Abstract
Epidemiologic studies from several different populations have demonstrated that prenatal insults, which adversely affect fetal growth, result in an increased incidence of hypertension when the offspring reaches adulthood. It is now becoming evident that low-birth-weight infants are also at increased risk for chronic kidney disease. To determine how prenatal insults result in hypertension and chronic kidney disease, investigators have used animal models that mimic the adverse events that occur in pregnant women, such as dietary protein or total caloric deprivation, uteroplacental insufficiency, and prenatal administration of glucocorticoids. This review examines the role of the kidney in generating and maintaining an increase in blood pressure in these animal models. This review also discusses how early postnatal adverse events may have repercussions in later life. Causes for the increase in blood pressure by perinatal insults are likely multifactorial and involve a reduction in nephron number, dysregulation of the systemic and intrarenal renin-angiotensin system, increased renal sympathetic nerve activity, and increased tubular sodium transport. Understanding the mechanism for the increase in blood pressure and renal injury resulting from prenatal insults may lead to therapies that prevent hypertension and the development of chronic kidney and cardiovascular disease.
Keywords: blood pressure, prenatal insults, sodium absorption, renin-angiotensin system
prenatal insults can result in offspring that are small for gestational age. Barker and colleagues (5, 8–10) were the first to demonstrate that small-for-gestational-age infants are at risk for developing diseases, such as coronary artery disease and hypertension as adults, independent of other risk factors. The association of small-for-gestational-age neonates and increased blood pressure in later life has been found in many different populations world-wide (6, 7, 26, 32, 33, 48, 79, 89, 119, 159). A low-protein intake relative to a proportionally high-carbohydrate intake, especially in the third trimester, is an important risk factor in the development of hypertension (26, 120). Many studies have shown that intrauterine growth retardation programs infants to develop not only hypertension and ischemic heart disease, but other components of the metabolic syndrome, such as hyperlipidemia, noninsulin-dependent diabetes mellitus, and a predisposition to chronic kidney disease (6, 7, 26, 32, 33, 44, 45, 48, 56–58, 78, 81, 88, 89, 102, 115, 117, 118, 133, 158). In addition to prenatal adverse events, there is increasing evidence that a suboptimal early postnatal environment may be a predisposing factor for renal injury and hypertension (21, 94, 124, 146). This review discusses how prenatal and early postnatal adverse events result in hypertension and renal injury in adults by focusing predominantly on animal models.
Animal Models of Prenatal Programming of Hypertension
Timing of maternal adverse events.
Our understanding of the pathogenesis of hypertension induced by prenatal insults results primarily from experiments performed in rats and sheep. The models employed have tried to recapitulate insults that are comparable to what is experienced in humans, such as maternal malnutrition, prenatal exposure to glucocorticoids, and uteroplacental insufficiency. Depending on the timing and severity, each of these insults is associated with small-for-gestational-weight infants. However, as recently reviewed by Dr. Moritz et al., (90) prenatal insults can result in programming a reduced nephron number and renal dysfunction in adulthood independent of a low birth weight.
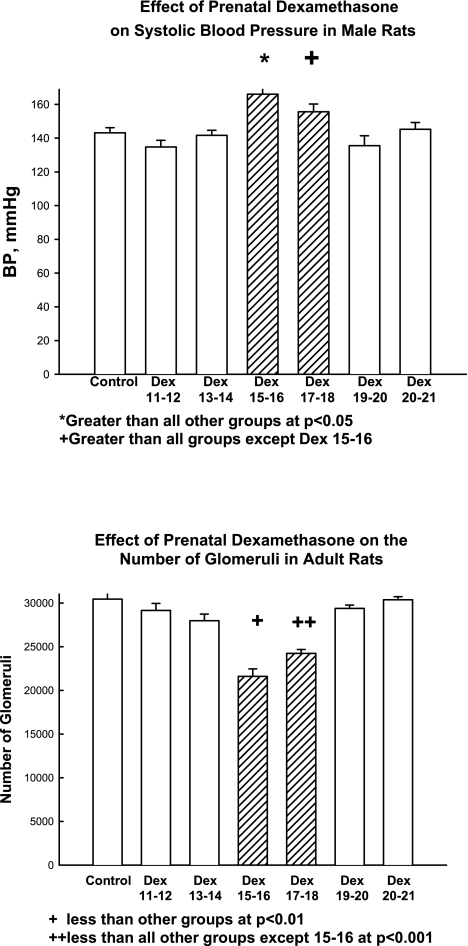
The timing of the prenatal insult can determine whether there will be programmed changes that affect the kidney. Studies have shown a reduction in glomerular number and elevated blood pressure in adult male offspring of pregnant rats that were fed a low-protein diet in the second half but not in the first half of pregnancy (155). Similarly, short-term moderate, maternal dietary protein deprivation (9% casein compared with 18% casein) on days 8–14 or 15–22 of the 22-day gestation in the rat reduced nephron number in offspring, but there was no effect of dietary protein deprivation when administered during the first 7 days of gestation (77). Glucocorticoids are often administered to pregnant women to accelerate fetal lung maturation. As shown in Fig. 1, prenatal dexamethasone resulted in hypertension and a reduction in nephron number in adult offspring of pregnant rats when administered daily on days 15 and 16 or 17 and 18 of gestation but not before or after this time (100, 101). In vitro studies have shown that dexamethasone inhibited branching morphogenesis and reduced glomerular number when added to media of day 14.5 and day 15.5 embryonic rat metanephroi (131). Thus, glucocorticoids decrease nephron number at the point in nephrogenesis in the rat when the ureteric bud invades the metanephric mesenchyme and when branching begins in earnest (91, 104, 131).
Fig. 1.
Effect of prenatal dexamethasone (Dex) on blood pressure and glomerular number in the rat. Prenatal dexamethasone was administered to pregnant rats on the specific days of gestation noted (0.2 mg/kg body wt daily for 2 days). Blood pressure (BP) and glomerular number were measured in adult male rats. As is evident, there was a discrete period of time when prenatal dexamethasone programs hypertension and a reduction in nephron number. [From Ortiz LA et al. (100).]
The results in the sheep are not as straight forward. One group found that administration of glucocorticoids at 22–29 days gestation in sheep, the time of initiation of nephrogenesis (40, 91), resulted in a reduction in nephron number and hypertension, while there was no effect when glucocorticoids were administered on 59–66 days of pregnancy (term ∼150 days) (40, 91). However, another group showed that the period of susceptibility to glucocorticoids was on days 80 and 81 of gestation, a time of active nephrogenesis (47). The disparity in these results cannot be explained by the dose of glucocorticoid administered. In summary, the period of active nephrogenesis is the point where the kidney is most susceptible to injury, resulting in a reduction in nephron number and programming hypertension in the adult offspring.
Sex differences in response to an adverse maternal event.
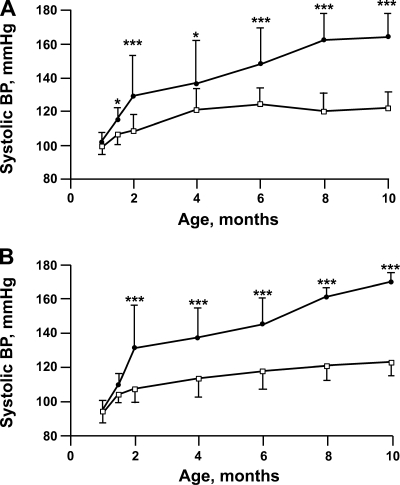
Prenatal insults have been shown to affect males and females differently in some studies. Moderate maternal dietary protein deprivation (8.5% protein compared with 19%) resulted in postnatal hypertension measured in chronically instrumented conscious male but not female rats (150, 151), unlike a prenatal 6% protein diet that affected blood pressure, measured by tail cuff, in both sexes (86, 137). As shown in Fig. 2, maternal dietary protein restriction (6% vs. 20% protein in the control group) caused a chronic increase in blood pressure in both male and female rat offspring (137). Administration of dexamethasone on days 14 and 15 of gestation resulted in male and female rats that had elevated blood pressure compared with controls at 2 mo of age (100), while only males were hypertensive at 6–9 mo of age (101). In summary, both males and females develop an increase in blood pressure with severe prenatal insults. Milder maternal insults either affect only males or have only a transient effect on blood pressure in females.
Fig. 2.
Effect of prenatal low-protein diet on blood pressure in rats of various ages. Systolic blood pressure was measured by tail cuff in adult male (top) and female (bottom) rats whose mothers were fed a low-protein diet (6%) during the last half of pregnancy and compared with rats fed a normal protein diet (20%). There was a progressive increase of blood pressure with age in the low-protein group. Data are means ± SE; *P < 0.05; ***P < 0.001 [From Vehaskari et al. (137).]
Recent studies have examined the cause for the difference in blood pressure between males and females resulting from prenatal insults. Male rats that had intrauterine growth retardation secondary to uteroplacental insufficiency were hypertensive as adults, measured using radiotelemetry (98). Castration of male offspring of mothers with uteroplacental insufficiency at 10 wk of age normalized the blood pressure to control levels when measured using radiotelemetry 2–6 wk after surgery, while the blood pressure of castrated control animals was comparable to intact rats (98). The hypertension seen in intrauterine growth retarded female offspring at 8 wk, which were the product of mothers with uteroplacental insufficiency, normalized at 12 wk of age when the rats were sexually more mature (1). While ovariectomy had no effect on blood pressure in control rats, ovariectomy resulted in an increase in blood pressure in offspring that were the product of mothers with uteroplacental insufficiency (97). The effect of ovariectomy on blood pressure was reversed by estrogen replacement (97). Enalapril administration to both intact males and ovariectomized females that were growth retarded at birth due to uteroplacental insufficiency normalized blood pressure to control levels consistent with the known effect of testosterone and estrogen on the renin-angiotensin system (97, 98). Thus, the susceptibility of males and relative protection of females from prenatal insults is likely due to testosterone and estrogen, respectively.
Common Mechanistic Pathways Leading to Prenatal Programming
There is evidence that injury to the developing fetus by maternal dietary protein deprivation is mediated by exposure to maternal steroids thereby linking the pathogenesis of these two prenatal insults. The fetus is protected from the relatively high concentration of maternal glucocorticoids by placental 11β-hydroxysteroid dehydrogenase type 2 that converts physiologic maternal glucocorticoids to inactive metabolites (16, 17, 41, 42, 76, 127). Rats fed a low-protein diet during pregnancy had lower placental 11β-hydroxysteroid dehydrogenase activity (16, 76). In humans, placental 11β-hydroxysteroid dehydrogenase activity was shown not to change substantively during gestation; however, it was significantly reduced in placentas of small-for-gestational-age infants (66). Inhibition of placental 11β-hydroxysteroid dehydrogenase with carbenoxolone during pregnancy in rats resulted in intrauterine growth retardation in offspring whose mothers were fed a normal protein diet (74, 80). Adult rats whose mothers received carbenoxolone during pregnancy developed hypertension that was prevented by maternal adrenalectomy demonstrating that exposure of the fetus to maternal steroids resulted in hypertension (80). Dexamethasone is able to cross the placenta unscathed since it is a poor substrate for 11β-hydroxysteroid dehydrogenase and thus can cause similar insults as dietary protein deprivation.
The effect of inhibition of maternal steroid synthesis on prenatal programming of hypertension in rats due to maternal dietary protein deprivation was examined by inhibiting glucocorticoid production with metyrapone (73). In this study dietary protein deprivation was administered throughout pregnancy, and metyrapone was administered during the first half of pregnancy but not during the period of active nephrogenesis. Nonetheless, the offspring of the mothers that received metyrapone and a low-protein diet had blood pressures comparable to controls at 7 wk of age. Unfortunately, neither the number of glomeruli nor renal function was measured in this study. Thus, both maternal dietary protein deprivation and prenatal dexamethasone (or betamethasone) administration result in exposure of the fetus to excess glucocorticoids potentially linking these two fetal insults to the same underlying mechanism.
The common variable between prenatal low-protein diet and maternal steroid administration may be related to reduced maternal food intake (153). Administration of dexamethasone to rats on days 15–20 of gestation resulted in hypertension in the offspring measured using chronic instrumentation in conscious adult rats (153). The dexamethasone-treated dams ate less and gained less weight than vehicle-treated controls. Pregnant rats pair fed to the intake of dexamethasone-treated dams also gained less weight than the controls. Of importance was the finding that the offspring of the pair-fed rats were equally hypertensive as the offspring of the dexamethasone-treated rats (153). Similarly, maternal weight gain was reduced by a chronic reduction in uterine perfusion pressure resulting in uteroplacental insufficiency (3). Thus reduced maternal food intake is likely an important factor in prenatal programming of hypertension in maternal dexamethasone exposure and uteroplacental insufficiency as it is in maternal low-protein diet. It is unknown at this point whether pregnant rats that were pair fed to dexamethasone-treated rats and rats with uteroplacental insufficiency had increased fetal glucocorticoid exposure. Nonetheless, it is likely that uteroplacental insufficiency, maternal dietary protein deprivation, and prenatal dexamethasone cause fetal injury by a common mechanism resulting in offspring with a similar phenotype but variable severity depending on the extent of the fetal insult.
Mechanism for Hypertension by Prenatal Programming
Glomerular number.
Brenner hypothesized that infants with a paucity of nephrons at birth develop an impaired ability to excrete sodium leading to systemic and glomerular hypertension and eventually to glomerular sclerosis and impaired renal function (23–25, 83). In support of this hypothesis is the fact that in most animal models of prenatal programming, there is a concordance of a reduction in nephron number with an increase in blood pressure measured in adult animals (27, 77, 100, 123, 137, 145, 145–147, 155, 161). A reduction in nephron number was demonstrated in human neonates with intrauterine growth retardation (56, 57, 84). Caucasians with hypertension had fewer nephrons compared with those without hypertension (62, 68); however, this was not true of African Americans (62). The study demonstrating that Caucasians with hypertension had a reduction in nephron number as adults does not resolve the issue of whether the reduction in nephron number caused the hypertension or the hypertension caused the reduction in nephron number. While it is appealing to infer causality and assume that the paucity of nephrons due to prenatal programming is the cause for the hypertension, the two results of prenatal programming may not be directly related.
In some animal models there is a disjoint between a reduction in nephron number and the development of hypertension. Adverse prenatal events in rats have resulted in offspring with hypertension, despite a normal complement of nephrons (101, 146). In a study that examined blood pressure and nephron number in the F2 progeny of litters that were the result of mating spontaneously hypertensive rats and normotensive Wistar Kyoto rats, there was no correlation between nephron number and blood pressure (19). Furthermore, it is hard to attribute the severity of hypertension in some models of prenatal programming to a relatively small decrease in glomerular number and normal glomerular filtration rate (100, 101, 153). On the other hand, unilateral nephrectomy in rats on the first day of life resulted in hypertension that was exacerbated by a high-salt diet (149, 154). These rats had a 26% reduction in glomerular filtration rate compared with control rats at 8 wk of age that decreased to 49% of control by 20 wk of age (154). In addition, nephrectomized rats developed proteinuria and lesions comparable to focal and segmental glomerulosclerosis (154). In summary, while it is clear from the neonatal nephrectomy data that a significant reduction in nephron number (50% in the rat) contributed to the development and maintenance of hypertension (149, 154), it is unlikely that a reduction in nephron number is the sole or even a major factor causing hypertension when the nephron deficit is 20–30% of control, and there is no change in glomerular filtration rate, as is the case in most studies involving offspring of mothers that had a intrauterine insult.
Renal sodium transport.
There is significant evidence that prenatal insults program an increase in renal sodium transport. Adult rats whose mothers were fed a low-protein diet or administered prenatal glucocorticoids had an increase in renal α1-, β1-Na+/K+-ATPase mRNA abundance (17, 157). Studies have also demonstrated that there was an in increase in renal bumetanide-sensitive cotransporter (NKCC2) and thiazide-sensitive cotransporter (NCC) but not the Na+/H+ exchanger 3 (NHE3) the apical proximal tubule NHE3, or any of the ENaC (epithelial sodium channel) subunit protein abundance in offspring of mothers that had dietary protein restriction compared with controls (85). Similar findings were demonstrated in adult offspring of rats that received prenatal dexamethasone (34–36). However, there was an increase in brush-border membrane vesicle NHE3 protein abundance in the offspring of the prenatal dexamethasone-treated rats (34, 36). The disparity between the absence of an effect of prenatal programming on total cellular NHE3 and the increase in brush-border membrane NHE3 abundance suggests that prenatal insults affect trafficking of the NHE3 to the apical membrane of the proximal tubule. The studies examining transporter abundance are consistent with an increase in sodium transport in the thick ascending limb and distal convoluted tubule in rats that were small for gestational age, but the protein was obtained from a renal cortical homogenate and it does not guarantee that there is increased expression on the apical membrane or an increase in sodium transport in that nephron segment.
Sodium transport via ENaC in the distal convoluted tubule and collecting tubule is regulated by aldosterone. Glucocorticoids bind to the mineralocorticoid receptor with equal affinity to aldosterone but are prevented from having an effect because of 11β-hydroxysteroid dehydrogenase type 2 in the distal nephron, which converts cortisol to cortisone in humans and corticosterone to inactive 11-dehydrocorticosterone in the rat. Renal mRNA expression of 11β-hydroxysteroid dehydrogenase type 2 was lower in adult rat and sheep kidneys that had a prenatal insult than in controls (17, 144). Thus, while whole kidney ENaC protein abundance may not be different in controls and rats with prenatal insults, there still may be increased surface ENaC expression leading to higher rates of sodium absorption. Whether there is an increase in ENaC activity in the offspring of animals that suffered a prenatal insult has yet to be determined directly.
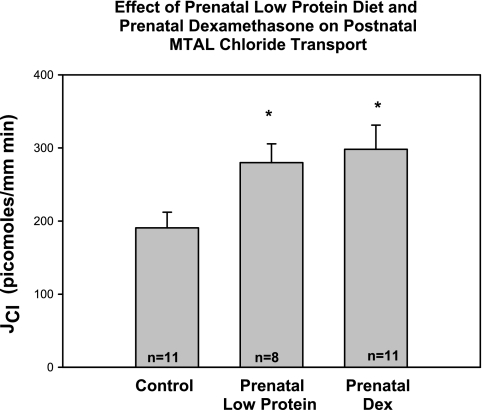
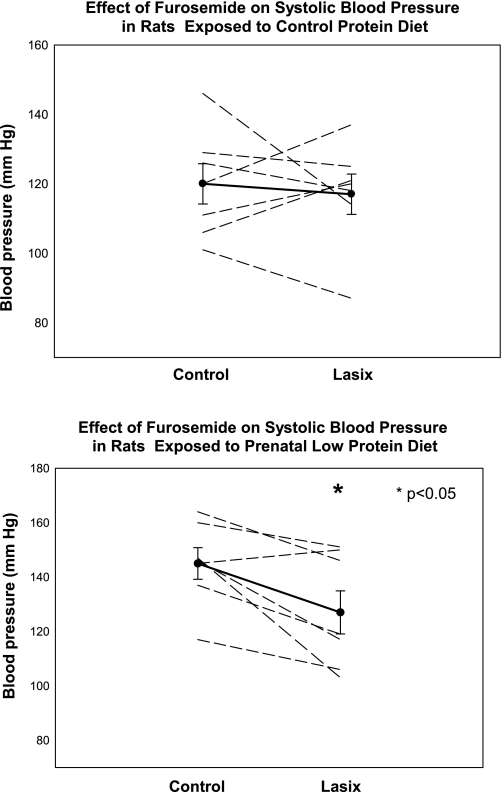
There is direct evidence that prenatal programming increases renal tubular sodium transport. Offspring of rats that received prenatal dexamethasone had an increase in proximal convoluted tubule volume absorption, an index of net sodium absorption in this segment, and apical NHE3 activity compared with controls when the tubules were dissected free and studied using in vitro microperfusion (34). As shown in Fig. 3, medullary thick ascending limbs from adult rats that received prenatal dexamethasone or dietary protein deprivation had a higher rate of chloride absorption and thus sodium absorption than control rats. Administration of furosemide to the drinking water for 1 day had no effect on blood pressure of control rats but caused a reduction in blood pressure in rats that had hypertension as a result of maternal ingestion of a low-protein diet (Fig. 4). Whether other nephron segments are affected or the regulation of sodium transport is different in other nephron segments will be of importance to understand the mechanism of hypertension in prenatal programming.
Fig. 3.
Effect of prenatal dexamethasone and prenatal dietary protein deprivation on thick ascending limb chloride transport. Medullary thick ascending limb (MTAL) chloride transport (JCl) from adult rats whose mothers were exposed either to prenatal dexamethasone (0.2 mg/kg body wt daily on days 15–18 gestation) or a low-protein diet during the last half of pregnancy. Both prenatal dexamethasone and dietary protein deprivation programmed an increase in MTAL chloride transport in adult rats compared with control when studied using in vitro microperfusion. [From Dagan et al. (35).]
Fig. 4.
Effect of furosemide on blood pressure control and rats whose mothers were fed a low-protein diet. Blood pressure was measured in control male rats and male rats whose mothers were fed a low-protein diet during the last half of pregnancy. Furosemide was then added to the drinking water, and the blood pressure was measured the next day. There was no effect of furosemide on blood pressure in control rats, but blood pressure decreased in the prenatal low-protein group. Veh, vehicle. [From Dagan et al. (35).]
Renin-angiotensin system.
All the components of the renin-angiotensin system are present in the fetus where it plays a vital role in the development of several organs, including the kidney (49, 52, 136). Pregnant women treated with an angiotensin converting enzyme inhibitor during the first trimester have been shown to be at increased risk for having children with central nervous system and cardiovascular disorders (31). Angiotensin converting enzyme inhibitors administered after the first trimester in humans resulted in severe renal damage leading to anuria and oligohydramnios (134). The prognosis for these children is usually grim. Similarly, rats treated with angiotensin converting enzyme inhibitors or angiotensin receptor blockers during nephrogenesis develop renovascular abnormalities, papillary atrophy, and a secondary renal concentrating defect (52). Rats administered losartan, an angiotensin II receptor blocker, during the perinatal days when nephrogenesis was still occurring, had a reduction in nephron number as well as a lower glomerular filtration rate, impaired renal concentrating ability, and increased blood pressure compared with controls (152). Mice with selective deletion of genes in the renin-angiotensin system had similar abnormalities (52). Thus the renin-angiotensin system plays a critical role in renal development, and disruption of the renin-angiotensin system leads to significant renal developmental abnormalities.
There is substantive evidence that the fetal renal renin-angiotensin system is affected by adverse maternal events. In rats, maternal dietary protein deprivation resulted in offspring with a reduction in renal renin mRNA, renal AT1-receptor protein, and mRNA abundance and renal angiotensin II levels, but higher AT2-receptor mRNA levels (138, 150, 151). There was also a reduction in renal renin and angiotensin II content in male pups whose mothers were fed an 8.5% protein diet (151). This reduction in renal renin and angiotensin II content was not seen in females in this study, but the mothers were fed only a moderately reduced protein diet that is not as low as the 6% protein diet used in some studies (151). Small-for-gestational-age rat pups that were the product of mothers that had uteroplacental insufficiency had lower intrarenal renin and angiotensinogen mRNA levels than controls (51). There are other factors besides the renin-angiotensin system that are affected by prenatal insults that could set the stage for postnatal hypertension. Administration of dexamethasone in vivo and in vitro altered gene expression of key mediators of renal branching morphogenesis including an increase in glial-derived neurotrophic factor and a decrease in both bone morphogenic protein-4 and transforming growth factor (132). In summary, prenatal insults likely result in renal injury by disturbing the renin-angiotensin system as well as other factors necessary for normal renal development.
Plasma levels of angiotensin II and aldosterone affect sodium homeostasis and blood pressure. Studies examining prenatal programming of the renin-angiotensin system have not yielded consistent results. Plasma renin activity was reduced in 4- and 8-wk-old rats whose mothers were fed a low-protein diet (87, 137) but increased at 4, 6, and 11 mo of age (86, 87). Similar increases in plasma renin activity were noted with prenatal dexamethasone in rats studied at 6 mo, long after the onset of hypertension (95). In summary, the elevated plasma renin noted in these studies were in older rats and did not correspond to the onset of the hypertension. Thus, the elevated plasma renin is likely the result of renal injury rather than the cause for the hypertension.
Other components of the renin-angiotensin system have also been examined in offspring of mothers that sustained a prenatal insult. Nine-week-old offspring of mothers fed a 6% protein diet had an increase in pulmonary angiotensin converting enzyme activity compared with controls, but this was not the case when the mothers were fed a 12% or a 9% protein diet (72, 75). The offspring of rats fed a 9% protein diet had an increase in plasma angiotensin converting enzyme activity compared with controls (75). However, maternal dietary protein deprivation had no effect on angiotensin II levels in the adult offspring (75, 138). Plasma aldosterone levels were higher in rats whose mothers were fed a low-protein diet compared with controls at 4 and 8 wk of age but not at 16 wk of age (87, 137). Thus, while there are changes in plasma angiotensin converting enzyme activity, none of the downstream mediators of the renin-angiotensin system are increased, so it is unlikely that the systemic renin-angiotensin system plays the primary role in generating or mediating the hypertension seen in neonates or adults that were small for gestational age.
In addition to the systemic renin-angiotensin system, there is an intrarenal renin-angiotensin system. The adult kidney has all the machinery to generate angiotensin II (69) and the intrarenal angiotensin II content was found to be higher than could be explained by circulating angiotensin II levels (22, 69, 92, 128). The proximal tubule luminal fluid angiotensin II concentration was determined to be about 100-fold higher than that in blood (22, 128). This renally produced angiotensin II has been shown to modulate transport in several nephron segments (30, 55, 70, 103, 105, 126, 142). Secreted angiotensin II has been shown to increase proximal tubule sodium absorption in vivo and in vitro (14, 109). In addition, the effect of the intrarenal renin-angiotensin system on proximal tubule transport was regulated by changes in the extracellular fluid volume and by renal nerves (14, 109–113). Luminal angiotensin II has been shown to increase sodium transport in the distal convoluted tubule as well (142). Finally, the collecting duct can convert angiotensin I to angiotensin II where it increased sodium transport by increasing ENaC activity (70). Thus the intrarenal renin-angiotensin system plays a significant role to modulate sodium transport in several nephron segments.
A number of studies have examined the effect of prenatal programming on the intrarenal renin-angiotensin system. Moderate maternal dietary protein deprivation (9% vs. 18% protein) had no effect on renal angiotensin converting enzyme activity in offspring at 4 wk and 20 wk of age compared with controls (93). However, maternal administration of dexamethasone from day 13 of gestation to term, a severe prenatal insult, increased renal angiotensin converting enzyme mRNA and renal renin mRNA levels in adult offspring compared with control rats (157). In 16-wk-old rats whose mothers had uteroplacental insufficiency, intrarenal angiotensin converting enzyme activity, renin, and angiotensinogen mRNA were higher than in control rats, but intrarenal angiotensin II levels were comparable to controls (51). Similarly, there was no increase in renal angiotensin I and angiotensin II levels in 28-day prehypertensive rats whose mothers were fed a 6% protein diet (138). Except for one study where the insult was the result of uteroplacental insufficiency, which used quantitative autoradiography, a method that is only reliable in detecting large changes (51), renal angiotensin II AT1 receptor mRNA and/or protein abundance was greater in rats whose mothers were fed a low-protein diet compared with controls (122, 138) and in small-for-gestational age piglets compared with their larger littermates (121). Despite the variability in results in prenatal programming of the intrarenal and systemic renin-angiotensin levels, which is likely the result of differences in the timing of the insult during gestation and the severity of the adverse event, several studies have demonstrated that treatment of rats that had a prenatal insult with an angiotensin converting enzyme inhibitor or angiotensin receptor blocker resulted in a reduction in blood pressure without a change in blood pressure in the control rats (28, 51, 75, 87, 129, 130). In summary, it is likely that the renin-angiotensin system plays a role in generating and mediating the hypertension in some animal models, but the lack of a consistent increase in the systemic or intrarenal angiotensin II levels indicate that it is not the predominant factor in mediating the hypertension caused by prenatal insults. As discussed above, there is augmentation of renal tubular sodium transport in several nephron segments consistent with an increase in extracellular fluid volume. Under these conditions, one would expect that the renin-angiotensin system would be downregulated. The fact that the systemic and intrarenal angiotensin II and aldosterone levels are normal and not suppressed is consistent with dysregulation of the renin-angiotensin system by prenatal programming.
Sympathetic nervous system.
The hypertension secondary to prenatal insults is exacerbated by stress. Prenatal dexamethasone induces hypertension in rats when measured by tail cuff (16, 27, 36, 95, 100, 101, 153). However, when blood pressure was measured by telemetry, the offspring of mothers that received prenatal dexamethasone actually had a lower blood pressure than controls (96). Prenatal dexamethasone-treated offspring monitored by telemetry had an augmented response to stress with a greater increase in blood pressure with restraint or intraperitoneal amphetamine administration (96). Similarly rats, whose mothers were fed a low-protein diet for 5 wk before and throughout pregnancy, had only a small increase in diastolic, but not in systolic, blood pressure and only during their awake cycle when blood pressure was measured by telemetry (135). However, the low-protein group had an augmented blood pressure response to stress (nasal ammonia) (135). Adult rats that were the product of mothers with uteroplacental insufficiency had elevated blood pressure when monitored by telemetry (97, 98, 125), and had higher systolic blood pressure in response to noxious nasal stimuli (125). Thus, blood pressure measured by telemetry has increased our understanding of prenatal programming of hypertension. Only severe prenatal insults from uteroplacental insufficiency result in hypertension in offspring when blood pressure is measured by telemetry in nonstressed animals. Offspring of mothers with prenatal dietary protein deprivation and prenatal dexamethasone have elevated blood pressure when measured even under the mild stress of a tail cuff, but have no significant increase when left undisturbed.
In human studies, adults that were small for gestational age had a higher resting pulse rate, a crude measure of sympathetic activity, than normal birth weight controls (106). Using more direct measures of sympathic nerve activity, such as the response of cardiac function to stress and peroneal nerve activity, there was an increase in sympathetic nerve activity in adults born small for gestational age compared with those of average birth weight (20, 63). However, resting peroneal nerve activity was lower in another study in subjects who were small for gestational age, but the small difference disappeared with breath holding and a cold pressor test (143). Nonetheless, in the latter study, the magnitude of the change in nerve activity was greater with an imposed stress, such as apnea or a cold pressor test, in subjects that were small for gestational age (143). Taken together the preponderance of data from human and animal studies demonstrate that the elevated blood pressure with prenatal programming is either due to or augmented by a stressful change in environment due to increased sympathetic nerve activity.
Renal nerves.
Recent studies have examined the role of renal nerves in the generation and maintenance of hypertension (2, 36, 99). The role of the renal sympathetic nervous system was first examined in rats whose mothers had uterine vessel ligation to produce uteroplacental insufficiency (2, 99). The hypertension induced by uteroplacental insufficiency was abrogated to control values with renal denervation (2, 99). Renal denervation did not affect blood pressure of control animals. Similarly, while renal denervation did not affect the blood pressure of control male rats, renal denervation normalized the blood pressure of male rats that had prenatal programming of hypertension due to maternal administration of dexamethasone (Fig. 5). In addition, as shown in Fig. 6, renal denervation decreased the protein abundance of NHE3, NKCl2, and NCC to levels seen in sham control rats (36). Control rats had no change in the NHE3, NKCC2, and NCC protein abundance with renal denervation. The epithelial sodium channel protein abundance was not affected by prenatal programming and was not affected by renal denervation in controls or in the prenatal dexamethasone group (36).
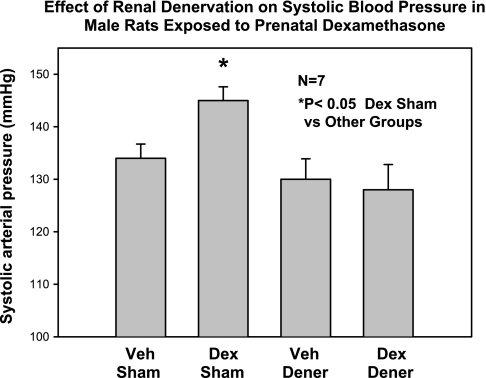
Fig. 5.
Effect of renal denervation on blood pressure in rats exposed to prenatal dexamethasone. Rats whose mothers were treated with prenatal dexamethasone (0.2 mg/kg body wt) daily on days 15–18 of gestation or vehicle underwent either a sham operation or renal denervation at 6 wk of age and blood pressure was measured at 8 wk. Prenatal dexamethasone caused an increase in blood pressure that was prevented by renal denervation. [From Dagan et al. (36).]
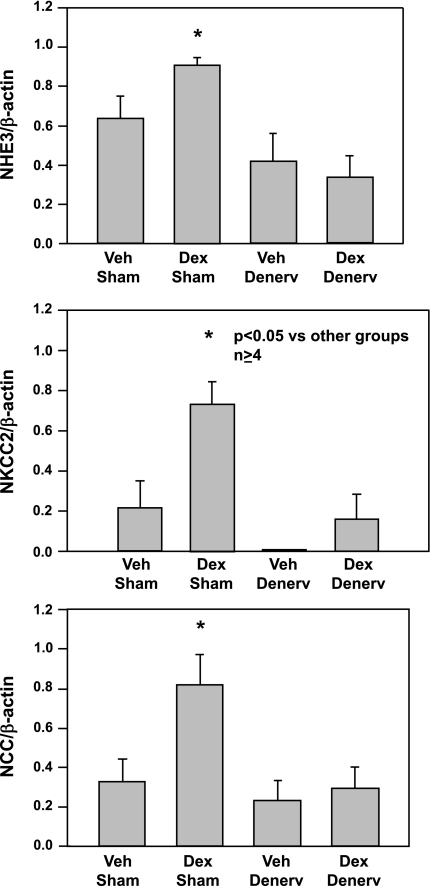
Fig. 6.
Effect of renal denervation on Na+/H+ exchanger (NHE3), bumetanide-sensitive cotransporter (NKCC2), and thiazide-sensitive cotransporter (NCC) protein abundance in rats exposed to prenatal dexamethasone. Adult rats whose mothers were treated with prenatal dexamethasone (0.2 mg/kg body wt daily) on days 15–18 of gestation or vehicle underwent either a sham operation or renal denervation at 6 wk of age. Renal NHE3, NKCC2, and NCC protein abundance were determined by immunoblot. Prenatal dexamethasone increased the abundance of each of these transporters in adult rats, and the abundance decreased to control levels with denervation. There was no effect of prenatal dexamethasone or denervation on epithelial sodium channel protein abundance (not shown). [From Dagan et al. (36).]
It is yet to be determined how renal sympathetic nerves regulate the expression of transporters. The effect of renal nerve activity could be a direct effect on sodium transport as renal nerves increase proximal tubule and thick ascending limb sodium reabsorption (4, 13, 15, 29, 107, 114, 148). It is also possible that the effect of renal nerves on sodium transport was indirect. Studies have demonstrated an interdependence between the renin-angiotensin system and renal nerves (37–39). The stimulatory effect of renal nerves on renal sodium reabsorption requires circulating angiotensin II (54, 64, 65). For example, the increase in renal sodium absorption accompanying renal nerve stimulation occurred only in the presence of circulating angiotensin II and was blunted by administration of captopril (64). Furthermore, circulating angiotensin II facilitated adrenergic transmission at the renal nerve-renal epithelial cell junction (54, 64, 65). We previously showed that the intrarenal renin-angiotensin system regulates proximal tubular transport (14, 109, 111, 112). The augmentation of proximal tubule sodium transport by the intrarenal renin-angiotensin system was dependent upon and regulated by renal nerves (113, 114). Thus, while renal nerves clearly play a role in causing an increase in blood pressure in the offspring of rats that had a maternal insult, the mechanism whereby they mediate this effect is not as yet clearly established.
Chronic Kidney Disease
Animal studies have demonstrated that prenatal insults are a risk factor for chronic kidney disease. Eight-week-old male, but not female rats, that were the offspring of mothers fed a low-protein diet (6% protein) had an elevated urinary protein/creatinine ratio, the harbinger of glomerular injury (86). Proteinuria has also been observed in 5-mo-old rats exposed to mild dietary protein deprivation in utero (9% protein) (93). A 50% maternal food restriction throughout pregnancy in rats resulted in offspring that had almost a 50% reduction in glomerular filtration rate when studied at 3 and 18 mo of age (82). These rats had progressive and marked histologic changes comparable to adult humans with severe chronic kidney disease, including glomerular sclerosis and tubular interstitial lesions (82, 116). Similarly, prenatal administration of dexamethasone on days 15 and 16 of gestation resulted in offspring with renal interstitial fibrosis, tubular atrophy, and glomerular sclerosis at 8 mo of age that was not apparent at 2 mo of age (100, 101). Thus, prenatal insults in rats result in offspring that develop progressive renal injury over time.
There is increasing evidence that humans born small for gestational age are at risk for developing chronic kidney disease. Indeed, small-for-gestational-age infants have many risk factors for kidney disease including a reduction in nephron number, diabetes, hypertension, and dyslipidemia (6, 7, 26, 32, 33, 44, 45, 48, 56, 57, 78, 81, 88, 89, 102, 115, 117, 118, 133, 158). Small-for-gestational-age infants have been shown to be born with fewer nephrons making a second insult potentially more likely to cause progressive renal injury (56, 61, 84, 108). In Aborigines, a population at high risk for chronic kidney disease, a birth weight of < 2.5 kg increased the risk of albuminuria almost threefold compared with those of higher birth weights (60). Similarly, 19-yr-old subjects from the Netherlands born small for gestational age had a higher prevalence of albuminuria than those born appropriate for gestational age (67). In addition, the estimated glomerular filtration rate was slightly, but significantly, lower in the group that was born small for gestational age (67). A similar finding of low normal glomerular filtration rates was found in a large study of Norwegian young adults who were small for gestational age, with a greater effect in men (53). A recent study examined renal biopsies of patients with a birth weight < 1,500 g. The renal biopsies of all six adolescent and adult patients evaluated for proteinuria showed focal and segmental glomerulosclerosis without any other predisposing factor for this lesion except low birth weight (59). It was not clear how many of these patients were small for gestational age or just premature. One must be mindful that a neonate born < 1,500 g is likely to have had a prolonged stay in the neonatal intensive care unit and be exposed to nephrotoxic drugs during the period of nephrogenesis. Whether it is prematurity or small for gestational age or both, neonates born < 2.5 kg have been shown to be at ∼1.5 greater risk of developing end stage renal disease than are children born at a normal weight of 3–3.5 kg (46, 71, 141). Thus, a history of prematurity or low birth weight should be considered when investigating potential factors leading to chronic kidney disease.
Effect of the Postnatal Environment on Glomerular Number and Blood Pressure
The rodent kidney is not fully developed at birth, and there are changes in glomerular filtration rate and tubular function that continue until at least the time of weaning (12). Nephrogenesis continues in humans until 34- to 36-wk gestation. Thus premature human neonates born before 34- to 36-wk gestation still have new nephrons forming after birth. The postnatal environment can have a negative impact on renal development and program changes in blood pressure. Recent evidence shows that postnatal nutrition and drugs can modulate prenatal programming of hypertension as well as the number of glomeruli. As an example of how the postnatal environment can have an adverse effect on the kidney, a reduction in food intake was induced by increasing the number of rat pups to 20 by fostering additional pups to a mother (124). These food-deprived neonates had postnatal growth retardation compared with control offspring of mothers that reared 10 pups. The food-restricted rats had a 25% reduction in nephron number and a concomitant increase in glomerular volume compared with rats that were reared in a normal litter size of ∼10 pups. Another example of postnatal programming was recently demonstrated in rats exposed to 80% oxygen from postnatal days 3–10. The oxygen-exposed rats developed vascular dysfunction, elevated blood pressure, and had 25% fewer nephrons compared with control rats when studied as adults (160). These findings have relevance to premature human neonates cared for in a neonatal intensive care unit where, even under the best of circumstances, neonates are not in a comparable environment as when they were in their mother's womb.
In most studies performed to date, the offspring suffering a prenatal insult were reared by their mother who was placed on a normal diet with the assumption that the insult has been removed after birth. However, prenatal insults to the mother have been shown to affect postnatal rearing and the composition of the mother's milk (94). Even the number of pups reared by the mother can affect composition of the mother's milk (94). To examine the importance of postnatal variables, Wlodek et al. (146) performed an elegant study where all the offspring were cross fostered. Normal neonates that were reared by mothers that had uterine vessel ligation on day 18 of gestation developed hypertension as adults. These rats, unlike the offspring of mothers with uteroplacental insufficiency and raised by mothers that had uteroplacental insufficiency, did not have a reduction in nephron number. Of significance, neonates that were the product of mothers who had uterine vessel ligation on day 18 and were raised by mothers that had a sham operation, did not develop hypertension or a reduction in nephron number as adults (146). Thus, some studies where the insult was assumed to be prenatal may actually be exacerbated by the postnatal environment.
Perinatal caloric excess can be deleterious as well. The offspring of a rat litter reduced to three pups on day 3 of life was compared with the offspring of mothers that bore and raised 10 pups (21). The rats that were fed excess milk gained more weight and both males and females had significantly more glomeruli than the control offspring. Nonetheless, male offspring that were overfed had higher blood pressure at 2 mo of age, proteinuria at 12 mo of age, and glomerulosclerosis at 22 mo of age. These changes were not seen in overfed females or control rats. Thus the early postnatal environment may be as important as the prenatal environment in programming hypertension and renal injury.
Prenatal programming may also be modified outside of the perinatal period. The effect of enalapril and a low-salt diet were examined in rats that were the products of mothers that were fed a low-protein diet. These rats developed hypertension as adults that was exacerbated by a high-salt diet (87). Administration of either a low-salt diet or enalapril to offspring of mothers fed a low-protein diet at the time of weaning for 3 wk prevented the development of hypertension. What was remarkable was the fact that the blood pressure remained at control levels even though the low-salt diet and enalapril were discontinued weeks before the final measurement (87). A similar persistent effect of early treatment followed by discontinuation of medication had been shown for losartan but not with the calcium channel blocker nifedipine (130). Thus, there are critical times during postnatal development where one could potentially intercede and prevent hypertension. Whether this is true in humans has not been determined.
The metabolic abnormalities seen with prenatal programming can also be modulated by the postnatal environment. Offspring of rats that were severely undernourished during pregnancy had hyperleptinemia, hyperinsulinemia, and hyperphagia, which was exacerbated by a high-fat diet (139, 140). The offspring of undernourished rats had decreased activity and excessive weight gain compared with controls. Treatment of neonates with leptin from days 3–13 of life had no significant effect on control rats, but normalized the activity levels, caloric intake, and body fat as well as the plasma insulin, glucose, and leptin levels in the offspring of underfed mothers (140). These findings are of clinical significance in view of the fact that there is a predisposition to develop a high body mass index in humans who were small for gestational age (11, 18).
Clinical Perspective
Epidemiological studies have found that prenatal adverse events predispose adult humans to develop hypertension and renal injury. Animal studies have validated these findings showing an association of prenatal insults and adult diseases and have provided important clues to the pathogenesis of these disorders. There are still many challenges to be met in view of these clinical and laboratory observations.
It is now clear that postnatal nutrition in rodents is critical. Either a deficiency or surplus of food in rats can cause renal injury similar to that seen with prenatal programming (21, 124). These findings may be applicable to premature human neonates born at an appropriate weight for gestational age before 34–36 wk when nephrogenesis is still occurring. These infants have an extended stay in the neonatal intensive care unit and are likely to have periods of suboptimal nutrition, hypoxia, hyperoxia, acidosis, infection, etc. that may place them at risk for the same adult diseases seen in neonates with prenatal adverse events leading to a low birth weight.
Finally, recent studies in rodents have shown that prenatal programming of hypertension and renal injury is not set in stone and can be modulated by the postnatal environment. The fact that optimal postnatal nutrition can potentially ameliorate or prevent programming of hypertension and renal injury in rats is of potential clinical importance (146). A recent study demonstrated that postnatal dietary supplementation with long-chain ω-3 fatty acids prevented the increase in blood pressure in adult rats whose mothers received dexamethasone during the last half of pregnancy (156). It must be recognized that postnatal overfeeding does not make up for prenatal malnutrition and can be deleterious. Administration of a hypercaloric diet after weaning to prenatally undernourished rats resulted in greater weight gain, higher plasma insulin levels, and higher blood pressure than prenatally undernourished rats fed a normal rat diet (139). The recent epidemiologic findings showing that low birth weight and an increase in body mass index after 2 years of age was a risk factor for hypertension, cardiovascular disease, and impaired glucose tolerance or diabetes in adulthood begs the question as to whether prevention of the rapid weight gain in early childhood would have altered the prognosis of these children (11, 18, 43). Clearly, much has yet to be learned about the factors that could potentially ameliorate the clinical consequences of prenatal insults.
DISCLOSURES
No conflicts of interest are declared by the author.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-41612, DK-078596, T32-DK-07257, and P30-DK-079328 (through the O'Brien Center).
REFERENCES
- 1.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension 45: 754–758, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension 37: 485–489, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Bailly C, Imbert-Teboul M, Roinel N, Amiel C. Isoproterenol increases Ca, Mg, and NaCl reabsorption in mouse thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1224–F1231, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ 301: 259–262, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJ, Godfrey KM, Osmond C, Bull A. The relation of fetal length, ponderal index and head circumference to blood pressure and the risk of hypertension in adult life. Paediatr Perinat Epidemiol 6: 35–44, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36: 62–67, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Barker DJ, Osmond C. Low birth weight and hypertension. BMJ 297: 134–135, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298: 564–567, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 2: 577–580, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Barker DJP, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med 353: 1802–1809, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Baum M, Quigley R, Satlin L. Seldin And Giebisch's The Kidney Physiology and Pathophysiology, edited by Alpern RJ, Hebert SC. London: Academic, 2008, p. 707–722 [Google Scholar]
- 13.Baum M, Quigley R. Inhibition of proximal convoluted tubule transport by dopamine. Kidney Int 54: 1593–1600, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baum M, Quigley R, Quan A. Effect of luminal angiotensin II on rabbit proximal convoluted tubule bicarbonate absorption. Am J Physiol Renal Physiol 273: F595–F600, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Bello-Reuss E. Effect of catecholamines on fluid reabsorption by the isolated proximal convoluted tubule. Am J Physiol Renal Fluid Electrolyte Physiol 238: F347–F352, 1980 [DOI] [PubMed] [Google Scholar]
- 16.Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet 341: 339–341, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11β-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology 142: 2841–2853, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Bhargava SK, Sachdev HS, Fall CHD, Osmond C, Lakshmy R, Barker DJP, Biswas SKD, Ramji S, Prabhakaran D, Reddy KS. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med 350: 865–875, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black MJ, Briscoe TA, Constantinou M, Kett MM, Bertram JF. Is there an association between level of adult blood pressure and nephron number or renal filtration surface area? Kidney Int 65: 582–588, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Boguszewski MC, Johannsson G, Fortes LC, Sverrisdottir YB. Low birth size and final height predict high sympathetic nerve activity in adulthood. J Hypertens 22: 1157–1163, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Boubred F, Buffat C, Feuerstein JM, Daniel L, Tsimaratos M, Oliver C, Lelievre-Pegorier M, Simeoni U. Effects of early postnatal hypernutrition on nephron number and long-term renal function and structure in rats. Am J Physiol Renal Physiol 293: F1944–F1949, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Braam BMKFJNL. Proximal tubular secretion of angiotensin II in rats. Am J Physiol Renal Fluid Electrolyte Physiol 264: F891–F898, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Brenner BM, Chertow GM. Congenital oligonephropathy: an inborn cause of adult hypertension and progressive renal injury? Curr Opin Nephrol Hypertens 2: 691–695, 1993 [PubMed] [Google Scholar]
- 24.Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23: 171–175, 1994 [PubMed] [Google Scholar]
- 25.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1: 335–347, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Campbell DM, Hall MH, Barker DJ, Cross J, Shiell AW, Godfrey KM. Diet in pregnancy and the offspring's blood pressure 40 years later. Br J Obstet Gynaecol 103: 273–280, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Celsi G, Kistner A, Aizman R, Eklof AC, Ceccatelli S, de Santiago A, Jacobson SH. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res 44: 317–322, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Ceravolo GS, Franco MCP, Carneiro-Ramos MS, Barreto-Chaves MLM, Tostes RCA, Nigro D, Fortes ZB, Carvalho MHC. Enalapril and losartan restored blood pressure and vascular reactivity in intrauterine undernourished rats. Life Sci 80: 782–787, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Cogan MG. Neurogenic regulation of proximal bicarbonate and chloride reabsorption. Am J Physiol Renal Fluid Electrolyte Physiol 250: F22–F26, 1986 [DOI] [PubMed] [Google Scholar]
- 30.Cogan MG. Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Hypertension 15: 451–458, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, Hall K, Ray WA. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 354: 2443–2451, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, Speizer FE, Stampfer MJ. Birth weight and adult hypertension and obesity in women. Circulation 94: 1310–1315, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation 94: 3246–3250, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Dagan A, Gattineni J, Cook V, Baum M. Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am J Physiol Regul Integr Comp Physiol 292: R1230–R1235, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dagan A, Habbib S, Gattineni J, Dwarakanath V, Baum M. Prenatal programming of rat thick ascending limb chloride transport by low protein diet and dexamethasone. Am J Physiol Regul Integr Comp Physiol 297: R93–R99, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol 295: F29–F34, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiBona GF. Nervous kidney. Interaction between renal sympathetic nerves and the renin-angiotensin system in the control of renal function. Hypertension 36: 1083–1088, 2000 [DOI] [PubMed] [Google Scholar]
- 38.DiBona GF. Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am J Physiol Regul Integr Comp Physiol 279: R1517–R1524, 2000 [DOI] [PubMed] [Google Scholar]
- 39.DiBona GF. Peripheral and central interactions between the renin-angiotensin system and the renal sympathetic nerves in control of renal function. Ann NY Acad Sci 940: 395–406, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 94: 149–155, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet 341: 355–357, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. 11β-Hydroxysteroid dehydrogenases: key enzymes in determining tissue-specific glucocorticoid effects. Steroids 61: 263–269, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Eriksson JG, Forsen TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension 49: 1415–1421, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Fall CH, Osmond C, Barker DJ, Clark PM, Hales CN, Stirling Y, Meade TW. Fetal and infant growth and cardiovascular risk factors in women. BMJ 310: 428–432, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fall CH, Stein CE, Kumaran K, Cox V, Osmond C, Barker DJ, Hales CN. Size at birth, maternal weight, and type 2 diabetes in South India. Diabet Med 15: 220–227, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Fan ZJ, Lackland DT, Lipsitz SR, Nicholas JS. The association of low birthweight and chronic renal failure among Medicaid young adults with diabetes and/or hypertension. Public Health Rep 121: 239–244, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res 58: 510–515, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Forrester TE, Wilks RJ, Bennett FI, Simeon D, Osmond C, Allen M, Chung AP, Scott P. Fetal growth and cardiovascular risk factors in Jamaican schoolchildren. BMJ 312: 156–160, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Friberg P, Sundelin B, Bohman SO, Bobik A, Nilsson H, Wickman A, Gustafsson H, Petersen J, Adams MA. Renin-angiotensin system in neonatal rats–induction of a renal abnormality in response to ace-inhibition or angiotensin-ii antagonism. Kidney Int 45: 485–492, 1994 [DOI] [PubMed] [Google Scholar]
- 50.Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med 5, Suppl A: S121–S132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, Speth RC, Brosnihan KB, Alexander BT. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 293: R804–R811, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guron G, Friberg P. An intact renin-angiotensin system is a prerequisite for normal renal development. J Hypertens 18: 123–137, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Hallan S, Euser AM, Irgens LM, Finken MJ, Holmen J, Dekker FW. Effect of intrauterine growth restriction on kidney function at young adult age: the Nord Trondelag Health (HUNT 2) Study. Am J Kidney Dis 51: 10–20, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Handa RK, Johns EJ. Interaction of the renin-angiotensin system and the renal nerves in the regulation of rat kidney function. J Physiol 369: 311–321, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris PJ, Young JA. Dose-dependent stimulation and inhibition of proximal tubular sodium reabsorption by angiotensin II in the rat kidney. Pflügers Arch 367: 295–297, 1977 [DOI] [PubMed] [Google Scholar]
- 56.Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, van Velzen D. The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol 99: 296–301, 1992 [DOI] [PubMed] [Google Scholar]
- 57.Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64: 777–784, 1991 [PubMed] [Google Scholar]
- 58.Hodgin JB, Rasoulpour M, Markowitz GS, D'Agati VD. Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 4: 71–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hodgin JB, Rasoulpour M, Markowitz GS, D'Agati VD. Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 4: 71–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoy WE, Rees M, Kile E, Mathews JD, Wang Z. A new dimension to the Barker hypothesis: low birthweight and susceptibility to renal disease. Kidney Int 56: 1072–1077, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Hughson M, Farris AB, III, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 63: 2113–2122, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the Southeastern United States. Kidney Int 69: 671–678, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Ijzerman RG, Stehouwer CD, de Geus EJ, van Weissenbruch MM, Delemarre-van de Waal HA, Boomsma DI. Low birth weight is associated with increased sympathetic activity: dependence on genetic factors. Circulation 108: 566–571, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Johns EJ. The role of angiotensin II in the antidiuresis and antinatriuresis induced by stimulation of the sympathetic nerves to the rat kidney. J Auton Pharmacol 7: 205–214, 1987 [DOI] [PubMed] [Google Scholar]
- 65.Johns EJ. Role of angiotensin II and the sympathetic nervous system in the control of renal function. J Hypertens 7: 695–701, 1989 [PubMed] [Google Scholar]
- 66.Kajantie E, Dunkel L, Turpeinen U, Stenman UH, Wood PJ, Nuutila M, Andersson S. Placental 11β-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. J Clin Endocrinol Metab 88: 493–500, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Keijzer-Veen MG, Schrevel M, Finken MJ, Dekker FW, Nauta J, Hille ET, Frolich M, van der Heijden BJ. Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol 16: 2762–2768, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Keller G, Zimmer G, Mall G, Ritz E, Amann K.Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension 42: 195–199, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med 160: 1472–1476, 2000 [DOI] [PubMed] [Google Scholar]
- 72.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 86: 217–222, 1994 [DOI] [PubMed] [Google Scholar]
- 73.Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens 15: 537–544, 1997 [DOI] [PubMed] [Google Scholar]
- 74.Langley-Evans SC. Maternal carbenoxolone treatment lowers birthweight and induces hypertension in the offspring of rats fed a protein-replete diet. Clin Sci (Lond) 93: 423–429, 1997 [DOI] [PubMed] [Google Scholar]
- 75.Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol 110: 223–228, 1995 [DOI] [PubMed] [Google Scholar]
- 76.Langley-Evans SC, Phillips GJ, Benediktsson R, Gardner DS, Edwards CR, Jackson AA, Seckl JR. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta 17: 169–172, 1996 [DOI] [PubMed] [Google Scholar]
- 77.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 64: 965–974, 1999 [DOI] [PubMed] [Google Scholar]
- 78.Law CM, Barker DJ, Bull AR, Osmond C. Maternal and fetal influences on blood pressure. Arch Dis Child 66: 1291–1295, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens 14: 935–941, 1996 [PubMed] [Google Scholar]
- 80.Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11β-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension 27: 1200–1204, 1996 [DOI] [PubMed] [Google Scholar]
- 81.Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell UB, Leon DA. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50–60 years. BMJ 312: 406–410, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lucas SRR, Miraglia SM, Gil FZ, Coimbra TM. Intrauterine food restriction as a determinant of nephrosclerosis. Am J Kidney Dis 37: 467–476, 2001 [DOI] [PubMed] [Google Scholar]
- 83.Mackenzie HS, Lawler EV, Brenner BM. Congenital oligonephropathy: The fetal flaw in essential hypertension? Kidney Int Suppl 55: S30–S34, 1996 [PubMed] [Google Scholar]
- 84.Manalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int 58: 770–773, 2000 [DOI] [PubMed] [Google Scholar]
- 85.Manning J, Beutler K, Knepper MA, Vehaskari VM. Upregulation of renal BSC1 and TSC in prenatally programmed hypertension. Am J Physiol Renal Physiol 283: F202–F206, 2002 [DOI] [PubMed] [Google Scholar]
- 86.Manning J, Vehaskari VM. Low birth weight-associated adult hypertension in the rat. Pediatr Nephrol 16: 417–422, 2001 [DOI] [PubMed] [Google Scholar]
- 87.Manning J, Vehaskari VM. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am J Physiol Regul Integr Comp Physiol 288: R80–R84, 2005 [DOI] [PubMed] [Google Scholar]
- 88.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85: 571–633, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Moore VM, Miller AG, Boulton TJ, Cockington RA, Craig IH, Magarey AM, Robinson JS. Placental weight, birth measurements, and blood pressure at age 8 years. Arch Dis Child 74: 538–541, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moritz KM, Singh RR, Probyn ME, Denton KM. Developmental programming of a reduced nephron endowment: more than just a baby's birth weight. Am J Physiol Renal Physiol 296: F1–F9, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Moritz KM, Wintour EM. Functional development of the meso- and metanephros. Pediatr Nephrol 13: 171–178, 1999 [DOI] [PubMed] [Google Scholar]
- 92.Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins I and II in anesthetized rats. J Am Soc Nephrol 5: 1153–1158, 1994 [DOI] [PubMed] [Google Scholar]
- 93.Nwagwu MO, Cook A, Langley-Evans SC. Evidence of progressive deterioration of renal function in rats exposed to a maternal low-protein diet in utero. Br J Nutr 83: 79–85, 2000 [PubMed] [Google Scholar]
- 94.O'Dowd R, Kent JC, Moseley JM, Wlodek ME. Effects of uteroplacental insufficiency and reducing litter size on maternal mammary function and postnatal offspring growth. Am J Physiol Regul Integr Comp Physiol 294: R539–R548, 2008 [DOI] [PubMed] [Google Scholar]
- 95.O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab 287: E863–E870, 2004 [DOI] [PubMed] [Google Scholar]
- 96.O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone 'programmes' hypotension, but stress-induced hypertension in adult offspring. J Endocrinol 196: 343–352, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension 50: 679–685, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 292: R758–R763, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Ojeda NB, Johnson WR, Dwyer TM, Alexander BT. Early renal denervation prevents development of hypertension in growth-restricted offspring. Clin Exp Pharmacol Physiol 34: 1212–1216, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int 59: 1663–1669, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41: 328–334, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect 108, Suppl 3: 545–553, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292: F914–F920, 2007 [DOI] [PubMed] [Google Scholar]
- 104.Perantoni AO, Williams CL, Lewellyn AL. Growth and branching morphogenesis of rat collecting duct anlagen in the absence of metanephrogenic mesenchyme. Differentiation 48: 107–113, 1991 [DOI] [PubMed] [Google Scholar]
- 105.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT1 receptors. J Am Soc Nephrol 13: 1131–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 106.Phillips DIW, Barker DJP. Association between low birthweight and high resting pulse in adult life: is the sympathetic nervous system involved in programming the insulin resistance syndrome? Diabet Med 14: 673–677, 1997 [DOI] [PubMed] [Google Scholar]
- 107.Plato CF. α-2 and β-Adrenergic receptors mediate NE's biphasic effects on rat thick ascending limb chloride flux. Am J Physiol Regul Integr Comp Physiol 281: R979–R986, 2001 [DOI] [PubMed] [Google Scholar]
- 108.Puddu M, Fanos V, Podda F, Zaffanello M. The kidney from prenatal to adult life: perinatal programming and reduction of number of nephrons during development. Am J Nephrol 30: 162–170, 2009 [DOI] [PubMed] [Google Scholar]
- 109.Quan A, Baum M. Endogenous production of angiotensin II modulates rat proximal tubule transport. J Clin Invest 97: 2878–2882, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Quan A, Baum M. Regulation of proximal tubule transport by angiotensin II. Semin Nephrol 17: 423–430, 1997 [PubMed] [Google Scholar]
- 111.Quan A, Baum M. Endogenous angiotensin II modulates rat proximal tubule transport with acute changes in extracellular volume. Am J Physiol Renal Physiol 275: F74–F78, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quan A, Baum M. Regulation of proximal tubule transport by endogenously produced angiotensin II. Nephron 84: 103–110, 2000 [DOI] [PubMed] [Google Scholar]
- 113.Quan A, Baum M. The renal nerve is required for regulation of proximal tubule transport by intraluminally produced ANG II. Am J Physiol Renal Physiol 280: F524–F529, 2001 [DOI] [PubMed] [Google Scholar]
- 114.Quan A, Baum M. Renal nerve stimulation augments effect of intraluminal angiotensin II on proximal tubule transport. Am J Physiol Renal Physiol 282: F1043–F1048, 2002 [DOI] [PubMed] [Google Scholar]
- 115.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351: 173–177, 1998 [DOI] [PubMed] [Google Scholar]
- 116.Regina S, Lucas R, Miraglia SM, Zaladek GF, Machado CT. Intrauterine food restriction as a determinant of nephrosclerosis. Am J Kidney Dis 37: 467–476, 2001 [DOI] [PubMed] [Google Scholar]
- 117.Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Gillman MW, Hennekens CH, Speizer FE, Manson JE. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med 130: 278–284, 1999 [DOI] [PubMed] [Google Scholar]
- 118.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Schroeder-Tanka JM, van Montfrans GA, Michels RP, Bleker OP. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart 84: 595–598, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roseboom TJ, van der Meulen JH, Ravelli AC, van Montfrans GA, Osmond C, Barker DJ, Bleker OP. Blood pressure in adults after prenatal exposure to famine. J Hypertens 17: 325–330, 1999 [DOI] [PubMed] [Google Scholar]
- 120.Roseboom TJ, van der Meulen JH, van Montfrans GA, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Maternal nutrition during gestation and blood pressure in later life. J Hypertens 19: 29–34, 2001 [DOI] [PubMed] [Google Scholar]
- 121.Ruster M, Sommera M, Stein G, Bauer K, Walter B, Wolf G, Bauer R. Renal angiotensin receptor type 1 and 2 upregulation in intrauterine growth restriction of newborn piglets. Cells Tissues Organs 182: 106–114, 2006 [DOI] [PubMed] [Google Scholar]
- 122.Sahajpal V, Ashton N. Renal function and angiotensin AT1 receptor expression in young rats following intrauterine exposure to a maternal low-protein diet. Clin Sci 104: 607–614, 2003 [DOI] [PubMed] [Google Scholar]
- 123.Schreuder MF. Unravelling perinatal programming of the kidney. Am J Physiol Regul Integr Comp Physiol 293: R2159, 2007 [DOI] [PubMed] [Google Scholar]
- 124.Schreuder MF, Nyengaard JR, Remmers F, van Wijk JAE, Delemarre-van de Waal HA. Postnatal food restriction in the rat as a model for a low nephron endowment. Am J Physiol Renal Physiol 291: F1104–F1107, 2006 [DOI] [PubMed] [Google Scholar]
- 125.Schreuder MF, van Wijk JAE, Delemarre-van de Waal HA. Intrauterine growth restriction increases blood pressure and central pulse pressure measured with telemetry in aging rats. J Hypertens 24: 1337–1343, 2006 [DOI] [PubMed] [Google Scholar]
- 126.Schuster VL, Kokko JP, Jacobson HR. Angiotensin II directly stimulates sodium transport in rabbit proximal convoluted tubules. J Clin Invest 73: 507–515, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seckl JR, Benediktsson R, Lindsay RS, Brown RW. Placental 11β-hydroxysteroid dehydrogenase and the programming of hypertension. J Steroid Biochem Mol Biol 55: 447–455, 1995 [DOI] [PubMed] [Google Scholar]
- 128.Seikaly MG, Arant BS, Jr, Seney FD., Jr Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest 86: 1352–1357, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sherman RC, Langley-Evans SC. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin Sci (Lond) 94: 373–381, 1998 [DOI] [PubMed] [Google Scholar]
- 130.Sherman RC, Langley-Evans SC. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin Sci (Lond) 98: 269–275, 2000 [PubMed] [Google Scholar]
- 131.Singh RR, Moritz KM, Bertram JF, Cullen-McEwen LA. Effects of dexamethasone exposure on rat metanephric development: in vitro and in vivo studies. Am J Physiol Renal Physiol 293: F548–F554, 2007 [DOI] [PubMed] [Google Scholar]
- 132.Singh RR, Moritz KM, Bertram JF, Cullen-McEwen LA. Effects of dexamethasone exposure on rat metaneprhic development: in vitro and in vivo studies. Am J Physiol Renal Physiol 293: F548–F554, 2007 [DOI] [PubMed] [Google Scholar]
- 133.Stein CE, Fall CH, Kumaran K, Osmond C, Cox V, Barker DJ. Fetal growth and coronary heart disease in south India. Lancet 348: 1269–1273, 1996 [DOI] [PubMed] [Google Scholar]
- 134.Tabacova S, Little R, Tsong Y, Vega A, Kimmel CA. Adverse pregnancy outcomes associated with maternal enalapril antihypertensive treatment. Pharmacoepidemiol Drug Saf 12: 633–646, 2003 [DOI] [PubMed] [Google Scholar]
- 135.Tonkiss J, Trzcinska M, Galler JR, Ruiz-Opazo N, Herrera VL. Prenatal malnutrition-induced changes in blood pressure: dissociation of stress and nonstress responses using radiotelemetry. Hypertension 32: 108–114, 1998 [DOI] [PubMed] [Google Scholar]
- 136.Tufro-McReddie A, Romano LM, Harris JM, Ferder L, Gomez RA. Angiotensin II regulates nephrogenesis and renal vascular development. Am J Physiol Renal Fluid Electrolyte Physiol 269: F110–F115, 1995 [DOI] [PubMed] [Google Scholar]
- 137.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int 59: 238–245, 2001 [DOI] [PubMed] [Google Scholar]
- 138.Vehaskari VM, Stewart T, Lafont D, Soyez C, Seth D, Manning J. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am J Physiol Renal Physiol 287: F262–F267, 2004 [DOI] [PubMed] [Google Scholar]
- 139.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279: E83–E87, 2000 [DOI] [PubMed] [Google Scholar]
- 140.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology 146: 4211–4216, 2005 [DOI] [PubMed] [Google Scholar]
- 141.Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 19: 151–157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F143–F149, 1996 [DOI] [PubMed] [Google Scholar]
- 143.Weitz G, Deckert P, Heindl S, Struck J, Perras B, Dodt C. Evidence for lower sympathetic nerve activity in young adults with low birth weight. J Hypertens 21: 943–950, 2003 [DOI] [PubMed] [Google Scholar]
- 144.Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early to midgestation programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11β-hydroxysteroid dehydrogenase isoforms, and type 1 angiotensin II receptor in neonatal sheep. Endocrinology 142: 2854–2864, 2001 [DOI] [PubMed] [Google Scholar]
- 145.Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol 549: 929–935, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM. Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am Soc Nephrol 18: 1688–1696, 2007 [DOI] [PubMed] [Google Scholar]
- 147.Wlodek ME, Westcott K, Siebel AL, Owens JA, Moritz KM. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int 74: 187–195, 2008 [DOI] [PubMed] [Google Scholar]
- 148.Wong KR, Berry CA, Cogan MG. α1-Adrenergic control of chloride transport in the rat S1 proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 270: F1049–F1056, 1996 [DOI] [PubMed] [Google Scholar]
- 149.Woods LL. Neonatal uninephrectomy causes hypertension in adult rats. Am J Physiol Regul Integr Comp Physiol 276: R974–R978, 1999 [DOI] [PubMed] [Google Scholar]
- 150.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49: 460–467, 2001 [DOI] [PubMed] [Google Scholar]
- 151.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol 289: R1131–R1136, 2005 [DOI] [PubMed] [Google Scholar]
- 152.Woods LL, Rasch R. Perinatal ANG II programs adult blood pressure, glomerular number, and renal function in rats. Am J Physiol Regul Integr Comp Physiol 275: R1593–R1599, 1998 [DOI] [PubMed] [Google Scholar]
- 153.Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol 289: R955–R962, 2005 [DOI] [PubMed] [Google Scholar]
- 154.Woods LL, Weeks DA, Rasch R. Hypertension after neonatal uninephrectomy in rats precedes glomerular damage. Hypertension 38: 337–342, 2001 [DOI] [PubMed] [Google Scholar]
- 155.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int 65: 1339–1348, 2004 [DOI] [PubMed] [Google Scholar]
- 156.Wyrwoll CS, Mark PJ, Mori TA, Puddey IB, Waddell BJ. Prevention of programmed hyperleptinemia and hypertension by postnatal dietary omega-3 fatty acids. Endocrinology 147: 599–606, 2006 [DOI] [PubMed] [Google Scholar]
- 157.Wyrwoll CS, Mark PJ, Waddell BJ. Developmental programming of renal glucocorticoid sensitivity and the renin-angiotensin system. Hypertension 50: 579–584, 2007 [DOI] [PubMed] [Google Scholar]
- 158.Yajnik CS, Fall CH, Vaidya U, Pandit AN, Bavdekar A, Bhat DS, Osmond C, Hales CN, Barker DJ. Fetal growth and glucose and insulin metabolism in four-year-old Indian children. Diabet Med 12: 330–336, 1995 [DOI] [PubMed] [Google Scholar]
- 159.Yiu V, Buka S, Zurakowski D, McCormick M, Brenner B, Jabs K. Relationship between birthweight and blood pressure in childhood. Am J Kidney Dis 33: 253–260, 1999 [DOI] [PubMed] [Google Scholar]
- 160.Yzydorczyk C, Comte B, Cambonie G, Lavoie JC, Germain N, Ting SY, Wolff J, Deschepper C, Touyz RM, Lelievre-Pegorier M, Nuyt AM. Neonatal oxygen exposure in rats leads to cardiovascular and renal alterations in adulthood. Hypertension 52: 889–895, 2008 [DOI] [PubMed] [Google Scholar]
- 161.Zeman FJ. The effect of prenatal protein-calorie malnutrition on kidney development in the rat. Prog Clin Biol Res 140: 309–338, 1983 [PubMed] [Google Scholar]