Abstract
Endurance training has been associated with increased orthostatic intolerance. The purpose of the present study was to test the hypothesis that endurance training reduces renal vasoconstriction to orthostatic stress. Blood pressure, heart rate, and renal blood flow velocity were measured during a 25-min 60° head-up tilt (HUT) test before and after 8 wk of endurance training in eight healthy sedentary subjects (26 ± 1 yrs). Training elicited a 21 ± 3% increase in peak oxygen uptake (V̇o2peak) and a reduction in heart rate at rest of 8 ± 2 beats/min. During HUT, heart rate progressively increased (∼20 beats/min) over the 25-min HUT trial both before and after training. Systolic arterial blood pressure during HUT was unchanged with training, whereas diastolic arterial blood pressure was lower at the end of HUT after training. Before training renal blood flow velocity (Δ14 ± 5 cm/s) and renal vascular conductance (Δ22 ± 7%) decreased during HUT, whereas after training renal blood flow velocity (Δ2 ± 5 cm/s) and renal vascular conductance (Δ1 ± 12%) did not change significantly during HUT. Renal blood flow velocity and vascular conductance responses to HUT did not change in control subjects during the 8-wk period. These results demonstrate that endurance training reduces renal vasoconstriction during an orthostatic challenge and may contribute to training-induced orthostatic intolerance.
Keywords: renal vascular conductance, head-up tilt
cardiac output declines ∼20% when humans assume an upright posture. Therefore, increases in peripheral resistance must occur to maintain blood pressure during standing. The renal vasculature is one of the most important vascular beds for increasing total peripheral resistance upon standing (17). Renal vascular resistance has been shown to increase ∼30% during head-up tilt (HUT) (8, 15). Another important role of the renal vasculature is observed during exercise, when renal and splanchnic vasoconstriction counteracts the vasodilation of the skeletal muscle to minimize a decrease in blood pressure (16).
Some previous cross-sectional studies suggest that highly trained endurance athletes appear to be more susceptible to orthostatic intolerance than healthy sedentary individuals. It has been hypothesized that this increased susceptibility to orthostatic intolerance could be caused by either increased aerobic capacity or a hereditary predisposition for both increased aerobic capacity and orthostatic intolerance. Although there is evidence to support the hypothesis that increased aerobic capacity is associated with orthostatic intolerance, a mechanism for this association is equivocal (4).
It has been established that trained humans and animals exhibit less vasoconstriction in both splanchnic and renal vasculature in response to the same acute dynamic exercise modality with which they were trained (5–7, 12, 17, 21). However, the response of human renal vasculature to HUT before and after training has not been examined. The aim of this study was to determine the effect of endurance training on renal vascular responses to orthostatic stress. It was hypothesized that renal vasoconstrictor responses to HUT would be attenuated after endurance training. This reduced renal vasoconstriction may contribute to orthostatic hypotension in endurance-trained athletes.
METHODS
Subjects.
Sixteen healthy, sedentary young volunteers (6 men and 10 women) participated in the study. Participants had a mean ± SE age of 26 ± 1 yr, height of 169 ± 3 cm, and weight of 68 ± 5 kg. All subjects were nonsmokers, nonobese, normotensive, not participating in any regular physical activity program, and not taking any medications that would influence the results of the study. Women taking oral contraceptives participated in the study, and all women were tested on or close to the same day during their menstrual cycle throughout the study; however, phase of menstrual cycle was not controlled for between subjects. Additionally, all subjects were fasted for at least 8 h before the experimental protocol. All subjects received a physical examination before participation. Written informed consent was obtained from all subjects after verbal explanation of the experimental protocol. The Institutional Review Board of the Pennsylvania State University College of Medicine approved this study.
Experimental protocol.
Training subjects (7 women and 1 man) were randomly assigned to 8 wk of either running or stationary cycling training. Endurance training consisted of running or biking 4 times/wk for 8 wk. Training sessions were 1 h. Subjects wore a Polar heart rate monitor (Polar S610; Polar Electro; New York, NY) during all exercising sessions to ensure that target heart rates were maintained. Training began by exercising for 20 min at a work rate sufficient to achieve 80% of subjects‘ maximum heart rate. Exercise duration increased to 60 min as training progressed. As training progressed, subjects ran faster or increased bike power output to maintain heart rate at 80% of maximum. In addition, subjects performed interval exercises after the first week of training (Table 1). Intervals consisted of increasing work rates to obtain subjects’ maximum heart rate throughout a given exercise period, with a recovery period between each high-intensity exercise bout. The running and stationary cycling training schedule for the subjects is presented in Table 1. Control subjects (3 women and 5 men) were told to maintain their current lifestyle throughout the study.
Table 1.
Exercise training programs for running and stationary cycling
| Week | Day 1 | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|
| Running | ||||
| 1 | 20-min Run | 20-min Run | 20-min Run | 20-min Run |
| 2 | 25-min Run | 25-min Run | 25-min Run | Hill intervals (3×) |
| 3 | 35-min Run | Hill intervals (4×) | 35-min Run | Sprint intervals (4×) |
| 4 | 45-min Run | Hill intervals (5×) | 45-min Run | Sprint intervals (5×) |
| 5 | 55-min Run | Hill intervals (5×) | 55-min Run | Sprint intervals (6×) |
| 6 | 60-min Run | Hill intervals (5×) | 60-min Run | Sprint intervals (6×) |
| 7 | 60-min Run | Hill intervals (6×) | 60-min Run | Sprint intervals (6×) |
| 8 | 60-min Run | Hill intervals (7×) | 60-min Run | Sprint intervals (6×) |
| Stationary cycling | ||||
| 1 | 20-min Bike | 20-min Bike | 20-min Bike | 20-min Bike |
| 2 | 25-min Bike | 25-min Bike | 25-min Bike | Intervals (3×) |
| 3 | 35-min Bike | Intervals (4×) | 35-min Bike | Intervals (4×) |
| 4 | 45-min Bike | Intervals (5×) | 45-min Bike | Intervals (5×) |
| 5 | 55-min Bike | Intervals (6×) | 55-min Bike | Intervals (6×) |
| 6 | 60-min Bike | Intervals (6×) | 60-min Bike | Intervals (6×) |
| 7 | 60-min Bike | Intervals (6×) | 60-min Bike | Intervals (6×) |
| 8 | 60-min Bike | Intervals (6×) | 60-min Bike | Intervals (6×) |
Sprint interval, = 30 s sprint, 1-min recovery, 1-min sprint, 2-min recovery performed on flat course; hill interval = uphill sprint and downhill recovery on a 0.1-mi course at 6% grade (weeks 2–5) or 10% grade (weeks 6–8); bike interval = increased power output for 30 s, 1-min recovery, 1-min increased power output, 2-min recovery.
V̇o2peak protocol.
Peak oxygen uptake (V̇o2peak) was determined by maximal graded exercise test on a cycle ergometer (Lode; workload increased 30 W every minute to fatigue). V̇o2 was measured continuously during the graded exercise test (True One 2400, Parvo Medics). V̇o2peak was accepted as the highest value obtained during the last 30 s of the graded exercise test. All experimental procedures were replicated for each subject at the end of 8 wk.
Head-up tilt test.
Before and after 8 wk of endurance training, subjects performed a 25-min 60° HUT test on a separate day from V̇o2peak testing. Renal blood flow velocity (RBFV) was determined during supine rest (baseline) and throughout the HUT test.
Subjects lay supine on a tilt table and were instrumented for the study. Subjects then rested in a dimly lit, quiet laboratory maintained at 21–23°C for 20 min. Baseline measurements (heart rate, arterial blood pressure, and RBFV) were made for 5 min, followed by a 25-min passive HUT and a 5-min recovery period in the supine position. The tilt test was terminated if the subject completed 25 min of HUT; if the subject began to feel presyncopal symptoms including lightheadedness, nausea, vomiting, excessive heat, sweating; if the subject‘s systolic blood pressure decreased >25 mmHg or to <70 mmHg; or if the subject’s diastolic blood pressure decreased >15 mmHg. Subjects who did not complete 25 min of HUT before training were excluded from this study.
Renal vascular responsiveness.
Renal vascular responsiveness to α-adrenergic stimulation was determined in five trained and four control subjects. RBFV was measured in the supine position by Doppler ultrasound before and after steady-state intravenous infusions of phenylephrine. Baseline measurements were taken for 5 min. Phenylephrine infusion commenced at a rate of 0.5 μg·kg−1·min−1 for 5 min and was increased by 0.5 μg·kg−1·min−1 increments every 5 min. Phenylephrine infusions were stopped if blood pressure increased >30 mmHg from baseline or if heart rate was <40 beats/min.
Measurements.
Resting heart rate and brachial arterial blood pressure were collected after 20 min of rest in the supine position in a dimly lit room (Dinamap, General Electric, Waukesha, WI).
Doppler ultrasound (HDI 5000, ATL Ultrasound, Bothell, WA) was used to measure RBFV. All arteries were scanned by the anterior abdominal approach with a curved-array transducer (2–5 MHz) with a 2.5-MHz pulsed Doppler frequency. The probe insonation angle to the artery was ≤60°. The focal zone was set at the depth of the artery. The transducer was held in the same place to record velocity tracings during each trial, and the data were obtained in the same phase of the respiratory cycle. Continuous measurements of RBFV were taken during baseline and during HUT at minutes 1–5, 9–10, 14–15, 19–20, and 24–25.
Continuous measurements of blood pressure and heart rate were made by finger plethysmography with a Finometer blood pressure monitor (Finapres Medical Systems).
Data analysis.
Blood pressure and heart rate were analyzed off-line with Chart software (ADInstruments, Newcastle, Australia). Doppler ultrasound tracings were analyzed with the software of the ATL to obtain mean blood velocity for each cardiac cycle. Because of technological limitations it is not possible to accurately measure the diameter of the arteries in the present study with the ATL Doppler ultrasound machine; therefore an index of renal vascular conductance (RVC) was calculated by dividing the respective artery blood velocity by mean arterial blood pressure of the given trial. For both trials 5 min of baseline were averaged together and reported as the baseline value for the respective trial. Heart rate, arterial blood pressure, and RBFV for each minute of HUT were averaged and reported. At least six RBFV tracings were used to calculate the average for RBFV per minute.
The data were analyzed with a two-within [training (before and after) × tilt time] repeated-measures analysis of variance for each group (control vs. training). Significance was set at P < 0.05. All data are presented as means ± SE.
RESULTS
V̇o2peak (ml·kg−1·min−1) was increased by 21 ± 3% after 8 wk of endurance training, with no significant change in body weight (Table 2). V̇o2peak did not change in the control subjects (Table 2). Resting heart rates were significantly decreased in trained subjects compared with control subjects after 8 wk (Δ8 ± 2 and Δ3 ± 2 beats/min, respectively; Table 2). Resting brachial blood pressure did not significantly change in either the trained or the control subjects (Table 2).
Table 2.
Baseline measurements in trained and control groups
| Trained (n = 8) |
Control (n = 8) |
|||
|---|---|---|---|---|
| Variable | Before | After | Before | After |
| V̇o2peak, ml · kg−1 · min−1 | 32 ± 1 | 39 ± 1* | 31 ± 2 | 31 ± 2† |
| Weight, kg | 63 ± 5 | 62 ± 5 | 73 ± 8 | 73 ± 8 |
| Systolic pressure, mmHg | 109 ± 3 | 104 ± 2 | 114 ± 3 | 109 ± 2 |
| Diastolic pressure, mmHg | 63 ± 2 | 60 ± 2 | 64 ± 2 | 62 ± 2 |
| MAP, mmHg | 78 ± 2 | 75 ± 2 | 80 ± 2 | 78 ± 2 |
| Heart rate, beats/min | 60 ± 3 | 52 ± 2* | 62 ± 3 | 59 ± 4† |
| RVC, cm · s−1 · mmHg−1 | 0.81 ± 0.09 | 0.68 ± 0.05* | 0.78 ± 0.08 | 0.78 ± 0.08 |
Values are means ± SE. V̇o2peak, peak oxygen uptake; MAP, mean arterial blood pressure; RVC, renal vascular conductance.
Significantly different from before training,
significantly different from trained (P < 0.05).
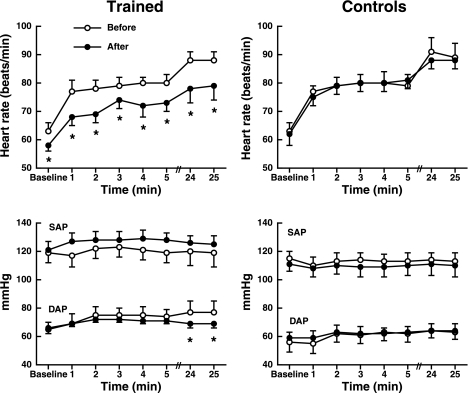
Heart rate significantly increased with HUT before and after training (Fig. 1), and heart rate responses to HUT were not altered with training. However, heart rate was significantly higher at baseline and during HUT before training compared with after training. Heart rate significantly increased during HUT in control subjects before and after 8 wk, but heart rate responses were not different between the two visits. Systolic arterial blood pressure was not significantly affected by training (train × tilt; P = 0.87; Fig. 1). However, diastolic arterial blood pressure was significantly lower during the end of HUT after training (77 ± 8 and 69 ± 3mmHg for before and after training, respectively, for minutes 24 and 25, P < 0.001; Fig. 1). Arterial blood pressure was not changed in the control group during the 8-wk period (Fig. 1).
Fig. 1.
Heart rate (top) and arterial blood pressure (bottom) differences before and after 8 wk in both trained and control groups during head-up tilt. Heart rate increases significantly from baseline during head-up tilt in both groups. However, heart rate responses were attenuated after training. Values are means ± SE. *Significantly different from before. SAP, systolic arterial pressure; DAP, diastolic arterial pressure.
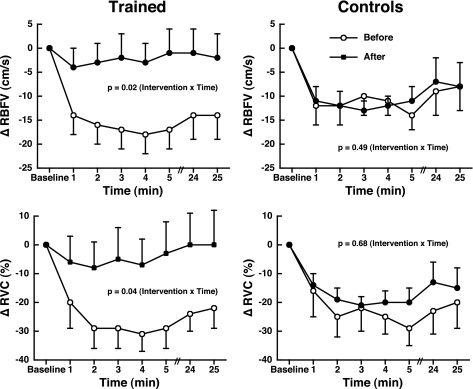
RBFV and RVC decreased during HUT before training (Δ −14 ± 5 cm/s and Δ −22 ± 7%, respectively) but did not change in response to HUT after training (Δ −2 ± 5 cm/s and Δ −1 ± 12%, respectively; Fig. 2). The decrease in RBFV and RVC in control subjects during HUT was not different before and after 8 wk (Fig. 2).
Fig. 2.
Renal blood flow velocity (RBFV; top) and renal vascular conductance (RVC; bottom) differences before and after 8 wk in both trained and control groups during head-up tilt. Values are means ± SE.
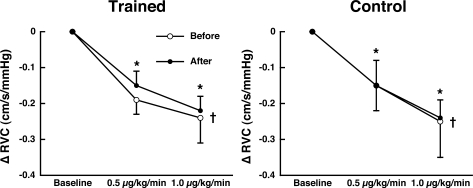
Only data from phenylephrine infusions at 0.5 and 1.0 μg·kg−1·min−1 are reported because most subjects did not tolerate higher infusion rates. Mean arterial pressure at rest in the trained (75 ± 5 and 74 ± 8 mmHg) and control (86 ± 5 and 82 ± 3 mmHg) groups were not significantly different before and after training, respectively. During phenylephrine infusion blood pressure increased as a function of dose for both control and training groups, but there were no differences in blood pressure response to phenylephrine before and after training (P = 0.36 and P = 0.44, respectively). RVC decreased as a function of dose during phenylephrine infusions in all subjects, but the response was not different before and after training for both the trained and control subjects (Fig. 3).
Fig. 3.
RVC to infusions of phenylephrine at 0.5 and 1.0 μg·kg−1·min−1. RVC was decreased as a function of drug dose. More importantly, RVC responses were not different before and after 8 wk in both trained (n = 5) and control (n = 4) groups. Values are means ± SE. *Significantly different from baseline (P < 0.05). †There was a linear dose response to phenylephrine (P = 0.0007 for trained and P = 0.009 for control groups).
DISCUSSION
The major finding of this study is that endurance training elicits a blunted vasoconstriction of the renal artery during HUT. This is the first study to report a significant attenuation in renal vasoconstriction to HUT in humans in response to endurance training. This insight into the effects of endurance training on the renal vasculature expands our understanding of the effects of exercise training on humans. Moreover, it provides a possible explanation why some endurance athletes have been reported to be more orthostatically intolerant than sedentary subjects (4).
These findings are consistent with both animal and human studies that have demonstrated decreased vasoconstriction of the renal vasculature during exercise in trained compared with untrained individuals (5–7, 12, 17, 21). Because both exercise and HUT activate the sympathetic nervous system, it is therefore likely that the same or a similar mechanism is responsible for the observed attenuation of vasoconstriction in both conditions. There are two plausible mechanisms for the effects of exercise training on renal blood flow during HUT. Less renal vasoconstriction in the trained state could be a result of decreased sympathetic outflow to the renal vasculature, or a decreased vascular response to the plasma norepinephrine (NE) concentration. Evidence that supports the former mechanism demonstrates lower plasma NE levels at the same exercise intensity after training (23). Meredith et al. (13) demonstrated that endurance training selectively lowered resting renal NE spillover, suggesting that the renal sympathetic nerves had reduced firing rates. This reduction in nerve activation supports our finding of decreased vasoconstriction in trained subjects. If the decrease in renal vasoconstriction is due to decreased vascular responsiveness to NE, it may be a result of training-induced decreases in α-adrenergic receptors or decreased intracellular signaling (5–7, 12, 17, 21). However, there was a comparable decrease in RVC to phenylephrine infusions before and after training, suggesting that renal vascular responsiveness to α-adrenergic receptor stimulation is not altered with training. It is possible that vascular responses to other substances may be altered with training (e.g., angiotensin II). Additionally, the increase in arterial blood pressure elicited by phenylephrine infusion could mediate a myogenic vascular response. The myogenic response to pressure changes could confound our interpretation. However, because blood pressure responses were comparable to phenylephrine infusion before and after training in both the control and training groups this would suggest that this mechanism is not likely to have influenced our results and interpretation with respect to exercise training.
Wallin et al. (22) demonstrated that there was a significant positive correlation between muscle sympathetic nerve activity (MSNA) and renal NE spillover in humans. This finding indicates that in healthy humans the resting renal sympathetic nerve activity (RSNA) is similar or proportional to MSNA. With this association between MSNA and RSNA established, the training-induced MSNA reduction during leg exercise training suggests that there is a concurrent reduction in RSNA during training (14). Additionally, other studies have found that training is associated with an attenuation of exercise-induced increases of MSNA (14, 19, 20). Therefore, the lack of renal vasoconstriction that we observed in the present study is likely a result of training-induced RSNA reductions.
DiCarlo and Bishop (1) demonstrated that 8 wk of endurance training in rabbits significantly attenuates arterial baroreflex control of RSNA. In a follow-up study they concluded that training enhances the inhibitory influence of cardiac afferents on the arterial baroreflex regulation of RSNA (2). Although their results were observed in resting animals, our present study in humans demonstrates a similar effect during HUT. Therefore, an attenuation of renal vasoconstriction during HUT may be related to changes in the sensitivity of cardiac afferents following training.
Another possible mechanism for the decrease in vasoconstriction is activation of the vestibulosympathetic reflex (VSR) with training. We have demonstrated (18) that engagement of the VSR can elicit renal vasoconstriction. If the VSR is attenuated after endurance training, this would cause less renal vasoconstriction.
Training reduced both heart rate and diastolic arterial pressure during HUT. One potential explanation for this lower blood pressure is that the decrease in renal vasoconstriction results in a decrease in total peripheral resistance at a time when cardiac output is lower, resulting in a lower blood pressure.
Our results are clinically relevant because the association we demonstrated between RVC during HUT and exercise training may help to further our understanding of orthostatic intolerance. Levine et al. (9) have provided evidence that endurance athletes may have a diminished tolerance to orthostatic stress compared with moderately fit or sedentary subjects. They suggest that maximal aerobic capacity is a poor predictor for orthostatic intolerance because the association does not seem to fit a simple, linear pattern. There are potentially multiple factors that contribute to cardiovascular regulation during orthostasis. Fu et al. (3) have expanded on this concept by suggesting that humans have an individual, intrinsic, and limited sympathetically mediated vasoconstrictor reserve that could affect the maintenance of orthostatic tolerance. Furthermore, they suggest that physical fitness may be one important factor underlying individual differences in vasoconstrictor reserve. Our study demonstrates that endurance training causes attenuated renal vasoconstriction; this is one factor that may contribute to endurance training-associated orthostatic intolerance.
In the present study Doppler ultrasound could not measure renal artery diameter. Thus we are unable to be certain that HUT did not elicit changes in renal diameter. There is some evidence to suggest that pharmacologicallly mediated renal vasoconstriction (11) and vasodilation (10) do not alter diameter of the renal artery. Furthermore, the vessel we examined was a conduit and not a resistance vessel. Therefore, it is unlikely that changes in renal artery diameter during HUT influenced the results of the study. However, if differences in renal diameters occur as the result of exercise training this could affect our results and subsequent interpretation.
In summary, the renal vasoconstrictor response to HUT was attenuated after 8 wk of endurance training in humans. This attenuation of renal vasoconstriction may be one contributing factor for endurance training-induced orthostatic intolerance when it occurs in susceptible subjects.
GRANTS
This study was supported by National Institutes of Health (NIH) Grants DC-006549 and P01-HL-077670 and National Space and Biomedical Research Institute Grant CA-00207. Additional support was provided by a NIH-sponsored General Clinical Research Center with National Center for Research Resources Grants M01-RR-10732 and C06-RR-016499.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We acknowledge Matthew L. Kearney, Damian J. Dyckman, and Nathan T. Kuipers for technical assistance in the study.
REFERENCES
- 1.DiCarlo SE, Bishop VS. Exercise training attenuates baroreflex regulation of nerve activity in rabbits. Am J Physiol Heart Circ Physiol 255: H974–H979, 1988. [DOI] [PubMed] [Google Scholar]
- 2.DiCarlo SE, Bishop VS. Exercise training enhances cardiac afferent inhibition of baroreflex function. Am J Physiol Heart Circ Physiol 258: H212–H220, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation 110: 2931–2937, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Geelen G, Greenleaf JE. Orthostasis: exercise and exercise training. Exerc Sport Sci Rev 21: 201–230, 1993. [PubMed] [Google Scholar]
- 5.Grimby G. Renal clearances at rest and during physical exercise after injection of bacterial pyrogen. J Appl Physiol 20: 137–141, 1965. [DOI] [PubMed] [Google Scholar]
- 6.Hohimer AR, Hales JR, Rowell LB, Smith OA. Regional distribution of blood flow during mild dynamic leg exercise in the baboon. J Appl Physiol 55: 1173–1177, 1983. [DOI] [PubMed] [Google Scholar]
- 7.Kenney WL, Ho CW. Age alters regional distribution of blood flow during moderate-intensity exercise. J Appl Physiol 79: 1112–1119, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Kuipers NT, Sauder CL, Carter JR, Ray CA. Neurovascular responses to mental stress in the supine and upright postures. J Appl Physiol 104: 1129–1136, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine BD, Buckey JC, Fritsch JM, Yancy CW, Jr, Watenpaugh DE, Snell PG, Lane LD, Eckberg DL, Blomqvist CG. Physical fitness and cardiovascular regulation: mechanisms of orthostatic intolerance. J Appl Physiol 70: 112–122, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Manoharan G, Pijls NH, Lameire N, Verhamme K, Heyndrickx GR, Barbato E, Wijns W, Madaric J, Tielbeele X, Bartunek J, De Bruyne B. Assessment of renal flow and flow reserve in humans. J Am Coll Cardiol 47: 620–625, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Marraccini P, Fedele S, Marzilli M, Orsini E, Dukic G, Serasini L, L'Abbate A. Adenosine-induced renal vasoconstriction in man. Cardiovasc Res 32: 949–953, 1996. [PubMed] [Google Scholar]
- 12.McAllister RM. Adaptations in control of blood flow with training: splanchnic and renal blood flows. Med Sci Sports Exerc 30: 375–381, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Meredith IT, Friberg P, Jennings GL, Dewar EM, Fazio VA, Lambert GW, Esler MD. Exercise training lowers resting renal but not cardiac sympathetic activity in humans. Hypertension 18: 575–582, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Ray CA. Sympathetic adaptations to one-legged training. J Appl Physiol 86: 1583–1587, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Ring-Larsen H, Hesse B, Henriksen JH, Christensen NJ. Sympathetic nervous activity and renal and systemic hemodynamics in cirrhosis: plasma norepinephrine concentration, hepatic extraction, and renal release. Hepatology 2: 304–310, 1982. [DOI] [PubMed] [Google Scholar]
- 16.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974. [DOI] [PubMed] [Google Scholar]
- 17.Rowell LB. Human Cardiovascular Control New York: Oxford Univ. Press, 1993. [Google Scholar]
- 18.Sauder CL, Conboy EE, Chin-Sang SA, Ray CA. Otolithic activation on visceral circulation in humans: effect of aging. Am J Physiol Renal Physiol 295: F1166–F1169, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinoway L, Shenberger J, Leaman G, Zelis R, Gray K, Baily R, Leuenberger U. Forearm training attenuates sympathetic responses to prolonged rhythmic forearm exercise. J Appl Physiol 81: 1778–1784, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Somers VK, Leo KC, Shields R, Clary M, Mark AL. Forearm endurance training attenuates sympathetic nerve response to isometric handgrip in normal humans. J Appl Physiol 72: 1039–1043, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Tidgren B, Hjemdahl P, Theodorsson E, Nussberger J. Renal neurohormonal and vascular responses to dynamic exercise in humans. J Appl Physiol 70: 2279–2286, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol 491: 881–887, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winder WW, Hickson RC, Hagberg JM, Ehsani AA, McLane JA. Training-induced changes in hormonal and metabolic responses to submaximal exercise. J Appl Physiol 46: 766–771, 1979. [DOI] [PubMed] [Google Scholar]