Abstract
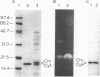
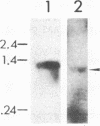
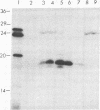
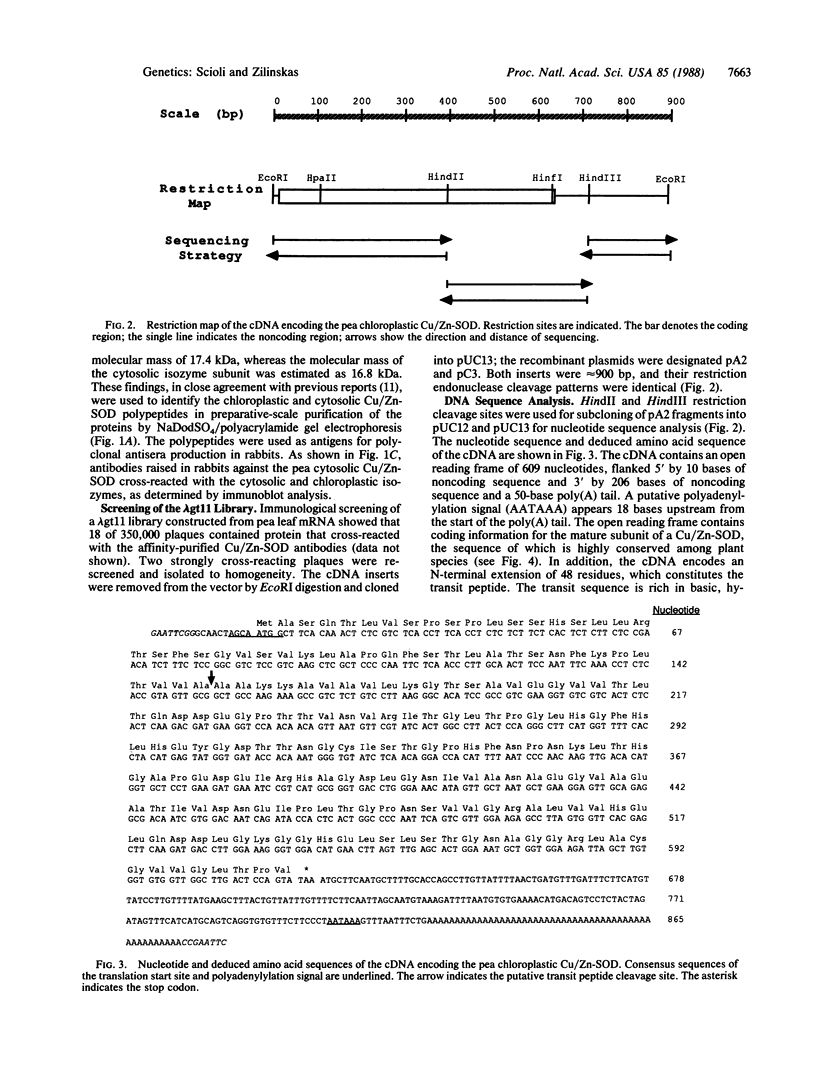
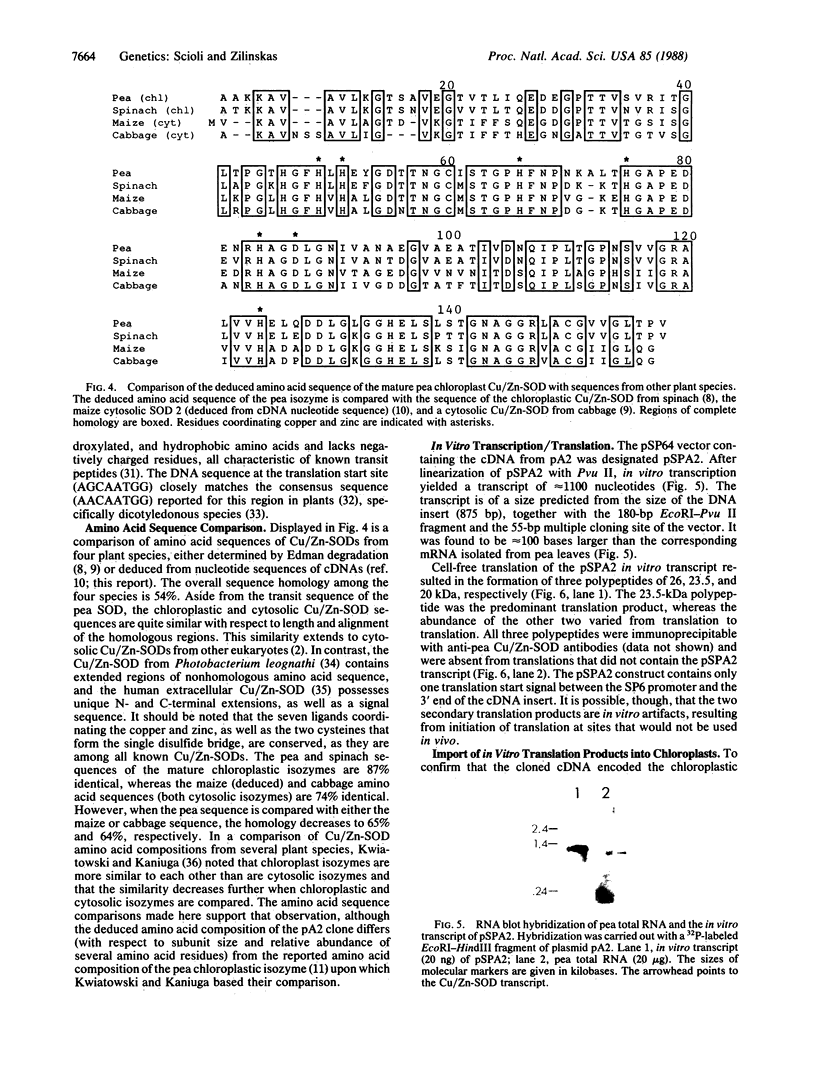
A cDNA encoding the chloroplastic copper/zinc-superoxide dismutase of pea (Pisum sativum L.) was isolated from a cDNA library constructed in lambda gt11 from leaf mRNA. Nucleotide sequence analysis of the 875-base-pair clone revealed that it contained the complete coding sequence of the mature superoxide dismutase isozyme subunit, along with sequence information for a 48-amino acid N-terminal transit peptide. The deduced amino acid sequence of the mature subunit proved to be 64-87% homologous with amino acid sequences of copper/zinc-superoxide dismutases from other plant species. In vitro transcription, followed by cell-free translation, of the cDNA resulted in the formation of a 23.5-kDa precursor polypeptide, which, upon incubation with isolated pea chloroplasts, was imported and processed to its mature subunit molecular mass of 17.4 kDa.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autor A. P. Biosynthesis of mitochondrial manganese superoxide dismutase in saccharomyces cerevisiae. Precursor form of mitochondrial superoxide dismutase made in the cytoplasm. J Biol Chem. 1982 Mar 10;257(5):2713–2718. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Bywater M., Bywater R., Hellman L. A novel chromatographic procedure for purification of bacterial plasmids. Anal Biochem. 1983 Jul 1;132(1):219–224. doi: 10.1016/0003-2697(83)90451-7. [DOI] [PubMed] [Google Scholar]
- Cannon R. E., White J. A., Scandalios J. G. Cloning of cDNA for maize superoxide dismutase 2 (SOD2). Proc Natl Acad Sci U S A. 1987 Jan;84(1):179–183. doi: 10.1073/pnas.84.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt J. S., Key J. L. Isolation of nuclear encoded plastid ribosomal protein cDNAs. Mol Gen Genet. 1986 Feb;202(2):186–193. doi: 10.1007/BF00331635. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C., Dench J., Moore A. L., Halliwell B., Foyer C. H., Hall D. O. Subcellular localisation and identification of superoxide dismutase in the leaves of higher plants. Eur J Biochem. 1978 Nov 15;91(2):339–344. doi: 10.1111/j.1432-1033.1978.tb12685.x. [DOI] [PubMed] [Google Scholar]
- Kitagawa Y., Tsunasawa S., Tanaka N., Katsube Y., Sakiyama F., Asada K. Amino acid sequence of copper,zinc-superoxide dismutase from spinach leaves. J Biochem. 1986 May;99(5):1289–1298. doi: 10.1093/oxfordjournals.jbchem.a135596. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marres C. A., Van Loon A. P., Oudshoorn P., Van Steeg H., Grivell L. A., Slater E. C. Nucleotide sequence analysis of the nuclear gene coding for manganese superoxide dismutase of yeast mitochondria, a gene previously assumed to code for the Rieske iron-sulphur protein. Eur J Biochem. 1985 Feb 15;147(1):153–161. doi: 10.1111/j.1432-1033.1985.tb08731.x. [DOI] [PubMed] [Google Scholar]
- Orozco E. M., Jr, Mullet J. E., Hanley-Bowdoin L., Chua N. H. In vitro transcription of chloroplast protein genes. Methods Enzymol. 1986;118:232–253. doi: 10.1016/0076-6879(86)18076-1. [DOI] [PubMed] [Google Scholar]
- Pain D., Blobel G. Protein import into chloroplasts requires a chloroplast ATPase. Proc Natl Acad Sci U S A. 1987 May;84(10):3288–3292. doi: 10.1073/pnas.84.10.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., Bridges S. M. Absence of the iron-containing superoxide dismutase in mitochondria from mustard (Brassica campestris). Biochem J. 1981 Apr 1;195(1):229–233. doi: 10.1042/bj1950229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. The transport of proteins into chloroplasts. Annu Rev Biochem. 1986;55:879–912. doi: 10.1146/annurev.bi.55.070186.004311. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens G. J., Bannister J. V., Bannister W. H., Flohé L., Günzler W. A., Kim S. M., Otting F. The primary structure of Cu-Zn superoxide dismutase from Photobacterium leiognathi: evidence for a separate evolution of Cu-Zn superoxide dismutase in bacteria. Hoppe Seylers Z Physiol Chem. 1983 Jun;364(6):675–690. doi: 10.1515/bchm2.1983.364.1.675. [DOI] [PubMed] [Google Scholar]
- Steffens G. J., Michelson A. M., Otting F., Puget K., Strassburger W., Flohé L. Primary structure of Cu-Zn superoxide dismutase of Brassica oleracea proves homology with corresponding enzymes of animals, fungi and prokaryotes. Biol Chem Hoppe Seyler. 1986 Oct;367(10):1007–1016. doi: 10.1515/bchm3.1986.367.2.1007. [DOI] [PubMed] [Google Scholar]
- White J. A., Scandalios J. G. In vitro synthesis, importation and processing of Mn-superoxide dismutase (SOD-3) into maize mitochondria. Biochim Biophys Acta. 1987 Oct 8;926(1):16–25. doi: 10.1016/0304-4165(87)90178-4. [DOI] [PubMed] [Google Scholar]