Abstract
Epithelial sodium channels (ENaC) are regulated by protein kinase A, in addition to a broad spectrum of other protein kinases. It is not clear whether cGMP/PKG signaling might regulate ENaC activity. We examined the responses of αβγ-ENaC channels expressed in Xenopus oocytes to 8-(4-chlorophenylthio)-cGMP (8-pCPT-cGMP), a cell-permeable cGMP analog. This compound stimulated human αβγ-ENaC activity in a dose-dependent fashion, but cell-impermeable cGMP had no effect. Similar stimulatory effects of cGMP were observed in oocytes expressing either mouse or rat αβγ-ENaC channels. The identical ion selectivity and amiloride sensitivity of the 8-pCPT-cGMP-activated currents to those of αβγ-ENaC channels suggest that the cGMP-activated currents are associated with expressed ENaC. The PKGI activator Sp isomer of β-phenyl-1,N2-etheno-8-bromo-cGMP did not elicit a rise in ENaC current and that the 8-pCPT-cGMP-induced activation of ENaC channels was blocked by incubating oocytes with a PKG inhibitor, but not with other cGMP-sensitive kinase inactivators for PKA, MEK, MAP, and PKC. Surprisingly, both site-directed mutation of putative consensus PKG phosphorylation sites and truncation of entire cytosolic NH2- and COOH-terminal tails did not alter the response to 8-pCPT-cGMP. The ENaC activity was activated to the same extent by 8-pCPT-cGMP in cells in which PKGII expression was knocked down using small interfering RNA. Analog to 8-CPT-cAMP, 8-pCPT-cGMP was capable of activating ENaC in the identical manner in cell-free outside-out patches. We conclude that the rapid upregulation of human αβγ-ENaC activity in oocytes by external 8-pCPT-cGMP and 4-chlorothiolphenol-cAMP depends on the para-chlorophenylthiol and the hydroxy groups, and 8-pCPT-cGMP may serve as a novel ENaC ligand in addition to activating PKG signal.
Keywords: amiloride; epithelial Na+ channel; guanosine 3′,5′-cyclic monophosphate; domain mapping
the channel activity of apically located epithelial Na+ channels (ENaC) has been reported to be regulated by a number of protein kinases. Protein kinase A (PKA) was first identified to facilitate αβγ-ENaC activity in epithelial cells and when heterologously expressed in Madin-Darby canine kidney cells (44). The ENaC lost the rapid response to the cAMP/PKA signal, however, when expressed in Xenopus oocytes (2, 25). By comparison, long-term incubation of oocytes with a cAMP-elevating reagent improved ENaC expression and activity (55). In addition to PKA, increasing evidence showed that G-protein-coupled receptor 2, casein kinase 2, PKD, and extracellular-regulated kinase (ERK) activate ENaC channels (3, 10, 32, 42–44, 55). Most of these kinases were able to phosphorylate putative consensus phosphorylation residues in the carboxy termini of ENaC subunits (3, 42–44, 55). In contrast, PKC exhibited a biphasic effect via unknown mechanisms (46, 49, 54).
Three PKG isoforms have been isolated, namely, PKGI-α, PKGI-β, and PKGII (39). Accumulating evidence from gene modulation models suggests that cardiovascular phenotypes are predominant for PKGI knockout, while PKGII deficiency leads to the dysfunction in epithelial tissues (17, 38). Both cytosolic guanylyl cyclases/nitric oxide (NO) and the transmembrane guanylyl cyclase [atrial natriuretic peptide (ANP) receptor] increased cell cGMP, which, in turn, evokes PKG activity. ANP activated amiloride-sensitive Na+ channel activity at the single-channel level in the epithelial cells of frog urinary bladder by an increment in cGMP and activation of PKA (52, 53). In addition, prolonged infusion of ANP increased apical α- and γ-ENaC expression in conscious, euvolumic rats (50). In contrast, contradictory observations have been reported regarding the regulation of both native and cloned ENaC by NO donors (45). Very recently, our laboratory has reported the PKGII-mediated regulation of ENaC function in human pleural mesothelial and Clara H441 cells (33, 34).
The potential regulation of heterologous ENaC channels by the cGMP/PKG signal has not been systematically examined. ENaC are localized at the luminal membrane of absorptive and secretory epithelium. As a central salt-absorptive system, ENaC is directly exposed to reactive species, including those released from inflammatory cells, pollutants, and endogenous NO produced by endothelial and epithelial cells. Numerous studies have documented the regulation of ENaC by the gaseous signal molecule NO and by reactive oxygen and nitrogen species (30). Obviously, the use of NO donors to study the regulation of ENaC by cGMP/PKG signaling has a number of limitations. For example, the half-life of NO is only 3∼5 s, and, in addition, some NO donors not only release NO, but, depending on the experimental conditions, may also generate other adducts (4, 48). Finally, cGMP is not the sole mediating effector of NO: numerous posttranslational modifications of proteins by NO and its adducts have been found (15).
There is evidence that toxic peptides released from a number of life-threatening pathogens activate the cGMP/PKG signal pathway, either by modifying the process, such as membrane guanylate cyclase modulators or due to their structural analogy to signal molecules (8, 29, 36, 56). Most of these viral and bacterial proteins cause fatal edematous illness, for instance, noncardiogenic pulmonary edema and enterotoxigenic diarrhea (23, 51). These pathogen peptides abnormally regulate major transepithelial pathways responsible for fluid secretion and resolution. Among the numerous known ionic and fluid transport systems, impaired ENaC and cystic fibrosis transmembrane conductance regulator have been shown to be pivotal pathogenic processes (9, 14, 21).
The aim of the present study was to investigate the direct regulation of heterologously expressed αβγ-ENaC activity in oocytes by the cGMP/PKG signals. Human αβγ-ENaC subunits were coexpressed in oocytes, and channel activity was measured using the two-electrode voltage-clamp technique. Our initial results showed that αβγ-ENaC can be activated by cell-permeable PKGII-specific cGMP in a reversible, dose-dependent fashion. However, subsequent mutagenesis studies suggested that the intracellular tails of ENaC may not be involved. This was corroborated by evidence that ENaC activity can be activated to the same extent following the PKGII knockdown with small interfering RNA (siRNA). Intriguingly, domain-mapping studies confirmed that both the para-chlorophenylthiol at the purine C8 and the hydroxy groups at ribose 2′-O positions contribute to the stimulating effects of 4-chlorothiolphenol (CPT)-cGMP, as well as 8-CPT-cAMP. 8-(4-Chlorophenylthio)-cGMP (8-pCPT-cGMP) activates αβγ-ENaC single-channel activity in the same manner as that for 8-CPT-cAMP, an external ENaC ligand in outside-out patches. Thus 8-pCPT-cGMP, in addition to being a signal molecule of the cGMP/PKG signal pathway, surprisingly behaves as a novel extracellular ligand of ENaC with potential pharmaceutical relevance.
METHODS
Site-directed and truncated mutagenesis.
Wild-type human, mouse, and rat α-, β-, and γ-ENaC cDNAs were gifts from Drs. Lingueglia (26), Kleyman (1), and Rossier (6), respectively. The human NH2-terminal (αΔ2-67, βΔ2-49, and γΔ2-53) and COOH-terminal truncation (αR586X, βR566X, and γR564X) and single-point mutants were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), following the manufacturer's instruction. All of the mutants were confirmed by sequencing.
Oocyte expression and voltage-clamp studies.
The use of Xenopus laevis was approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler. Oocytes were surgically removed from appropriately anesthetized adult female Xenopus laevis (Xenopus Express) and cRNAs for full-length and mutated α-, β-, and γ-ENaC were prepared as described previously (20). Briefly, the ovarian tissue was removed from frogs under anesthesia by ethyl 3-aminobenzoate methanesulfonate salt (Sigma) through a small incision in the lower abdomen. Ovary lobes were removed and digested in OR-2 calcium-free medium (in mM: 82.5 NaCl, 2.5 KCl, 1.0 MgCl2, 1.0 Na2HPO4, and 10.0 HEPES, pH 7.5) with the addition of 2 mg/ml collagenase (Roche Indianapolis). Defolliculated oocytes were cytosolically injected with ENaC cRNAs (25 ng per oocyte in 50 nl of RNase-free water with a ratio of 1α:1β:1γ subunit) and incubated in half-strength L-15 medium at 18°C. The two-electrode voltage-clamp technique was used to record whole cell currents 48 h postinjection. Oocytes were impaled with two electrodes filled with 3 M KCl, having resistances of 0.5–2 MΩ. A TEV-200 voltage-clamp amplifier (Dagan) was used to clamp oocytes with concomitant recording of currents. Two reference electrodes were connected to the bath. The continuously perfused bathing solution was ND96 medium (in mM: 96.0 NaCl, 1.0 MgCl2, 1.8 CaCl2, 2.5 KCl, and 5.0 HEPES, pH 7.5). Whole cell currents were recorded as previously reported (22). Experiments were controlled by pCLAMP 10.1 software (Molecular Devices), and currents at −40, −100, and +70 mV were continuously monitored, with recordings at an interval of 10 s. Data were sampled at the rate of 200 Hz and filtered at 500 Hz. Cyclic nucleotides were purchased from BIOLOG. Other chemicals were from Sigma-Aldrich.
To examine the ion selectivity of the 8-pCPT-cGMP-activated current associated with human αβγ-ENaC, NaCl in the external bath solution was replaced with equivalent concentrations of LiCl or KCl in place of the 96 mM NaCl in regular ND-96 medium.
Single-channel patch-clamp assay.
Oocytes were placed in a hyperosmotic medium (420 mmol/kg) with the following composition (in mM): 200 K-aspartate, 20 KCl, 1 MgCl2, 5 EGTA, 10 HEPES, pH 7.4. The vitelline membrane was then manually removed from the shrunken cells using fine forceps. The devitellinized oocyte was immediately transferred to the recording chamber. The outside-out configuration was used to record single-channel currents at the holding potential of −60 mV, using an Axopatch 200B amplifier (Molecular Devices). Patch pipettes were made of borosilicate glass (WPI, Sarasota, FL) and pulled with a P-97 multistepped micropipette puller (Flaming/Brown). The electrode tips were fire-polished with Micro Forge MF-830 (Narishige, Tokyo, Japan). The resistance of the electrode was 3–5 MΩ when filled with pipette solution (in mM: 100 LiCl, 10 HEPES, 10 EGTA, pH 7.5). The composition of the bath solution was (in mM): 100 LiCl, 10 HEPES, 1 CaCl2, pH 7.5. Single-channel currents were filtered at 0.1 kHz and digitized at 1 kHz using a Digidata 1440A converter (Molecular Devices). Single-channel currents were analyzed using CLAMPFIT 10.1 (Molecular Devices). Single-channel activity was calculated as N · Po = ILi/iLi, where N is the number of channels, Po is open probability, ILi is the mean current over the period of observation, and iLi is the unitary current measured as the peak-to-peak interval in the current amplitude histogram.
Reverse transcription-PCR.
For analysis of PKG mRNA expression, total RNA was isolated from oocytes using TRIzol reagent (Invitrogen), according to the manufacturer's instructions. The RNA concentration was measured spectrophotometrically. The ratios of 260-nm/280-nm absorbency were routinely above 1.95. RNA quality was also verified by denatured agarose gel electrophoresis. First-strand cDNA synthesis was performed using random hexamers and SuperScript II RT (Invitrogen). PCR was performed on a mastercycler gradient thermocycler (Eppendorf Scientific). Primers for PCR are as follows: PKGI, 5′-gagcgtcaagttatgcacca-3′ (sense), 5′-gccctttgcctgattatgtact-3′ (antisense); PKGII, 5′-ccatcccagtgtggacaac-3′ (sense), 5′-cacggtctagagcccatgtt-3′ (antisense). Sequence accession numbers in GenBank are BE576517 and BC072999 for the PKGI and PKGII, respectively. The corresponding product sizes are 300 bp (PKGI) and 108 bp (PKGII). Custom primers specific for PKGI and PKGII were synthesized by Sigma Genosys and used at a final concentration of 0.6 μM.
Two microliters of first-strand cDNA product were used as template in each 50-μl PCR reaction. PCR conditions were optimized using the PCR Optimizer kit (Invitrogen). The cycling parameters consisted of one cycle at 95°C for 3 min and then 30 cycles at 95°C for 30 s, 52–62°C (depends on the primers) for 30 s, and 72°C for 30 s, followed by a single 10-min extension period at 72°C. RT-PCR products were subsequently separated on a 1.5% agarose gel using 100-bp markers (Promega) as a standard to identify the product size. PCR products were purified using QIAquick gel extraction kit (Qiagen) and subcloned into the pCR-2.1 vector (Invitrogen). Positive clones were selected by blue/white screening and followed by digestion with EcoRI (Promega) to verify incorporation of the insert of the correct size. PCR product identities were then confirmed by sequencing (DNA Sequencing Core, University of Alabama at Birmingham).
siRNA knockdown of PKGII.
Three custom-designed siRNAs against amphibian PKGII were purchased from Qiagen. The concentration and expression of these siRNAs were optimized using Western blot assays. The most potent siRNA was microinjected into oocyte cytosol. The PKG knockdown oocytes were used to record whole cell currents and then homogenized for Western blot.
Western blotting of PKG isoforms.
To detect PKG isoforms, homogenized proteins were solubilized in a solution containing the following (in mM): 150 NaCl, 2 EDTA, 2 EGTA, 50 Tris, pH 7.4 (adjusted with HCl), 1% Triton X-100, and complete proteinase inhibitors (Roche Applied Science). Protein concentrations were assayed by the Lowry method. Samples containing 30∼60 μg of protein were resolved by SDS-PAGE on a 7.5% acrylamide gel. Mouse brain tissue extract (20 μg) (Stressgen) and 0.075 μg PKGII purified protein from bovine lung (Sigma) were used as positive control for PKGI and PKGII, respectively. Proteins were subsequently transferred electrophoretically onto polyvinylidene difluoride membranes (Millipore), and the membrane was blocked with 0.2% I-blocker (Tropix) and probed with anti-PKGI (Stressgen, 1:2,000) or anti-PKGII antibody (Santa Cruz, 1:2,000). Blots were then overlaid with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Pierce, 1:10,000), and labeled bands were visualized by enhanced chemiluminescent detection (Pierce).
PKG activity assay.
Oocytes 24-h postinjection of αβγ-cRNAs were incubated with 8-pCPT-cGMP (0.2 mM), Rp-8-bromo-cGMP (Rp-8-Br-cGMP) (0.5 mM), or both for 1 h. Cells were mashed with 75 μl prechilled lysis buffer (Sigma). Lysates were centrifuged at 3,500 rpm for 5 min at 4°C to collect cytosol. To measure PKG activity, cytosol (10 μl) was added to the microplate of the Cyclex PKG Assay Kit following the manufacturer's instruction (MBL International).
Data analysis.
Electrophysiological data from the two-electrode voltage-clamp studies were primarily analyzed with the Clampfit 10.1 (Molecular Devices). The measurements were then imported into Origin 8.0 (OriginLab) for statistical computation and graph plotting. The EC50 values of 8-pCPT-cGMP activation were calculated by fitting the dose-response curves to the Hill equation:
where the current level (I) was plotted as a function of 8-pCPT-cGMP concentration (CPT-cGMP); n is the Hill slope. To evaluate the 8-pCPT-cGMP sensitivity, the ratio of the basal current level and the maximal activated level in the presence of 8-pCPT-cGMP was computed (ICPT-cGMP/Ibasal).
All results are presented as means ± SE of currents. One-way ANOVA computation combined with the Bonferroni test was used to analyze the difference of means for significance. A probability level of ≤0.05 was considered significant.
RESULTS
Activation of human αβγ-ENaC channels expressed in oocytes by 8-pCPT-cGMP.
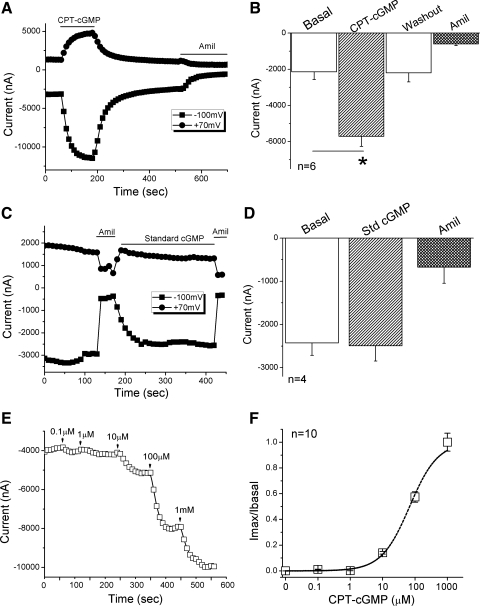
To examine the effects of cGMP on human αβγ-ENaC expressed in oocytes, cell-permeable 8-pCPT-cGMP (0.2 mM) was superfused onto oocytes. As shown in Fig. 1A, both inward and outward whole cell currents were elicited and reached a stable plateau in a few minutes. The activation by 8-pCPT-cGMP was reversible, as indicated by the return of current amplitude to the basal level post-wash out of 8-pCPT-cGMP. The activated current at −100 mV was approximately threefold that of the basal level, a statistically significant change (Fig. 1B, P < 0.05, n = 6). In the presence of amiloride (10 μM), which completely inhibits αβγ-ENaC activity, however, the same amount of 8-pCPT-cGMP had no stimulatory effect (data not shown). In contrast to the results using cell-permeable 8-pCPT-cGMP, membrane-impermeable standard cGMP did not stimulate the αβγ-ENaC channel, even at higher concentrations up to 1 mM (Fig. 1, C and D).
Fig. 1.
Activation of human αβγ-epithelial Na+ channels (ENaC) by 8-(4-chlorophenylthio)-cGMP (8-pCPT-cGMP) in Xenopus oocytes. 8-pCPT-cGMP Na salts and amiloride (Amil) were continuously perfused on oocytes. Whole cell currents were monitored at 10-s intervals. A: representative inward and outward current traces in the presence of 8-pCPT-cGMP (CPT-cGMP) and amiloride. Currents were digitized at both −100 and +70 mV. Application of drugs is indicated by solid horizontal lines. B: average currents at −100 mV (means ± SE). *P < 0.05 compared with basal level. n = 6. C: representative current trace showing no effect of cell-impermeable standard cGMP (Std cGMP). Amiloride was applied at the end of experiments. D: corresponding average currents at −100 mV (means ± SE). E: application of 8-pCPT-cGMP ranged from 0.1 to 1,000 μM. Current trace was recorded at −100 mV. F: dose-response curve. The dashed line was created by fitting the raw data with the Hill equation. n = 10.
To further characterize the stimulatory effects of 8-pCPT-cGMP on human αβγ-ENaC channels, a series of increasing concentrations, ranging from 0.1 μM to 1,000 μM, were applied to bath solutions (Fig. 1E). 8-pCPT-cGMP activated human αβγ-ENaC in a dose-dependent manner with an EC50 value of 101 μM (Fig. 1F, n = 10). These results suggest that the regulation of human αβγ-ENaC by 8-pCPT-cGMP is rapid, reversible, cell permeability dependent, and concentration dependent.
Upregulation of mouse and rat αβγ-ENaC by 8-pCPT-cGMP in oocytes.
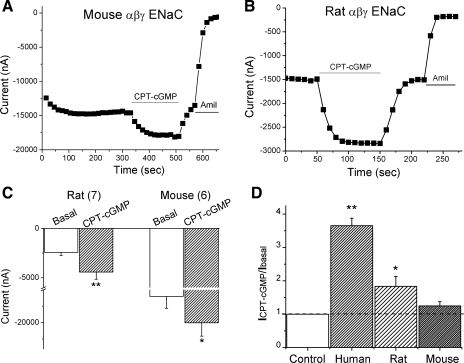
To extend our observations to other species, we studied the effects of 8-pCPT-cGMP on mouse and rat ENaC activities (Fig. 2). Although the expression levels of the mouse and rat ENaCs in oocytes were not identical to that of human ENaC, 8-pCPT-cGMP displayed reversible stimulatory effects on ENaC channels (Fig. 2, A and B). Both the basal and activated current levels at −100 mV were averaged in Fig. 2C. The sensitivity to 8-pCPT-cGMP differed among these species, as reflected by the ratio of currents recorded in the presence and absence of 8-pCPT-cGMP (Fig. 2D). Clearly, regulation of ENaC by 8-pCPT-cGMP is in a nonspecies-specific process. Moreover, the sensitivity of ENaC to repeated application of 8-pCPT-cGMP gradually decreased, suggesting the existence of desensitization (not shown).
Fig. 2.
8-pCPT-cGMP activates mouse and rat αβγ-ENaC heterologously expressed in oocytes. A and B: representative current traces showing the effects of 8-pCPT-cGMP (CPT-cGMP) on mouse and rat ENaC channels, respectively. Membrane potential was −100 mV. C: summary of current levels. The numbers in parentheses are the number of oocytes examined for each group. *P < 0.05, **P <0.01. D: the ratio of activated and basal currents (ICPT-cGMP/Ibasal) in the presence and absence of 8-pCPT-cGMP. *P < 0.05, **P <0.01.
Effects of 8-pCPT-cGMP on ENaC currents in oocytes bathed in Li+- or K+-rich solution.
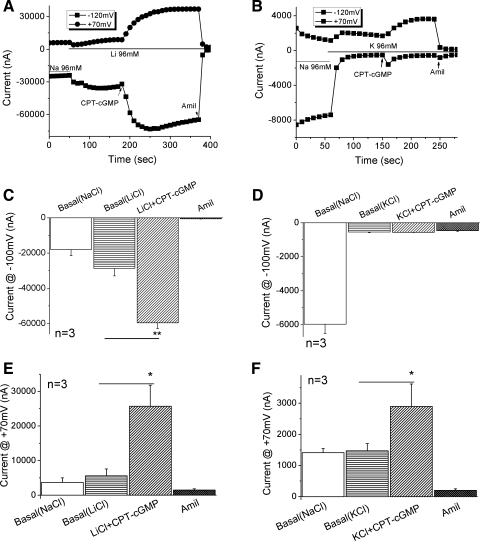
αβγ-ENaC channels expressed in oocytes are slightly more permeable to Li+, but much less to K+ ions (Na/K > 20) than to Na+ ions (6, 31, 41). If 8-pCPT-cGMP-activated current fractions are associated with ENaC, then one would predict that the inward currents carried by Li+ and K+ ions should be greater and lesser, respectively, than that by Na+ ions. Both inward and outward currents increased when the bath solution was switched from NaCl to LiCl-rich medium (Fig. 3A). In contrast, only the inward current was markedly reduced when the bath salt was changed to KCl-rich medium (Fig. 3B). This is consistent with our laboratory's previous reports (20, 22). Perfusion of 8-pCPT-cGMP led to an increment in both inward (2-fold) and outward Li+ currents (5-fold) (Fig. 3, A, C, and E). In contrast to the results for the Li+ current, only the outward K+ current showed a twofold increase (Fig. 3, B, D, and F). Of note, the outward currents in Na+ preloaded oocytes most likely represent the efflux of Na+ ions; the outward currents at +70 mV in cells bathed in Li+-rich or K+-rich solutions cannot be used for characterizing ion permeability. The 8-pCPT-cGMP-activated inward currents carried by Li+, Na+, and K+ ions support our premise that 8-pCPT-cGMP-activated currents are mediated through αβγ-ENaC.
Fig. 3.
8-pCPT-cGMP-activated currents associated with human αβγ-ENaC in oocytes perfused with alkali ions. To ascertain that the 8-pCPT-cGMP-activated current was associated with αβγ-ENaC, the effects of 8-pCPT-cGMP (CPT-cGMP) were examined when oocytes were bathed in LiCl and KCl solutions. A and B: representative whole cell current traces elicited in LiCl and KCl media, respectively. Bath solution was switched to LiCl or KCl solution from regular ND-96 medium containing 96 mM NaCl. 8-pCPT-cGMP was perfused to the bath followed by the perfusate with amiloride. C and D: summary of corresponding inward currents at −100 mV. **P <0.01. E and F: summary of corresponding outward currents at +70 mV. *P < 0.05. n = 3.
Effects of PKG isoform-specific modulators.
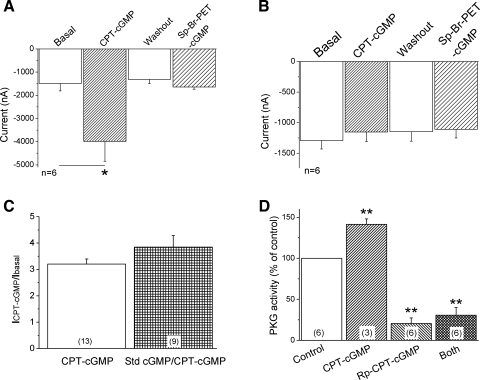
Three PKG isoforms have so far been identified, namely, PKGI-α, PKGI-β, and PKGII (27). 8-pCPT-cGMP predominantly activates PKGII with an EC50 value of 1.8 μM (12). This leads to the question of whether various PKGI isoform activators may also be able to activate ENaC channels. To compare the effects of PKGI and PKGII activators, we first applied 8-pCPT-cGMP to stimulate channel activity, washed it out, and then added Sp isomer of β-phenyl-1,N2-etheno-8-bromo-cGMP (Sp-8-Br-PET-cGMP), a specific PKGI activator. Figure 4A showed that 8-pCPT-cGMP, but not Sp-8-Br-PET-cGMP, was capable of increasing ENaC current level (P < 0.05, n = 6). Sp-8-Br-PET-cGMP slightly stimulated current to a nonsignificant level (P > 0.05). We also repeated these experiments in reverse by applying Sp-8-Br-PET-cGMP first, washing it out, and then adding 8-pCPT-cGMP. Again, Sp-8-Br-PET-cGMP had no significant effect, while 8-pCPT-cGMP stimulated the ENaC activity (data not shown). This is not an artifact caused by their cell permeabilities, as Sp-8-Br-PET-cGMP is more membrane permeable (its lipophilicity scale is 180 vs. 55 for 8-pCPT-cGMP), and the EC50 values for both are similar. Apparently, PKGII may mediate the activation of ENaC channels by 8-pCPT-cGMP in the oocyte model.
Fig. 4.
Regulation of αβγ-ENaC activity by PKG isoform-specific modulators. A: effects of PKGI and PKGII activators on ENaC activity. 8-pCPT-cGMP (CPT-cGMP) was a cell-permeable PKG activator, predominantly activating PKGII isoform. Sp isomer of β-phenyl-1,N2-etheno-8-bromo-cGMP (Sp-8-Br-PET-cGMP) was a specific PKGI (both I-α and I-β) activator. Oocytes were perfused with 8-pCPT-cGMP, and then it was washed out, followed by application of Sp-8-Br-PET-cGMP. *P < 0.05. n = 6. B: blockade of the stimulating effect in oocytes pretreated with PKG inhibitor. Cells were pretreated with 250 μM Rp-8-Br-cGMP to block both PKGI and PKGII for 1 h at room temperature. n = 6. C: competition of standard cGMP (Std cGMP) and 8-pCPT-cGMP. The activation efficacy of 8-pCPT-cGMP (ICPT-cGMP/Ibasal) was compared in the absence and presence of standard cGMP. P > 0.05. D: PKG enzymatic activity. Normalized PKG activity in oocytes was incubated with vehicle (Control), 8-pCPT-cGMP (0.2 mM), Rp-8-Br-cGMP (0.5 mM), or both for 1 h. **P < 0.01 compared with control. Numbers in parentheses, number of oocytes examined for each group.
If PKGII mediates the upregulation of ENaC activity by 8-pCPT-cGMP, these observations should not be seen in oocytes pretreated with a PKG antagonist. To test this conjecture, oocytes expressing αβγ-ENaC were incubated with Rp-8-Br-cGMP (250 μM) for 1 h to inactivate both PKGI and PKGII activity (40). Pretreated oocytes were then voltage clamped to detect cGMP-mediated activation of ENaC channels. Under these conditions, no activation of ENaC activity was observed in oocytes incubated with PKG inactivator (Fig. 4B). To exclude the possible competition between 8-pCPT-cGMP and other analogs, we compared the stimulating efficacy of 8-pCPT-cGMP on ENaC in the absence and presence of standard cGMP (Fig. 4C). Standard cGMP instead of Rp-8-Br-cGMP was used due to its cell impermeability and without influence of PKG activity. The current ratio of 8-pCPT-cGMP activated and basal levels in the presence of standard cGMP did not differ from that in the absence of this drug. Furthermore, corresponding PKG activity was significantly augmented in oocytes incubated with 8-pCPT-cGMP (141.6 ± 6.6% of control) and markedly but not completely reduced in oocytes incubated with Rp-8-Br-cGMP alone (20.5 ± 6.9%) and both drugs (30.7 ± 9.4%) (Fig. 4D).
Exclusion of cross talk with other protein kinases.
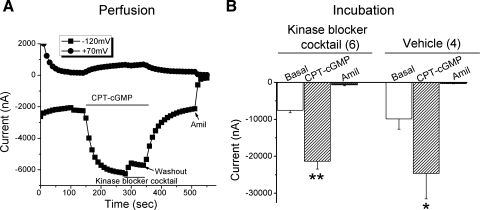
A number of reports previously described that cyclic GMP regulates PKA, PKC, MEK, and MAP kinases (39), all of which can upregulate ENaC activity. To narrow down the involvement of these signal pathways, oocytes were first perfused with 8-pCPT-cGMP, and, once the current had plateaued, a kinase inhibitor cocktail (containing the PKA inhibitor, Rp-cAMP; the PKC inhibitor, acryriarubin A; the MEK inhibitor, PD98059; and the p38 MAP inhibitor SB-202190) was perfused to the bath. This kinase inhibitor cocktail only transiently and slightly reduced the inward current (Fig. 5A). A second series of experiments was conducted by pretreating oocytes with the kinase inhibitor cocktail for 1 h at room temperature and then applying 8-pCPT-cGMP to voltage-clamped oocytes. The ENaC currents in these pretreated oocytes were elevated to the same extent (P < 0.01, n = 6) as in control oocytes, which were treated with vehicle (Fig. 5B). These data imply that these four protein kinases are not involved in the regulation of ENaC by 8-pCPT-cGMP in oocytes.
Fig. 5.
Acute and chronic effects of kinase blocker cocktail on the 8-pCPT-cGMP-mediated stimulation of αβγ-ENaC activity. A: current trace from an oocyte perfused with 8-pCPT-cGMP (CPT-cGMP) and followed by the kinase inhibitor cocktail (PKA inhibitor, Rp-cAMP 100 μM; PKC inhibitor, acryriarubin A 2 μM; MEK inhibitor, PD98059 20 μM; and p38 MAP inhibitor, SB-202190 30 μM). 8-pCPT-cGMP (CPT-cGMP) and the kinase inhibitor cocktail were washed out with ND-96 medium before addition of amiloride. B: pretreatment of oocytes with kinase blocker cocktail. Cells were incubated with the kinase blocker cocktail for 1 h at room temperature, and then the responses of ENaC channels to 8-pCPT-cGMP and amiloride were recorded. Data are represented as means ± SE; n = 4–6. *P < 0.05, **P <0.01.
Identification of potential phosphorylation sites in cytosolic tails of ENaC subunits.
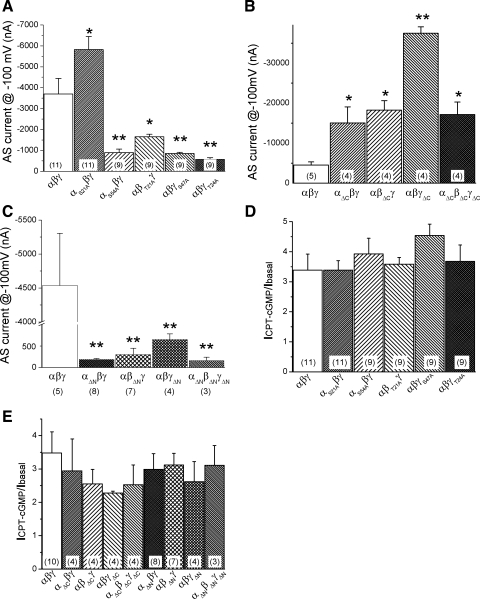
The fact that only cell-permeable 8-pCPT-cGMP is able to activate PKGII-evoked ENaC prompted us to ask the question: what amino acid residues are crucial for PKG to phosphorylate ENaC proteins? Five potential phosphorylation sites were identified within the NH2-termini using NetPhos 2.0 Server (www.cbs.dtu.dk/services/NetPhos). These potential phosphorylatable amino acids were substituted with alanines. All of these single-point mutants, except αS21Aβγ, had lower channel activity (Fig. 6A). Surprisingly, these mutants showed a similar response to 8-pCPT-cGMP as that of wild-type αβγ-ENaC (Fig. 6D).
Fig. 6.
Response of potential PKG phosphorylation mutants and cytosolic N- and C-tail truncation mutants to 8-pCPT-cGMP. A–C: amiloride-sensitive (AS) currents associated with single (A) and COOH- (B) and NH2-terminal tail truncation mutants (C). *P < 0.05 and **P < 0.01 compared with wild-type channels. Holding potential, −100 mV. D: single-point mutation of intrinsic PKG phosphorylation sites. The current ratios of activated and basal current levels were shown. E: sensitivity of intracellular COOH- and NH2-terminal tail truncation mutants to 8-pCPT-cGMP. Numbers in parentheses, number of oocytes examined for each group.
We then constructed intracellular terminal tail truncation mutants to broaden our search range. The COOH- and NH2-terminal tail truncated mutants of each α-, β-, and γ-ENaC subunit were coexpressed with two full-length counterparts of the other two subunits, or all three truncation mutants were coexpressed together. As previously reported (11, 16, 19, 37), the NH2-terminal truncated mutants produced detectable lower currents, while the COOH-terminal truncated Liddle mutants-associated currents were greater over those of wild-type channels (Fig. 6, B and C). Unexpectedly, both the COOH-terminal and NH2-terminal tail truncation mutants retained their sensitivities to 8-pCPT-cGMP (Fig. 6E).
Knockdown of PKGII isoforms in oocytes.
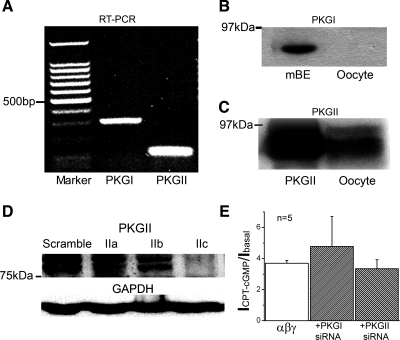
To ascertain that PKG isoforms are expressed in oocytes and do not mediate the 8-pCPT-cGMP-induced activation of ENaC, we first performed RT-PCR to detect the expression of PKG mRNA (Fig. 7A). Although both PKGI and PKGII isoforms were expressed in oocytes at the transcriptional level, only PKGII protein was detected by immunoblotting analysis (Figs. 7, B and C). Subsequently, we knocked down the PKGII protein expression over 90% by injecting a potent siRNA (2c) into oocytes (Fig. 7D). However, the same sensitivity of ENaC to 8-pCPT-cGMP was observed in siRNA-injected oocytes (Fig. 7E). Obviously, 8-pCPT-cGMP-mediated activation of heterologously expressed human αβγ-ENaC unlikely depends on the well-known cGMP/PKG signal pathway, at least in the Xenopus oocytes.
Fig. 7.
Knockdown of PKG expression in oocytes. A: expression of PKG isoforms at the mRNA level. Typical mRNA blot shows PKGI (300 bp) and PKGII (108 bp) expression in oocytes. B and C: PKG isoform I (PKGI) and II (PKGII) expressed at the protein levels. Mouse brain extract (mBE) and purified PKGII protein from bovine lung served as positive controls for PKGI and PKGII, respectively. n = 4. D: knockdown of PKGII. The knockdown effects of three small interfering RNAs (siRNAs; 30 ng/cell) against PKGII were tested using Western blot. GAPDH was used as loading control. E: activation of 8-pCPT-cGMP on ENaC in PKGI and PGKII knockdown oocytes. n = 5.
Critical GMP domains for activating ENaC.
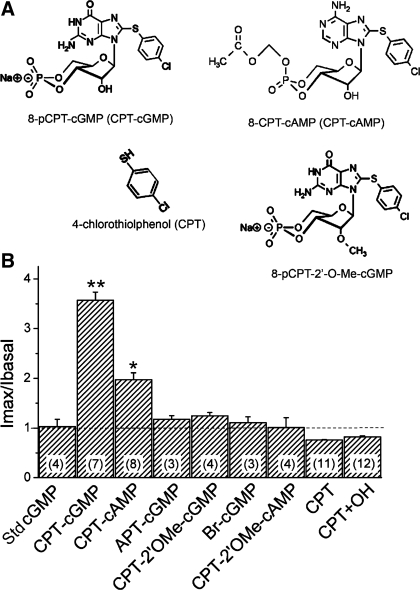
8-pCPT-cGMP may activate ENaC as an external ligand, as several luminal reagents, for example, amiloride, protons, and zinc ions, have all been considered to regulate ENaC by interacting with ectoloop of the channel proteins (18). On the other hand, it has been proposed that 8-CPT-cAMP and 8-pCPT-cGMP may activate ENaC and cystic fibrosis transmembrane conductance regulator, respectively, not via the widely accepted cAMP/PKA and cGMP/PKG pathways (35, 47). If this is also a scenario for 8-pCPT-cGMP to activate ENaC, a defined cGMP structure is required. By comparing 8-pCPT-cGMP with the standard natural signal molecule cGMP, the hydrogen in position C8 of the heterocyclic nucleobase is replaced by the lipophilic para-chlorophenylthio moiety. Analogs substituted with bromine (Sp-8-Br) or 2-aminophenylthio group at the same position did not activate ENaC, as shown in Fig. 8. Indeed, 8-CPT-cAMP also activated ENaC activity. These observations are in good agreement with previous reports (7, 35).
Fig. 8.
Domain-dependence of CPT-cGMP and CPT-cAMP analogs. A: modified positions are shown for 8-pCPT-cGMP and 8-CPT-cAMP. The responses of human αβγ-ENaC to these compounds (0.2 mM) were compared: 8-pCPT-cGMP (CPT-cGMP), 8-CPT-cAMP (CPT-cAMP), 2-aminophenylthio (APT)-cGMP, 8-pCPT-methylated ribose 2′-hydroxy (2′-O-Me)-cGMP (CPT-2′OMe-cGMP), Sp-8-Br-cGMP (Br-cGMP), 8-CPT-2′-O-Me-cAMP (CPT-2′OMe-cAMP), 4-chlorothiolphenol (CPT), and the mixture of CPT and KOH (CPT+OH). The current ratios after and before application of these compounds were compared with that of the standard cGMP molecule (Std cGMP). **P < 0.01, *P < 0.05. Numbers in parentheses are oocytes examined for each group.
The role of the ribose 2′-hydroxy group was also examined. Neither 8-pCPT-2′-O-Me-cGMP nor 8-CPT-2′-O-Me-cAMP, analogs with the methylated (2′-O-Me) ribose 2′-hydroxy domain, activated ENaC (Fig. 8). Taken together, both the lipophilic para-chlorophenylthio moiety and the ribose 2′-hydroxy group of guanosine appear to be critical for activation of ENaC.
The new question raised from aforementioned results is if the para-chlorophenylthio group itself could activate ENaC? To address this issue, we applied CPT, which has the same structure as the para-chlorophenylthio moiety of the 8-pCPT-cGMP. The same dose (0.2 mM) of CPT caused a slight reduction in the ENaC current in the absence and presence of OH− (0.2 mM KOH) (Fig. 8), suggesting that the neither CPT side-chain and hydroxyl group nor their combination can open ENaC channels. In another word, the nucleoside and base frame, as well as other moieties, are required for binding and activating ENaC.
8-pCPT-cGMP increases single-channel activity in outside-out patches.
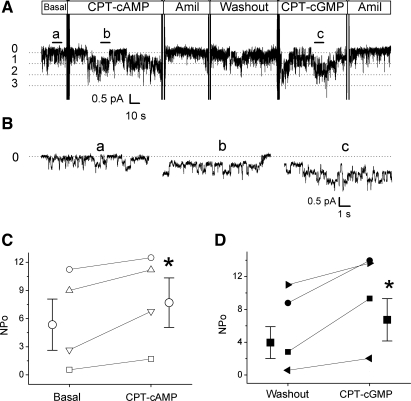
We further asked the question: does 8-pCPT-cGMP serves as an external ENaC ligand to stimulate ENaC? To answer this question, we used cell-free outside-out mode to exclude the involvement of soluable cytosolic signal molecules. 8-CPT-cAMP has been recently confirmed as a ligand to intermolecularly regulate the extracellular domains of cloned ENaC in oocytes (18, 35). As a positive control (Fig. 9, A and B), 0.2 mM 8-CPT-cAMP markedly increased the number of opening levels from 2.3 ± 0.9 to 4.0 ± 1.1 (P < 0.05, n = 4) without alteration of unitary current level (before 0.5 ± 0.04 pA vs. after 0.5 ± 0.05 pA). Similarly, subsequent application of 8-pCPT-cGMP (0.2 mM) to the washout patches evoked the number of opening levels to 4.5 ± 1.1 (P < 0.05, n = 4), whereas the unitary current was not altered. The computed single-channel activity (NPo) was significantly increased by both 8-CPT-cAMP (from 5.9 ± 2.5 to 8.0 ± 2.4, P < 0.05) and 8-pCPT-cGMP (4.0 ± 1.9 to 6.7 ± 2.6, P < 0.05) to a similar extent (Fig. 9, C and D). Of note, four of six outside-out patches responded to 8-pCPT-cGMP. The nonresponsive patches were not included to calculate single-channel activity. These results strongly suggest that the activation of 8-pCPT-cGMP on human αβγ-ENaC may share the same mechanism for 8-CPT-cAMP as an ENaC activator.
Fig. 9.
Stimulatory effects of 8-pCPT-cGMP and 8-CPT-cAMP on human αβγ-ENaC single-channel activity. A: representative outside-out recording. The patch potential was −60 mV. Closed (0) and open (1–3) levels were marked. Perfusion of 8-CPT-cAMP (CPT-cAMP, 0.2 mM) and subsequently 8-pCPT-cGMP (CPT-cGMP, 0.2 mM) and amiloride (Amil, 10 μM) was indicated. B: expanded segments for basal (a), 8-CPT-cAMP activated (b), and 8-pCPT-cGMP activated (c) current traces. C: single-channel activity (NPo). *P < 0.05 compared with basal or washout level using paired t-test.
DISCUSSION
The highly structural similarity between cAMP and cGMP and the cross talk generally observed between PKA and PKG signal pathways make it extremely difficult to clearly distinguish the roles of these two signal pathways. To circumvent this problem, we have utilized heterologously expressed ENaC channel activity in Xenopus oocytes, which is not acutely regulated by PKA, in sharp contrast to native ENaC and those expressed in Madin-Darby canine kidney cells (2, 44). Our results show that the cell-permeable PKGII isoform-specific activator, 8-pCPT-cGMP, acutely stimulates human, rat, and mouse αβγ-ENaC reversibly in a concentration-dependent manner. Surprisingly, mutation and knockdown studies suggest that intraoocyte domains of ENaC subunits and traditional soluble protein kinases may not be involved in the activation of cloned αβγ-ENaC by 8-pCPT-cGMP in oocytes. Instead, we identified that the para-chlorophenylthio moiety and ribose 2′-hydroxy group of 8-pCPT-cGMP are crucial for activating ENaC. Finally, both 8-pCPT-cGMP and CPT-cAMP activated the single-channel activity in outside-out patches in the same pattern.
The ENaC/degenerin family members have been proposed to function as a ligand-gated channel (18). In fact, several cell-impermeable reagents were found to upregulate ENaC activity luminally two decades ago (13), such as p-chloromercuribenzoate, benzimidazolylguanidine, and protons. These reagents alter self-inhibition of ENaC channels (13). Very recently, a small molecule, S6939, has been reported to activate ENaC as an ENaC channel opener (28). These ENaC upregulating compounds generally increase channel open probability, but affect neither the unitary conductance nor the recruitment of new channel proteins (28). The acute action of 8-pCPT-cGMP on activating both whole cell and single-channel ENaC activity and the reversibility strongly suggest that 8-pCPT-cGMP shares the same mechanism.
The observations on the regulation of cloned human αβγ-ENaC activity in oocytes are inconsistent with our recent publication (33). Pharmacological evidence suggests that native ENaC activity in H441 cells is acutely evoked by specific PKGII activator. It is reminiscent of us the similar divergent observations between the regulation of native and cloned ENaC by cAMP/PKA signal. In sharp contrast to the native cAMP-activated ENaC, heterologously expressed αβγ-ENaC in oocytes has been considered to be cAMP/PKA independent, particularly for acute regulation (55). Furthermore, a critical extracellular domain has recently been identified as 8-CPT-cAMP binding site (35), indicating that 8-CPT-cAMP is an extracellular ligand for ENaC (18, 35). Combined with our similar diverse observations for the activation of native and cloned ENaC by 8-pCPT-cGMP, 8-pCPT-cGMP may serve as a new ENaC ligand to regulate channel activity extracellularly. Our domain mapping and single-channel studies support this notion. Moreover, the divergent affinities of αβγ- (EC50, 101 μM) and δαβγ-ENaC channels (5 μM) for 8-pCPT-cGMP may not be an appropriate explanation for the responsive diversities between native and cloned ENaC.
In addition to absence of soluble cGMP/PKG signal molecules, the outside-out patches assumingly do not have the entire working machinery for protein synthesis and exocytosis; 8-pCPT-cGMP, therefore, most likely stimulates ENaC by increasing Po and awakening electrically “silent” ENaC channels. A comparable study clearly indicated that ∼90% of immunobiochemically detectable ENaC proteins on cell surface are unable to be electrically recorded in oocytes (24). On the other hand, proteases proteolytically activate near “silent” native ENaC (5). Taken together, 8-pCPT-cGMP does not likely increase ENaC activity by recruiting more new synthesized proteins from missing cytosol during the process of outside-out patch formation. Instead, 8-pCPT-cGMP may acts as an ENaC ligand as 8-CPT-cAMP for activating single-channel activity (Fig. 9).
Based on the similarity in structure, 8-pCPT-cGMP may competitively bind to the same site as other cGMP analogs. However, observations in oocytes preincubated with the standard cGMP did not support this notion. It is conceivable that the cGMP moieties required for binding to the channel proteins may differ from those converting ENaC to an opening conformational status. Another possibility is Rp-8-Br-cGMP may prevent the process of conformational change caused by the intermolecular association of ENaC and 8-pCPT-cGMP.
We cannot completely exclude the roles of cGMP/PKG signal in the effects of 8-pCPT-cGMP on ENaC expressed in oocytes. The incomplete inhibition of PKG activity by specific inactivator and <100% knockdown of PKG expression using siRNA imply the potential involvement of residual PKG expression and activity. The specific mammalian PKGII inhibitor appears unable to completely inhibit amphibian PKGII activity (Fig. 4D), possibly due to the diversity of PKGII between these species. On the other hand, there may be more than one site that can be phosphorylated by PKG, e.g., one in the NH2-terminal and another in the COOH-terminal. Furthermore, modification of other ENaC inhibitor/activator by cGMP/PKG signal cannot be completely ruled out, even though recognized ENaC modifying pathways, including PKA, PKC, ERK, and p38 MAP, appear not to be involved.
Activation of single-channel activity by 8-pCPT-cGMP in outside-out patches suggests a PKG-independent mechanism exists, based on the fact that this configuration does not have natural cytosolic cGMP/PKG signal molecules. In addition, multiple intracellular microinjections of 8-pCPT-cGMP do not alter ENaC current, and subsequent external perfusion of equal dose of the drug rapidly activates the channels (unpublished data). The coexistence of PKG-dependent and -independent mechanisms may also be the case for prolonged incubation of oocytes with CPT-contained analogs. Yang and coworker identified two putative ERK-consensus motifs, which were dephosphorylated by long-term incubation of oocytes with a cAMP-elevating reagent (55). Our preliminary data show that overnight incubation of ENaC oocytes with 8-pCPT-cGMP activates ENaC activity to a significant extent (unpublished observations). This may be due to the long-lasting acute stimulatory effect and the coexistence of cGMP/PKG-mediated upregulation of channel activity.
The inconsistent responses to 8-pCPT-cGMP among human, rat, and mouse ENaC are not unique. Chraibi and coworkers reported that guinea pig α-ENaC, when coexpressed with rat β- and γ-subunits, can be activated by 8-CPT-cAMP (7). These results cannot be produced in rat and Xenopus ENaC. The specific domain responsible for the cAMP to activate guinea pig ENaC has been identified (35). However, the amino acid residue for cAMP is identical between human and mouse α-ENaC. Besides, 8-pCPT-cGMP stimulates both rat and human ENaC in oocytes. These data do not support that 8-pCPT-cGMP shares the same interactive domains with 8-CPT-cAMP. Identification of these external domains interactive with 8-pCPT-cGMP awaits further studies.
As revealed by the extremely diverse affinities (101 μM for ENaC and 1.8 μM for PKGII), responsiveness, and reversibility, our studies for the first time suggest that 8-pCPT-cGMP activates ENaC acutely by serving as an external ligand, in addition to being a down-stream effector of the traditional cGMP/PKG signal pathway. The strict requirement of both para-chlorophenylthio and the ribose 2′-hydroxy groups may help clarify the inconsistent results for the regulation of ENaC by a variety of cGMP analogs. On the other hand, our results provide a new explanation for the activation of ENaC by 8-CPT-cAMP previously described (7, 35). Importantly, the observation that specific moieties of 8-pCPT-cGMP are required for activating ENaC may provide pivotal information for developing potential ENaC channel openers of pharmaceutical value.
In summary, the present study demonstrates that the 8-pCPT-cGMP activates αβγ-ENaC expressed in Xenopus oocytes, probably by acting as a channel ligand, but not a cytosolic signal molecule. Domain mapping studies indicate that both the para-chlorophenylthio moiety at the position C8 and the ribose 2′-hydroxy group are critical in the acute stimulation of ENaC activity. These findings may have clinical relevance for developing potent new ENaC openers structurally related to 8-pCPT-cGMP, which would be extremely useful for combating diseases associated with lower ENaC function.
GRANTS
The work was supported by National Institutes of Health (NIH) Grants HL87017 and HL095435, and American Heart Association Grant 0635355N to H.-L. Ji and NIH Grant DK072154 to J.-B. Peng.
DISCLOSURES
I am not aware of financial conflict(s) with the subject matter or materials discussed in this manuscript with any of the authors, or any of the authors’ academic institutions or employers.
ACKNOWLEDGMENTS
The authors thank Dr. Mark Atkinson for proofreading of the manuscript and Dr. Peter Smith (Physiology, University of Alabama at Birmingham) for superb technical support.
Present address of H.-G. Nie: School of Pharmaceutical Sciences, China Medical University, Liaoning, Shenyang 110001, China (e-mail: niehg@hotmail.com).
REFERENCES
- 1.Ahn YJ, Brooker DR, Kosari F, Harte BJ, Li J, Mackler SA, Kleyman TR. Cloning and functional expression of the mouse epithelial sodium channel. Am J Physiol Renal Physiol 277: F121–F129, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Awayda MS, Ismailov II, Berdiev BK, Fuller CM, Benos DJ. Protein kinase regulation of a cloned epithelial Na+ channel. J Gen Physiol 108: 49–65, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachhuber T, Almaca J, Aldehni F, Mehta A, Amaral MD, Schreiber R, Kunzelmann K. Regulation of the epithelial Na+ channel by the protein kinase CK2. J Biol Chem 283: 13225–13232, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman JS, Koppenol WH. Nitric oxide, superoxide, peroxynitrite: the good, the bad, and ugly. Am J Physiol Cell Physiol 271: C1424–C1437, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol 288: L813–L819, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Chraibi A, Schnizler M, Clauss W, Horisberger JD. Effects of 8-cpt-cAMP on the epithelial sodium channel expressed in Xenopus oocytes. J Membr Biol 183: 15–23, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Cowley S, Ko M, Pick N, Chow R, Downing KJ, Gordhan BG, Betts JC, Mizrahi V, Smith DA, Stokes RW, Av-Gay Y. The Mycobacterium tuberculosis protein serine/threonine kinase PknG is linked to cellular glutamate/glutamine levels and is important for growth in vivo. Mol Microbiol 52: 1691–1702, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Davis IC, Xu A, Gao Z, Hickman-Davis JM, Factor P, Sullender WM, Matalon S. Respiratory syncytial virus induces insensitivity to beta-adrenergic agonists in mouse lung epithelium in vivo. Am J Physiol Lung Cell Mol Physiol 293: L281–L289, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinudom A, Fotia AB, Lefkowitz RJ, Young JA, Kumar S, Cook DI. The kinase Grk2 regulates Nedd4/Nedd4–2-dependent control of epithelial Na+ channels. Proc Natl Acad Sci USA 101: 11886–11890, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci USA 93: 15370–15375, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamm DM, Francis SH, Angelotti TP, Corbin JD, Uhler MD. The type II isoform of cGMP-dependent protein kinase is dimeric and possesses regulatory and catalytic properties distinct from the type I isoforms. J Biol Chem 270: 27380–27388, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Garty H, Benos DJ. Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev 68: 309–373, 1988 [DOI] [PubMed] [Google Scholar]
- 14.Golin-Bisello F, Bradbury N, Ameen N. STa and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am J Physiol Cell Physiol 289: C708–C716, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol 287: L262–L268, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton RP. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet 11: 76–82, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 86: 1–23, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Horisberger JD, Chraibi A. Epithelial sodium channel: a ligand-gated channel? Nephron Physiol 96: p37–p41, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Ji HL, Fuller CM, Benos DJ. Peptide inhibition of constitutively activated epithelial Na+ channels expressed in Xenopus oocytes. J Biol Chem 274: 37693–37704, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Ji HL, Parker S, Langloh AL, Fuller CM, Benos DJ. Point mutations in the post-M2 region of human α-ENaC regulate cation selectivity. Am J Physiol Cell Physiol 281: C64–C74, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Ji HL, Song W, Gao Z, Su XF, Nie HG, Jiang Y, Peng JB, He YX, Liao Y, Zhou YJ, Tousson A, Matalon S. SARS-CoV proteins decrease levels and activity of human ENaC via activation of distinct PKC isoforms. Am J Physiol Lung Cell Mol Physiol 296: L372–L383, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji HL, Su XF, Kedar S, Li J, Barbry P, Smith PR, Matalon S, Benos DJ. Delta-subunit confers novel biophysical features to alpha beta gamma-human epithelial sodium channel (ENaC) via a physical interaction. J Biol Chem 281: 8233–8241, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kaur T, Ganguly NK. Modulation of gut physiology through enteric toxins. Mol Cell Biochem 253: 15–19, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Kellenberger S, Hoffmann-Pochon N, Gautschi I, Schneeberger E, Schild L. On the molecular basis of ion permeation in the epithelial Na+ channel. J Gen Physiol 114: 13–30, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett 318: 95–99, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Lohmann SM, Walter U. Tracking functions of cGMP-dependent protein kinases (cGK). Front Biosci 10: 1313–1328, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lu M, Echeverri F, Kalabat D, Laita B, Dahan DS, Smith RD, Xu H, Staszewski L, Yamamoto J, Ling J, Hwang N, Kimmich R, Li P, Patron E, Keung W, Patron A, Moyer BD. Small molecule activator of the human epithelial sodium channel. J Biol Chem 283: 11981–11994, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Mann EA, Jump ML, Wu J, Yee E, Giannella RA. Mice lacking the guanylyl cyclase C receptor are resistant to STa-induced intestinal secretion. Biochem Biophys Res Commun 239: 463–466, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Matalon S, Hardiman KM, Jain L, Eaton DC, Kotlikoff M, Eu JP, Sun J, Meissner G, Stamler JS. Regulation of ion channel structure and function by reactive oxygen-nitrogen species. Am J Physiol Lung Cell Mol Physiol 285: L1184–L1189, 2003 [DOI] [PubMed] [Google Scholar]
- 31.McDonald FJ, Snyder PM, McCray PB, Jr, Welsh MJ. Cloning, expression, and tissue distribution of a human amiloride-sensitive Na+ channel. Am J Physiol Lung Cell Mol Physiol 266: L728–L734, 1994 [DOI] [PubMed] [Google Scholar]
- 32.McEneaney V, Harvey BJ, Thomas W. Aldosterone regulates rapid trafficking of epithelial sodium channel subunits in renal cortical collecting duct cells via protein kinase D activation. Mol Endocrinol 22: 881–892, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nie HG, Chen L, Han DY, Li J, Song WF, Wei SP, Fang XH, Gu X, Matalon S, Ji HL. Regulation of epithelial sodium channels by cGMP/PKGII. J Physiol 587: 2663–2676, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie HG, Tucker T, Su XF, Na T, Peng JB, Smith PR, Idell S, Ji HL. Expression and regulation of epithelial Na+ channels by nucleotides in pleural mesothelial cells. Am J Respir Cell Mol Biol 40: 543–554, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renauld S, Allache R, Chraibi C. Ile481 from the guinea pig alpha-subunit plays a major role in the activation of ENaC by cpt-cAMP. Cell Physiol Biochem 22: 101–108, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Scherr N, Honnappa S, Kunz G, Mueller P, Jayachandran R, Winkler F, Pieters J, Steinmetz MO. Structural basis for the specific inhibition of protein kinase G, a virulence factor of Mycobacterium tuberculosis. Proc Natl Acad Sci USA 104: 12151–12156, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schild L, Canessa CM, Shimkets RA, Gautschi I, Lifton RP, Rossier BC. A mutation in the epithelial sodium channel causing Liddle disease increases channel activity in the Xenopus laevis oocyte expression system. Proc Natl Acad Sci USA 92: 5699–5703, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlossmann J, Feil R, Hofmann F. Insights into cGMP signalling derived from cGMP kinase knockout mice. Front Biosci 10: 1279–1289, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Schlossmann J, Feil R, Hofmann F. Signaling through NO and cGMP-dependent protein kinases. Ann Med 35: 21–27, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Schlossmann J, Hofmann F. cGMP-dependent protein kinases in drug discovery. Drug Discov Today 10: 627–634, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Sheng S, McNulty KA, Harvey JM, Kleyman TR. Second transmembrane domains of ENaC subunits contribute to ion permeation and selectivity. J Biol Chem 276: 44091–44098, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Shi H, Asher C, Chigaev A, Yung Y, Reuveny E, Seger R, Garty H. Interactions of beta and gamma ENaC with Nedd4 can be facilitated by an ERK-mediated phosphorylation. J Biol Chem 277: 13539–13547, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Shi H, Asher C, Yung Y, Kligman L, Reuveny E, Seger R, Garty H. Casein kinase 2 specifically binds to and phosphorylates the carboxy termini of ENaC subunits. Eur J Biochem 269: 4551–4558, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Shimkets RA, Lifton R, Canessa CM. In vivo phosphorylation of the epithelial sodium channel. Proc Natl Acad Sci USA 95: 3301–3305, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song W, Matalon S. Modulation of alveolar fluid clearance by reactive oxygen-nitrogen intermediates. Am J Physiol Lung Cell Mol Physiol 293: L855–L858, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Stockand JD, Bao HF, Schenck J, Malik B, Middleton P, Schlanger LE, Eaton DC. Differential effects of protein kinase C on the levels of epithelial Na+ channel subunit proteins. J Biol Chem 275: 25760–25765, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Sullivan SK, Agellon LB, Schick R. Identification and partial characterization of a domain in CFTR that may bind cyclic nucleotides directly. Curr Biol 5: 1159–1167, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Thippeswamy T, McKay JS, Quinn JP, Morris R. Nitric oxide, a biological double-faced janus–is this good or bad? Histol Histopathol 21: 445–458, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Volk KA, Husted RF, Snyder PM, Stokes JB. Kinase regulation of hENaC mediated through a region in the COOH-terminal portion of the α-subunit. Am J Physiol Cell Physiol 278: C1047–C1054, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Wang W, Li C, Nejsum LN, Li H, Kim SW, Kwon TH, Jonassen TE, Knepper MA, Thomsen K, Frokiaer J, Nielsen S. Biphasic effects of ANP infusion in conscious, euvolumic rats: roles of AQP2 and ENaC trafficking. Am J Physiol Renal Physiol 290: F530–F541, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med 353: 2788–2796, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Yamada T, Konno N, Matsuda K, Uchiyama M. Frog atrial natriuretic peptide and cGMP activate amiloride-sensitive Na+ channels in urinary bladder cells of Japanese tree frog, Hyla japonica. J Comp Physiol [B] 177: 503–508, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Yamada T, Matsuda K, Uchiyama M. Frog ANP increases the amiloride-sensitive Na+ channel activity in urinary bladder cells of Japanese tree frog, Hyla japonica. Gen Comp Endocrinol 152: 286–288, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Yamagata T, Yamagata Y, Masse C, Tessier MC, Brochiero E, Dagenais A, Berthiaume Y. Modulation of Na+ transport and epithelial sodium channel expression by protein kinase C in rat alveolar epithelial cells. Can J Physiol Pharmacol 83: 977–987, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Yang LM, Rinke R, Korbmacher C. Stimulation of the epithelial sodium channel (ENaC) by cAMP involves putative ERK phosphorylation sites in the C termini of the channel's beta- and gamma-subunit. J Biol Chem 281: 9859–9868, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Zhang W, Mannan I, Schulz S, Parkinson SJ, Alekseev AE, Gomez LA, Terzic A, Waldman SA. Interruption of transmembrane signaling as a novel antisecretory strategy to treat enterotoxigenic diarrhea. FASEB J 13: 913–922, 1999 [DOI] [PubMed] [Google Scholar]