to horseradish peroxidase (Pierce). The reaction products were detected with a chemiluminescence kit. The signal was detected by exposure to Fuji RX film and quantified by densitometry.
Adenovirus expressing dominant-negative Rac1.
Dominant-negative Rac1 was generated and provided by Dr. Gary M. Bokoch (Scripps Research Institute, La Jolla, CA). One point mutation at Thr17 was inserted into the full-length Rac1, preventing its activation by phosphorylation (2).
Recombinant replication-deficient adenoviruses encoding for dominant-negative Rac1 under the control of a cytomegalovirus promoter were constructed by ViraQuest (North Liberty, IA), as described previously (21, 22). The complete DNA sequence encoding for dominant-negative Rac1 plus the upstream cytomegalovirus promoter was inserted into a shuttle plasmid (pVQAd5CMVK-NpA) provided by ViraQuest, which contains a cloning site for insertion of a heterologous gene and a polyadenylation signal flanked by adenoviral sequences 5′ and 3′. The plasmids were transfected into permissive HEK-293 host cells, along with a nearly full-length adenoviral DNA that was restricted to remove the E1 region. Virus isolates were plaque-purified, propagated in HEK-293 cells, isolated, concentrated, and titered. The final titer of the adenovirus was 1.9 × 1012 particles/ml.
In vivo adenovirus-mediated kidney transduction.
We performed in vivo adenovirus-mediated transduction of TALs as described previously (21, 22). Briefly, rats were anesthetized with ketamine (60 mg/kg ip) and xylazine (20 mg/kg ip) before surgery. The left kidney was exposed via a left flank incision, the fatty tissue around the renal pole was removed, and the renal artery and vein were clamped. Then four 20-μl injections of 1.9 × 1012 particles/ml were made using a custom-made 30-gauge needle attached to polyethylene (PE-10) tubing, which was connected to a Harvard syringe pump set at 20 μl/min. The needle was inserted perpendicular to the renal capsule, parallel to the medullary rays, and directed toward the medulla. Injections were made along the longitudinal axis of the kidney. Injection points were separated by 2.5 mm. To avoid bleeding and leakage of the virus, the needle remained in place for 30 s after infusion was complete. The clamp was removed from the renal artery and vein after 6.5–8 min. After renal blood flow was reestablished, the kidney was returned to the abdominal cavity and the incision was sutured. We previously showed that ≥91% of the TALs can be efficiently transduced using this technique (15). Since we found that peak dominant-negative Rac1 expression occurred 3–5 days after the injection, experiments were performed at this time point.
Determination of protein content.
Total protein content was determined using Coomassie Plus reagent (Pierce, Rockford, IL), based on Bradford's colorimetric method.
Statistics.
Values are means ± SE. Differences in means were analyzed using Student‘s t-test for paired experiments or an unpaired t-test, with application of Hochberg’s adjustment when appropriate to determine significance. Statistical analysis was performed by the Henry Ford Hospital Department of Biostatistics and Epidemiology.
RESULTS
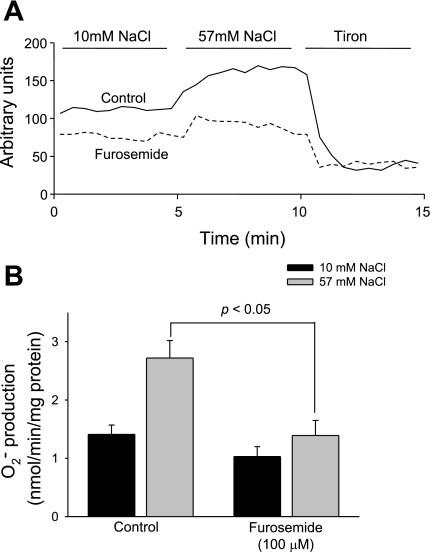
To determine whether increasing extracellular NaCl in the absence of luminal flow stimulates O2− generation in TALs, we measured O2− generation after changing NaCl from 10 to 57 mM. We found that O2− generation increased from 1.41 ± 0.16 to 2.72 ± 0.30 nmol O2−·min−1·mg protein−1 (P < 0.05, n = 6; Fig. 1).
Fig. 1.
Effect of increased NaCl on thick ascending limb (TAL) O2− generation. A: results from a representative experiment. B: NaCl-induced O2− generation was completely blocked in the presence of the Na-K-2Cl cotransporter inhibitor furosemide.
To investigate whether NaCl-induced O2− generation is due to increased luminal NaCl and transport by the Na-K-2Cl cotransporter, we measured O2− generation in the presence of furosemide (100 μM). We found that increasing NaCl from 10 to 57 mM stimulated O2− generation from 1.03 ± 0.16 to only 1.40 ± 0.25 nmol O2−·min−1·mg protein−1, representing 73% inhibition (P < 0.05 vs. control, n = 6; Fig. 1). Taken together, these data indicate that physiological increases of NaCl stimulate O2− generation in the TAL via the Na-K-2Cl cotransporter. They also show that NaCl per se can enhance O2− production without the need for luminal flow.
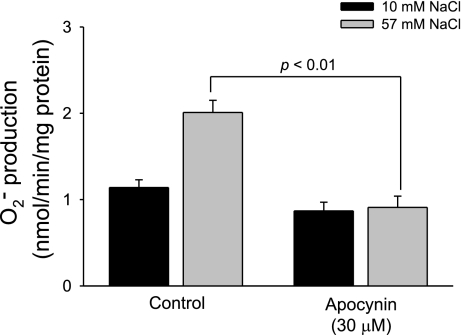
We used apocynin to test whether NADPH oxidase activation is involved in NaCl-induced O2− generation. In the absence of apocynin, increasing NaCl concentration from 10 to 57 mM stimulated O2− generation from 1.14 ± 0.10 to 2.01 ± 0.14 nmol O2−·min−1·mg protein−1. In contrast, in the presence of apocynin (30 μM), increasing NaCl concentration from 10 to 57 mM did not significantly enhance O2− generation (from 0.87 ± 0.10 to 0.91 ± 0.13 nmol O2−·min−1·mg protein−1, P < 0.01 vs. control, n = 6; Fig. 2).
Fig. 2.
Effect of increased NaCl on TAL O2− generation during inhibition of NADPH oxidase. Increased NaCl concentration in the presence of the NADPH oxidase inhibitor apocynin inhibited O2− generation (n = 6).
Others have postulated that apocynin is a O2− scavenger, rather than an NADPH oxidase inhibitor. Therefore, using xanthine oxidase (10 mU) and hypoxanthine (500 μM) as sources of O2− in a cell-free system, we tested whether apocynin was acting as a scavenger in our preparations. We found that, in the absence of apocynin, O2− generation was 5.49 ± 0.45 arbitrary light units. Even after addition of apocynin, it remained essentially unchanged (5.47 ± 0.43 arbitrary light units, n = 5, not significant). These data indicate that, at least under the conditions of our experiment, apocynin does not act as a O2− scavenger. Taken together, they show that increased NaCl enhances O2− generation by activating NADPH oxidase.
In several types of cells, Rac1 regulates O2− generation (11, 14), and increasing osmolality with NaCl enhances Rac1 activation (4). Thus we first tested whether increases in NaCl concentration in the physiological range stimulate Rac1 activity in the TAL in isotonic conditions. In cells exposed to physiological saline (290 mosmol/kgH2O) containing 10 mM NaCl, Rac1 in the membrane-enriched fraction was 6.8 ± 0.8% of total Rac1. In contrast, after NaCl was increased to 57 mM in isotonic conditions, Rac1 in the membrane-enriched fraction increased to 11.7 ± 2.4% of total Rac1 (P < 0.05, n = 7; Fig. 3).
Fig. 3.
Effect of increased NaCl on Rac1 activity. Increasing NaCl from 10 to 57 mM increased Rac1 activity in medullary TAL suspensions measured by translocation (n = 7). Sol, soluble; Par, particulate.
To show that the effect described above was due to increased intracellular Na, rather than some nonspecific effect, we used the Na ionophore nystatin. Addition of nystatin (300 U/ml) under isotonic conditions increased the percentage of Rac1 in the particulate fraction by 481 ± 110% (P < 0.01, n = 6). Taken together, these data indicate that increased intracellular Na stimulates Rac1 translocation in the TAL and that increased NaCl in the physiological range enhances Rac1 activity in this nephron segment.
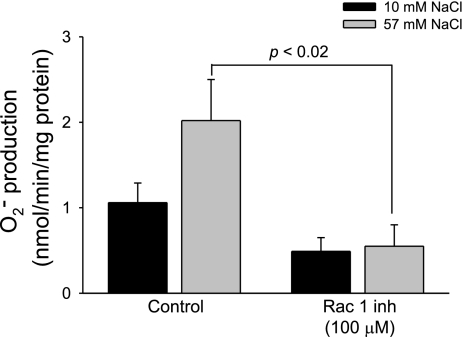
To test whether Rac1 activation is required for NaCl-induced O2− generation, we measured O2− generation in the presence and absence of the Rac1-selective inhibitor. We found that, in the absence of the Rac1 inhibitor, raising the NaCl concentration from 10 to 57 mM increased O2− generation from 1.07 ± 0.23 to 2.02 ± 0.48 nmol O2−·min−1·mg protein−1. In contrast, in the presence of the Rac1 inhibitor (100 μM), increasing NaCl concentration from 10 to 57 mM did not stimulate O2− generation (0.49 ± 0.16 vs. 0.55 ± 0.25 nmol O2−·min−1·mg protein−1, P < 0.02 vs. control, n = 6; Fig. 4).
Fig. 4.
Effect of increased NaCl on TAL O2− generation during inhibition of Rac1. Increased NaCl concentration in the presence of the Rac1 inhibitor (Rac1 inh) inhibited O2− generation (n = 6).
To confirm the results with the pharmacological Rac1 inhibitor, we used a dominant-negative Rac1, with β-galactosidase serving as a control. Adenovirus containing the sequences encoding for dominant-negative Rac1 or β-galactosidase were injected into the outer medulla of the left kidney while the right kidney was used as noninjected control. We found that, in TALs from noninjected kidneys, increasing NaCl from 10 to 57 mM enhanced O2− generation by 1.06 ± 0.20 nmol O2−·min−1·mg protein−1. Similarly, in TALs from kidneys injected with adenovirus encoding for β-galactosidase, increasing NaCl from 10 to 57 mM enhanced O2− generation by 0.81 ± 0.17 nmol O2−·min−1·mg protein−1. In contrast, in TALs from kidneys injected with adenovirus encoding for the dominant-negative Rac1, increasing NaCl from 10 to 57 mM enhanced O2− generation by only 0.33 ± 0.04 nmol O2−·min−1·mg protein−1, representing 60% inhibition (P < 0.05 vs. adenovirus injected with β-galactosidase, n = 6; Fig. 5). These data indicate that activation of Rac1 is required for NaCl-induced O2− generation in TALs.
Fig. 5.
Effect of increased NaCl on TAL O2− generation in adenoviral (Adv) control and dominant-negative (dn) Rac1-injected kidneys. NaCl-induced O2− generation was significantly lower in dominant-negative Rac1-transduced TALs than in adenoviral control (Adv-Lacz)-transduced TALs (n = 6).
DISCUSSION
We hypothesized that increasing NaCl per se within the physiological range activates Rac1 and NADPH oxidase and, thereby, increases O2− production. We found that increasing NaCl from 10 to 57 mM enhanced O2− generation in suspensions of TALs, and this increase was blocked by 80% by the Na-K-2Cl cotransporter inhibitor furosemide. These data indicate that increasing NaCl within the range seen in the lumen of the TAL under physiological conditions: 1) enhances O2− generation; and 2) involves increased Na transport by the luminal Na-K-2Cl cotransporter.
Our finding that increasing NaCl from 10 to 57 mM in the lumen of the TAL stimulates O2− generation is similar to previous findings reported by us (10) and others (1). However, the concentrations used in these previous studies were significantly greater and likely outside the physiological range, given that values from ∼15 to 50 mM have been reported (3, 7, 17, 19). Additionally, the data presented here are the first to show that increasing NaCl absorption via the Na-K-2Cl cotransporter stimulates O2− generation in the absence of luminal flow, demonstrating that NaCl can augment O2− production on its own.
We also found that apocynin completely blocked NaCl-enhanced O2−. We conclude that these data indicate that the source of O2− was NADPH oxidase. Our data are consistent with other reports showing that the major source of O2− in the TALs is NADPH oxidase (12). Such conclusions have recently been challenged because of a report that apocynin can act as a O2− scavenger (8). We tested this by measuring O2− produced from hypoxanthine by xanthine oxidase in a cell-free system. We found that 30 μM apocynin did not scavenge O2−.
The small GTPase Rac1 is an accessory protein that regulates O2− generation by NADPH oxidase. To determine whether Rac1 mediates NaCl-induced O2− generation in the TAL, we first tested the ability of NaCl to activate Rac1. We found that NaCl doubled Rac1 translocation. Because Rac1 translocates when activated (14), we concluded that increasing luminal NaCl from 10 to 57 mM activates Rac1.
To confirm that Rac1 activation is necessary for NaCl-induced O2− generation in the TAL, we measured O2− generation in the presence of a Rac1-selective inhibitor, as well as dominant-negative Rac1. We found that the Rac1 inhibitor blocked NaCl-induced O2− generation. Because the effects of the Rac1 inhibitor may not be 100% specific, we measured O2− generation in TALs transduced with a dominant-negative Rac1. We found a decreased ability to stimulate O2− after increased NaCl in TALs transduced with dominant-negative Rac1 compared with TALs transduced with the control gene (β-galactosidase) or not transduced. Thus we concluded that Rac1 mediates the effect of increased NaCl on O2− production. We believe this is the first report indicating that Rac1 acts as an NaCl-responsive element in the TAL. Given the absorptive nature of the TAL and the stimulatory effect of O2− on NaCl reabsorption (23), this finding is critical to understand the physiology and the adaptative responses of this nephron segment to increased NaCl.
Our data support the findings of others indicating that Rac1 could be activated by an increase in extracellular NaCl. In other nonepithelial cells such as fibroblasts, NaCl-mediated cell shrinkage increases apoptosis via a mechanism mediated by Rac1 in T cells (4). Similarly, other reports indicate that Rac1 is activated by NaCl-induced changes in osmolality. The protein complex involving Rac1, osmolality, and MEKKs has been shown to activate proteins such as p38 and caspase-3 in response to increased NaCl or hypertonic stimuli. Therefore, it has been suggested that the protein complex involving Rac1 is implicated in sensing changes in osmolality in mammalian cells (25). Increased NaCl could activate Rac1 by a number of mechanisms. Increased NaCl activates the luminal Na-K-2Cl cotransporter, which increases Na and Cl entry into TAL cells. Increased intracellular Cl stimulates basolateral Cl exit and depolarizes the cells (6). It could also cause them to swell. Any or all of these mechanisms might cause Rac1 activation and O2− generation in the TAL after NaCl increases. However, because Rac1 activity is increased during swelling of fibroblasts (4) and depolarization of endothelial cells (24), both of these processes likely play an important role in Rac1 activation and, hence, O2− generation in this nephron segment.
In summary, we conclude that increased luminal NaCl concentration in the physiological range stimulates O2− generation in the TAL by a mechanism that requires intracellular Na-dependent Rac1 activation.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-090550-01 and HL-028982-27 (to J. L. Garvin) and American Heart Association-Greater Midwest Grant 0615718Z (to G. B. Silva).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Abe M, O'Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW., Jr Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol Renal Physiol 291: F350–F357, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Dharmawardhane S, Schurmann A, Sells MA, Chernoff J, Schmid SL, Bokoch GM. Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell 11: 3341–3352, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DuBose TD, Jr, Seldin DW, Kokko JP. Segmental chloride reabsorption in the rat nephron as a function of load. Am J Physiol Renal Fluid Electrolyte Physiol 234: F97–F105, 1978 [DOI] [PubMed] [Google Scholar]
- 4.Friis MB, Friborg CR, Schneider L, Nielsen MB, Lambert IH, Christensen ST, Hoffmann EK. Cell shrinkage as a signal to apoptosis in NIH 3T3 fibroblasts. J Physiol 567: 427–443, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greger R. Physiology of renal sodium transport. Am J Med Sci 319: 51–62, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Greger R, Bleich M, Schlatter E. Ion channels in the thick ascending limb of Henle's loop. Renal Physiol Biochem 13: 37–50, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Gutsche HU, Peterson LN, Levine DZ. In vivo evidence of impaired solute transport by the thick ascending limb in potassium-depleted rats. J Clin Invest 73: 908–916, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Holstein-Rathlou NH, Marsh DJ. Oscillations of tubular pressure, flow, and distal chloride concentration in rats. Am J Physiol Renal Fluid Electrolyte Physiol 256: F1007–F1014, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol 292: F993–F998, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res 98: 453–462, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NADH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol 282: F1111–F1119, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Majid DS, Nishiyama A. Nitric oxide blockade enhances renal responses to superoxide dismutase inhibition in dogs. Hypertension 39: 293–297, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Moldovan L, Mythreye K, Goldschmidt-Clermont PJ, Satterwhite LL. Reactive oxygen species in vascular endothelial cell motility. Roles of NAD(P)H oxidase and Rac1. Cardiovasc Res 71: 236–246, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Mori T, Cowley AW., Jr Renal oxidative stress in medullary thick ascending limbs produced by elevated NaCl and glucose. Hypertension 43: 341–346, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Ortiz PA, Hong NJ, Plato CF, Varela M, Garvin JL. An in vivo method for adenovirus-mediated transduction of thick ascending limbs. Kidney Int 63: 1141–1149, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Peterson LN, de Rouffignac C, Sonnenberg H, Levine DZ. Thick ascending limb response to dDAVP and atrial natriuretic factor in vivo. Am J Physiol Renal Fluid Electrolyte Physiol 252: F374–F381, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Schmidt-Nielsen B. The renal concentrating mechanism in insects and mammals: a new hypothesis involving hydrostatic pressures. Am J Physiol Regul Integr Comp Physiol 268: R1087–R1100, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Schnermann J, Briggs J, Schubert G. In situ studies of the distal convoluted tubule in the rat. I. Evidence for NaCl secretion. Am J Physiol Renal Fluid Electrolyte Physiol 243: F160–F166, 1982 [DOI] [PubMed] [Google Scholar]
- 20.Silva GB, Garvin JL. Angiotensin II-dependent hypertension increases Na transport-related oxygen consumption by the thick ascending limb. Hypertension 52: 1091–1098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva GB, Garvin JL. TRPV4 mediates hypotonicity-induced ATP release by the thick ascending limb. Am J Physiol Renal Physiol 295: F1090–F1095, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva GB, Garvin JL. Akt1 mediates purinergic-dependent NOS3 activation in thick ascending limbs. Am J Physiol Renal Physiol 297: F646–F652, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension 48: 467–472, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Sohn HY, Keller M, Gloe T, Morawietz H, Rueckschloss U, Pohl U. The small G-protein Rac mediates depolarization-induced superoxide formation in human endothelial cells. J Biol Chem 275: 18745–18750, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Uhlik MT, Abell AN, Johnson NL, Sun W, Cuevas BD, Lobel-Rice KE, Horne EA, Dell'Acqua ML, Johnson GL. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol 5: 1104–1110, 2003. [DOI] [PubMed] [Google Scholar]