Abstract
Renal blood flow (RBF) autoregulation is mediated by at least two mechanisms, the fast acting myogenic response (∼5 s) and slow acting tubuloglomerular feedback (TGF; ∼25 s). Previous studies suggest epithelial Na+ channel (ENaC) family proteins, β-ENaC in particular, mediate myogenic constriction in isolated renal interlobar arteries. However, it is unknown whether β-ENaC-mediated myogenic constriction contributes to RBF autoregulation in vivo. Therefore, the goal of this investigation was to determine whether the myogenic mediated RBF autoregulation is inhibited in a mouse model of reduced β-ENaC (m/m). To address this goal, we evaluated the temporal response of RBF and renal vascular resistance (RVR) to a 2-min step increase in mean arterial pressure (MAP). Pressure-induced changes in RBF and RVR at 0–5, 6–25, and 110–120 s after step increase in MAP were used to assess the contribution of myogenic and TGF mechanisms and steady-state autoregulation, respectively. The rate of the initial increase in RVR, attributed to the myogenic mechanism, was reduced by ∼50% in m/m mice, indicating the speed of the myogenic response was inhibited. Steady-state autoregulation was similar between β-ENaC +/+ and m/m mice. Although the rate of the secondary increase in RVR, attributed to TGF, was similar in β-ENaC +/+ and m/m mice, however, it occurred over a longer period (+10 s), which may have allowed TGF to compensate for a loss in myogenic autoregulation. Our findings suggest β-ENaC is an important mediator of renal myogenic constriction-mediated RBF autoregulation in vivo.
Keywords: epithelial Na+ channel, ion channel, degenerin, renal blood flow autoregulation, renal injury, myogenic constriction
autoregulation of blood flow describes the function of a vascular bed to maintain a constant flow despite variations of the level of arterial pressure by regulating vascular resistance. In the kidney, at least two mechanisms promote autoregulatory adjustments to vascular resistance, a fast acting myogenic constriction and a slow acting tubuloglomerular feedback (TGF) (21, 22). In addition to a role in renal blood flow (RBF) autoregulation, the myogenic mechanism may also play a protective role against pressure-related injury. Despite the physiological importance of the myogenic response, the molecular identity of the elements transducing vascular stretch into a cellular event remains unclear.
Several candidates have been considered as transducers of vascular stretch into intracellular signaling including integrins, transient receptor potential channels, and epithelial Na+ channel (ENaC) proteins (3, 14, 25). ENaC proteins are candidates because they are related to a family of mechanosensitive proteins thought to form the ion channel pore of a mechanosensor modeled in the nematode, Caenorhabditis elegans, termed degenerins. Previous studies suggest a specific ENaC protein, β-ENaC, is essential to transduction of myogenic constriction in vitro (10, 11, 27). Transient gene silencing using siRNA or dominant-negative constructs demonstrates silencing of β-ENaC alone is sufficient to abolish myogenic constriction (10) in mouse renal interlobar arteries. Furthermore, myogenic constriction in isolated middle cerebral arteries is abolished in a mouse model with reduced levels of β-ENaC (27). However, equivocal findings on the importance of ENaC in myogenic constriction of preglomerular resistance vessels (6, 28), the major site of renal vascular resistance (RVR) and thus RBF regulation (1), raise the question of whether β-ENaC contributes to whole kidney RBF autoregulation.
Therefore, the goal of the current investigation was to test that hypothesis that loss of β-ENaC leads to loss of myogenic whole kidney autoregulation. To address this goal, we evaluated myogenic RBF autoregulation to a step increase in mean arterial pressure (MAP) in a mouse model with reduced levels of β-ENaC developed by gene-targeting methods. The animal model was originally generated with the intention of creating a Liddle's mouse model by the insertion of a premature stop codon at R566. However, the presence of the selection marker (neomycin) disrupted the β-ENaC gene locus resulting in low levels of β-ENaC expression (23). Mice homozygous for the mutation 1) express low levels of β-ENaC in the lung, kidney, and vascular smooth muscle cells (VSMCs), 2) show delayed lung-liquid clearance, and 3) show reduced colonic ENaC-mediated transport, all findings that suggest reduced ENaC function. The major finding of the current investigation indicates that β-ENaC is required for normal renal myogenic constriction-mediated RBF autoregulation in vivo.
METHODS
Animals
Heterozygous β-ENaC (+/m) mating pairs (generously provided by E. Hummler and B. Rossier, University of Lausanne, Switzerland) were used to generate wild-type (+/+) and homozygous mutant (m/m) animals (23). Animals were fed a standard rodent chow with free access to tap water and were kept on a 12:12-h light-dark cycle. All experiments were conducted at the University of Mississippi Medical Center in accordance with the Guide for the Care and Use of Research Animals and approved by the local Institutional Animal Care and Use Committee. Mice were genotyped as previously described (27). All mice were studied between the ages of 16 and 21 wk of age. Mice in both groups were of similar age (19 ± 2 vs. 19.1 ± 1 wk), body weight (25.1 ± 0.5 vs. 25.7 ± 1.2 g), and left kidney weight (0.16 ± 0.02 vs. 0.13 ± 0.01 g) for β-ENaC +/+ (n = 9) and m/m (n = 7) mice, respectively.
Western Blot Analysis
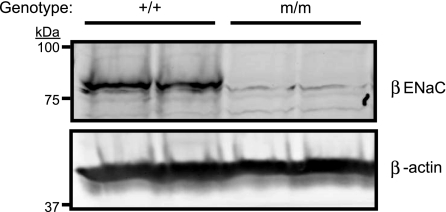
Kidneys were harvested from isoflurane-anesthetized mice and homogenized directly into 2× Lamelli buffer containing DTT. Proteins were separated on 7.5% SDS-PAGE gels where appropriate (Bio-Rad, Hercules, CA) and transferred to nitrocellulose membranes. To confirm knockdown of β-ENaC in m/m mice, membranes were incubated with rabbit anti-β-ENaC antibody (1:1,000) (10, 11, 27), an antibody directed to the COOH-terminal region of β-ENaC. Mouse anti-β-actin antibody (1:5,000; Abcam, Cambridge, MA) was used as a loading control. Following, membranes were incubated with donkey anti-rabbit IR700 (1:2,000; Rockland Immunologicals, Rockland, IL) and donkey anti-mouse IR800 (1:2,000). Antibody binding was visualized using the Odyssey Infrared Imaging System (LiCor, Lincoln, NE). As shown in Fig. 1, a band between 75 and 100 kDa present in the +/+ kidney is absent in the m/m kidney.
Fig. 1.
β-Epithelial Na+ channel (ENaC) PHA-1 mouse model. Western blot detection of β-ENaC in whole kidney lysates from homozygous (m/m) mutant mice and wild-type control (+/+). β-ENaC expression is substantially reduced in m/m when compared with +/+. β-Actin loading control is shown.
RBF Autoregulation
Catheterization.
Mice were maintained under isoflurane anesthesia on a heating pad to maintain body temperature at 37°C for the duration of the study. The depth of anesthesia was monitored by the response to toe pinching. Mice were instrumented with a left carotid artery catheter (Rena pulse 040, Braintree Scientific) to directly monitor MAP. Pressure was measured using fluid-filled catheters attached to a force displacement transducer.
RBF measurements.
The left kidney was approached retroperitoneal through a flank incision. The renal artery and celiac and mesenteric arteries were freed from adjacent tissue. A silk ligature was placed around the celiac and superior mesenteric arteries. RBF was measured using a perivascular flow probe (0.5 PSB, Transonic, Ithaca, NY) positioned on the left renal artery, using a micromanipulator, in anesthetized mice. Twenty minutes were allowed for stabilization after surgery. RBF was measured using an ultrasound transit-time flowmeter (TS420, Transonic, low-pass filter 30–40 Hz) recorded, along with pressure, with a computerized chart recorder (LabChart 6.0, PowerLab, ADInstruments, Colorado Springs, CO). After stability was achieved, RBF was recorded for 10 min. The ligature around the celiac and mesenteric arteries was tightened to achieve a ∼20-mmHg increase in MAP and RBF was recorded for another 10 min. At the conclusion of the experiments, the animals were euthanized by an overdose of isoflurane followed by decapitation. MAP and RBF data were recorded at 1,000 Hz. RBF was normalized to left kidney weight and reported as milliliters per minute per gram of kidney weight.
Data Analysis
RVR was calculated as MAP/RBF and reported as resistance units (RU = mmHg·ml−1·min·g kidney wt). Conductance was calculated as RBF/MAP and reported as percent of baseline (conductance units = ml·min−1·g kidney wt−1·mmHg−1). To quantify renal autoregulation, we continuously recorded MAP and RBF 10 s before and 2 min after a step increase in MAP. Data were extracted at 1-s intervals in Chart and exported to Excel for analysis. The first time point where MAP increased 5% or more above baseline was used to identify the zero time point.
The speed of myogenic and TGF mechanisms was assessed using linear regression. The speed of the myogenic response was assessed by slope of the time RVR and time conductance % baseline relationship at 0–5 s. The slope of the time RVR and time conductance % baseline relationship at 6–25 s assessed the speed of the TGF response. The contribution of the myogenic and TGF mechanisms to the autoregulatory response was determined by the correction of RVR at 4–8 and 20–30 s, respectively, following the pressure step. At these intervals, the rate of change in RVR (first derivative of RVR) approached negative values suggesting a transition between mechanisms and is similar to those used by others (12, 13). The contribution of the myogenic mechanism to total autoregulatory efficiency was calculated as % AR efficiency = [(RVR4–8s − RVR0–1s)/(RVR60–120s − RVR0–1s) × 100]. The contribution of the TGF mechanism to total autoregulatory efficiency was calculated as % AR efficiency = [(RVR20–30s − RVR4–8s)/(RVR60–120s − RVR0–1s) × 100].
Steady-state autoregulation at 2 min was quantified using the autoregulatory index (AI) = [(RBF2 − RBF1)/RBF1]/[(MAP2 − MAP1)/MAP1], where RBF1 and MAP1are the mean values during the 10 s before change in pressure, and RBF2 and MAP2are the average values during 110–120 s after the pressure step. An AI = 0 indicates perfect autoregulation, and AI = 1 indicates that the vessels act as passive conduits for blood flow. An AI < 1 indicates “over” autoregulation.
Statistical Analysis
All data are presented as means ± SE. Independent one-tailed t-tests were used to make comparisons. One-tailed t-tests were applied because the results of the experiments were predicted beforehand. Statistical significance was determined as P < 0.05.
RESULTS
Baseline Hemodynamic Parameters
Baseline MAP, RBF, RVR, and conductance under basal conditions are summarized in the Table 1. Although baseline MAP and RBF are not different, RVR tends to be lower in β-ENaC m/m mice (P = 0.0656) and renal conductance is higher in β-ENaC m/m mice (P = 0.0443).
Table 1.
Baseline hemodynamics in β-ENaC mice
| Group | MAP, mmHg | RBF, ml·min−1·g−1 | RVR | Conductance | n |
|---|---|---|---|---|---|
| +/+ | 77 ± 3 | 5.3 ± 0.4 | 14.8 ± 1.0 | 0.070 ± 0.004 | 9 |
| m/m | 71 ± 3 | 5.9 ± 0.4 | 12.5 ± 0.9 | 0.082 ± 0.006 | 7 |
| P value | 0.1956 | 0.1722 | 0.0655 | 0.0443 |
Data are means ± SE. ENaC, epithelial sodium channel; MAP, mean arterial pressure; RBF, renal blood flow; RVR, renal vascular resistance; n, number of animals. P < 0.05 considered significantly different.
Time Course of MAP, RBF, RVR, and Conductance (% of Baseline) to Step Increase in Pressure
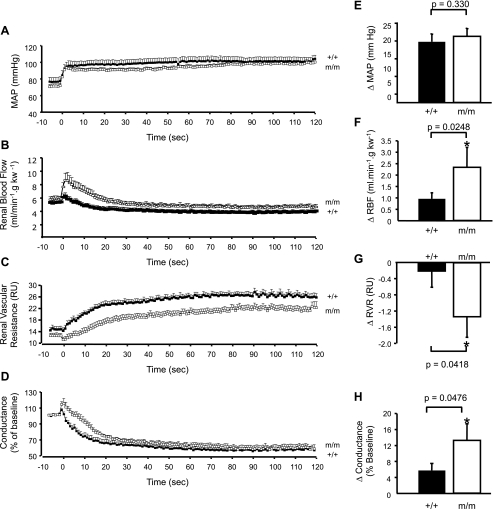
The temporal responses of MAP, RBF, RVR, and conductance (% of baseline) are shown in Fig. 2. MAP increases ∼20 mmHg and remains elevated in +/+ and m/m groups (Fig. 2A). The peak increase in MAP is similar in +/+ and m/m (Fig. 2E; 19 ± 2 vs. 21 ± 3 mmHg, P = 0.3304, respectively). RBF increases transiently with the increase in MAP in both +/+ and m/m mice (Fig. 2B). The peak increase in RBF with the step increase in MAP is nearly 2.5-fold greater in the β-ENaC m/m mice (Fig. 2F; P = 0.0248), but returns to control levels within 5–20 s, eventually dropping below baseline RBF (Fig. 2B). Although RBF returns to baseline levels within approximately ∼6 s in +/+, it requires ∼20 s in m/m mice. RVR falls transiently with the step increase in MAP (Fig. 2C). The peak decrease in RVR is greater in β-ENaC m/m mice (Fig. 2G; P = 0.0418). In +/+ animals, the decrease in RVR is followed by an initial rapid increase within the first 5 s, followed by a secondary increase that begins at ∼5 s and slows down at 20 s. In m/m animals, the distinction between the first and secondary increases in RVR is less obvious and secondary increase slows ∼30 s. In Fig. 2D, the step increase in MAP initially increases conductance (% baseline) to a greater extent in m/m mice (P = 0.0476; Fig. 2H). Following this initial increase, conductance (% baseline) decreases in a manner similar to RVR where conductance (% baseline) decreases rapidly in the first 5 s, followed by a secondary decrease that slows between 20 and 30 s. Despite the similar MAP responses between groups, the RBF, RVR, and conductance (% baseline) responses are largely delayed in m/m mice.
Fig. 2.
Time course of renal blood flow (RBF) correction. Time course of the autoregulatory response of mean arterial pressure (MAP; A), RBF (B), renal vascular resistance (RVR; C), and conductance as % of baseline (D) to a step increase in renal arterial pressure (RAP) in +/+ (■, n = 9) and mutant (m/m; □, n = 7). Peak change in RAP (E), RBF (F), RVR (G), and conductance as % of baseline (H) following step increase in MAP are shown. P values for analysis with t-test are provided for E–H. Data are means ± SE. *Significantly different from +/+ control animals, P < 0.05.
Importance of β-ENaC in Speed of Myogenic and TGF Mechanisms of Whole Kidney Autoregulation
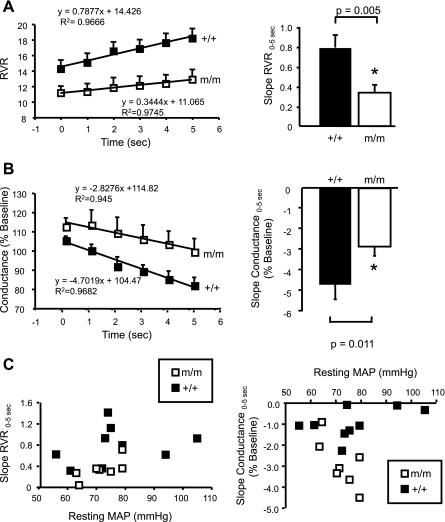
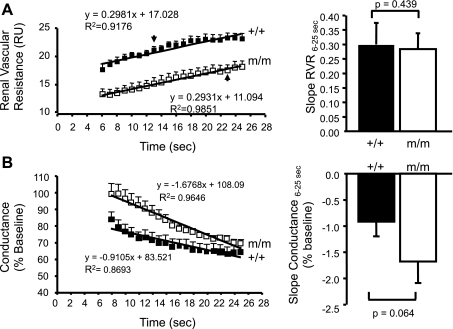
The multiphase RVR response is very similar to previous studies in which the initial autoregulatory response at 0–5 s reflects the speed/strength of the myogenic response and the secondary response between 6 and 25 s estimates TGF (12, 13). To investigate the speed of each mechanism to autoregulation, we calculated the slope of the linear regression lines for RVR and conductance (% baseline) between 0 and 5 s and 6 and 25 s. The speed of the myogenic mechanism is reduced in β-ENaC m/m (RVR0–5s, 0.34 ± 0.07 vs. 0.79 ± 0.13 RU/s, P = 0.005; conductance (% baseline)0–5s −2.83 ± 0.44 vs. −4.70 ± 0.72 RU/s, P = 0.011; Fig. 3, A and B). The dependence of slope of RVR0–5s and conductance (% baseline)0–5s on baseline MAP is shown in Fig. 3C. The relationship between MAP and slope RVR0–5s and conductance (% baseline)0–5s is relatively flat, suggesting that the reduced speed of myogenic correction of RVR following a step increase in MAP is not directly related to a decreased resting MAP in β-ENaC m/m mice. In contrast to the reduced myogenic speed, we found the TGF speed (slope for RVR6–25s, 0.30 ± 0.07 vs. 0.28 ± 0.05 RU/s, P = 0.439; conductance (% baseline)6–25s, −0.91 ± 0.29 vs. −1.68 ± 0.40 RU/s, P = 0.063) was similar between +/+ and m/m mice (Fig. 4, A and B). These data suggest that the speed of the myogenic, but not TGF, mechanism is reduced by more than 50% in β-ENaC m/m mice.
Fig. 3.
Contribution of the myogenic response to RBF autoregulation following a step increase in MAP. The slope of the linear regression RVR (A) and conductance (B) from time 0–5 s was used to determine the speed of myogenic RBF autoregulation in +/+ (■, n = 9) and m/m (□, n = 7). Group data are shown at right. C: scatter plots of baseline MAP vs. slope RVR0–5s and MAP vs. slope conductance0–5s. This graph demonstrates that the reduced RVR0–5s and conductance0–5s slope is not a function of baseline MAP. Data are means ± SE. *Significantly different from +/+ control animals, P < 0.05.
Fig. 4.
Contribution of tubuloglomerular feedback (TGF) to RBF autoregulation following a step increase in RAP. The slope of the linear regression RVR (A) and conductance (B) from time 6–25 s was used to determine the speed of TGF-mediated RBF autoregulation in +/+ (■, n = 9) and m/m (□, n = 7). Group data are shown at right. Data are means ± SE. P values for analysis with t-test are provided.
Importance of β-ENaC in Steady-State Whole Kidney Autoregulation
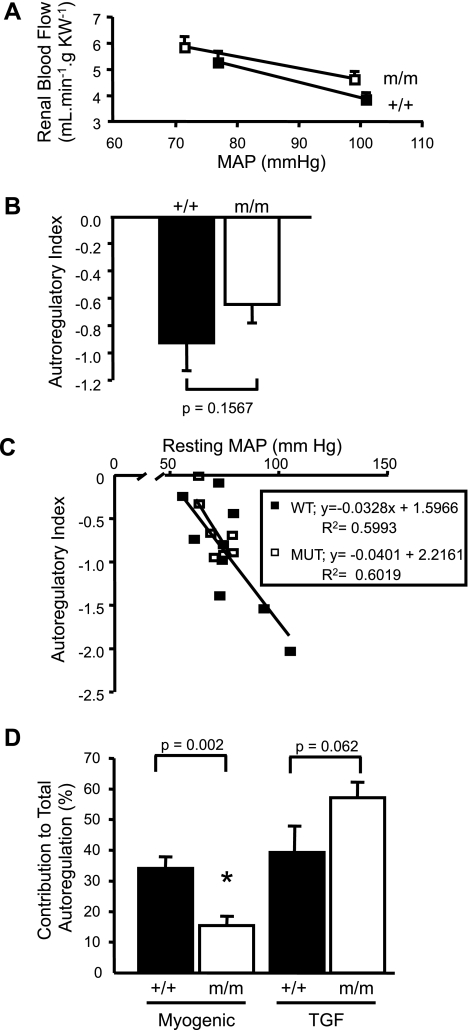
In addition to the dynamic responses, we also evaluated steady-state autoregulation of RBF by comparing MAP-RBF relationship before and 2 min after a step increase in MAP. As shown in Fig. 5A, the shift in the MAP-RBF relationship is similar in β-ENaC +/+ and m/m mice. The AI, an estimate of autoregulatory efficiency, is not different between +/+ and m/m groups (m/m: −0.64 ± 0.13 and +/+: −0.92 ± 0.21, P = 0.1567; Fig. 5B). Since autoregulatory efficiency can be influenced by blood pressure, we evaluated the relationship between resting MAP and AI. Although resting MAP influences AI, the relationship between resting MAP and AI is identical in +/+ and m/m mice. These findings suggest steady-state RBF autoregulation is not different between +/+ and m/m mice.
Fig. 5.
Steady-state RBF autoregulation. A: MAP-RBF relationship before and 2 min after a step increase in MAP in +/+ (■, n = 9) and m/m (□, n = 7). The step increase in pressure was similar between groups (see Fig. 2E). B: autoregulatory index, an indicator of the efficiency of steady-state autoregulation, was not significantly different between groups. C: scatter plot of resting MAP vs. autoregulatory index in β-ENaC mice. Although resting MAP influences the autoregulatory index, there is no difference in this relationship between β-ENaC +/+ and m/m mice. D: contribution of myogenic and TGF mechanisms to autoregulation. The reduced contribution of the myogenic mechanism in β-ENaC m/m mice tends to be compensated by enhanced TGF. Data are means ± SE. P values for analysis with t-test are provided. *Significantly different from +/+ control animals, P < 0.05.
Does TGF Compensate For Loss of Myogenic Mechanism?
Since steady-state autoregulation is similar in +/+ and m/m mice, it is likely that TGF compensates for the reduced myogenic autoregulation to maintain steady-state autoregulation. As mentioned previously, we noted the plateau of the secondary increase in RVR occurred near 20 s in +/+ but was delayed to 30 s in m/m mice, suggesting the mechanism responsible for the secondary increase in RVR took longer in m/m mice. Since TGF speed is not increased in m/m mice, we considered the possibility that TGF might be active for a longer period to allow adjustment of RVR. To quantify this, we calculated the time required for RVR to reach 90% of steady-state RVR (RVR during the last 10 s of the pressure step, RVR110–120s). We selected 90% since previous studies suggest myogenic and TGF mechanisms account for ∼90% of renal autoregulation (12, 13). It took almost twice as long to reach 90% of RVR110–120s (steady-state RVR) in m/m mice (25 ± 4 vs. 42 ± 6 s, P = 0.0204, data not shown in graph format). This finding suggests that although the TGF speed is not different in m/m mice, it may be active for a longer period, permitting RVR adjustment. With this in mind, we quantified the contribution of the myogenic and TGF mechanisms to total autoregulatory adjustments in RVR. As shown in Fig. 5D, the contribution of the myogenic mechanism was inhibited by 54% in m/m mice (33.6 ± 3.7 vs. 15.4 ± 5.1%, P = 0.002). The contribution of the TGF mechanism tended to be increased in m/m mice (38.8 ± 8.6 vs. 57.1 ± 5.1%, P = 0.062). These findings suggest TGF compensates for the reduced myogenic response, perhaps by prolonging the time TGF is active.
DISCUSSION
Importance of β-ENaC in Myogenic Constriction
Recent in vitro studies point to an important role for β-ENaC in the myogenic mechanism. ENaC inhibition, using pharmacological inhibitors and gene-silencing approaches, abolishes myogenic constriction in isolated middle cerebral and renal interlobar arteries (4, 10, 11, 27). However, the role of β-ENaC (as well as other ENaC proteins) in afferent arterioles, the major site of RVR, is not clear. A recent study by Wang et al. (28) suggests ENaC proteins do not contribute to myogenic constriction in renal afferent arterioles using the hydronephrotic kidney. In contrast, Guan et al. (6) recently demonstrated that myogenic constriction in afferent arterioles in the perfused juxtamedullary kidney is sensitive to ENaC inhibition. Since afferent arterioles are the major site of RVR, and thus RBF regulation, the question of whether ENaC proteins play an important enough role in myogenic constriction to control whole kidney RVR remains. The current study addresses this mechanistic question. Therefore, the goal of the current investigation was to determine whether β-ENaC contributes to myogenic whole kidney autoregulation. To address this goal, we evaluated the myogenic component of RBF autoregulation to a step increase in MAP in a mouse model of reduced β-ENaC expression. Results from our study indicate that the speed and contribution of the myogenic mechanism to renal autoregulation are reduced by ∼50% in β-ENaC m/m mice. Steady-state RBF autoregulation is unchanged in β-ENaC m/m mice, which may be due to compensation by the TGF mechanism. These data suggest β-ENaC plays an important role in myogenic mediated whole kidney autoregulation.
Mouse Model of Reduced β-ENaC
In the present study, we used a mouse model with reduced β-ENaC levels to determine the importance of β-ENaC in whole kidney myogenic autoregulation. The animal model was generated using standard gene-targeting approaches in the course of generating a model of Liddle's syndrome (increased β-ENaC) by inserting a premature stop codon in the COOH-terminus coding region. However, the presence of the neomycin selection marker disrupts the β-ENaC gene locus resulting in reduced β-ENaC expression. Thus, a mouse model that under- rather than overexpresses β-ENaC was generated. Mice homozygous for the mutation (m/m) express very low levels of β-ENaC transcripts and protein in the lung and kidney as well as reduced β-ENaC protein in cerebral VSMCs (23, 27). Consistent with these findings, we show β-ENaC protein in whole kidney lysates is greatly reduced in β-ENaC m/m mice.
Contribution of Myogenic Constriction to Whole Kidney Autoregulation
Autoregulation of blood flow describes the function of a vascular bed to maintain a constant flow despite variations of the level of arterial pressure by regulating vascular resistance. With increasing pressure, flow is maintained by vasoconstriction; with decreasing pressure, flow is maintained by vasodilation. In the kidney, at least two mechanisms promote autoregulatory adjustments to vascular resistance; myogenic constriction and TGF (21, 22). The TGF mechanism is mediated by increases in sodium chloride delivery to the macula densa in the early distal tubule, which leads to vasoconstriction of the afferent arteriole (24). TGF operates at a low frequency (<0.05 Hz) and requires ∼20–25 s for TGF signaling to regulate vascular resistance. The myogenic mechanism is an inherent mechano-dependent property of VSMCs in resistance arteries and arterioles and is initiated by pressure-induced vessel stretch (3). Vascular stretch activates a mechano-dependant signaling mechanism leading to vasoconstriction. The myogenic mechanism operates at a higher frequency (0.2–0.3 Hz) and adjusts vascular resistance approximately within 5–10 s (2, 8, 18, 19). The early onset of the myogenic mechanism allows its contribution to whole kidney RBF autoregulation to be isolated. Therefore, adjustments in RVR occurring with 5 s can be attributed to myogenic responses. Although adjustments occurring after 5 s reflect a combination of myogenic and TGF responses, the time period of 6–25 s has been used as an estimate of TGF speed (12, 13).
Importance of β-ENaC to Myogenic Whole Kidney Autoregulation
Three findings from the present investigation demonstrate that β-ENaC protein contributes to the myogenic mechanism of RBF autoregulation. First, the step increase in MAP elicited a greater peak increase in RBF (P = 0.0248) and decrease in RVR (P = 0.0418) in β-ENaC m/m mice, indicating a greater passive response to the initial step increase in MAP. Second, the rate of increase in RVR during the first 5 s after the step increase in MAP, an estimate of the contribution of the myogenic mechanism to RBF autoregulation, was inhibited by 56% in β-ENaC m/m mice. Third, the contribution of the myogenic mechanism to total autoregulation of RVR was inhibited by 54% in β-ENaC m/m mice. These findings support the conclusion that the myogenic mechanism is weakened β-ENaC m/m mice.
Since the rate of increase in RVR during the 6–25 s after the step increase in MAP, an estimate of TGF speed, was not different between β-ENaC +/+ and m/m mice, our findings also suggest that TGF-mediated RBF autoregulation is not dependent on β-ENaC. This is consistent with previous studies demonstrating the importance of the furosemide-sensitive Na+-K+-2Cl− cotransporter in the TGF mechanism (17). Despite the delay in the speed of the myogenic mechanism, steady-state autoregulation at 2 min was not different between +/+ and m/m mice, suggesting β-ENaC-dependent myogenic constriction does not play a dominant role in steady-state RBF autoregulation. It is likely that other autoregulatory mechanisms, such as TGF or the “third mechanism,” produce the resistance changes required for maintenance of steady-state RBF (12). In support of this, we found that the 1) contribution of TGF to total autoregulation tended to be increased (P = 0.062) and 2) second phase of increased RVR, which is associated with the TGF response, continued for a longer duration in β-ENaC m/m mice. Thus, increased duration of TGF response, rather than increased speed, may have compensated for the loss of myogenic control to maintain steady-state autoregulation.
Importance of Baseline MAP on Renal Autoregulation
RBF autoregulation is influenced by blood pressure and active in the pressure range between 50 and 150 mmHg, and pressures outside of the autoregulatory range are associated with a passive response to pressure. In our study, both baseline MAP and the magnitude of the step increase in pressure were similar between +/+ and m/m mice. Although autoregulatory control of RBF falls off at lower pressures, RBF autoregulation is mediated by myogenic and TGF mechanisms, and myogenic responsiveness is still active in the lower pressure ranges to varying extents (9–13, 16, 18, 26). Furthermore, one in vivo report suggests that in the absence of TGF, the myogenic speed is unaffected by a drop in resting MAP from 110 to 50 mmHg (12). The lack influence of resting MAP on the estimated speed of the myogenic response [slope RVR0–5s, slope conductance (% baseline)] in β-ENaC m/m mice is shown in Fig. 3C. This figure demonstrates that although MAP distribution is narrower and shifted toward lower resting MAP in m/m mice, for any given resting MAP, the slope of RVR0–5s and conductance (% baseline)0–5s tend to be lower in m/m mice. These findings suggest the lower slope RVR0–5s, and thus slower myogenic speed, found in β-ENaC m/m was not dependent on a lower resting pressure, per se. However, since conscious resting blood pressures were not determined in the current study, we cannot rule out the possibility that β-ENaC m/m mice may experience a greater decline in resting blood pressure with anesthesia, which may contribute to the reduced myogenic speed in β-ENaC m/m mice.
How Might β-ENaC Contribute To Myogenic Constriction?
The myogenic response is a mechano-dependent response initiated by conversion of a mechanical stimulus (pressure) into a cellular event (depolarization/constriction) in VSMCs (3). Mechano-activation causes VSM membrane depolarization and subsequent calcium influx via voltage-gated calcium channels induces constriction (7, 15, 20). Although several classes of proteins are considered candidates for the VSMC mechanosensor, we speculate that β-ENaC is a critical component. β-ENaC is a member of a large family of proteins termed the degenerin/ENaC family. Members of this family are thought to act as mechanosensors in a diverse range of species (nematode, fly, mammals) and tissues (sensory neurons, skeletal muscle, VSMC). Because β-ENaC is closely related to the nematode mechanosensor degenerins, we speculate that β-ENaC is a component of a similar mechanosensor in VSMCs. It is likely that β-ENaC interacts with other mammalian degenerin proteins such as γ-ENaC and acid-sensing ion channel 2 to form the pore of a VSMC mechanosensor (5, 10). The compensatory activity of these remaining degenerin proteins may account for the remaining myogenic response (only 50% of the slope RVR0–5s was inhibited in β-ENaC m/m).
In summary, our data support the hypothesis that β-ENaC is an important mediator of whole kidney myogenic autoregulation. Although loss of whole kidney myogenic autoregulation is present in reduced β-ENaC mice, steady-state renal autoregulation is maintained probably by compensatory increases in TGF.
GRANTS
This work was supported by National Institutes of Health Grant HL-086996 (to H. Drummond) and American Heart Association Grant 0725322B (to S. C. Grifoni).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank J. M. doCarmo for assistance with surgical technique and laboratory colleagues for excellent discussion.
REFERENCES
- 1.Carmines PK, Inscho EW, Ortenberg JM, Cook AK. Determinants of renal microvascular autoregulatory behavior in normal and hypertensive rats. Kidney Int Suppl 32: S89–S93, 1991 [PubMed] [Google Scholar]
- 2.Cupples WA, Novak P, Novak V, Salevsky FC. Spontaneous blood pressure fluctuations and renal blood flow dynamics. Am J Physiol Renal Fluid Electrolyte Physiol 270: F82–F89, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension 44: 643–648, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Gannon KP, Vanlandingham LG, Jernigan NL, Grifoni SC, Hamilton G, Drummond HA. Impaired pressure-induced constriction in mouse middle cerebral arteries of ASIC2 knockout mice. Am J Physiol Heart Circ Physiol 294: H1793–H1803, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Guan Z, Cook AK, Hobbs JL, Inscho EW. Effect of epithelial sodium channel blockade on the myogenic response of rat juxtamedullary afferent arterioles. Hypertension in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res 55: 197–202, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Holstein-Rathlou NH, Wagner AJ, Marsh DJ. Dynamics of renal blood flow autoregulation in rats. Kidney Int Suppl 32: S98–S101, 1991 [PubMed] [Google Scholar]
- 9.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Renal autoregulation in P2X1 knockout mice. Acta Physiol Scand 181: 445–453, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous β and γENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol 289: F891–F901, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Just A, Arendshorst WJ. Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol 285: R619–R631, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Just A, Arendshorst WJ. A novel mechanism of renal blood flow autoregulation and the autoregulatory role of A1 adenosine receptors in mice. Am J Physiol Renal Physiol 293: F1489–F1500, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508: 199–209, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol Heart Circ Physiol 269: H348–H355, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Lai EY, Welch WJ, Wilcox CS. Myogenic responses of mouse isolated perfused renal afferent arterioles: effect of renal mass. FASEB J 23: 804.3, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leyssac PP, Holstein-Rathlou NH. Effects of various transport inhibitors on oscillating TGF pressure responses in the rat. Pflügers Arch 407: 285–291, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res 90: 1316–1324, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Loutzenhiser R, Bidani AK, Wang X. Systolic pressure and the myogenic response of the renal afferent arteriole. Acta Physiol Scand 181: 407–413, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Meininger GA, Davis MJ. Cellular mechanisms involved in the vascular myogenic response. Am J Physiol Heart Circ Physiol 263: H647–H659, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Navar LG. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol Renal Fluid Electrolyte Physiol 234: F357–F370, 1978 [DOI] [PubMed] [Google Scholar]
- 22.Navar LG, Marsh DJ, Blantz RC, Hall J, Ploth DW, Nasjletti A. Intrinsic control of renal hemodynamics. Fed Proc 41: 3022–3030, 1982 [PubMed] [Google Scholar]
- 23.Pradervand S, Barker PM, Wang Q, Ernst SA, Beermann F, Grubb BR, Burnier M, Schmidt A, Bindels RJ, Gatzy JT, Rossier BC, Hummler E. Salt restriction induces pseudohypoaldosteronism type 1 in mice expressing low levels of the beta-subunit of the amiloride-sensitive epithelial sodium channel. Proc Natl Acad Sci USA 96: 1732–1737, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnermann J, Traynor T, Yang T, Arend L, Huang YG, Smart A, Briggs JP. Tubuloglomerular feedback: new concepts and developments. Kidney Int Suppl 67: S40–S45, 1998 [DOI] [PubMed] [Google Scholar]
- 25.VanBavel E, Wesselman JP, Spaan JA. Myogenic activation and calcium sensitivity of cannulated rat mesenteric small arteries. Circ Res 82: 210–220, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ. Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol Regul Integr Comp Physiol 276: R855–R863, 1999 [DOI] [PubMed] [Google Scholar]
- 27.VanLandingham LG, Gannon KP, Drummond HA. Pressure-induced constriction is inhibited in a mouse model of reduced βENaC. Am J Physiol Regul Integr Comp Physiol 297: R723–R728, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Takeya K, Aaronson PI, Loutzenhiser K, Loutzenhiser R. Effects of amiloride, benzamil, and alterations in extracellular Na+ on the rat afferent arteriole and its myogenic response. Am J Physiol Renal Physiol 295: F272–F282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]