Abstract
To examine the functional interaction between superoxide dismutase (SOD) and NADPH oxidase activity, we assessed renal responses to acute intra-arterial infusion of ANG II (0.5 ng·kg−1·min−1) before and during administration of a SOD inhibitor, diethyldithiocarbamate (DETC, 0.5 mg·kg−1·min−1), in enalaprilat-pretreated (33 μg·kg−1·min−1) rats (n = 11). Total (RBF) and regional (cortical, CBF; medullary; MBF) renal blood flows were determined by Transonic and laser-Doppler flowmetry, respectively. Renal cortical and medullary tissue NADPH oxidase activity in vitro was determined using the lucigenin-chemiluminescence method. DETC treatment alone resulted in decreases in RBF, CBF, MBF, glomerular filtration rate (GFR), urine flow (V), and sodium excretion (UNaV) as reported previously. Before DETC, ANG II infusion decreased RBF (−18 ± 3%), CBF (−16 ± 3%), MBF [−5 ± 6%; P = not significant (NS)], GFR (−31 ± 4%), V (−34 ± 2%), and UNaV (−53 ± 3%). During DETC infusion, ANG II also caused similar reductions in RBF (−20 ± 4%), CBF (−19 ± 3%), MBF (−2 ± 2; P = NS), and in GFR (−22 ± 7%), whereas renal excretory responses (V; −12 ± 2%; UNaV; −24 ± 4%) were significantly attenuated compared with those before DETC. In in vitro experiments, ANG II (100 μM) enhanced NADPH oxidase activity both in cortical [13,194 ± 1,651 vs. 20,914 ± 2,769 relative light units (RLU)/mg protein] and in medullary (21,296 ± 2,244 vs. 30,597 ± 4,250 RLU/mg protein) tissue. Application of DETC (1 mM) reduced the basal levels and prevented ANG II-induced increases in NADPH oxidase activity in both tissues. These results demonstrate that renal excretory responses to acute ANG II administration are attenuated during SOD inhibition, which seems related to a downregulation of NADPH oxidase in the deficient condition of SOD activity.
Keywords: superoxide dismutase, NADPH oxidase, diethyldithiocarbamate, renal hemodynamics, sodium excretion
ang ii is a powerful vasoconstrictor agent that contributes to the regulation of renal function and blood pressure (22, 23, 26, 30). Elevated intrarenal ANG II level causes alterations in the renal function, leading to sodium retention and thus is involved in the development and maintenance of hypertension (22, 23). ANG II is known to induce superoxide (O2−) generation via activation of NADPH oxidase activity (5). An increase in oxidative stress has been strongly suggested to be involved in the development and maintenance of ANG II-dependent hypertension (26, 30).
O2− is a product of cellular oxidative metabolism, and NADPH oxidase is one of the major sources of O2− in living tissue (1). Reactive O2− needs to be instantly reduced by the enzyme superoxide dismutase (SOD) (25). It had been demonstrated that the pretreatment with a SOD mimetic, tempol, attenuates renal hemodynamics and excretory responses to both acute (16) and chronic (9) administration of ANG II, indicating that the renal vascular and tubular actions of ANG II are partially mediated by concomitant generation of O2−. Furthermore, the renal excretory responses to acute administration of an inhibitor of nitric oxide (NO) synthase were also shown to be partially attenuated by tempol treatment, indicating that NO blockade enhances O2− activity in the kidney (17). On the other hand, SOD inhibition with sodium diethyldithiocarbamate (DETC) in anesthetized dogs caused decreases in both renal cortical and medullary blood flow as well as urinary sodium excretion, which were significantly enhanced during NO synthase inhibition (15). Similar reductions in regional blood flow and sodium excretion were also noted when DETC was administered directly in the renal medulla (33). However, the role of SOD inhibition in the renal responses to ANG II administration has not yet been investigated.
We hypothesized that the activity of a SOD enzyme limits ANG II-induced increases in NADPH oxidase activity and thus prevents exacerbation of the renal vascular and tubular actions of ANG II in the condition of enhancement of the renin-angiotensin system. In the present study, we examined this hypothesis by conducting experiments in rats with consideration of two specific aims: 1) to investigate in vivo the renal responses to ANG II administration during inhibition of SOD in anesthetized rats and 2) to assess in vitro the NADPH oxidase activity in response to ANG II treatment during inhibition of SOD in rat renal tissue. Anesthetized rats pretreated with an angiotensin-converting enzyme inhibitor, enalaprilat, were used in these experiments to minimize the possible influence of alterations in endogenous ANG II during the experimental period (24). In these enalaprilat-pretreated rats, renal hemodynamic and excretory responses to acute intra-arterial administration of ANG II directly in the kidney were assessed before and during SOD inhibition with DETC administration. NADPH oxidase activity was assessed in vitro in renal tissues collected from these anesthetized rats pretreated with enalaprilat.
METHODS
All of the experimental procedures described in this study were approved by and performed in accordance with the guidelines and practices established by the Tulane University Animal Care and Use Committee. These experiments were conducted in male Sprague-Dawley rats weighing 250–300 g body wt (8 wk old) that were purchased from Charles River Laboratories (Wilmington, MA), housed at least 3 days for acclimatization in a temperature- and light-controlled room, and allowed free access to a standard diet (Ralston-Purina, St. Louis, MO) and tap water. On the day of experiments, these rats were anesthetized with thiobutabarbital sodium (Inactin; Sigma-Aldrich) at a dose of 100 mg/kg ip. Supplemental doses of anesthesia were administered throughout the experimental period as required. These animals were surgically prepared for acute measurements of renal hemodynamics and excretory parameters as described earlier (10, 11). The rats were placed on a servo-controlled surgical table that maintained body temperature at 37°C. Tracheostomy was performed, and the animals were continuously provided with a mixture of 95% O2 and 5% CO2 through the endotracheal tube (PE-200). The right jugular vein was catheterized for intravenous administration of an isotonic saline solution containing 1% albumin and 7.5% inulin (Inutest, Laevosan, Linz/Donau, Austria). The right femoral artery was catheterized, and the cannula was connected to a pressure transducer to allow continuous monitoring of arterial blood pressure using the AcqKnowledge data acquisition system (Biopac). The left kidney was exposed via a flank incision and placed in a Lucite cup, and the ureter was cannulated with a PE-10 catheter for urine collection. Another PE-10 catheter (OD, 0.61 mm) was tapered (40–50%) and inserted 2–3 mm deep in the left renal artery from the aorta via the left femoral artery to allow administration of drugs directly in the kidney at a rate of 5 μl/min. This catheter was kept patent by a continuous infusion of heparinized isotonic saline. This procedure of inserting a tapered catheter does not compromise the renal blood flow (RBF) because in pilot experiments it was observed that there was no significant difference in baseline RBF before or after the catheter insertion. Moreover, this technique was successfully used in several earlier studies conducted in our laboratory (10, 11, 20). An ultrasonic flow probe (Transonic System) was placed on the left renal artery to measure total RBF. Laser-Doppler flow (LDF) probes (500 μm OD, Periflex 4001; Perimed) were used to measure the regional blood flow in the kidney. Cortical blood flow (CBF) was measured by a probe placed on the surface of the kidney, and medullary blood flow (MBF) was measured by a needle probe, carefully inserted ∼3 mm deep in the kidney. Zero flow recordings with these LDF probes were determined when the renal artery was completely occluded at the end of the experiment.
Experimental protocol.
After completion of the surgery and a 60-min stabilization period, the experimental protocol was started with a 30-min control clearance urine collection. A schematic diagram showing the experimental protocol is provided in Fig. 1. After the control collection period, a continuous infusion of enalaprilat (33 μg·kg−1·min−1) was started and maintained for the rest of the experimental period (24). After a 20-min stabilization period, and a 30-min urine collection period, a blood sample was collected during enalaprilat infusion alone. At the end of this collection, ANG II infusion at a rate of 0.5 ng·kg−1·min−1 (16) was started, and, after a 20-min stabilization period, a 30-min urine sample followed by a blood sample was collected. ANG II infusion was then stopped and, after a 20-min of stabilization period, another 30-min urine sample (control collection before DETC administration) and blood sample were taken. DETC infusion at a dose of 0.5 μg·kg−1·min−1 (15) was then started, and, after a 20-min stabilization period, a 30-min urine sample and a blood sample were taken. Next, ANG II infusion was restarted in the presence of DETC, and again, after a 20-min stabilization period, a 30-min urine sample and a blood sample collection were carried out. Additional experiments were also conducted in these enalaprilat-pretreated rats (n = 5) to examine the time-dependent changes in the renal responses to repeated infusions of ANG II at an interval period that matched exactly the lapsed time between two infusions of ANG II before DETC administration in these experiments.
Fig. 1.
Schematic description of the experimental protocol. EN, enalaprilat; DETC, diethyldithiocarbamate.
All blood and urine samples were kept frozen (−20°C) until analyzed. Urine samples for 8-isoprostane were preserved with 0.005% butylhydroxytoluene prepared in ethanol, whereas those for nitrate/nitrite were preserved in isopropanol before being kept frozen at −80°C. The left kidney was removed, stripped of all surrounding tissue, blotted dry, and weighed so that the calculated parameters could be expressed per gram of kidney weight.
In vitro measurement of renal NADPH oxidase activity.
In a separate set of experiments, kidney samples were collected from anesthetized rats pretreated with enalaprilat used in time control experiments (n = 7). After collection, these samples were snap-frozen and then kept at −800C for in vitro measurement of NADPH oxidase activity. Determination of renal cortical and medullary NADPH oxidase activity was carried out in vitro by the lucigenin-chemiluminescence assay as described earlier (21). The NADPH oxidase activity is expressed as relative light unit/mg protein (RLU/mg protein). Protein content of the renal tissues was determined by the Bradford method using a commercially available assay kit (Bio-Rad). DETC-induced changes in luminescence in vitro were also tested in a protein-free solution to determine its nonspecific effects unrelated to O2− generation.
Analytical procedures, calculations, and statistical analysis.
The collected blood and urine samples were analyzed for inulin and sodium/potassium concentrations. Inulin was determined by spectrophotometry, and sodium/potassium concentrations were determined by flame photometry. The value of inulin clearance was considered as glomerular filtration rate (GFR). The formula for measurement of GFR was as follows: GFR (inulin clearance) = (urinary concentration of inulin × urine volume)/plasma inulin concentration. Renal vascular resistance (RVR) was calculated by dividing the value of mean arterial pressure (MAP) with the value of RBF. Urinary concentrations of 8-isoprostane and nitrate/nitrite were determined by enzyme immunoassay and colorimetric methods using commercially available assay kits (Cayman Chemical). All values were normalized per gram of kidney weight. Data are expressed as means ± SE. Statistical comparisons of differences in the responses were carried out by one-way ANOVA followed by appropriate (Holm-Sidak) post hoc tests. Comparison of the percent responses to ANG II infusion before and during DETC infusion was made using unpaired Student's t-test. Differences in the mean values were deemed significant at P <0.05.
RESULTS
Effects of enalaprilat administration alone on renal hemodynamics and excretory function.
These effects are summarized in Table 1. Infusion of enalaprilat in the renal artery in these rats (n = 11) did not significantly influence MAP. However, there were mild to moderate increases in renal hemodynamic and excretory parameters during enalaprilat administration in these rats. Although the changes in RBF, CBF, MBF, or urine flow (V) did not reach statistical significance, the increases in GFR and sodium excretion (UNaV) were significant. The urinary excretion of 8-isoprostane (UIsoV) and nitrate/nitrite (UNOXV) were not significantly influenced by enalaprilat infusion.
Table 1.
Renal hemodynamic and excretory responses to ANG II before and during administration of DETC in enalaprilat-pretreated rats (n = 11)
| Parameters | Control | EN (Pre-ANG II) | EN + ANG II | EN (Post-ANG II) | EN + DETC | EN + DETC + ANG II |
|---|---|---|---|---|---|---|
| Mean arterial pressure, mmHg | 107 ± 1.7 | 102 ± 1 | 100 ± 2 | 100 ± 2 | 101 ± 2 | 100 ± 2 |
| Renal blood flow, ml·min−1·g−1 | 5.4 ± 0.2 | 6.0 ± 0.3 | 4.7 ± 0.3† | 5.2 ± 0.3 | 4.6 ± 0.3‡ | 3.7 ± 0.2§ |
| Cortical blood flow, PU/min | 328 ± 20 | 346 ± 21 | 288 ± 18† | 315 ± 18 | 287 ± 16‡ | 233 ± 19§ |
| Medullary blood flow, PU/min | 172 ± 22 | 172 ± 24 | 152 ± 15 | 148 ± 15 | 131 ± 11 | 127 ± 10 |
| Glomerular filtration rate, ml·min−1·g−1 | 0.7 ± 0.03 | 0.9 ± 0.06* | 0.6 ± 0.06† | 0.7 ± 0.05 | 0.5 ± 0.04‡ | 0.4 ± 0.02§ |
| Urine flow, μl·min−1·g−1 | 9.7 ± 0.7 | 10.7 ± 0.6* | 7.1 ± 0.5† | 8.6 ± 0.6 | 8.0 ± 0.6 | 7.1 ± 0.6 |
| Urinary excretion of sodium, μmol·min−1·g−1 | 1.2 ± 0.1 | 1.4 ± 0.1* | 0.7 ± 0.06† | 1.0 ± 0.1 | 0.7 ± 0.05‡ | 0.5 ± 0.06 |
| Fractional excretion of sodium, % | 1.3 ± 0.1 | 1.2 ± 0.1 | 0.75 ± 0.06† | 0.9 ± 0.04 | 0.9 ± 0.04 | 0.9 ± 0.04 |
| Urinary excretion of 8-isoprostane, pg·min−1·g−1 (n = 7) | 3.5 ± 0.3 | 3.0 ± 0.2 | 6.0 ± 0.6† | 5.0 ± 0.6 | 5.0 ± 0.3 | 3.0 ± 0.3§ |
| Urinary excretion of nitrate/nitrite, nmol·min−1·g−1 (n = 7) | 1.6 ± 0.07 | 1.7 ± 0.1 | 0.7 ± 0.08† | 1.2 ± 0.2 | 1.0 ± 0.1 | 1.1 ± 0.2 |
Values are means ± SE; n, no. of rats. EN, enalaprilat; DETC, diethyldithiocarbamate; PU, perfusion unit. P < 0.05 vs. control (*), vs. EN (Pre-ANG II) (†); vs. EN (Post-ANG II) (‡), and vs. EN + DETC (§).
Effect of DETC on renal hemodynamics and excretory function.
Table 1 also summarized these results. Intra-arterial infusion of DETC directly in the kidney for 50 min decreases renal hemodynamic and excretory parameters, mostly similar to those reported in other studies (15, 32). However, there were no apparent changes in UNOXV or UIsoV during DETC treatment alone in these enalaprilat-pretreated rats.
Renal responses to infusion of ANG II before and during DETC treatment.
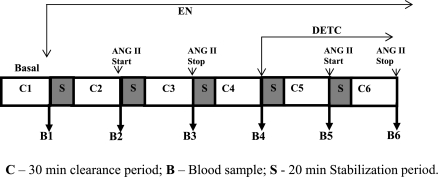
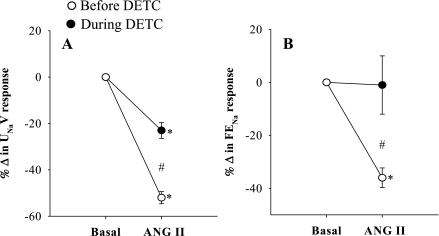
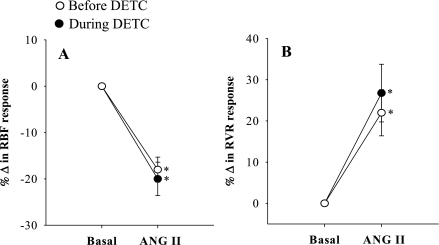
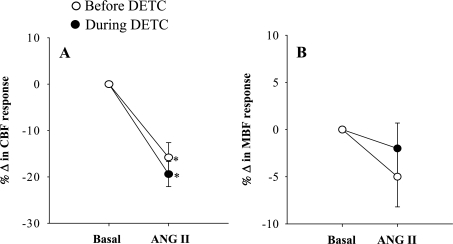
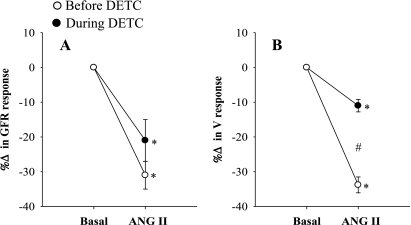
The summarized absolute results from these experiments are shown in Table 1. Comparison of the percentage responses to ANG II on renal hemodynamics and excretory functions before and during DETC infusion were shown in Figs. 1–5. It is observed that the reduction of RBF by ANG II was similar before and during DETC treatment (−18 ± 3% vs. −20 ± 4%) (Fig. 2A). The increases in RVR caused by ANG II administration before and during DETC treatment (22 ± 6 vs. 27 ± 8%) were also not significantly different (Fig. 2B). The magnitudes of the percent reduction of CBF and MBF responses to ANG II before and during DETC treatment were also not statistically different (CBF, −16 ± 3% vs. −19 ± 3%; MBF, −5 ± 6% vs −2 ± 2%) (Fig. 3, A and B). The difference between the magnitudes of percent GFR responses to ANG II before and during DETC treatment (−31 ± 4% vs. −22 ± 7%) (Fig. 4A) was also not significant. However, the marked antidiuretic and antinatriuretic responses to ANG II before administration of DETC were seen markedly attenuated during DETC treatment [V, −34 ± 3% vs. −12 ± 2% (Fig. 4B) and UNaV, −53 ± 3% vs. −24 ± 4% (Fig. 5A)]. It was noted that the decrease in fractional excretion of sodium (FENa) in response to ANG II before DETC infusion (−36 ± 4%) was markedly attenuated during DETC infusion (−6 ± 11%) (Fig. 5B). However, there were no such reductions, rather slightly increased, in the responses to the second administration of ANG II compared with the first administration on renal parameters in the corresponding time-controlled experiments (n = 5). The changes in renal excretory parameters in response to the first and the second administrations of ANG II were as follows: V, 9.4 ± 0.3 to 5.4 ± 0.6 μl·min−1·g−1 (−42.5 ± 4.8%) and 7.4 ± 0.7 to 3.8 ± 0.2 μl·min−1·g−1 (−47.5 ± 3.8%); UNaV, 1.3 ± 0.1 to 0.6 ± 0.04 μmol·min−1·g−1 (−53.5 ± 3.4%) and 0.9 ± 0.1 to 0.4 ± 0.04 μmol·min−1·g−1 (−58 ± 2.3%); and FENa, 0.8 ± 0.03 to 0.4 ± 0.02% (−47.6 ± 1.8%) and 0.6 ± 0.03 to 0.3 ± 0.03% (−53.5 ± 3.5%). There were also similar changes in the renal hemodynamic parameters during the first and the second administrations of ANG II in these time-controlled experiments. The changes in renal hemodynamic parameters in response to the first and the second administrations of ANG II were as follows: RBF, 6.9 ± 0.6 to 4.7 ± 0.3 ml·min−1·g−1 (−31.5 ± 3.8%) and 5.8 ± 0.7 to 3.2 ± 0.3 ml·min−1·g−1 (−44.1 ± 3.5%); CBF, 391 ± 14 to 324 ± 21 perfusion units (PU); (−17.5 ± 2.9%) and 334 ± 19 to 221 ± 16 PU (−34 ± 2.3%); MBF 122 ± 9.3 to 118 ± 8 PU (−2.7 ± 2.0%) and 121 ± 9 to 102 ± 17 PU (−15 ± 13%); and GFR 1.1 ± 0.1 to 0.9 ± 0.01 ml·min−1·g−1 (−23.6 ± 2.2%) and 1.0 ± 0.1 to 0.6 ± 0.03 ml·min−1·g−1 (−38.6 ± 2.7%). These findings demonstrate that the observed reductions in the renal responses to ANG II in the presence of DETC were unlikely to be contributed by the time-dependent changes in the present study.
Fig. 5.
Percentage (%) changes in urinary sodium excretion (UNaV; A) and fractional excretion of sodium (FENa; B) responses to acute ANG II in the presence and absence of SOD inhibition by DETC (n = 11). P < 0.05 vs. basal (*) and vs. before DETC (#).
Fig. 2.
Percentage (%) changes in renal blood flow (RBF; A) and renal vascular resistance (RVR; B) responses to acute ANG II in the presence and absence of superoxide dismutase (SOD) inhibition by DETC (n = 11 experiments). *P < 0.05 vs. basal.
Fig. 3.
Percentage (%) changes in cortical blood flow (CBF; A) and medullary blood flow (MBF; B) responses to acute ANG II in the presence and absence of SOD inhibition by DETC (n = 11). *P < 0.05 vs. basal.
Fig. 4.
Percentage (%) changes in glomerular filtration rate (GFR; A) and urine flow (V; B) responses to acute ANG II in the presence and absence of SOD inhibition by DETC (n = 11). P < 0.05 vs. basal (*) and before DETC (#).
UISOV and UNOxV in response to ANG II before and during DETC treatment.
Table 1 summarized the results in UISOV and UNOxV obtained from seven rats tested in these experiments. ANG II administration markedly increases UISOV before the infusion of DETC. However, during DETC infusion, UISOV is decreased by ANG II administration. ANG II administration caused a decrease in UNOxV before to DETC infusion but caused no change during DETC infusion.
Renal cortical and medullary NADPH oxidase activity in response to ANG II before and during DETC treatment.
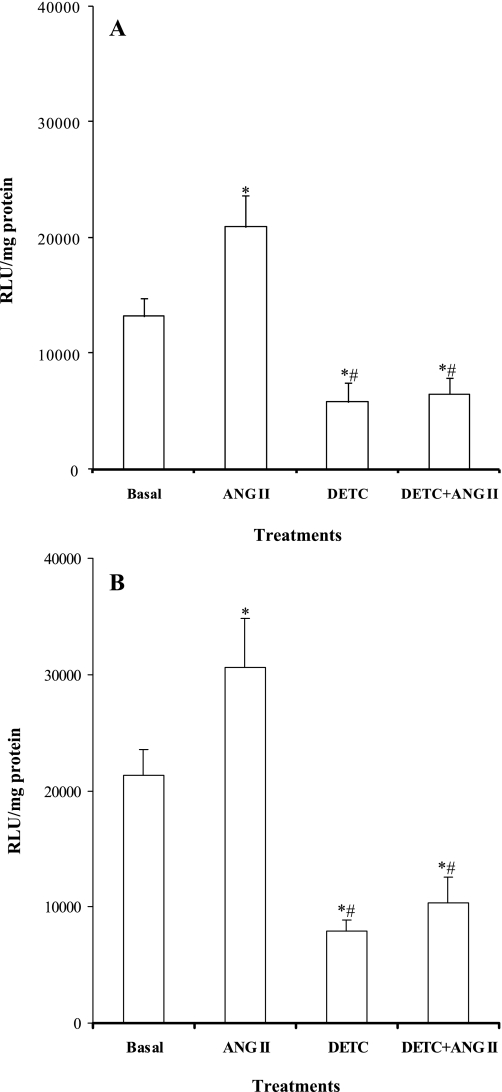
Figure 6 shows the summarized results obtained from in vitro studies. ANG II enhanced the NADPH oxidase activity in both cortical and medullary tissues (cortex, 13,194 ± 1,651 vs. 20,914 ± 2,769 RLU/mg protein and medulla, 21,296 ± 2,244 vs. 30,597 ± 4,250 RLU/mg protein) (Fig. 6, A and B). DETC treatment decreased NADPH oxidase activity both in the cortex (5,805 ± 1,688 RLU/mg protein) and in the medulla (7,921 ± 960 RLU/mg protein). These reductions in NADPH activity could not be considered being a nonspecific effect of DETC to reduced luminescence by a mechanism unrelated to O2− generation in vitro, since DETC did not reduce the luminescence value in a protein-free solution but rather increased this value (control, 84 ± 7 RLU vs. DETC, 422 ± 16 RLU; n = 4). However, when the tissues were treated with ANG II in the presence of DETC, the ANG II-mediated enhancement in NADPH oxidase activity was attenuated in both the cortex (6,494 ± 1,438 RLU/mg protein) and medulla (10,315 ± 2,261 RLU/mg protein) (Fig. 6, A and B).
Fig. 6.
Renal cortical (A) and medullary (B) NADPH oxidase activity in tissues treated with ANG II, DETC, and their combination (n = 7). RLU, relative light units. P < 0.05 vs. basal (*) and vs. ANG II (#).
DISCUSSION
The results from the present investigation demonstrate that SOD inhibition in the kidney does not alter the responses to acute administration of ANG II on RBF or GFR but shows significant attenuation of its antinatriuretic effect in rats pretreated with enalaprilat. Because SOD effectively reduces the reactive molecule of O2−, its inhibition is expected to increase the tissue level of this oxygen radical, particularly during administration of ANG II, which stimulates NADPH oxidase activity to generate this radical compound in biological tissue (1). As a well-known effective inhibitor of SOD (8), the metal ion-chelating agent DETC was used in many previous studies (15, 18, 19, 33) to assess the role of cellular O2− formation and thus the role of enhanced oxidative stress in regulating different organ function, including the kidney. It was reported that administration of DETC caused decreases in RBF, GFR, and UNaV when infused intra-arterially in dogs (15) or intrarenally in rats (33), and these responses to DETC were attributed to enhancement of O2− activity in the kidney. In the present study, DETC administration alone in the kidney also induced similar renal effects as reported previously (15), indicating that the dose of DETC used here was effective as an inhibitor of SOD. However, contrary to our hypothesis, SOD inhibition by DETC in these present experiments did not exacerbate the renal actions of ANG II but rather attenuated its antinatriuretic action. In addition, it was also observed that DETC treatment attenuated the ANG II-induced increases in both UISOV (marker for endogenous O2− activity) in vivo and the renal tissue NADPH oxidase activity in vitro. To our knowledge, this is the first in vivo study that evaluated the renal responses to acute administration of ANG II in the condition of inhibited SOD activity.
Previous studies using O2− scavenger or inhibitor (6, 10, 16) indicated an involvement of concomitant generation of O2− in mediating renal vasoconstrictor and excretory responses to acute administration of ANG II (6, 16). However, the contribution of possible enhancement in O2− activity is not reflected in the renal responses to acute ANG II during SOD inhibition in the present study. This is mainly due to the fact that SOD inhibition by DETC attenuates the tissue NADPH oxidase activity and thus limits O2− production in response to ANG II. The findings in our earlier studies (15, 16, 17) demonstrated that the changes in O2− activity in response to acute ANG II administration or NO inhibition in the kidney exert comparatively greater influence on renal tubular than on the vascular function. Thus it is expected that the renal action of ANG II is more attenuated in excretory function than in hemodynamic function due to reductions in NADPH oxidase activity in response to SOD inhibition in the present study. These findings demonstrate that a functional deficiency in SOD activity resulted in attenuation of both basal and ANG II-stimulated increases in tissue NADPH oxidase activity in the kidney, which also correlates with the functional data showing attenuation of renal responses to ANG II. Thus these interesting results strongly suggest an interactive role between SOD and NADPH oxidase enzymes in limiting each other's activity in the state of an enhanced renin-angiotensin system and oxidative stress.
Although an existence of such cross link between the activities of these two enzymes (NADPH oxidase and SOD) is not clearly known in the current literature, an earlier study in vitro (8) also reported that application of DETC in rat aortic rings inhibited the stimulated production of O2− by blocking the catalytic activity of xanthine oxidase while not affecting the basal production of vascular O2−. The findings in the present study in vivo also demonstrate that DETC treatment inhibited the stimulated production of O2− by ANG II, since it is observed that UISOV decreased during ANG II infusion in the presence of DETC, whereas it was increased before DETC administration. SOD inhibition by DETC treatment had been shown to reduce intracellular reactive oxygen species production in ANG II-treated cardiac fibroblasts (12). Supporting these observations, it was also reported that chronic administration of ANG II for 2 wk in extracellular SOD (ecSOD) knockout mice resulted in a downregulation of NADPH oxidase activity both in aortic tissue (4) and in the kidney (31). Increases in renal tissue O2− production and UISOV because of chronic ANG II administration were also shown to be absent in ecSOD knockout mice (31). Thus these cumulative data strongly suggest that, in a deficient condition of SOD enzyme, a downregulation of NADPH oxidase activity occurs that would prevent the stimulated production of O2− and would limit the possible exacerbation of renal functional responses to an enhanced renin-angiotensin system.
The exact mechanism of how SOD inhibition causes attenuation of NADPH oxidase activity is not clear yet. However, it had been reported that decreases in cellular SOD activity by administration of DETC in vitro decrease nuclear transcription factor-κB (NF-κB) (27, 32). In fact, the dithiocarbamate compounds such as pyrrolidine dithiocarbamates and DETC were used in previous studies (2, 13, 14) as an inhibitor of NF-κB activation to evaluate its role in mediating inflammatory responses to various tissue injury. Because ANG II-induced activation of NADPH oxidase has been linked to an upregulation of NF-κB activity in many studies (7, 28, 29), it is conceivable that an inhibition of such nuclear transcription factor by DETC may suppress the activity of NADPH oxidase observed in the present study. It is also possible that, while administration of DETC effectively inhibits ecSOD, it could also be less effective in inhibiting the cytosolic or intracellular isoform of SOD (Cu-Zn SOD). It was reported that chronic ANG II administration in gene knockout mice lacking the ecSOD gene upregulated the intracellular isoform of SOD, whereas this isoform was downregulated in the corresponding wild-type strain of mice (4, 31). In an earlier study, it is also observed that, in mice lacking ecSOD, the upregulation of cytosolic isoforms of SOD offset ANG-II mediated enhancement of NAPDH oxidase activity (30). It is to be noted here that rats are usually known as a species that lacks vascular ecSOD activity (3). Thus, in the present study in rats, ANG II administration may have increased the cytosolic isoform of SOD, which offset the enhanced activity of NADPH oxidase to limit the production of tissue O2− during DETC administration. However, further experiments with a specific protocol to examine the activity of different isoforms of SOD in response to acute ANG II administration need to be conducted to clarify this issue.
In conclusion, these results demonstrate that renal excretory responses to acute ANG II administration are attenuated during SOD inhibition. These data suggest that such attenuation of renal functional responses to ANG II is related to a downregulation of NADPH oxidase activity in the deficient condition of SOD enzyme.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-66432 and the Tulane Enhancement Fund.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol 285: R117– R124, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Elks CM, Mariappan N, Haque M, Guggilam A, Majid DS, Francis J. Chronic NF-κB blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am J Physiol Renal Physiol 296: F298– F305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukai T, Folz RJ, Landmesser U, Harrison DG. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res 55: 239– 249, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension 48: 473– 481, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase role in cardiovascular biology and disease. Circ Res 86: 494– 501, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Just A, Olson AJ, Whitten CL, Arendshorst WJ. Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide. Am J Physiol Heart Circ Physiol 292: H83– H92, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res 82: 503– 512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kober T, König I, Weber M, Kojda G. Diethyldithiocarbamate inhibits the catalytic activity of xanthine oxidase. FEBS Lett 551: 99– 103, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Kopkan L, Majid DS. Enhanced superoxide activity modulates renal function in NO-deficient hypertensive rats. Hypertension 47: 568– 572, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Kopkan L, Castillo A, Navar LG, Majid DS. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol 290: F80– F86, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Kopkan L, Husková Z, Vanourková Z, Thumová M, Skaroupková P, Cervenka L, Majid DS. Superoxide and its interaction with nitric oxide modulates renal function in prehypertensive Ren-2 transgenic rats. J Hypertens 25: 2257– 2265, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Lijnen P, Petrov V, van Pelt J, Fagard R. Inhibition of superoxide dismutase induces collagen production in cardiac fibroblasts. Am J Hypertens 21: 1129– 1136, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation 100: 1330– 1337, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Maio R, Sepodes B, Figueiredo N, Pinto R, McDonald M, Thiermermann C, Costa P, Mota-Filipe H. Effects of diethyldithiocarbamate (DETC) on liver injury induced by ischemia-reperfusion in rats. Transplant Proc 39: 365– 368, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Majid DS, Nishiyama A. Nitric oxide blockade enhances renal responses to superoxide dismutase inhibition in dogs. Hypertension 39: 293– 297, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Majid DS, Nishiyama A, Jackson KE, Castillo A. Superoxide scavenging attenuates renal responses to ANG II during nitric oxide synthase inhibition in anesthetized dogs. Am J Physiol Renal Physiol 288: F412– F419, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Majid DS, Nishiyama A, Jackson KE, Castillo A. Inhibition of nitric oxide synthase enhances superoxide activity in canine kidney. Am J Physiol Regul Integr Comp Physiol 287: R27– R32, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Makino A, Skelton MM, Zou AP, Cowley AW., Jr Increased renal medullary H2O2 leads to hypertension. Hypertension 42: 25– 30, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW., Jr Increased renal medullary oxidative stress produces hypertension. Hypertension 39: 667– 672, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Matavelli LC, Kadowitz PJ, Navar LG, Majid DS. Renal hemodynamic and excretory responses to intra-arterial infusion of peroxynitrite in anesthetized rats. Am J Physiol Renal Physiol 296: F170– F176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller AA, Drummond GR, De Silva TM, Mast AE, Hickey H, Williams JP, Broughton BR, Sobey CG. NADPH oxidase activity is higher in cerebral versus systemic arteries of four animal species: role of Nox2. Am J Physiol Heart Circ Physiol 296: H220– H225, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 39: 316– 322, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navar LG, Kobori H, Prieto-Carrasquero M. Intrarenal angiotensin II and hypertension. Curr Hypertens Rep 5: 135– 143, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omoro SA, Majid DSA, El-Dahr SS, Navar LG. Kinin influences on renal regional blood flow responses to angiotensin-converting enzyme inhibition in dogs. Am J Physiol Renal Physiol 276: F271– F277, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Qin Z, Reszka KJ, Fukai T, Weintraub NL. Extracellular superoxide dismutase (ecSOD) in vascular biology: an update on exogenous gene transfer and endogenous regulators of ecSOD. Translational Res 151: 68– 78, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol 284: R893– R912, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Schreck R, Meier B, Mannel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med 175: 1181– 1194, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J 10: 709– 720, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-κB activation via NADPH oxidase. Am J Physiol Endocrinol Metab 294: E345– E351, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol 288: H22– H28, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill PS, Aslam S, Wang X, Ji H, Sandberg K, Jose P, Wilcox CS. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension 48: 934– 941, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat 111: 419– 427, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou AP, Li N, Cowley AW., Jr Production and actions of superoxide in the renal medulla. Hypertension 37: 547– 553, 2001. [DOI] [PubMed] [Google Scholar]