Abstract
Inhibitors of histone deacetylases, including suberoylanilide hydroxamic acid (SAHA) and trichostatin A (TSA), are emerging anticancer agents. In the current study, we examined the cytoprotective effects of these agents. Cisplatin induced 40–50% apoptosis in rat kidney proximal tubular cells in 18 h, which was suppressed to 20–30% by 1–5 μM SAHA or 0.1 μM TSA. Consistently, SAHA partially prevented cisplatin-induced caspase activation. The cytoprotective effects of SAHA and TSA were associated with long-term cell survival. During cisplatin treatment, Bax translocated to mitochondria, leading to cytochrome c release. Both Bax translocation and cytochrome c release were ameliorated by SAHA. Mechanistically, SAHA inhibited and TSA delayed p53 phosphorylation, acetylation, and activation during cisplatin incubation. At the upstream signaling level, SAHA blocked cisplatin-induced phosphorylation of Chk2, a key DNA damage response kinase. Interestingly, in HCT116 colon cancer cells, SAHA suppressed cisplatin-induced p53 activation, but enhanced apoptosis. The results suggest that inhibitors of histone deacetylases can protect against cisplatin nephrotoxicity by attenuating DNA damage response and associated p53 activation.
Keywords: cisplatin nephrotoxicity, suberoylanilide hydroxamic acid, trichostatin A
cisplatin is being used for the treatment of a variety of cancers or tumors. A well-recognized side effect of cisplatin-based chemotherapy is nephrotoxicity, leading to acute kidney injury in cancer patients (2, 24). Currently, the only available approach to reduce cisplatin nephrotoxicity is excessive hydration; nevertheless, the effect is partial and still over a quarter of patients experience renal problems or deficiency (2, 24). Research during the last few years has significantly advanced the mechanistic understanding of cisplatin nephrotoxicity. Especially, multiple signaling pathways have been implicated in cisplatin-induced renal cell injury and death (17–20, 23, 26–29, 31).
One of the major signaling pathways for cisplatin nephrotoxicity involves p53 (13). p53 Is activated early during cisplatin incubation of renal tubular cells and induces the expression of proapoptotic genes, leading to apoptosis (15, 30, 32). Inhibition of p53 by pharmacological inhibitors or dominant negative mutants blocks cisplatin-induced apoptosis in tubular cells (7, 11, 14, 17, 35). Moreover, cisplatin induces significantly lower kidney injury in p53-null mice than their wild-type littermates (35), further supporting a role for p53 signaling in cisplatin nephrotoxicity. Our recent work further revealed a robust DNA damage response involving ATR and Chk2 that is largely responsible for cisplatin-induced p53 activation in renal tubular cells and tissues (25). These observations suggest that it is possible to block p53 signaling to ameliorate cisplatin-induced nephrotoxicity during chemotherapy. In a very recent study, Molitoris and colleagues (22) reported that siRNA downregulation of p53 affords impressive renoprotective effects in animal models of renal ischemia-reperfusion and cisplatin nephrotoxicity.
Histone deacetylase (HDAC) inhibitors, including suberoylanilide hydroxamic acid (SAHA) and trichostatin A (TSA), are emerging anti-cancer agents (3, 5, 21, 36). These small molecule chemicals can be structurally different and can either selectively inhibit specific HDACs or be general inhibitors of several HDACs. Interestingly, recent work by Arany et al. (1) demonstrated an impressive cytoprotective effect of TSA during cisplatin treatment of renal tubular cells, whereas we showed that HDAC inhibitors can be cytotoxic to renal tubular cells after overnight treatment at relatively higher concentrations (9). The current study further examined the cytoprotective effects of SAHA and TSA in cultured renal proximal tubular cells. Especially, we tested the hypothesis that HDAC inhibitors may block the DNA damage response and associated p53 activation during cisplatin treatment, resulting in suppression of tubular cell apoptosis.
MATERIALS AND METHODS
Materials.
The rat kidney proximal tubular cell (RPTC) line was originally obtained from Dr. Hopfer (Case Western Reserve University, Cleveland, OH) and maintained as described previously (9, 14, 15, 17). HCT116 colon cancer cell line was purchased from American Type Culture Collection (ATCC; Manassas, VA) and cultured in McCoy's 5A medium as described previously (25). Antibodies were from the following sources: rabbit polyclonal anti-p53, anti-phospho(serine-15)-p53, anti-Chk2, and anti-phospo-H2AX antibodies from Cell Signaling Technology (Beverly, MA); monoclonal mouse anti-Bax from NeoMarkers (Fremont, CA); mouse monoclonal anti-cytochrome c from BD Pharmingen; mouse monoclonal anti-β-actin antibody from Sigma (St. Louis, MO); rabbit polyclonal anti-PUMA from Dr. Yu at University of Pittsburgh; all secondary antibodies from Jackson ImmunoResearch (West Grove, PA). Carbobenzoxy-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin (DEVD.AFC) and 7-amino-4-trifluoromethyl coumarin (AFC) for caspase assay were purchased from Enzyme Systems Products (Dublin, CA). Other reagents and chemicals including cisplatin were purchased from Sigma.
Treatment of RPTC cells.
In this study, RPTC cells were pretreated with SAHA or TSA and then further incubated with cisplatin in the presence of the agents. 1) For 5-μM SAHA pretreatment, cells were plated at a density of 1 × 106 cells/dish in 35-mm dishes to reach confluence by the next day. SAHA was then added to the cells at a final concentration of 5 μM for 6 h of pretreatment. After pretreatment, the cells were incubated with 20 μM cisplatin in the presence of 1 μM SAHA. 2) For 1-μM SAHA pretreatment, cells were plated at a density of 0.5 × 106 cells/dish in 35-mm dishes. In the next day, 1 μM SAHA was added to the cells for overnight pretreatment. After overnight pretreatment, the cells were incubated with 20 μM cisplatin in the presence of 1 μM SAHA. 3) For TSA experiments, an identical protocol of overnight pretreatment was followed, except that 0.1 μM TSA (instead of 1 μM SAHA) was used.
Morphological examination of apoptosis.
Apoptotic cells were identified by their morphology as described previously (6, 9, 14, 15, 17). Briefly, cells were stained with 10 μg/ml Hoechst 33342 staining for 10 min and then examined by phase contrast and fluorescence microscopy. Apoptotic cells showed a characteristic morphology including shrunken configuration, apoptotic blebs or bodies, and condensed and fragmented nucleus. Four fields with ∼200 cells per field were examined in each dish to estimate the percentage of apoptosis. Representative images were also recorded.
Measurement of caspase activity.
Caspase activity in cell lysate was measured as previously using DEVD.AFC, a fluorogenic peptide substrate (6, 9, 14, 15, 17). Briefly, cells were extracted with 1% Triton X-100. The lysates of 25 μg protein were added to enzymatic reactions containing 50 μM DEVD.AFC. After 1 h of incubation at 37°C, fluorescence at Ex 360 nm/Em 530 nm was measured. To indicate caspase activity, the fluorescence reading from each sample was calculated into the nanomolar amount of liberated AFC per milligram of protein, based on a standard curve constructed using free AFC.
Cellular fractionation.
To determine the subcellular localization of Bax and cytochrome c, cells were fractionated using digitonin as previously (6, 15, 33). Briefly, cells were permeabilized with 0.05% digitonin in an isotonic buffer for 2–5 min at room temperature to collect soluble extract as cytosolic fraction. The digitonin insoluble part was further dissolved in 2% SDS buffer as membrane-bound organellar fraction enriched with mitochondria. These two fractions were analyzed for Bax and cytochrome c by immunoblot analysis.
Immunoblot analysis.
A standard protocol of immunoblot analysis was followed. Briefly, the protein concentration of cell lysate was determined using the bicinchoninic acid reagent (BCA) reagent (Pierce, Rockford, IL). Equal amounts of protein were loaded in each lane for reducing SDS-gel electrophoresis and then electroblotted onto PVDF membranes. The blots were incubated with blocking buffer, a specific primary antibody, and horseradish peroxidase-conjugated secondary antibodies. Antigens on the blots were revealed using the ECL kit from Pierce.
Statistics.
Qualitative data including cell images and immunoblots are representatives of at least three experiments. Quantitative data were expressed as means ± SD. Statistical analysis was conducted using the GraphPad Prism software. Statistical differences between two groups were determined by two-tailed unpaired Student's t-test. P < 0.05 was considered significantly different.
RESULTS
Inhibition of cisplatin-induced apoptosis by SAHA and TSA in RPTC cells.
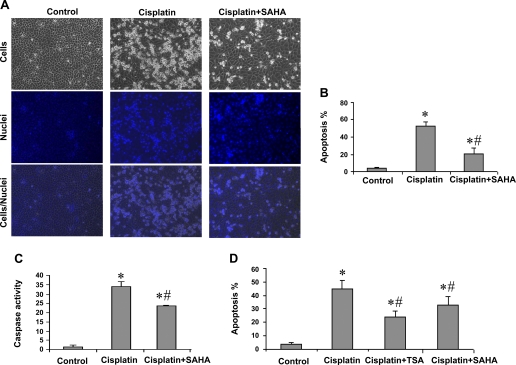
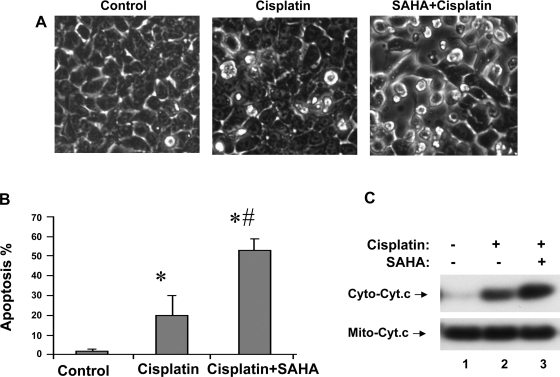
To determine the effects of SAHA on cisplatin-induced apoptosis in renal tubular cell, we pretreated RPTC cells with 5 μM SAHA for 6 h and then subjected the cells to 20 μM cisplatin treatment for 18 h in the presence of 1 μM SAHA. Another group of cells was treated with cisplatin without SAHA exposure, whereas the control was not exposed to either SAHA or cisplatin. As shown in Fig. 1A, control cells showed normal healthy morphology with minimal apoptosis (left). Cisplatin treatment for 18 h induced massive apoptosis, showing cellular and nuclear condensation and fragmentation (Fig. 1A, middle). The apoptosis was markedly suppressed by 5-μM SAHA pretreatment and the inclusion of 1 μM SAHA during cisplatin treatment (Fig. 1A, right). Of note, pretreatment or inclusion of SAHA during cisplatin incubation alone had some inhibitory effects on apoptosis (not shown), but the combination had the most consistent effects. Quantification by cell counting indicated that cisplatin induced 53% apoptosis, which was suppressed to 21% by SAHA (Fig. 1B). Consistently, cisplatin-induced caspase activation was also suppressed by SAHA, although the inhibition appeared less extensive than apoptosis (Fig. 1C). We further confirmed the cytoprotective effects of TSA that were reported previously (1). Overnight TSA pretreatment and the presence of TSA during cisplatin incubation reduced apoptosis from 45 to 28% in RPTC cells (Fig. 1D). In the same experiment, we also examined the effects of overnight pretreatment with 1 μM SAHA. As shown in Fig. 1D, overnight pretreatment with and inclusion of 1 μM SAHA during cisplatin incubation had significant inhibitory effects on cisplatin-induced apoptosis in RPTC cells.
Fig. 1.
Inhibition of cisplatin-induced apoptosis in renal proximal tubule cells (RPTC) by suberoylanilide hydroxamic acid (SAHA) and trichostatin A (TSA). A, B, and C: RPTCs were pretreated for 6 h without or with 5 μM SAHA and then incubated for 18 h with 20 μM cisplatin or 20 μM cisplatin plus 1 μM SAHA. D: cells were pretreated for overnight without or with 1 μM SAHA or 0.1 μM TSA and then incubated with 20 μM cisplatin or 20 μM cisplatin plus 1 μM SAHA or 0.1 μM TSA for 24 h. Control cells were not exposed to SAHA, TSA, or cisplatin. A: morphology. Cells were stained with 10 μg/ml Hoechst33342 to record nuclear and cellular morphology by fluorescence and phase-contrast microscopy. B: percentage of apoptosis. Apoptotic cells were counted by morphology to determine % apoptosis. C: caspase activity. Cell lysate was collected to measure caspase activity in an enzymatic assay. D: percentage of apoptosis. Cells were evaluated by morphology to determine % apoptosis. B, C, and D: data are expressed as means ± SD (n = 3–4). *Statistically significantly different from the control. #Statistically significantly different from the cisplatin-alone group.
Effects of SAHA and TSA on long-term survival of RPTC cells following cisplatin treatment.
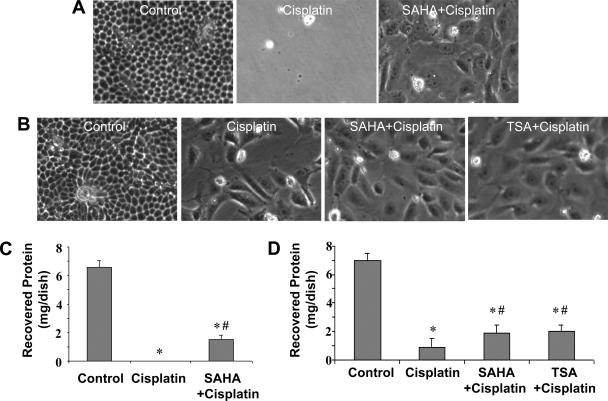
To determine whether SAHA and TSA have long-term effects on cell survival, we examined the cells at 48 h after cisplatin treatment. Specifically, we recorded the morphology of recovered cells and measured the cell protein recovered from each condition. We first determined the effect of 6 h of SAHA pretreatment. As shown in Fig. 2A, very few cells survived in the cisplatin group, whereas some cells in the SAHA+cisplatin group did survive. Compared with control, the surviving cells in the SAHA+cisplatin group appeared large and flat. Consistently, virtually no protein was recovered from the cisplatin group, but significant amounts of proteins were recovered from the SAHA+cisplatin dishes (Fig. 2C). We then examined the effects of overnight pretreatment with SAHA and TSA. As shown in Fig. 2B, by morphology more cells survived in the SAHA+cisplatin and TSA+cisplatin groups than the cisplatin-only group. The pretreated groups also recovered more proteins (Fig. 2D). Together with the apoptosis analyses (Fig. 1), these observations demonstrate the cytoprotective effects of SAHA and TSA against cisplatin-induced apoptosis in renal tubular cells.
Fig. 2.
Effects of SAHA and TSA on long-term survival of RPTCs following cisplatin treatment. A, C: RPTCs were pretreated for 6 h without or with 5 μM SAHA and then incubated for 18 h with 20 μM cisplatin or 20 μM cisplatin plus 1 μM SAHA. The cells were then changed to fresh culture medium for 24 h of recovery. Cell morphology was recorded by phase-contrast microscopy (A) and whole cell lysate was collected to determine the amount of recovered protein in each condition (C). B, D: RPTCs were pretreated for overnight without or with 1 μM SAHA or 0.1 μM TSA and then incubated with 20 μM cisplatin or 20 μM cisplatin plus 1 μM SAHA or 0.1 μM TSA for 24 h. The cells were then changed to fresh culture medium for 24 h of recovery to record cell morphology (B) and collect whole cell lysate to determine the amount of recovered protein (D). Data in C and D are expressed as means ± SD (n = 3). *Statistically significantly different from the control. #Statistically significantly different from the cisplatin-alone group.
Suppression of cisplatin-induced Bax translocation and cytochrome c release in RPTC cells.
Cisplatin activates the mitochondrial pathway of apoptosis, which is characterized by the translocation of Bax (a proapoptotic Bcl2 protein) from cytosol to mitochondria and consequent release of cytochrome c from the organelles (14, 15, 26). Our recent work further demonstrated a role for Bax activation in cisplatin nephrotoxicity in vivo using gene knockout mice (34). Thus, to understand the mechanism of cytoprotection by HDAC inhibitors, we initially examined the effects of SAHA on cisplatin-induced Bax translocation and cytochrome c release. To this end, RPTC cells were treated with cisplatin in the absence or presence of SAHA. The cells were then fractionated into cytosolic and membrane-bound fractions enriched with mitochondria for immunoblot analysis. As shown in Fig. 3A, Bax was almost completely cytosolic in control cells (lane 1) and after cisplatin incubation, a portion of Bax accumulated to the mitochondrial fraction (lane 2). Bax accumulation was partially suppressed by SAHA (lane 3). For cytochrome c (Fig. 3B), the majority was in the mitochondrial fraction in control cells as expected. However, significant cytochrome c was detected after cisplatin treatment. Cisplatin-induced release of cytochrome c was partially prevented by SAHA. Analysis of the immunoblots by densitometry indicates that mitochondrial accumulation of Bax during cisplatin treatment was reduced 32% by SAHA. Consistently, cisplatin-induced cytochrome c release was reduced 65% by SAHA. The results suggest that HDAC inhibitors may prevent cisplatin-induced apoptosis in tubular cells at upstream signaling levels.
Fig. 3.
Inhibition of cisplatin-induced Bax translocation and cytochrome c release by SAHA. RPTCs were pretreated for 6 h without or with 5 μM SAHA and then incubated for 18 h with 20 μM cisplatin or 20 μM cisplatin plus 1 μM SAHA. Control cells were not exposed to SAHA or cisplatin. The cells were fractionated into cytosolic and mitochondrial fractions for immunoblot analysis of Bax (A) and cytochrome c (B).
Suppression of cisplatin-induced p53 phosphorylation, acetylation, and activation by SAHA and TSA.
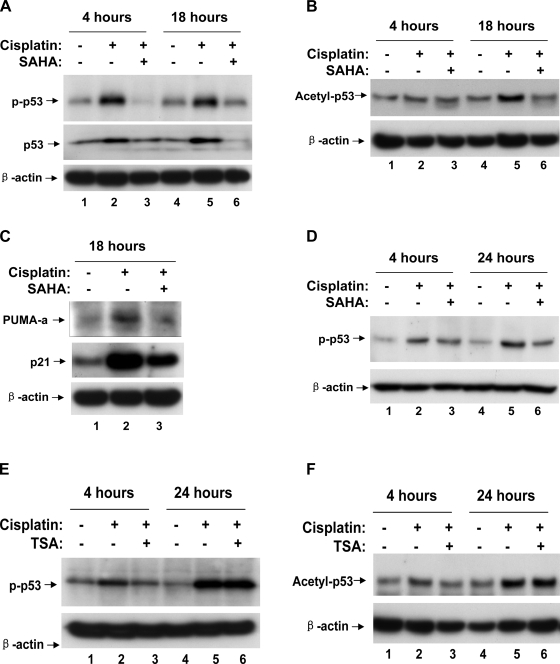
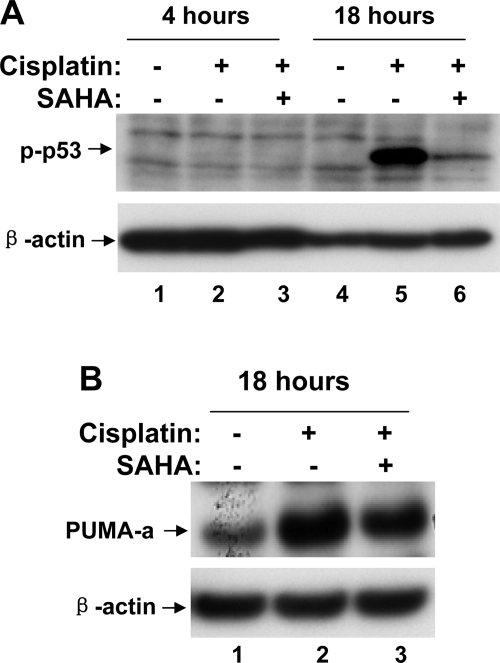
A major signaling pathway leading to cisplatin-induced renal cell apoptosis and nephrotoxicity involves DNA damage response and p53 activation (13, 25, 35). Because HDAC inhibitors are expected to block the structural alterations of chromosomes that are mediated by HDAC, we reasoned that the observed cytoprotective effects of SAHA and TSA might be related to DNA damage response and p53 activation during cisplatin treatment. To test this possibility, we first examined p53 phosphorylation and accumulation. As shown in Fig. 4A, cisplatin treatment for 4 and 18 h induced p53 phosphorylation at serine-15 (lanes 2, 5), which was attenuated by 6-h pretreatment with 5 μM SAHA (lanes 3, 6). Consistently, cisplatin-induced total p53 accumulation was also ameliorated by SAHA (Fig. 4A: lanes 2, 5 vs. 3, 6). Cisplatin treatment also led to p53 acetylation, another posttranslational mechanism of p53 regulation. As shown in Fig. 4B, p53 acetylation at lysine-379 was marginally increased after 4 h and marked increased after 18 h of cisplatin treatment (lanes 2, 5). p53 Acetylation during cisplatin treatment was diminished by SAHA pretreatment (Fig. 4B: lanes 2, 5 vs. 3, 6). We further analyzed the expression of PUMA-α and p21, two downstream target genes of p53. It was shown that SAHA partially but significantly suppressed the expression of these genes during cisplatin treatment of RPTC cells (Fig. 4C: lane 3 vs. 2).
Fig. 4.
Suppression of cisplatin-induced p53 activation by SAHA. A, B, C: RPTCs were subjected to 6 h of pretreatment without or with 5 μM SAHA. The cells were then incubated for 4 or 18 h with 20 μM cisplatin or 20 μM cisplatin plus 1 μM SAHA. Cell lysate was collected for immunoblot analysis of phospho-p53(serine-15), p53, acetyl-p53(lysine-379), PUMA-α, p21, and β-actin. D, E, F: RPTCs were subjected to overnight pretreatment with or without 1 μM SAHA or 0.1 μM TSA. The cells were then incubated for 4 or 24 h with 20 μM cisplatin or 20 μM cisplatin plus 1 μM SAHA or 0.1 μM TSA. Cell lysate was collected for immunoblot analysis of phospho-p53(serine-15), acetyl-p53(lysine-379), and β-actin.
Overnight pretreatment with 1 μM SAHA also showed inhibitory effects on cisplatin-induced p53 phosphorylation (Fig. 4D: lanes 2, 5 vs. 3, 6). In addition, overnight pretreatment with 0.1 μM TSA could block p53 activation at 4 h but not at the late time point of 24 h (Fig. 4D: lanes 2, 5). Similarly, TSA pretreatment suppressed cisplatin-induced p53 acetylation at 4 h (Fig. 4F: lane 2 vs. 3) but not at 24 h, suggesting that TSA can delay or slow down p53 activation during cisplatin treatment. Collectively, the results indicate that HDAC inhibitors can suppress cisplatin-induced p53 activation.
Blockade of cisplatin-induced activation of Chk2, but not H2AX, by SAHA.
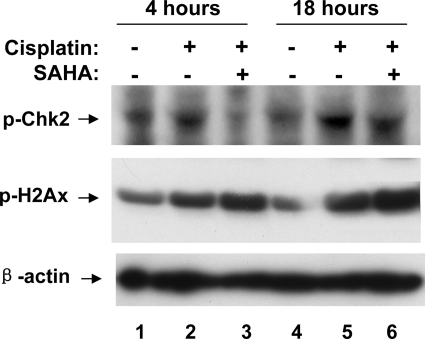
Cisplatin stimulates a rapid and robust DNA damage response, which is largely responsible for p53 activation during cisplatin-induced tubular cell apoptosis and nephrotoxicity (25). As discussed, the DNA damage response may not necessarily be caused by double- or single-strand breaks, rather by stalled replication stress due to cisplatin-mediated cross-linking of DNA (24). In this response, we detected the phosphorylation of H2AX, accumulation of H2AX and ATR at discrete nuclear foci or DNA damage sites, marginal increase of ATR activity, and phosphorylation/activation of Chk2, finally resulting in p53 phosphorylation/activation (25). To determine whether HDAC inhibitors suppress p53 activation by interrupting the DNA damage response, we specifically analyzed H2AX and Chk2 phosphorylation. As shown in Fig. 5, cisplatin induced Chk2 and H2AX phosphorylation at 4 h, which became evident at 18 h (lanes 2, 5). SAHA ameliorated Chk2 phosphorylation at both time points, but it did not suppress H2AX phosphorylation at either time points. The results suggest that SAHA does not reduce cisplatin-induced DNA damage stress and associated H2AX activation, but it interferes with the activation of downstream signaling molecules including Chk2, resulting in the inhibition of p53 activation.
Fig. 5.
Suppression of cisplatin-induced Chk2, but not H2AX, phosphorylation by SAHA. RPTCs were pretreated for 6 h without or with 5 μM SAHA and then incubated for 4 or 18 h with 20 μM cisplatin or 20 μM cisplatin plus 1 μM SAHA. Cell lysate was collected for immunoblot analysis of phospho-Chk2, phospho-H2AX, and β-actin.
SAHA enhances cisplatin-induced apoptosis in HCT116 cells.
The observed cytoprotective effects of SAHA and TSA in renal tubular cells suggest that these agents may be used for renoprotection during cisplatin-based chemotherapy. However, an effective renoprotective approach should not diminish the anti-cancer efficacy of cisplatin in tumors. To address this issue, we examined the effects of SAHA on cisplatin-induced apoptosis in HCT116 colon cancer cells. Cell morphology is shown in Fig. 6A. Quantification of apoptosis showed that 80 μM cisplatin induced ∼20% apoptosis in HCT116 cells in 18 h, which was increased to 53% by SAHA (Fig. 6B). Consistently, SAHA+cisplatin induced higher cytochrome c release than cisplatin-only group (Fig. 6C: lane 3 vs. 2), further suggesting the SAHA can enhance cisplatin-induced apoptosis in HCT116 cells.
Fig. 6.
SAHA enhances cisplatin-induced apoptosis in HCT116 colon cancer cells. HCT116 cells were pretreated for 6 h without or with 5 μM SAHA and then incubated for 4 or 18 h with 80 μM cisplatin or 80 μM cisplatin plus 1 μM SAHA. A: cell morphology. B: cells were evaluated by morphology to determine the percentage of apoptosis. Data are expressed as means ± SD (n = 3). *Statistically significantly different from control. #Statistically significantly different from the cisplatin-only group. C: after treatment, the cells were fractionated into cytosolic and mitochondrial fractions for immunoblot analysis of cytochrome c.
Inhibition of cisplatin-induced p53 activation by SAHA in HCT116 cells.
The results present above indicate that SAHA can protect renal tubular cells, but enhance cisplatin-induced injury and death in cancer cells. Because SAHA interfered with p53 signaling in RPTC cells (Figs. 4 and 5), we hypothesized that SAHA might augment p53 signaling in HCT116 cells to increase apoptosis during cisplatin incubation. To test this possibility, we analyzed p53 (serine-15) phosphorylation in HCT116 cells. As shown in Fig. 7A, cisplatin induced a marked p53 phosphorylation at 18 h, but not at the early time point of 4 h. Notably, cisplatin-induced p53 phosphorylation was abrogated by SAHA (Fig. 7A: lane 6 vs. 5). Further analysis showed that cisplatin-induced PUMA-α expression was suppressed by SAHA (Fig. 7B: lane 3 vs. 2). Together, these results indicate that HDAC inhibitors may inhibit p53 signaling in normal as well as cancerous cells.
Fig. 7.
Inhibition of cisplatin-induced p53 activation by SAHA in HCT116 colon cancer cells. HCT116 cells were pretreated for 6 h without or with 5 μM SAHA and then incubated for 4 or 18 h with 80 μM cisplatin or 80 μM cisplatin plus 1 μM SAHA. Cell lysate was collected for immunoblot analysis of phospho-p53 (serine-15) and β-actin (A) or PUMA-α and β-actin (B).
DISCUSSION
In 2008, Arany et al. (1) demonstrated the cytoprotective effects of TSA against cisplatin-induced apoptosis in cultured renal tubular cells, suggesting a new strategy for renoprotection. The current study confirmed the cytoprotective effects of TSA and extended the findings by demonstrating the protective effects of SAHA. Importantly, this study further showed that these agents can interfere with the DNA damage response during cisplatin treatment, resulting in the suppression of p53 activation, a major signaling pathway for tubular cell apoptosis during cisplatin nephrotoxicity. By contrast, SAHA enhances cisplatin-induced apoptosis in HCT116 colon cancer cells. Together, the results provide further support for the use of HDAC inhibitors for renoprotection during cisplatin and related platinum compound-based chemotherapy.
Interestingly, our previous study demonstrated cytotoxicity of SAHA in RPTC cells. It was shown that SAHA induces significant apoptosis at 5 μM or higher concentrations (9). It is noteworthy that the effect of SAHA depends not only on the SAHA concentrations used but also the treatment time or duration. During 18 h of treatment, SAHA induces apoptosis dose dependently with significant apoptosis induced at 5, 10, and 20 μM (dose curve shown in Ref. 9). However, shorter (e.g., 6 h) treatment with SAHA at these concentrations does not induce significant apoptosis (data not shown). In contrast, as shown in the current study, 6-h pretreatment with 5 μM SAHA protects against cisplatin-induced apoptosis.
Our results suggest that SAHA and TSA may protect renal tubular cells by blocking cisplatin-induced DNA damage response and p53 signaling. However, Arany et al. (1) suggested that TSA could increase CREB phosphorylation and restore CREB-mediated gene transcription to prevent cell death during cisplatin treatment. These two possibilities or mechanisms are not mutually exclusive; instead, they may be related and cooperate to underlie the cytoprotection by HDAC inhibitors. Interestingly, CREB and p53 share the CBP/p300 coactivator. Thus, it is possible that by increasing binding to CREB, HDAC inhibitors might reduce CBP/p300 binding to p53 during cisplatin treatment, leading to the suppression of p53 activation and consequent transcription of apoptotic genes. Alternatively, under conditions of DNA damage or genotoxic stress, CREB is phosphorylated via the upstream DNA damage sensor kinases, ATM and ATR (8). Cisplatin also induces a rapid CREB phosphorylation in renal tubular cells (1), suggesting that CREB phosphorylation induced by cisplatin may be a result of DNA damage signaling. The functional relationship between CREB and p53 activation during cisplatin injury has yet to be determined. However, CREB, a transcription factor with roles in cell survival, is inactivated during cisplatin treatment (1). It is speculated that CREB inactivation would facilitate cell killing by p53-mediated pathways. On the other hand, when HDAC inhibitors are present, the gene transcription and survival function of CREB are restored and p53 activation is suppressed in the same cells, culminating in cytoprotection and cell survival during cisplatin treatment. Further research needs to examine these possibilities to understand the cytoprotection by HDAC inhibitors.
The cisplatin-induced DNA damage response may not be primarily induced by double- or single-strand breaks. Rather, we believe it is a result of replication stalling stress due to cisplatin crosslinking of DNA strands (24). This type of genotoxic stress evokes a robust DNA damage response. Our recent work showed that cisplatin induces a rapid phosphorylation of H2AX and the accumulation of this protein to nuclear foci or DNA damage sites. Concurrently, ATR is recruited to these sites, leading to the activation of Chk2. Then, Chk2 can phosphorylate p53 for its activation (25). In the current study, we showed that H2AX phosphorylation is not affected by SAHA, but Chk2 is. The observation suggests that HDAC inhibitors do not prevent cisplatin crosslinking and initial DNA damage response, but they interfere with the subsequent signaling. Based on the DNA damage response, we speculate that SAHA may affect the assembly of the protein complexes at the DNA damage sites and prevent the activation of signaling protein kinases such as ATR. The binding of cisplatin to DNA and the crosslinking of the strands may be affected by alterations in chromatin structure. We speculate that HDAC-mediated deacetylation of histones, the main structural proteins in chromatin, may participate in the structural changes of chromatin and ensuing DNA damage signaling during cisplatin-induced cytotoxicity. While this possibility remains to be investigated in the future, it is noteworthy that the deacetylation substrates of HDACs are not limited to histones (4, 10, 12). Thus, the effects of HDAC inhibitors may as well be caused by the prevention of deacetylation of other proteins.
This and the earlier study by Arany et al. suggest that it is possible to use HDAC inhibitors to prevent renal damage during cisplatin chemotherapy. Interestingly, our results further show that SAHA can enhance apoptosis during cisplatin treatment of HCT116 colon cancer cells. Mechanistically, we showed that the increased apoptosis is associated with higher cytochrome c release from mitochondria, suggesting that SAHA may enhance the killing signals at the levels upstream of mitochondrial damage. We further showed that as in RPTC cells, SAHA suppresses cisplatin-induced p53 activation in HCT116 cells. The results indicate that the death-enhancing effects of SAHA in HCT116 cells are not due to an increased DNA damage response. Thus, currently the mechanism of the death-enhancing effects of SAHA in HCT116 cells remains unclear. Nonetheless, these studies indicate that normal cells/tissues and malignant cells may respond very differently to HDAC inhibitors. The protective effects in normal renal tubular cells and the death-enhancing effects in cancerous cells support the possibility of the in vivo use of HDAC inhibitors for renoprotection during cisplatin chemotherapy. Further studies need to test this possibility and extend the findings using in vivo models, preferably tumor-bearing animals.
GRANTS
The study was supported in part by grants from the National Institutes of Health and Department of Veterans Affairs (VA). Z. Dong is a Research Career Scientist of the VA.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Arany I, Herbert J, Herbert Z, Safirstein RL. Restoration of CREB function ameliorates cisplatin cytotoxicity in renal tubular cells. Am J Physiol Renal Physiol 294: F577–F581, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol 23: 460–464, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J Clin Oncol 23: 3971–3993, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem 73: 417–435, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5: 769–784, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Brooks C, Wei Q, Cho S, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther 302: 8–17, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Dodson GE, Tibbetts RS. DNA replication stress-induced phosphorylation of cyclic AMP response element-binding protein mediated by ATM. J Biol Chem 281: 1692–1697, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Dong G, Wang L, Wang CY, Yang T, Kumar V, Dong Z. Induction of apoptosis in renal tubular cells by histone deacetylase inhibitors, a family of anticancer agents. J Pharmacol Exp Ther 325: 978–984, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene 26: 5420–5432, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Han X, Yue J, Chesney RW. Functional TauT protects against acute kidney injury. J Am Soc Nephrol 20: 1323–1332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodawadekar SC, Marmorstein R. Chemistry of acetyl transfer by histone modifying enzymes: structure, mechanism and implications for effector design. Oncogene 26: 5528–5540, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Jiang M, Dong Z. Regulation and pathological role of p53 in cisplatin nephrotoxicity. J Pharmacol Exp Ther 327: 300–307, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Jiang M, Pabla N, Murphy RF, Yang T, Yin XM, Degenhardt K, White E, Dong Z. Nutlin-3 protects kidney cells during cisplatin therapy by suppressing Bax/Bak activation. J Biol Chem 282: 2636–2645, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang M, Wei Q, Wang J, Du C, Yu J, Zhang L, Dong Z. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene 25: 4056–4066, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Jiang M, Yi X, Hsu S, Wang CY, Dong Z. Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am J Physiol Renal Physiol 287: F1140–F1147, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Li S, Wu P, Yarlagadda P, Vadjunec NM, Proia AD, Harris RA, Portilla D. PPAR alpha ligand protects during cisplatin-induced acute renal failure by preventing inhibition of renal FAO and PDC activity. Am J Physiol Renal Physiol 286: F572–F580, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Lieberthal W, Triaca V, Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am J Physiol Renal Fluid Electrolyte Physiol 270: F700–F708, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Baliga R. Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol 16: 1985–1992, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol 25: 84–90, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol 20: 1754–1764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowak G. Protein kinase C-alpha and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J Biol Chem 277: 43377–43388, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Pabla N, Huang S, Mi QS, Daniel R, Dong Z. ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem 283: 6572–6583, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Park MS, De Leon M, Devarajan P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol 13: 858–865, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Price PM, Safirstein RL, Megyesi J. Protection of renal cells from cisplatin toxicity by cell cycle inhibitors. Am J Physiol Renal Physiol 286: F378–F384, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol 289: F166–F174, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 285: F610–F618, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP. p53-Dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury. J Biol Chem 280: 31230–31239, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, Nick HS, Agarwal A. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol 278: F726–F736, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Tsuruya K, Yotsueda H, Ikeda H, Taniguchi M, Masutani K, Hayashida H, Hirakata H, Iida M. Involvement of p53-transactivated Puma in cisplatin-induced renal tubular cell death. Life Sci 83: 550–556, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline upregulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279: 19948–19954, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Wei Q, Dong G, Franklin J, Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int 72: 53–62, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 293: F1282–F1291, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26: 5541–5552, 2007. [DOI] [PubMed] [Google Scholar]