Abstract
Insulin-like growth factor binding protein-5 (IGFBP-5) mediates mesangial cell migration through activation of cdc42, and laminin421 binding to α6β1-integrin (Berfield AK, Hansen KM, Abrass CK. Am J Physiol Cell Physiol 291: C589–C599, 2006). Because glomerular expression of laminin β2 is reduced in diabetic rats (Abrass CK, Spicer D, Berfield AK, St. John PL, Abrahamson DR. Am J Pathol 151: 1131–1140, 1997), we directly examined the effect of hyperglycemia on mesangial cell migration and laminin β2 expression. Migration mediated by IGFBP-5 is impaired in the presence of 25 mM glucose. This reduction in migration was found to result from a loss in mesangial cell synthesis of laminin421, and IGFBP-5-induced migration could be restored by replacing laminin421. Additional studies showed that there was selective reduction in mRNA translation of laminin β2 in the presence of high glucose. Preserved synthesis of laminin β1 indicates that not all proteins are reduced by high glucose and confirms prior data showing that laminin411 cannot substitute for laminin421 in IGFBP-5-mediated migration. Given the importance of mesangial migration in the reparative response to diabetes-associated mesangiolysis, these findings provide new insights into abnormalities associated with diabetic nephropathy and the potential importance of differential control of protein translation in determination of alterations of protein expression.
Keywords: diabetic nephropathy, protein translation
extracellular matrix (ECM) accumulation is a hallmark of diabetic nephropathy; yet the mechanisms responsible for altered accumulation of matrix, as well as the functional consequences of matrix accumulation remain to be elucidated. We previously demonstrated that not only does the amount of ECM in each of the renal compartments, glomerular basement membrane (GBM), tubular basement membrane, and mesangial matrix increase, but the composition of each compartment is altered (5). Furthermore, reductions in critically important proteins (20) may occur and contribute to organ dysfunction and disease progression. In addition to ECM accumulation and a change in ECM composition, mesangiolysis is a prominent feature of diabetic nephropathy (35). Repair following mesangiolysis requires that the mesangium be repopulated through migration of cells from the extraglomerular mesangium (15). Studies in the Thy1.1 model show that recurrent mesangiolysis accompanied by inadequate repopulation of mesangial cells (MC) leads to progressive glomerulosclerosis (34). Thus alterations in mesangial migration in the diabetic milieu or as a consequence of alterations in the composition of the ECM substrate may limit this important repair mechanism and thereby contribute to progressive loss of kidney function in diabetic nephropathy.
Insulin-like growth factor binding protein-5 (IGFBP-5) modulates the effects of IGF-1, as well as has IGF-1-independent effects. IGFBP-5 is normally expressed by MC only during kidney development (23), but it is increased in diabetic kidneys (27), and by MC exposed to high glucose concentration in vitro (27). In prior studies we showed that IGFBP-5 induces mesangial migration that requires the expression of laminin421 (LN421; α4β2γ1) (7), which in part is associated with new transcription of laminin β2 (lamb2) mRNA (7). Based on these observations, we would predict that laminin β2 synthesis and content might be increased in diabetic glomeruli. However, using fetal kidney grafting into the eyes of rats with streptozotocin (STZ)-diabetes, we found that developing glomeruli failed to express laminin β2, and this was associated with delayed appearance of MC and delayed glomerular maturation (2). The delay in population of glomeruli with MC in hyperglycemic animals would suggest that hyperglycemia-associated losses in expression of laminin β2 might be responsible for impaired IGFBP-5-mediated MC migration during glomerular development. Although laminin β2 was found to be reduced in vivo in diabetic rats, a direct causal link to hyperglycemia had not been established. The unexpected loss of laminin β2 protein in the face of increased levels of IGFBP-5 and modest increases in lamb2 mRNA suggested the possibility that elevated glucose concentrations altered lamb2 mRNA translation. Recently, in a series of publications, Fliers, Kasinath and colleagues (20, 21) have shown that despite reductions in mRNA expression for laminin subunits α5, β1, and γ1, protein accumulates as a result of enhanced translation by glucose and insulin-mediated enhancement of phosphatidylinositol 3-kinase, Akt, and mammalian target of rapamycin (mTOR) activities. These studies have been confirmed in the db/db mouse at 2 wk, in the insulin-deficient diabetic rat 5 days after STZ injection, and in proximal tubular cells in culture within 1 h. Our findings that laminin β2 is reduced suggest that hyperglycemia may have different effects on the translation of different proteins.
To further understand the role of glucose in mediating changes in ECM composition and cell function, we undertook studies of migratory function and laminin β2 synthesis by MC exposed to high glucose for periods up to 7 days in culture. These studies show that elevated glucose concentration is responsible for impaired MC migration, loss of laminin β2 protein, and that the loss of laminin β2 protein is responsible for the impaired MC migration in response to IGFBP-5. Also, despite the presence of lamb2 mRNA, protein levels fall with a consequent reduction in LN421. Furthermore, we show that laminin β2 translation is reduced with chronic exposure to high glucose (HG). These data suggest that although some matrix proteins are increased by hyperglycemia (e.g., laminin β1, collagen I, fibronectin), others may fall and that the change in ECM composition results in important changes in cell function. Loss of critical proteins may be equally important to progressive kidney disease as is over-expression of others.
METHODS
Materials.
Heparin (H-3393) and cycloheximide (CHX) were purchased from Sigma (St. Louis, MO). Antibodies included mouse monoclonal anti-LNβ2 (C4, DSHB, University of Iowa), rabbit anti-EHS laminin (7), rabbit anti-IGFBP-5 (Upstate, Lake Placid, NY), rabbit anti-GAPDH (Abcam, Cambridge, MA), and rabbit anti-tubulin and anti-β-actin (Sigma).
MC culture.
Rat glomerular MC were cultured by modification (3, 4) of routine methods (18). In brief, minced rat kidney cortex was sieved. Isolated glomeruli were plated in medium containing a 1:1 mix of 20% FCS-RPMI 1640 and previously collected glomerular conditioned medium. Insulin routinely added to supplement MC cultures was omitted. MC outgrowths were harvested and passed in this medium for an additional week, after which the conditioned medium was omitted. MC were cloned and studied at passages 8–12.
Cell proliferation and viability.
Cells were counted using a Coulter counter. Viability was determined by trypan blue exclusion, and proliferation was determined by a One Solution Cell Proliferation Assay (Promega, Madison, WI) according to the manufacturer's directions.
Migration.
MC migration was measured with and without IGFBP-5201-218 (30 μg/ml) in a wounding assay as described previously (1). In brief, MC (1 × 106 cells) were plated in 60-mm tissue culture dishes in 20% FCS-DMEM [200 mg/dl (11.1 mM) glucose] or 20% DMEM with varying glucose concentrations [5.5 mM (LG), 5.5 mM+19.5 mM mannitol (LM), 25 mM (HG)] for up to 10 days (chronic), growth arrested in 2% FCS-RPMI, and scraped with a sterile razor blade to create a linear wound across the dish. IGFBP-5201-218 peptide was added to the cultures, and migration was examined 48 h later. Controls were maintained in 2% FCS-RPMI medium. Cultures were fixed with 2% paraformaldehyde, stained with toluidine blue, and migrating cells along 1 mm length of the wound edge were counted in 0.1-mm increments. Three to five replicate plates were prepared for each condition, and five areas were examined per sample. Experiments were repeated three times. Data were analyzed as the total number of migrating cells and were expressed as a percentage of control. Individual control samples are also divided by the average for all controls and expressed as a percentage of control. An SD for these results is calculated and shown in the graph to indicate the variability of repeat control experiments, as well as experimental conditions. In previous experiments, we have compared the IGFBP-5 peptide (201–218), which represents the heparin-binding domain (1), to intact IGFBP-5, IGFBP-3, and IGFBP-4. IGFBP-5 (intact or the peptide) is unique in stimulating this form of migration. Because IGFBP-5201-218 does not bind to IGF-1, the effects of the peptide represent an IGF-1-independent effect, and it is not interfered with by endogenous IGF-1; thus we have used this peptide for the present studies. Because all of our prior studies were conducted in RPMI, this served as a control for the response, and all studies done in DMEM with varying glucose concentrations were expressed as a percentage of the RPMI control. In some experiments, MC were plated in varying concentrations of glucose (5.5, 11.1, 16.7, 22.2, 25, 27.7, 33.3 mM) for 1 or 3 days before the migration assay. For replacement of LN421, wells were coated with matrix isolated from MC as described (7).
RT-PCR.
mRNA was estimated by semiquantitative RT-PCR and confirmed by quantitative real-time PCR (qPCR) using an ABI-7900HT machine and SYBR green-sensing dT (Bioline, Taunton, MA). Primers for lamb1, lamb2, and gapdh were purchased from Qiagen (Valencia, CA). qPCR was also performed to assess mRNA association with polysomes. In those experiments, Escherichia coli RNA was added as a carrier to follow recovery and amplified using the following primers: forward primer (Ec16S-QA): 5′TGCAACGCGAAGAACCTTAC3′ and reverse primer (Ec16S-QB): 5′GCTCGTTGCGGGACTTAAC3′ (gift from Dr. Vivian McKay, University of Washington, Seattle, WA).
Protein content.
For Western blots, MC were lysed in PBS+0.1% SDS, 0.5% Triton X-100, and protease inhibitors (Sigma-Aldrich). Protein was quantified by BCA protein assay (Pierce, Rockford IL). Equal amounts of protein were subjected to SDS-PAGE electrophoresis and transferred to nitrocellulose. The membranes were incubated with anti-LNβ2 antibodies followed by peroxidase-conjugated anti-rabbit IgG antibodies. Blots were developed with SuperSignal West Pico chemiluminescence substrate (Pierce) and exposed onto X-O-mat film. The expression level of laminin was quantified using a Kodak EDAS camera system and 1D software (Eastman Kodak, Rochester, NY). These experiments were conducted on four separate occasions after 10 days’ exposure to 5.5, 25, or 5.5 mM glucose plus mannitol. Load controls were assessed using total protein, or staining for GAPDH, β-actin, or tubulin. Under the conditions of this study, there were no differences in the expression levels of any of these control proteins.
Fluorescence microscopy.
MC were grown in slide flasks in LG, LM, or HG medium with or without the addition of IGFBP-5201-218 as described above. Immunostaining was performed as previously described (6, 8). To establish cdc42 activation, 1 h following the addition of IGFBP-5201-218 cells were stained with antibody to cdc42GAP (Transduction Laboratories, Lexington, KY), a protein that associates with activated cdc42 (6). To visualize changes in the cytoskeleton, cells were dual stained with phalloidin (Molecular Probes, Eugene, OR) and antibody to vinculin (Sigma). Slides were examined using a Zeiss Axiophat fluorescence microscope equipped with epi-illumination and photographed using a Zeiss camera and Zeiss Axiovision software.
Protein translation.
mRNA translation was determined by assessing the proportion of mRNA that was associated with polysomes as described (31) using a wash (20 mM Tris · HCl, pH 8.5, 15 mM MgCl2, 140 mM KCl, 0.5 mM DTT, 0.1 mM CHX) and lysis (wash; 0.5 mg/ml heparin, 0.5% NP-40) buffer adapted (39). In brief, MC were grown for 7 days in HG, LG, or LM as described above. After 5-min incubation with 100 μg/ml of CHX, 5 × 106 cells were rinsed, lysed, dounced on ice, and clarified by centrifugation. The supernatant was loaded onto a 7–47% sucrose gradient and centrifuged at 130,000 g for 90 min at 4°C. Twelve 1-ml fractions were collected. mRNA was precipitated, reverse transcribed, and assayed by qPCR for lamb1, lamb2, and gapdh mRNA. Load and recovery were followed by the addition of carrier E. coli mRNA that was also assayed by qPCR. Primers for the genes of interest are listed above.
Statistical methods.
All samples were run in triplicate and repeated on a minimum of three separate occasions. Results are expressed as the group means ± SD. Group means were compared by ANOVA. P < 0.05 was considered statistically significant.
RESULTS
MC exposed to HG have a reduced migratory response to IGFBP-5.
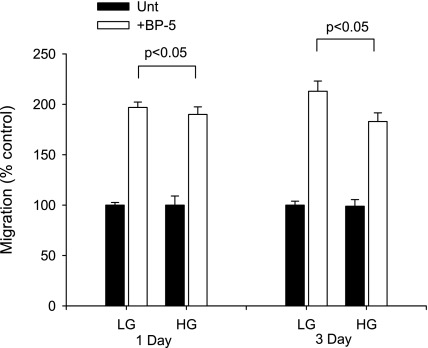
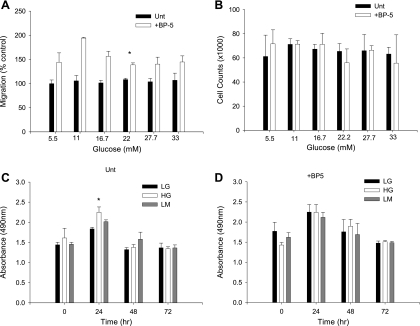
To examine the role of glucose concentration in modulating MC migration, MC were exposed to HG (25 mM) or LG (5.5 mM) and assayed for migratory response to IGFBP-5 in a wounding assay. HG had no effect after 1 day of exposure, but migration was impaired after 3 days of HG (Fig. 1). These data suggested that the effect of acute and chronic changes in glucose concentration might differ. Assessment of MC migration in response to IGFBP-5 after 3 days of exposure to various glucose concentrations showed that maximal inhibition of migration was achieved with 22 mM glucose with no further inhibition at 27.7 or 33.3 mM (Fig. 2A). Because changes in cell proliferation might affect the quantification of migrating cells, we also assessed cell number (Fig. 2B) and cell metabolism (Fig. 2C) in response to increasing glucose concentrations and IGFBP-5. No significant differences in cell number were observed in cultures exposed to various concentrations of glucose with or without IGFBP-5201-218 after 3 days. Cell proliferation as determined by the One Solution Cell Proliferation Assay requires that general cell metabolism be normal, and this was unaffected by the experimental conditions. Glucose concentration in the medium had no effect on the percentage of cells that took up trypan blue (2–6%, P > 0.05, ANOVA). These studies confirmed that the effects of HG on IGFBP-5-induced MC migration were not due to a change in proliferation rate or cell viability.
Fig. 1.
Effect of acute and chronic exposure to high glucose (HG). Mesangial cells (MC) were exposed to HG for 1 or 3 days before testing migratory response to insulin-like growth factor binding protein-5 (IGFBP-5). Differences in migratory response were only observed after 3 days of exposure to HG. LG, low glucose. *P < 0.05, ANOVA.
Fig. 2.
Glucose dose-response experiment. Effect is shown of 3 days of increasing glucose concentration on cell migration (A), cell numbers (B), and cell metabolism with (D) and without IGFBP-5 (C). LM, low-glucose+mannitol. *P < 0.05, ANOVA.
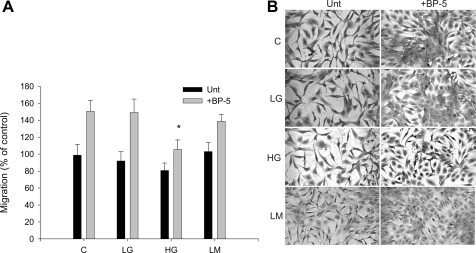
To confirm that the effects of HG concentrations were not merely due to an osmotic effect, subsequent experiments compared 5.5 mM glucose (LG), 25 mM glucose (HG), and 5.5 mM glucose+mannitol (LM) to achieve the same osmolality as 25 mM glucose. Migration was unaffected by the mannitol control; however, after 7 days of exposure to HG, the migratory response to IGFBP-5 was reduced further compared with 3 days of exposure to HG (Fig. 3). This observation suggests that the changes in cellular responses that occur with HG are persistent, increase over time, and are likely to be relevant to changes that occur in vivo in the presence of persistent hyperglycemia.
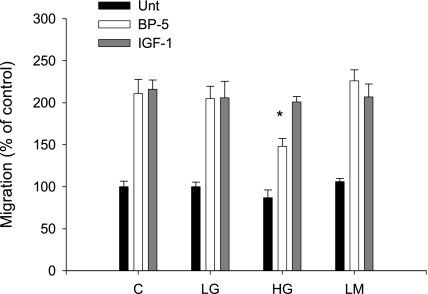
Fig. 3.
MC migration after 7 days in HG medium. MC were cultured for 7 days in control (C; 200 mg/dl glucose), 5.5 mM glucose (LG), 25 mM glucose (HG), or 5.5 mM glucose+mannitol (LM) medium before assessment of migration with and without IGFBP-5201-218 (30 μg/ml). A: migration was expressed as a % of control. *P < 0.05, ANOVA. B: photomicrograph showing cells filling the wounded area.
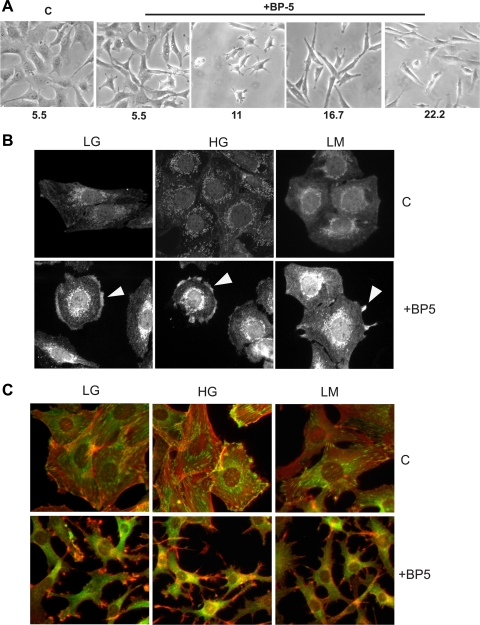
Previously, we reported that IGFBP-5-treated MC develop a characteristic spider-like morphology with unique focal adhesions (6, 8). As seen in Fig. 4A, increasing concentrations of glucose below 33 mM did not prevent the development of an elongated phenotype in response to IGFBP-5201-218. MC exposed to 33.3 mM glucose had a more rounded appearance and did not develop the characteristic filopodia in response to IGFBP-5 (not shown). Others have reported that MAPK activation and cytoskeletal reorganization are diminished by HG in cells subjected to various chemotactic stimuli (43). To determine whether IGFBP-5-mediated activation of signal transduction cascades and cytoskeletal reorganization were altered by HG as a cause for impaired migration, we examined cdc42 activation and cytoskeletal reorganization. When cdc42 is activated, cdc42GAP binds to it and becomes clustered in areas of activation. Similar to our previous data (6), by 1 h following treatment with IGFBP-5201-218, the intensity of staining was increased and distinct clusters of cdc42GAP were visible at the cell edge in areas of membrane reorganization (Fig. 4B). This response was unaffected by glucose concentration. Following cdc42 activation, the actin cytoskeleton reorganizes (6), as shown in Fig. 4C. In untreated cultures, cells are spread with distinct arrays of vinculin just under the membrane at the cell surface. F-actin forms parallel arrays that span the cell. Following treatment with IGFBP-5201-218, there is distinct reorganization of the cytoskeleton with development of a spider-like morphology with long actin-positive filopodial projections. The typical array of vinculin staining is lost as the cell is no longer in a spread phenotype. This change was unaffected by glucose content in the medium. These findings indicate that the initial signal transduction is activated and the subsequent cytoskeletal reorganization occurs; yet, as shown above, the cells have a marked reduction in actual migration. These findings suggest that something subsequent to these steps is altered and responsible for impaired migration in the presence of HG.
Fig. 4.
Cell phenotype, cdc42 activation, and cytoskeletal reorganization. A: MC were cultured in various concentrations of glucose for 3 days before addition of IGFBP-5201-218 and were photographed with phase-contrast microscopy 48 h later. In keeping with previous studies, the change in phenotype was unaffected by glucose concentration. B: MC were cultured for 7 days in 5.5 mM glucose (LG), 25 mM glucose (HG), or 5.5 mM glucose+mannitol (LM) medium before addition of IGFBP-5201-218 (30 μg/ml). One hour later, cells were stained with antibody to cdc42GAP. The activated cdc42-cdc42GAP complex becomes clustered and is detected in areas where the membrane is beginning to reorganize (arrowheads). C: 24 h after the addition of IGFBP-5201-218, F-actin (red) and vinculin (green) staining were performed. In untreated cultures in all 3 media, cells are spread and vinculin is stained in a submembrane array. F-actin fibers span the cell in both parallel and overlapping patterns. Following the addition of IGFBP-5201-218, cells have developed a spider-like morphology with long filopodia. The submembrane array of vinculin is lost, and F-actin has reorganized and extends along the filopodial projections. These responses to IGFBP-5 were not altered by changes in glucose concentration in the medium.
To further establish that basic migratory responses were not affected by HG, MC were exposed to altered glucose concentrations for 3 days and stimulated with either IGFBP-5201-218 or IGF-1. As seen in Fig. 5, the migratory response to IGF-1 was unaffected by HG, and LM, the osmotic control, had no effect on the migratory response to either stimulus. In previous studies, we showed that MC migratory response to stimulation with IGFBP-5 requires binding to LN421, and engagement of α6β1-integrin (7). This type of migration differs from that stimulated with IGF-1, which requires Rac1 activation, and adhesion to fibronectin/vitronectin via αvβ3-integrins (7). These findings suggest that mediators unique to the IGFBP-5 response are likely to be responsible for the reduction in migration in the presence of HG.
Fig. 5.
MC migration in response to IGFBP-5201-218 and IGF-1. MC migration was determined in control (C; 200 mg/dl glucose), 5.5 mM glucose (LG), 25 mM glucose (HG), or 5.5 mM glucose+mannitol (LM) with and without IGFBP-5201-218 (30 μg/ml) or IGF-1 (100 nM). MC were maintained in the experimental medium for 3 days before the migration assay. Bar graphs show results expressed as a % of control. *P < 0.05, ANOVA.
Impaired MC migration in HG is due to selective loss of laminin β2.
IGFBP-5-mediated MC migration requires LN421, and the migratory response cannot be maintained in the presence of only LN411 or other matrix elements. Because we had previously shown that laminin β2 was reduced in diabetic animals (2), we wondered whether the concentration or distribution of LN421 expression was altered directly by HG. Western blot analysis shows that HG was associated with a marked reduction in laminin β2 protein (Fig. 6, A and B). Because total LN and chains associated with LN411 were not reduced, we postulated that a selective loss in LN421 was responsible for the loss in IGFBP-5-mediated MC migration. MC normally produce IGFBP-5 that is increased by HG (unpublished observations and Ref. 27). Consistent with those studies; chronic HG was also associated with an increase in IGFBP-5 protein (Fig. 6B); thus it is unlikely that a loss in endogenous IGFBP-5 resulted in impaired migration. As basal migration in the presence of HG was not increased, it is unlikely that the basal response to endogenous IGFBP-5 was maximal, thereby preventing augmentation with added IGFBP-5201-218.
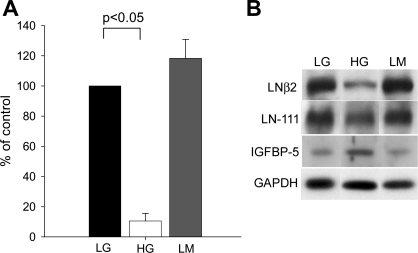
Fig. 6.
Laminin (LN) β2 protein. A: Western blot of proteins extracted from cells exposed to LG, HG, or LM for 10 days. Densitometric analysis of Western blots for laminin β2 was corrected for loading controls. Experiments were repeated on 4 separate occasions. *P < 0.05, ANOVA. B: representative Western blot for laminin β2, showing comparison with staining for laminin β1/γ1 as detected by antibodies to LN111, IGFBP-5, and GAPDH.
To establish that loss of LN421 was responsible for the loss of migratory response to IGFBP-5, MC previously exposed to different concentrations of glucose were examined with replacement of LN421. Replacement with a matrix that contained LN421 restored IGFBP-5-induced migration in MC exposed to HG (Fig. 7). Because HG-MC continue to express LN411, LN511, and collagens (data not shown), and we have previously shown that LN111, LN411, and LN511/521 are unable to restore the migratory response in the absence of LN421 (7), we conclude that the lack of these other proteins is not contributing to alterations in MC migration. Restoration of migration with LN421 indicates that loss of laminin β2 is responsible for our findings and supports the suggestion from data described above that indicates that signal transduction cascades and cytoskeletal responses are intact.
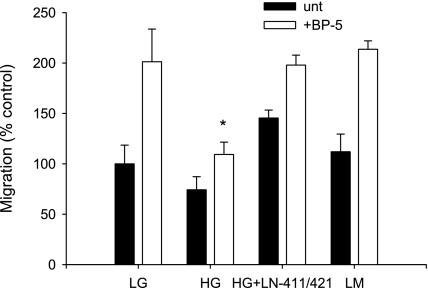
Fig. 7.
Restoration of IGFBP-5-induced MC migration by plating on LN421. MC were exposed to varying concentrations of glucose for 10 days followed by assessment of migration in response to IGFBP-5201-218 after plating on the substrates noted. The reduction in MC migration in HG was restored when MC were plated on matrix containing LN421 in addition to LN411. *P < 0.05, ANOVA.
Loss of laminin β2 is due to reduced lamb2 mRNA translation.
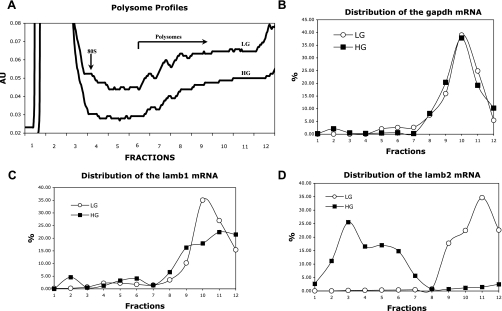
To explore the causes of loss of laminin β2 protein in HG-treated MC, first we examined lamb2 mRNA levels by qPCR. At both 3 (data not shown) and 7 days of exposure to HG, lamb2 mRNA is minimally increased by HG compared with LG (Fig. 8); thus a significant reduction in transcription did not account for the loss of laminin β2 protein. This indicated that there might be enhanced protein degradation or a loss of lamb2 mRNA translation. Because prior studies have shown that HG is associated with reduced ECM degradation (37), we elected to examine lamb2 mRNA translation. When mRNAs are being actively translated, attachment of multiple ribosomes to the mRNA increases their molecular weight in discrete increments. Fractionation of ribosome-mRNA complexes on a sucrose gradient with detection of mRNA in each fraction establishes polysome profiles for each mRNA of interest. Polysome-rich mRNAs that are capable of being translated are clustered in the high-molecular-weight fractions, whereas those with no or a single ribosome that cannot be translated are clustered in low-molecular-weight fractions. As seen in Fig. 9A, HG is associated with a slight reduction in the total proportion of material that is in the polysome-rich fractions, suggesting that total protein translation is reduced. This observation was made in repeated experiments. Despite this modest global reduction, specific mRNAs had very distinct profiles. The distribution of gapdh mRNA into the polysome-rich fractions is consistent with continued translation of this protein in the presence of HG (Fig. 9B). Although increases in gapdh mRNA and protein have been reported to occur following short-term exposure to HG (30), we did not find an increase in our studies at 7–10 days of exposure to HG. In keeping with reports from short-term studies by others (20), lamb1 mRNA levels (Fig. 8) were unaffected by HG and lamb1 mRNA translation was maintained despite chronic exposure to HG (Fig. 9C). The modest reduction in the height of the peak in high-numbered fractions is compensated for by the widening of the peaks over the high-numbered fractions, indicating a broader spectrum of the number of attached ribosomes and overall similar translation rates for lamb1 mRNA in both HG and LG conditions. These results were in sharp contrast to those seen for lamb2 mRNA (Fig. 9D). In MC cultured in LG for 7 days, lamb2 mRNA is associated with polysomes consistent with active translation, whereas in response to HG, lamb2 mRNA association with polysomes disappears, as all of the lamb2 mRNA was detected in the free or monosome fractions. Although a ribosome-mRNA association does not ensure that those mRNAs will be translated, failure to associate with ribosomes precludes translation. These results indicate that loss of laminin β2 protein in the presence of HG is due to impaired translation. Increased rates of degradation of preexisting or a small amount of newly synthesized laminin β2 protein cannot be excluded; however, it is difficult to investigate a possible change in the rate of degradation of laminin β2 when it is no longer detectable or being synthesized. Our observation that lamb1 and lamb2 mRNA translation are differentially controlled by chronic HG is a new finding.
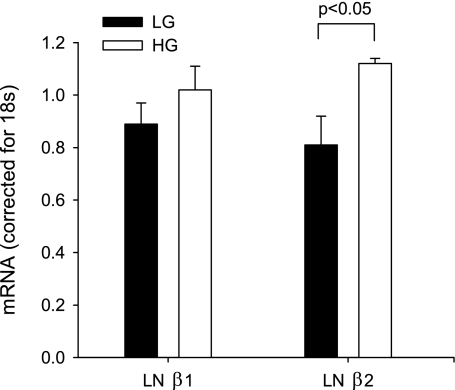
Fig. 8.
Quantification of laminin β1 and laminin β2 mRNA. MC were exposed to LG or HG for 10 days. LNβ1 and LNβ2 mRNA content were determined by quantitative real-time PCR. mRNA content corrected for 18S is shown; n = 3/condition, each run in triplicate. *P < 0.05, ANOVA.
Fig. 9.
mRNA association with polysomes. MC were cultured in LG or HG for 7 days before extraction. Polysomes were fractionated on a sucrose gradient, and each fraction was assayed for specific mRNA content by qPCR. In A, a slight reduction is shown in the total amount of polysomes in the high-molecular-weight fractions in HG-treated MC compared with LG, which is consistent with a reduction in total protein synthesis. Monosomes are at the left, and polysomes of increasing size are to the right. The profiles for gapdh (B), lamb1 (C), and lamb2 (D) are shown. Note that for both gapdh and lamb1, curves are similar in both LG and HG. In contrast, lamb2 mRNA is not detected in high-molecular-weight polysome fractions in HG, indicating that translation of this mRNA is impaired by HG.
DISCUSSION
We have previously established that MC migration induced by IGFBP-5 requires LN421 (7). In this report, studies show that chronic exposure to HG leads to marked reduction in MC migration. This reduction does not appear to be due to abnormalities in cell number or viability, or in MC capacity to reorganize the cytoskeleton, as migratory responses to IGF-1 are unaffected. Previously, we showed that IGF-1-mediated migration differs from that induced by IGFBP-5, as it requires Rac activation, and αvβ3-integrin bound to fibronectin/vitronectin (1). In contrast, IGFBP-5-mediated migration requires LN421 bound to integrin-α6β1; thus we postulated that loss of migration in response to IGFBP-5 might be due to an alteration in expression of LN421 (6, 7). In keeping with this postulate, we found that laminin β2 was lost with chronic exposure to HG. Laminin subunits α4, β1, and γ1 were unaffected; thus abundant amounts of LN411 were still present. Previously, we showed that LN411 alone was unable to support MC migration induced by IGFBP-5. The present studies support this finding, as do studies showing that migration was restored in HG-MC in which LN421 was added back. Mesangiolysis is a common finding in diabetic nephropathy, and it requires repair through proliferation and migration of extraglomerular MC into the peripheral capillary loops. Recovery in the Thy1.1 model of mesangiolysis is impaired in diabetic rats, where injury leads to sclerosis compared with healing in nondiabetic rats (40). Elevated levels of IGFBP-5 in diabetic nephropathy (27) might contribute to this repair; however, loss of LN421 would limit the effectiveness of this response.
In addition to its role in MC biology, laminin β2 is abundant in GBM and is critical to podocyte function. As a component of LN521, onset of expression of laminin β2 marks a critical transition in maturation of the glomerulus (24). Loss of laminin β2 causes severe nephrotic syndrome and death at ∼2 wk of age in null mutant mice (26). These observations led to a search for laminin β2 mutations in human disease, and now a number of different mutations have been described in patients classified as having Pierson's syndrome (9). Although different mutations have somewhat variable phenotypes, they most often display proteinuria, mesangial sclerosis, and eye defects (42). Recently, using techniques that produce graded reductions in laminin β2 expression in the GBM, Jarad and colleagues (16) reported that minor losses in GBM laminin β2 content can lead to proteinuria. These observations are relevant to our studies, as HG-mediated reductions in laminin β2 synthesis in podocytes might result in reduced laminin β2 content in GBM and diabetes-associated microalbuminuria. Previously, we showed that diabetes is associated with delayed transition from laminin β1 to laminin β2, and marked reductions in the total amount of laminin β2 in the developing GBM when fetal kidney grafts are placed into the eyes of rats with STZ-induced diabetes (2). The lack of laminin β2 expression was also associated with reduced population of the glomerulus by MC, which further indicates relevance of the present studies to the glomerulus in vivo in diabetic nephropathy. Results of the present studies establish that HG per se, as opposed to other abnormalities in the diabetic milieu, are responsible for the change in laminin β2. Furthermore, they establish that the loss of laminin β2 is due to alterations in mRNA translation. Additional studies are needed to specifically evaluate the effect of HG on laminin β2 synthesis in podocytes; but these findings and those in vivo indicate that HG per se may influence the development of microalbuminuria in diabetic nephropathy through changes in laminin β2 synthesis.
The effects of HG on laminin β2 protein content are contrasted with reports of laminin γ1 in which both short- and long-term exposure to HG is associated with increased mRNA and protein for laminin γ1 (29). Comparison of the changes in laminin γ1 expression under a variety of stimuli led the authors to conclude that both enhanced transcription and translation influenced the protein levels in the presence of HG. The effects on laminin β2 are also contrasted with those reported for laminin β1 in which mRNA levels were reduced or unaffected, but translation was activated by acute exposure to HG (20). Although we did not observe increases in laminin β1 translation in the face of chronic HG, translation was preserved at a level comparable to control MC. These findings show that each of these laminin subunits is differentially affected by HG, which may explain the change in the laminin isoforms that accumulate in diabetic kidney disease.
Our data showing that chronic HG leads to nearly complete loss of lamb2 mRNA association with polysomes indicates that translation of this laminin subunit is abrogated by chronic HG and controlled differently than lamb1. Protein synthesis consists of three phases, initiation, elongation, and termination. Each of these phases is regulated by a variety of mechanisms, although translation initiation is frequently the limiting step (33). Control of translation initiation is influenced by the structure of the 3′- and 5′-untranslated regions (UTR) and is regulated by stimulatory [e.g., mTOR, internal ribosome entry site (IRES)] and inhibitory factors [e.g., microRNA (miRNA)] (33). The 5′-UTR of gapdh mRNA is uncomplicated and allows ribosomes to be recruited passively whether regulatory pathways are active or not, thus accounting for relatively stable levels of this abundant protein. Initiation of translation of mRNAs such as lamb1 and lamb2 which have highly structured 5′-UTRs can be influenced by activity of the mTOR cascade. mTOR activation can increase translation of both lamb1 (20) and lamb2 (10), and short-term (up to 96 h) exposure to HG activates the mTOR pathway in mouse cortical tubule cells (20–22), podocytes (19), and mouse MC (25); thus one would expect enhanced translation of both of these mRNAs. Some studies have suggested that mTOR activity is reduced after prolonged exposure to HG (41); thus loss of mTOR activation could contribute to the reduction in lamb2 translation that we observed after chronic exposure to HG. mRNAs bearing an IRES allow translation initiation directly at the start codon, abolishing the need for scanning and activation by mTOR (33). The presence of an IRES in lamb1 mRNA (28) could preserve translation of this protein independently of fluctuating levels of mTOR activity and may be stimulated by HG as occurs in STZ-treated mice for FGF2 mRNA, which also contains an IRES (11, 36). Lack of an IRES in lamb2 mRNA precludes this form of control, rendering lamb2 translation susceptible to control by mTOR activity and microRNA. Translation initiation can also be regulated by binding of miRNA to the 3′-UTR of specific mRNAs which targets that mRNA for degradation. Although these small, noncoding, single-stranded RNA molecules are involved in control of cellular processes in the kidney (12, 14, 32), and alterations in the level of some miRNAs have been reported in diabetes and diabetic nephropathy (13, 17, 38), changes in a specific miRNA that target lamb2 mRNA have not been defined.
In these studies, we show that chronic exposure to HG leads to impaired MC migration in response to IGFBP-5 as a result of loss of laminin β2 protein. The profound reduction in lamb2 mRNA translation indicates that reductions in translation contribute in large part to the loss of laminin β2. These data show that the consequence of HG to protein translation differs by the duration of exposure to glucose and for the specific protein involved. Additional studies are needed to determine the role of mTOR activation, miRNAs, and other factors that might control lamb2 mRNA translation. The majority of previous studies have focused on increases in ECM protein synthesis in HG states. These data indicate that the composition of the protein synthetic profile differs, with some proteins being increased whereas other critical proteins are lost. Furthermore, the selective loss of laminin β2, which influences function of the filtration barrier and MC migratory responses necessary to glomerular repair, may have important consequences to glomerular function in diabetic nephropathy.
GRANTS
This material is the result of work supported with resources and the use of facilities at the VA Puget Sound Health Care System (Seattle, WA). This material is based upon work supported in part by the Office of Research and Development Medical Research Service, Department of Veterans Affairs (Merit Review Award to C. K. Abrass), and the National Institute of Diabetes and Digestive and Kidney Diseases (Grant R01-DK49771 to C. K. Abrass).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical assistance of Anne Berfield. The contents of this report do not represent the views of the Department of Veterans Affairs or the United States Government.
REFERENCES
- 1.Abrass CK, Berfield AK, Andress DL. Heparin binding domain of insulin-like growth factor binding protein-5 stimulates mesangial cell migration. Am J Physiol Renal Physiol 273: F899–F906, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Abrass CK, Spicer D, Berfield AK, St. John PL, Abrahamson DR. Diabetes induces changes in glomerular development and laminin b2 (s-laminin) expression. Am J Pathol 151: 1131–1140, 1997 [PMC free article] [PubMed] [Google Scholar]
- 3.Abrass CK, Spicer D, Raugi GJ. Insulin induces a change in extracellular matrix glycoproteins synthesized by rat mesangial cells in culture. Kidney Int 46: 613–620, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Abrass CK, Spicer D, Raugi GJ. Induction of nodular sclerosis by insulin in rat mesangial cells in vitro: studies of collagen. Kidney Int 47: 25–37, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Bell L, Madri JA. Influence of the angiotensin system on endothelial and smooth muscle cell migration. Am J Pathol 137: 7–12, 1990 [PMC free article] [PubMed] [Google Scholar]
- 6.Berfield AK, Andress DL, Abrass CK. IGFBP-5201–218 stimulates Cdc42 aggregation and filopodia formation in migrating mesangial cells. Kidney Int 57: 1991–2003, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Berfield AK, Hansen KM, Abrass CK. Rat glomerular mesangial cells require laminin-9 to migrate in response to insulin-like growth factor binding protein-5. Am J Physiol Cell Physiol 291: C589–C599, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Berfield AK, Spicer D, Abrass CK. Insulin-like growth factor I (IGF-I) induces unique effects in the cytoskeleton of cultured rat glomerular mesangial cells. J Histochem Cytochem 45: 583–593, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Choi HJ, Lee BH, Kang JH, Jeong HJ, Moon KC, Ha IS, Yu YS, Matejas V, Zenker M, Choi Y, Cheong HI. Variable phenotype of Pierson syndrome. Pediatr Nephrol 23: 995–1000, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Genolet R, Araud T, Maillard L, Jaquier-Gubler P, Curran J. An approach to analyse the specific impact of rapamycin on mRNA-ribosome association. BMC Med Genomics 1: 1–33, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Herrera IG, Prado-Lourenco L, Teshima-Kondo S, Kondo K, Cabon F, Arnal JF, Bayard F, Prats AC. IRES-dependent regulation of FGF-2 mRNA translation in pathophysiological conditions in the mouse. Biochem Soc Trans 34: 17–21, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, McManus MT, Benzing T, Miner JH. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol 19: 2150–2158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennessy E, O'Driscoll L. Molecular medicine of microRNAs: structure, function and implications for diabetes. Expert Rev Mol Med 10: 1–19, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Ho J, Ng KH, Rosen S, Dostal A, Gregory RI, Kreidberg JA. Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. J Am Soc Nephrol 19: 2069–2075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hugo C, Shankland SJ, Bowen-Pope DF, Couser WG, Johnson RJ. Extraglomerular origin of the mesangial cell after injury. J Clin Invest 223: 72–82, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities in Lamb2−/− mice, implicating glomerular basement membrane as an albumin barrier. J Clin Invest 116: 2272–2279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci USA 104: 3432–3437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreisberg JI, Karnovsky MJ. Glomerular cells in culture. Kidney Int 23: 439–447, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 292: F617–F627, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Mariappan MM, Feliers D, Mummidi S, Choudhury GG, Kasinath BS. High glucose, high insulin, and their combination rapidly induce laminin-b1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes 56: 476–485, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Mariappan MM, Lee MJ, Feliers D, Choudhury GG, Barnes JL, Kasinath BS. Regulation of elongation phase of mRNA translation in diabetic nephropathy: amelioration by rapamycin. Am J Pathol 171: 1733–1742, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariappan MM, Shetty M, Sataranatarajan K, Choudhury GG, Kasinath BS. Glycogen synthase kinase 3b is a novel regulator of high glucose- and high insulin-induced extracellular matrix protein synthesis in renal proximal tubular epithelial cells. J Biol Chem 283: 30566–30575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsell DG, Delhanty PJD, Stepaniuk O, Goodyer C, Han VKM. Expression of insulin-like growth factor and binding protein genes during nephrogenesis. Kidney Int 46: 1031–1042, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Miner JH. Building the glomerulus: a matricentric view. J Am Soc Nephrol 16: 857–861, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Nagai K, Matsubara T, Mima A, Sumi E, Kanamori H, Iehara N, Fukatsu A, Yanagita M, Nakano T, Ishimoto Y, Kita T, Doi T, Arai H. Gas6 induces Akt/mTOR-mediated mesangial hypertrophy in diabetic nephropathy. Kidney Int 68: 552–561, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin b2: nephrosis despite molecular compensation by laminin b1. Nat Gen 10: 400–406, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Park IS, Kiyomoto H, Alvarez F, Xu YC, Abboud HE, Abboud SL. Preferential expression of insulin-like growth factor binding proteins-1, -3, -5 during early diabetic renal hypertrophy in rats. Am J Kidney Dis 32: 1000–1010, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Petz M, Kozina D, Huber H, Siwiec T, Seipelt J, Sommergruber W, Mikulits W. The leader region of Laminin B1 mRNA confers cap-independent translation. Nucleic Acids Res 35: 2473–2482, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips SL, DeRubertis FR, Craven PA. Regulation of the laminin C1 promoter in cultured mesangial cells. Diabetes 48: 2083–2089, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Roche E, Assimacopoulos-Jeannet F, Witters LA, Perruchoud B, Yaney G, Corkey B, Asfari M, Prentki M. Induction by glucose on genes coding for glycolytic enzymes in a pancreatic beta-cell line (INS-1). J Biol Chem 272: 3091–3098, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Sampath P, Pritchard DK, Pabon L, Reinecke H, Schwartz SM, Morris DR, Murry CE. A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell 2: 448–460, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Shi S, Yu L, Chiu C, Sun Y, Chen J, Khitrov G, Merkenschlager M, Holzman LB, Zhang W, Mundel P, Bottinger EP. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J Am Soc Nephrol 19: 2159–2169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136: 731–745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stahl RA, Thaiss F, Wenzel U, Helmchen U. Morphologic and functional consequences of immune-mediated mesangiolysis: development of chronic glomerular sclerosis. J Am Soc Nephrol 2: S144–S148, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Stout LC, Kumar S, Whorton EB. Focal mesangiolysis and the pathogenesis of the Kimmelstiel-Wilson nodule. Hum Pathol 24: 77–89, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Teshima-Kondo S, Kondo K, Prado-Lourenco L, Gonzalez-Herrera IG, Rokutan K, Bayard F, Arnal JF, Prats AC. Hyperglycemia upregulates translation of the fibroblast growth factor 2 mRNA in mouse aorta via internal ribosome entry site. FASEB J 18: 1583–1585, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Thrailkill KM, Bunn CR, Fowlkes J. Matrix metalloproteinases: their role in the pathogenesis of diabetic nephropathy. Endocrine 35: 1–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q, Wang Y, Minto AW, Wang J, Shi Q, Li X, Quigg RJ. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J 22: 4126–4135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wewer UM, Iba K, Durkin ME, Nielsen FC, Loechel F, Gilpin BJ, Kuang W, Engvall E, Albrechtsen R. Tetranectin is a novel marker for myogenesis during embryonic development, muscle regeneration, and muscle cell differentiation in vitro. Dev Biol 200: 247–259, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Yoshida F, Isobe K, Matsuo S. In vivo effects of hyperglycemia on the outcome of acute mesangial injury in rats. J Lab Clin Med 125: 46–55, 1995 [PubMed] [Google Scholar]
- 41.Zdychova J, Vesela J, Kazdova L, Komers R. Renal activity of Akt kinase in experimental type 1 diabetes. Physiol Res 57: 709–715, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wühl E, Cochat P, Bouvier R, Kraus C, Mark K, Maldon H, Dötsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin b2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13: 2625–2632, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Zhou Z, Hurst RD, Templeton D, Whiteside CI. High glucose alters actin assembly in glomerular mesangial and epithelial cells. Lab Invest 73: 372–383, 1995 [PubMed] [Google Scholar]