Abstract
Classical conditioning of the eyeblink reflex is a form of motor learning that is uniquely dependent on the cerebellum. The cerebellar learning hypothesis proposes that plasticity subserving eyeblink conditioning occurs in the cerebellum. The major evidence for this hypothesis originated from studies based on the telecommunications network metaphor of eyeblink circuits. These experiments inactivated parts of cerebellum-related networks during the acquisition and expression of classically conditioned eyeblinks in order to determine sites at which the plasticity occurred. However, recent evidence revealed that these manipulations could be explained by a network performance hypothesis which attributes learning deficits to a non-specific tonic dysfunction of eyeblink networks. Since eyeblink conditioning is mediated by a spontaneously active, recurrent neuronal network with strong tonic interactions, differentiating between the cerebellar learning hypothesis and the network performance hypothesis represents a major experimental challenge. A possible solution to this problem is offered by several promising new approaches that minimize the effects of experimental interventions on spontaneous neuronal activity. Results from these studies indicate that plastic changes underlying eyeblink conditioning are distributed across several cerebellar and extra-cerebellar regions. Specific input interactions that induce these plastic changes as well as their cellular mechanisms remain unresolved.
The cerebellar learning hypothesis and classical conditioning of eyeblink responses
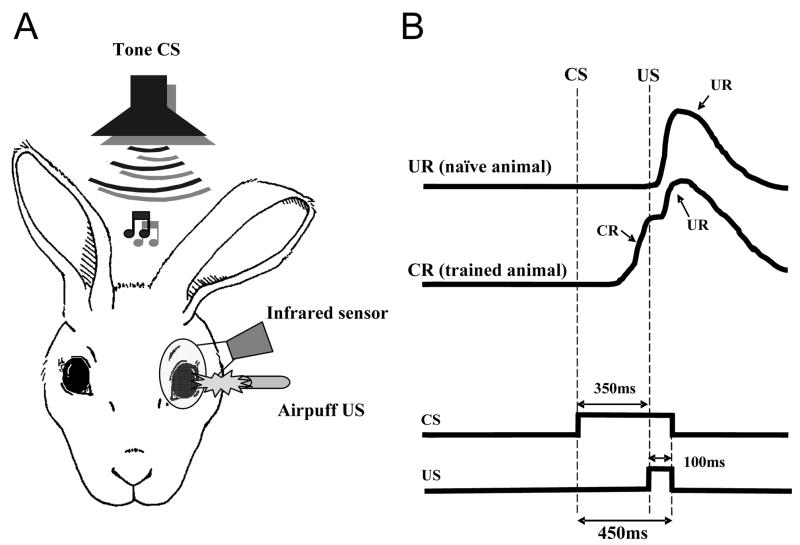
The classically conditioned eyeblink or nictitating membrane reflex is a unique type of associative learning in which the cerebellum plays a major role (Thompson, 1986, for review). In the delay conditioning paradigm, the conditioned stimulus (CS), a stimulus that normally does not evoke the reflex, is paired over successive trials and at a specific interval with an ensuing unconditioned stimulus (US), which is capable of eliciting the unconditioned response (UR) before the conditioning is initiated. In each trial the CS and US co-terminate (Fig. 1). As conditioning continues, a new eyeblink response, the conditioned response (CR), gradually develops in the interstimulus interval, and the peak of this response becomes progressively time-locked to the onset of the US. In addition, once acquired, the CR can be evoked by applying the CS alone.
Fig. 1.
Schematic of the eyeblink conditioning paradigm. A. Rabbits are presented with a paired tone conditioned stimulus (CS) and airpuff unconditioned stimulus (US). Evoked eyeblinks are recorded with an infrared sensor. B. Idealized eyeblink records in naive and trained animals and the pulse diagram denoting the timing of stimuli. In the delay classical conditioning paradigm, the onset of the CS precedes the onset of the US and the stimuli co-terminate. Naive animals don’t respond to the CS, but the US evokes reliably the hard-wired trigeminal unconditioned blink (UR, top eyeblink trace). Over time, rabbits associate the CS with the US, and they learn to blink in anticipation of the upcoming aversive US. These associatively learned responses are called conditioned responses (CR, the second trace from the top).
In the early eighties of the last century, a very exciting observation was reported implicating the cerebellum in this type of learned behavior. Lesioning a specific region of the cerebellar nuclei disrupted the performance of the classically conditioned eyeblink reflex in the rabbit (Clark et al., 1984; Yeo et al., 1985). Subsequently, several reports were published demonstrating that the modulation of neurons in the critical regions of the cerebellar cortex and nuclei is associated with the CS, US and CR (Berthier and Moore, 1986; Berthier and Moore, 1990). In addition, substantial cerebellar involvement in this type of learning has been shown in other species (e.g. Skelton, 1988; Chen et al., 1996; Voneida et al., 1990), including humans (Lye et al., 1988; Solomon et al., 1989). Since these seminal observations, temporary and permanent lesion experiments have implicated the cerebellum in multiple processes underlying the classical conditioning of this reflex system, including acquisition, retention and consolidation (Bracha and Bloedel, 1996; Christian and Thompson, 2003; De Zeeuw and Yeo, 2005, for review). Finally, this dependence was found to extend to other types of conditioned reflexes. Manipulations of the cerebellar circuitry or permanent lesions in cerebellar patients disrupted instrumentally conditioned eyelid closure (Bracha et al., 2001) and classically conditioned withdrawal reflexes in the extremities of cats (Kolb et al., 1997; Bracha et al., 1999) as well as humans (Timmann et al., 2000). Because of the extensive data from several laboratories dealing with the classically conditioned eyeblink reflex in the rabbit, our review will focus on data acquired from this species.
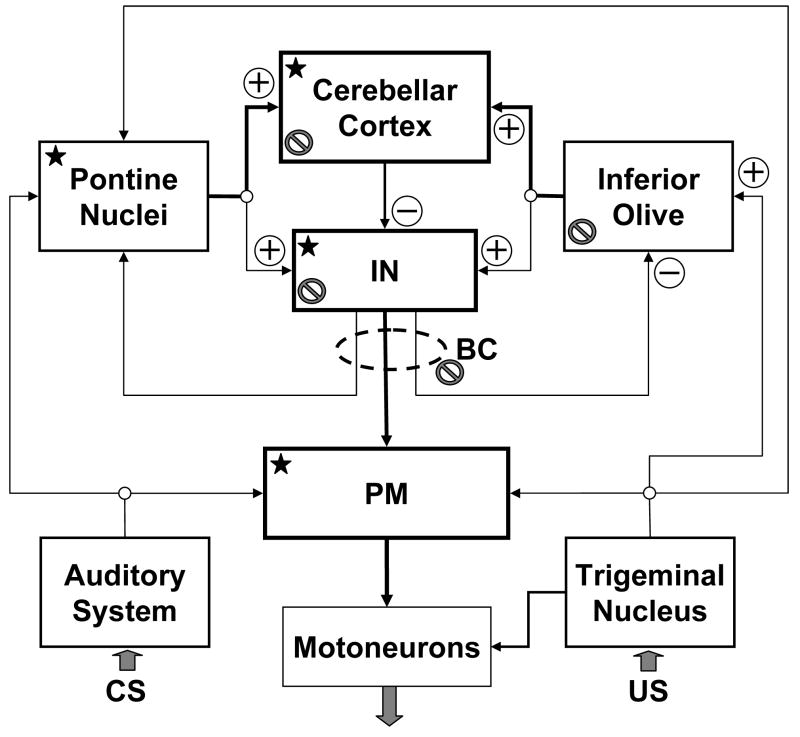
It is generally agreed that eyeblink conditioning in the delay paradigm is controlled by a combination of brainstem eyeblink reflex circuits and the intermediate cerebellar network, which is super-imposed over the UR system (Fig. 2). It has been proposed that the ipsilateral cerebellar interposed nuclei (IN) and the cerebellar cortex are essential and perhaps sufficient sites of plastic changes for generating the cerebellar CR motor command. This theoretical position, the cerebellar learning hypothesis, has been extensively reviewed (Thompson, 1986; Christian and Thompson, 2003; Ohyama et al., 2002; Attwell et al., 2002b; Bracha and Bloedel, 1996), and therefore, it will only be briefly outlined here. The primary tenet of this hypothesis is derived from original concepts posited by Albus (1971) and Marr (1969), who deduced testable predictions based on the cerebellum’s unique anatomical structure and synaptic organization. It is assumed that information about the CS and US arrives to the cerebellum via two distinct routes. The CS is conveyed through mossy fibers originating in pontine nuclei, whereas the US is coded in the discharge of climbing fibers originating in the inferior olive (IO). Information from the mossy and climbing fibers eventually converges on cortical Purkinje cells and cerebellar nuclear neurons. It is presumed that the hetero-synaptic interaction at the points of convergence triggers local cellular plastic processes resulting in the changed responsiveness of Purkinje and/or nuclear cells. These plastic changes cause the network to respond to the CS mossy fiber signal by issuing a cerebellar nuclear “motor command” that triggers the CR.
Fig. 2.
A conceptual block diagram of the cerebellum-related circuitry involved in acquisition and expression of classically conditioned eyeblinks in the rabbit. This diagram is a highly simplified representation of relevant structures and connectivity. Information regarding the conditioned stimulus (CS) and unconditioned stimulus (US) information enters the network via auditory and sensory trigeminal systems. These inputs are supplied in parallel to the serially connected pontine nuclei, cerebellar cortex, cerebellar interposed nuclei (IN) and brainstem nuclei contributing to projections to eyeblink premotoneurons and supplying motor commands to them. Since all these sites (labeled with a star) receive CS and US information, they should be considered as putative sites of learning. Output of eyeblink premotoneurons supplies motor commands to eyeblink motoneurons. Backslashed circles denote nodes at which inactivation during training disrupts CR acquisition. Boxes with bold borders represent structures among which are in our view distributed plastic changes underlying eyeblink conditioning. BC – brachium conjunctivum; PM – nuclei containing eyeblink premotoneurons that include the red nucleus. The plus symbols mark excitatory glutamatergic inputs and minus signs label inhibitory GABA-ergic inputs.
Despite almost three decades of research examining the cerebellum’s contribution to the acquisition, retention, and expression of the classically conditioned eyeblink reflex, a consensus regarding how this structure plays its important role in this behavior has not been reached. For example, in spite of numerous optimistic claims, specific contributions of the cerebellar cortex, cerebellar nuclei and extra-cerebellar substrates to plasticity that underlies learning are not known. This fundamental issue remains unresolved, mostly because of the lack of tools needed to interfere with learning without affecting both the local and global properties intrinsic to underlying circuits.
In this review we will first examine the conceptual underpinnings of experiments that tested the cerebellar learning hypothesis using local inactivation or manipulations of neurotransmitter signaling. We will outline the telecommunications network metaphor of eyeblink circuits, and we will show that some of the findings from studies that were designed based on this metaphor seem to disprove the cerebellar learning hypothesis or at the very least challenge some of its basic tenets. Then we will demonstrate that traditional cerebellar manipulations affect the spontaneous activity of neurons at the site of intervention and downstream from it, and that tonic interactions associated with this change can radically alter the functional state of the entire network. The tonic interactions in cerebellar systems have been largely overlooked in most discussions of the cerebellar learning hypothesis. We will argue that some of the pivotal observations that were declared to support this hypothesis can be ascribed to the effects that experimental manipulations had on the tonic activity of cerebellar circuitry and/or to methodological aspects of the experiments on which this view is based. We will present promising new data further supporting these arguments, and lastly, we will discuss approaches that could be used to address the function of eyeblink conditioning circuits more effectively.
The telecommunications network metaphor of eyeblink circuits
In most discussions of the neural substrates for the classically conditioned eyeblink reflex, it is often implicitly assumed that cerebellum-related eyeblink conditioning circuits (Fig. 2) operate as a telecommunications network. Telecommunications networks consist of links and nodes arranged so that messages may be passed from one part of the network to another over multiple links and through various nodes. To employ the metaphor, eyeblink circuits consist of nodes (nuclei) and links (inter-connecting axons). Nodes in eyeblink conditioning circuits, individual nuclei or parts of the cerebellar cortex, are viewed as input-output processing units that transform input messages into output signals. Importantly, properties of this signal transformation can change during learning. Based on this metaphor, experimental interventions in eyeblink circuits, such as local inactivation, are viewed as means for disrupting local information processing and for depriving the rest of the circuit of locally generated or transmitted task-specific signals. However, this concept neglects the fact that nodes in eyeblink networks also exchange a continuous stream of spontaneous activity that shapes the functional state of the network. In our view, this omission has led to severalsurprising and misleading conclusions.
The telecommunications network metaphor can be implicated in several of the early inactivation studies. For example, Krupa et al. (1993) proposed that systematically inactivating individual nodes in the network during training sessions could be used to find places where learning-induced plasticity occurs. They speculated that successful CR acquisition during inactivation of a particular node would signify that plastic changes develop not at this node, but at some other up-stream parts of the network. On the other hand, failure of CR acquisition would mean that learning occurred either at the manipulated node or at some of its down-stream efferent targets. With this logical reasoning one could methodically examine individual nodes until all potential sites of learning were found. Over time, it has been shown that blocking glutamate neurotransmission in the cerebellar cortex (Attwell et al., 2001) or inactivating cerebellar nuclei (Krupa et al., 1993; Hardiman et al., 1996) during learning prevents CR acquisition. In contrast, inactivating a major intermediate cerebellar efferent target, the red nucleus, had no effect on learning (Clark and Lavond, 1993; Krupa et al., 1993). These findings suggested that a significant site of plasticity related to CR acquisition is contained in the ipsilateral cerebellum. However, this conclusion was strongly contradicted by our recent discovery that a carefully placed, more extensive inactivation of the brachium conjunctivum (outgoing axons of deep cerebellar nuclei, BC) actually prevented CR acquisition (Nilaweera et al., 2006). Using the logic derived from the metaphor, this surprising finding leads to an important inference: the learning essential for CR acquisition occurs most likely outside of the cerebellum, in extra-rubral cerebellar efferent targets! Before accepting this unexpected scenario, the possible role of cerebellar feedback circuits has to be considered.
Besides projections to pre-motoneuronal parts of eyeblink circuits, the BC contains axons projecting to cerebellar afferent sources in the IO and in the pontine nuclei and thus participates in cerebellar feedback loops (Fig. 2). It is known that inactivating the IN does not prevent the pontine nuclei from transmitting CS signals (Cartford et al., 1997). Therefore, inactivating the BC should not affect learning via its effect on CS signals from the pontine nuclei. Perhaps more important are the implications derived from the cerebello-olivary-cerebellar feedback loop. The cerebellar learning hypothesis assumes that the IO supplies the cerebellum with a learning-inducing US signal (error signal) and that the inhibitory projection to the IO from the cerebellar nuclei suppresses this signal when learning is near completion (e.g. Medina et al., 2002). If so, then inactivating the BC would disinhibit the IO, thereby preventing the suppression of US signals. This condition, however, should not prevent learning (Kim at al., 1998). Consequently, it is unlikely that the effects of BC inactivation on CR acquisition were related to changes in transmission of US signals by the IO. At a minimum, we can conclude that employing all the assumptions underlying the telecommunications network metaphor, together with data from our recent BC inactivation studies, implicate extra-cerebellar sites as additional structures subserving eyeblink conditioning.
A second unexpected conclusion emerged from studies that inactivated the IO during CR expression. As reviewed above, the cerebellar learning hypothesis postulates that cerebellar plasticity is induced by inferior olivary US error signals that enter the IO via glutamatergic projections from the trigeminal nuclei. Correspondingly, lesioning or inactivating the IO prevents CR acquisition (McCormick et al., 1985; Welsh and Harvey, 1998). Given the assumption that IO error signals are required for the maintenance of cerebellar plasticity, the cerebellar learning hypothesis predicts that blocking US responses in the IO should lead to the gradual suppression of CRs – an “unlearning” of CRs analogous to CR extinction training in which the CS is repeatedly presented without the US (McCormick et al., 1985). The seemingly ultimate support for this concept came from Medina et al. (2002), who reported that blocking glutamate neurotransmission in the IO indeed produces the predicted extinction-like suppression of CRs. However, in our investigation of neurophysiological mechanisms of this phenomenon we found that the gradual suppression of CRs following the block of glutamate receptors in the IO is related to the gradual diffusion of the drug and not to unlearning (Zbarska et al., 2007; Zbarska et al., 2008). Moreover, we determined that precise injections of glutamate antagonists in the IO suppress CRs immediately. These behavioral results are clearly inconsistent with the cerebellar learning hypothesis.
The above findings, when viewed through the lens of the telecommunications network metaphor, contradict the cerebellar learning hypothesis. In the following sections we will argue that, before these contradictions can be considered as solid evidence favoring an alternative view incorporating extra-cerebellar sites of plasticity, a major flaw inherent in this metaphor needs to be exposed: the metaphor ignores the fact that experimental manipulations of cerebellar circuitry at the nodes of the eyeblink conditioning network generate coupled modifications of spontaneous activity capable of altering the functional state both within and beyond the targeted node, potentially cascading throughout the network. The inclusion of these tonic interactions in models of eyeblink circuits offers an alternate interpretation of the available experimental data.
Tonic interactions in cerebellar circuits
A fundamental feature of cerebellum-related eyeblink conditioning circuits is their spontaneous activity that can be observed in the absence of overt stimuli or movements. The spontaneous activity of individual neurons is a collective product of the intrinsic ability of some neurons (e.g. Purkinje cells, IO and IN neurons) to self-generate action potentials and of the drive from excitatory, inhibitory and modulatory synaptic inputs (Hausser et al., 2004). Spontaneous firing rates differ across individual nodes of the network. For example, the typical spontaneous firing rates of Purkinje cells, IN neurons and IO neurons are about 50 Hz, 10–40 Hz and 1–2 Hz, respectively. The spontaneous activity of individual neurons is propagated through the network affecting cells in other nuclei, and these effects are further sculpted by a number of excitatory and inhibitory recurrent loops. These dynamic, non-linear processes determine the self-regulating functional state of the network.
The importance of tonic activity in cerebellar circuits to the learning and execution of a specific motor behavior should not be a surprise. Several experimental studies over decades of research demonstrated that lesions within the cerebellum or its afferent or efferent systems produce significant tonic effects throughout the motor system, including modifications of a variety of spinal reflexes (for reviews see Dow and Moruzzi, 1958; MacKay and Murphy, 1979; Bloedel and Bracha, 1995). This is particularly clear when manipulating the olivo-cerebellar projection. The early experiments of Carrea et al. (1947) demonstrated that the effects of IO lesions on behavior are so profound that they actually mimic the effects of ablating major portions of the cerebellum itself. Later studies revealed that IO lesions and IO cooling have profound effects on the spontaneous activity of Purkinje cells. Considering the very low firing rate of IO neurons, it was surprising that removing the IO excitatory input to Purkinje cells led to a high, long lasting increase of their spontaneous discharge (e.g., Montarolo et al., 1982). Central to the arguments we will present, this tonic effect emerging from IO inactivation affected the spontaneous firing rate of cells at downstream sites. Since Purkinje cells are GABA-ergic, their sustained high activity was shown to suppress activity in their target cerebellar nuclear neurons (Batini et al., 1985). In turn, the decreased firing of nuclear neurons suppressed activity in the red nucleus (Billard et al., 1988), which is the main target of excitatory IN projections. In summary, these studies uncovered two important principles:
spontaneous activity in cerebellar network nodes can regulate tonic activity in their efferent targets;
suppressing spontaneous activity in one node can trigger related tonic changes capable of spreading through large portions of the network, negatively impacting its general functional state.
How relevant are these principles to eyeblink conditioning research?
Tonic cerebellar interactions in classically conditioned rabbits
Although speculations had been made in a number of studies regarding tonic phenomena and their importance (e.g. Bracha and Bloedel, 1996; Welsh and Harvey, 1998; Attwell et al., 2001), their experimental demonstration in the rabbit eyeblink conditioning model was reported only recently. Characterizing tonic interactions in cerebellar circuits requires combining local circuit manipulations with recording of neuronal activity. For that purpose, we developed a unique, microwire-based, multi-channel recording system that is well suited for long-term isolation of single units. The long-term stability of unitary recording is paramount for experiments that require monitoring cellular activity for at least 1–2 hours in animals with a freely moving head. In our initial studies, we focused on analyzing electrophysiological consequences of neurotransmitter manipulations in the IO and IN. Results of these experiments offer illuminating insights into the mechanisms through which cerebellar manipulations affect eyeblink conditioning.
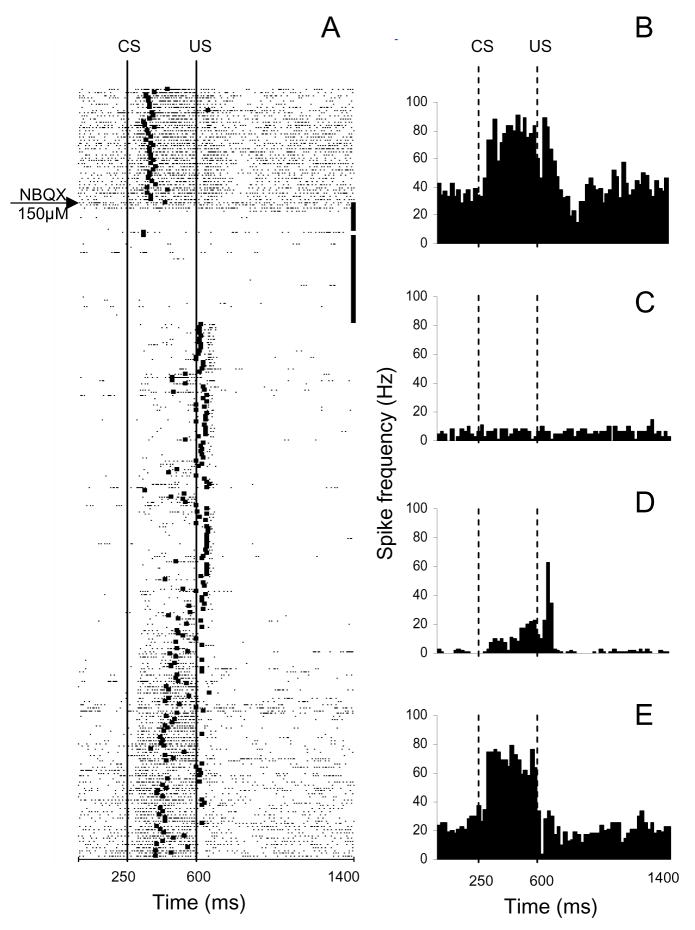
As explained in previous sections, demonstrating that US signals from the IO are required for the maintenance of CRs constitutes a pivotal test of the cerebellar learning hypothesis. In a frequently cited study, Medina et al. (2002) proposed that this prediction could be tested by blocking trigeminal projections to the IO by infusing the IO of trained rabbits with a fast glutamate receptor blocker, NBQX. They reported that NBQX indeed produced an extinction-like, gradual suppression of CRs. To investigate the neurophysiological mechanisms of this process, we injected NBQX in the IO of trained rabbits while simultaneously recording single-unit activity of IN neurons (Zbarska et al., 2008). Based on the prediction of Medina et al., which invokes the telecommunications network metaphor, one would expect NBQX to gradually “extinguish” CRs with a correlated gradual decrease of the CR-related modulation of neuronal activity in the IN. On the other hand, if NBQX decreases the spontaneous IO firing rate (Lang, 2002), one could also expect tonic suppression of IN activity. We not only found that NBQX immediately abolishes CRs without the need for CS presentations (a condition required for extinction), but this behavioral response coincided with the immediate suppression of both spontaneous IN activity and task-related modulation (Fig. 3). In the framework of the telecommunications network metaphor, the behavioral part of our study argued against the cerebellar learning hypothesis. However, our electrophysiological data revealed that this metaphor had a major shortcoming affecting the interpretation of the findings – its failure to recognize the importance of a fundamental variable, the tonic interactions in cerebellar networks. The intent of the above IO studies was to observe the consequences of blocking the IO error signal to the cerebellum. However, the IO injection of NBQX also made the cerebellar cortex and nuclei dysfunctional by blocking the cerebellar output, which in turn results in the abolition of CRs, precluding any conclusions about the mechanisms and sites involved in establishing plasticity.
Fig. 3.
An example of the parallel effects of inferior olivary NBQX infusion on CR performance and on the activity of a task-modulated IN cell. This experiment consisted of 260 trials. After 40 baseline trials, NBQX was injected at the beginning of a 40-trial no-stimulation period. A, Raster plot of IN cell activity during this experiment. The experiment starts at the top with each row representing one trial, and each dot marking the occurrence of an action potential. The black square in each row corresponds to the onset of the eyeblink in that particular trial. Consequently, CRs have onset markers between lines denoting the CS and US onsets. Eyeblinks initiated past the US onset occur in trials in which the animal failed to produce the CR. Black squares at the ends of the 40 trials following the NBQX injection marker denote the no-stimulation waiting period that was inserted to allow for drug diffusion. Before the injection, this cell responded with excitation during the CS–US interval and with a combined excitatory/inhibitory response to the US. During the drug diffusion period, the firing rate of this cell’s activity precipitously declined. When stimulation was resumed, CRs were abolished immediately as evidenced by the blink onset marks on the right side of the US onset line. The baseline activity remained suppressed, and modulation during the CS–US interval was severely reduced whereas the relative excitatory modulation to the US became more distinct. The neuronal activity gradually recovered toward the end of the experiment in parallel with the recovery of behavioral CRs. B–E, Peri-stimulus histograms of the same IN unit constructed for 40 trials before the injection (B), for 40 post-injection drug diffusion trials when stimulation was paused (C), for 40 trials following the waiting period when stimulation was resumed (D), and for the last 40 trials from the remaining 140 trials of the experiment (E). Bin width for histograms in B–E is 20 ms. CS, onset of conditioned stimulus; US, onset of unconditioned stimulus. (Reprinted with permission from Zbarska et al., 2008)
Additional evidence for cerebellar tonic interactions following manipulations of the cerebellar circuitry emerged when we examined GABA and glutamate neurotransmission in the IN of trained rabbits. Individual contributions of the cerebellar cortex and IN to CR acquisition and expression have been the subject of a long-standing debate. In an attempt to resolve this issue, some investigators proposed that nuclear components of learning could be revealed by blocking GABA-ergic projections of Purkinje cells to the IN (Medina et al., 2001; Ohyama et al., 2006). They reported that blocking GABA-A receptors either with picrotoxin or with gabazine shortens the latency of CRs. The authors proposed that short-latency CRs are a manifestation of nuclear plasticity that is revealed in the absence of cerebellar cortical input. In their computer simulations, Medina et al. (2001) predicted that injecting picrotoxin in the IN should affect the time profile of IN neuronal responses but should have no effect on their spontaneous activity. Relevant to the subject of this review, these simulations are based on the telecommunications network metaphor because they did not consider tonic interactions.
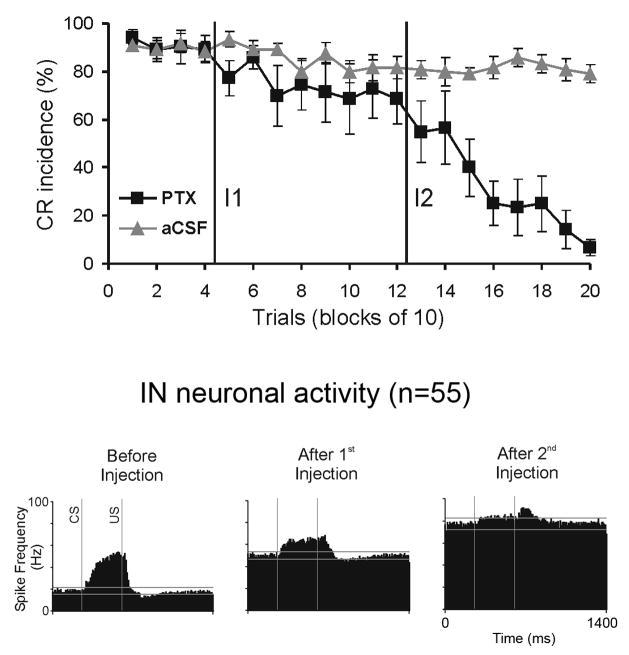
To examine these proposals, we injected the IN of trained rabbits with GABA agonists and antagonists and then measured their effect on IN single-unit activity and on CRs (Aksenov et al., 2004a). In our experiments, we could not confirm their prediction that effects would be limited to IN neuronal response timing. Instead, we found that a partial block of chloride channels with picrotoxin dramatically increased IN tonic activity. A more complete block of GABA neurotransmission with larger amounts of picrotoxin further increased IN spontaneous activity. It became so high that practically all modulation was suppressed, and behavioral CRs were abolished (Fig. 4). Although the goal of these experiments was to block signals embedded in Purkinje cell firing without altering normal IN activity, our recordings demonstrated that the functional state of the IN was dramatically altered. Furthermore, it is also likely that the excitability of IN efferent targets was also modified. This distributed functional abnormality prevented any conclusions about the cerebellar cortical and IN roles in CR expression. We conclude that simply blocking GABA neurotransmission in the IN can not address these questions.
Fig. 4.
Effects of injecting the IN with the chloride channel blocker, picrotoxin (PTX), on the expression of CRs and on IN neuronal activity. Top panel: CR incidence in 15 injection experiments in which two injections of PTX were applied to the IN. The first injection (I1) had only a small effect on the frequency of CRs. A more extensive block of GABA-ergic neurotransmission with the second PTX injection (I2) gradually abolished CRs. Control injections of vehicle (aCSF) did not affect CR incidence. Bottom panel: population peri-stimulus histograms of 55 neurons recorded during PTX injections. Before injections, this population exhibited approximately a 25 Hz spontaneous firing rate and an excitatory response in the CS-US interval. The first PTX injection doubled the spontaneous discharge of IN neurons and reduced their CS-related modulation. Following the second injection, the spontaneous activity further increased, and the responses in the CS-US interval were almost completely attenuated. Two horizontal lines in each histogram denote tolerance limits used for detecting significant levels of neuronal modulation relative to mean baseline activity (Adapted from Aksenov et al., 2004).
The examples of tonic interactions in cerebellar circuits demonstrate that local inactivation and pharmacological manipulation are imperfect tools when used to interfere with task-related signals or to block local information processing. This is because local interventions will inevitably alter normal spontaneous activity of manipulated structures, and this abnormality will spread to down-stream parts of the network. This problem is further exacerbated by participation of feedback loops that provide a path for the propagation of the tonic change to up-stream parts of the network. The spread of tonic changes via the cerebello-olivary-cerebellar feedback loop has been documented. Hesslow and colleagues recorded a dramatic reduction of spontaneous Purkinje cell activity when inactivating IN axons in the brachium conjunctivum of decerebrate ferrets (Svensson et al., 2005; Bengtsson et al., 2004), findings which are consistent with results from our laboratory. We found that inactivating the BC in classically conditioned rabbits elevates the tonic activity of upstream IN neurons (Nilaweera et al., 2002). In summary, combined microinjection and recording studies demonstrate that local manipulations alter spontaneous activity and that this change can spread via tonic interactions to both downstream and up-stream parts of the network.
The down-stream and recurrent propagation of tonic changes is highly pertinent to the interpretation of studies in which components of the cerebellar circuits are inactivated during learning. In previous sections we have shown that, when viewed through the perspective of the telecommunications network metaphor, the collective results of experiments in which this methodology was used point to the existence of extra-cerebellar sites for plasticity underlying this behavior. However, given the potential for recurrent and downstream spread of tonic changes, the failure to acquire CRs during BC inactivation does not prove extra-cerebellar learning. It is possible that this treatment also affected cerebellar learning via tonic malfunction within the cerebello-olivary-cerebellar feedback loop. As a result, despite the initial optimism regarding the use of reversible lesions, inactivating parts of cerebellar circuits during training actually tells us surprisingly little about the location of plastic changes.
Cerebellar learning vs. network performance hypothesis
As argued above, the telecommunications network metaphor can’t explain the results of experiments that inactivate neurons or block neurotransmission in eyeblink conditioning circuits. A more realistic conceptualization of how these circuits function has to include tonic phenomena. Eyeblink circuits should be viewed not only as a network of nodes that process and transmit task-related signals but also as a spontaneously active, recurrent neuronal network with strong tonic interactions.
Nevertheless, addressing such concepts as the cerebellar learning hypothesis can not proceed without using intervention methods if we are to understand the fundamental mechanisms responsible for motor learning phenomena like the classically conditioned eyeblink reflex. Only interfering with information processing within the putative learning-related substrates can confirm or disprove this view and evaluate its importance for learning at a systems level. The problem is that traditional intervention experiments, such as local inactivation or blocking specific neurotransmitter systems, up- or down-regulate spontaneous neuronal activity, thus complicating the interpretation of findings. For example, based on the telecommunications network metaphor, our study in which CR acquisition was blocked by BC inactivation (Nilaweera et al., 2006) suggests that extra-cerebellar learning definitely occurs. However, when the associated tonic changes of spontaneous activity are considered, an alternate, more parsimonious explanation – the network performance hypothesis – emerges as a basis for the majority of observations. This hypothesis proposes that the effects of manipulating cerebellar circuits are related to a non-specific, wide-spread malfunction of the networks responsible for acquiring and/or expressing CRs. Interestingly, we know that in the case of BC inactivation, the network performance hypothesis is correct, because blocking the BC alters tonic activity in the IO, cerebellar cortex (Bengtsson et al., 2004) and IN (Nilaweera et al., 2002), and most likely also in mesencephalic targets of cerebellar efferents. Does this mean that these BC inactivation studies disprove the cerebellar learning hypothesis? Not necessarily. In fact, both hypotheses could be correct because the cerebellar learning and performance hypotheses are not mutually exclusive nor are they truly antithetical. Rather, the network performance hypothesis proposes that the relevant findings are due to an abnormal functional state of the cerebellar circuitry. Consequently, no definite conclusions about cerebellar learning can be inferred. Advancing our understanding of the neural circuitry subserving eyeblink conditioning will require intervention methods that eliminate the abnormalities on which the network performance hypothesis is based.
Dissociating learning from network performance-related phenomena
An obvious solution for dissociating learning from network performance abnormalities would be the use of approaches that preserve spontaneous neuronal activity or that target processes insensitive to tonic changes. In our view, three promising research strategies are compatible with these goals.
In theory, not all cerebellar learning-related processes have to be sensitive to cerebellar tonic malfunction. A possible candidate for such a process could be memory consolidation which takes place after signaling events that induce learning in eyeblink circuits have already occurred and therefore could not be perturbed by tonic phenomena. Attwell et al. (2002a) reported that infusions of muscimol in the eyeblink area of the cerebellar cortex following training sessions prevented CR acquisition by interfering with memory consolidation. Interestingly, injecting muscimol in the deep cerebellar nuclei did not affect CR consolidation. Based on these results, Attwell at al. concluded that a muscimol-sensitive memory consolidation process in the cerebellar cortex is required for eyeblink conditioning. This finding, however, does not exclude consolidation of plastic changes in the IN or in extra-cerebellar sites that could be insensitive to IN inactivation and associated tonic changes in the circuit.
Another promising approach involves methods that could interfere with the putative cellular substrates of learning without affecting normal neuronal activity. An example of the successful use of this strategy is our study in which we blocked the synthesis of new proteins in the IN during CR acquisition sessions (Bracha et al., 1998). We found that infusing the IN with anisomycin suppressed CR acquisition. Since anisomycin has been reported to have minimal effects on spontaneous neuronal activity, this finding can be considered among the best evidence for cerebellar learning. Besides their potential for success, tools targeting possible cellular mechanisms of learning also have their limitations. The most important limitation of methods in this category is that they are not suited for analyzing the role of task-related signals during learning. For instance, blocking protein synthesis in the IN can’t determine which inputs to the IN trigger the protein synthesis-dependent learning mechanism.
The third category of approaches that could separate learning from abnormalities in network performance consists of combined applications of receptor agonists and antagonists. The tremendous potential of this approach was shown for the first time by Bao et al. (2002), who discovered that CRs suppressed by infusing a GABA-A agonist, muscimol, in the IN can be recovered by the subsequent infusion of the chloride channel blocker, picrotoxin. We were able to replicate these data (Aksenov et al., 2004b; Aksenov et al., in preparation). Our single-unit recordings in injected animals confirmed that muscimol-induced CR suppression was accompanied by inhibition of IN neurons: their spontaneous firing was suppressed. The subsequent infusion of picrotoxin reduced the inhibition and restored spontaneous firing to its near normal rate, but the amplitude of event-related responses was reduced (Aksenov et al., 2004b). Surprisingly, this group of observations was paralleled by the partial restoration of CRs. Because these studies blocked Purkinje cell input to the IN without markedly disrupting IN spontaneous activity, they were the first to eliminate the network performance hypothesis in experiments blocking network communication. The fundamental implication of these observations is that the IN can support CR expression in the absence of cerebellar cortical inputs. Did the remaining modulation of IN neurons generate the recovered CRs? To examine this issue, we again infused the IN with muscimol to block cerebellar cortical input, but in addition, a fast glutamate receptor blocker DGG was injected to block direct IN input from collaterals of mossy and climbing fibers. This treatment suppressed both IN activity and CR expression. Follow-up injections of PTX restored IN spontaneous firing, but all event-related modulation was suppressed. Yet, even in this condition CRs were partially restored (Aksenov et al., 2004b). This finding strongly suggests that the modulation of IN neurons is not required for the expression of these residual CRs. Such CRs do not seem to require motor commands from the cerebellum, supporting the argument that these CRs are most likely controlled by extra-cerebellar components of eyeblink circuits.
Conclusion
In conclusion, we have shown that the basis for the dependency of eyeblink conditioning on cerebellar circuits is not completely understood. The most investigated concept, the cerebellar learning hypothesis, assumes learning occurs in the cerebellar cortex, the deep cerebellar nuclei, or more recently – in both of these locations. However, because of difficulties in dissociating the learning and network performance hypotheses, the cerebellar learning hypothesis has not been supported by unequivocal, direct evidence from lesion or inactivation studies. Unless future intervention studies successfully manipulate putative conditioned eyeblink substrates without altering levels of spontaneous activity, the specific roles of the cerebellum in acquiring, expressing, and retaining classically conditioned eyeblinks will remain elusive.
Recent developments have identified new approaches that could minimize the impact of cerebellar tonic phenomena. Although the development of these new tools is still in its infancy, efforts in that direction have already revealed very promising results indicating plasticity in several cerebellar and extra-cerebellar parts of eyeblink circuits. Previously we proposed, mostly based on indirect evidence, that plastic changes supporting eyeblink conditioning are distributed across several components of eyeblink conditioning networks (Bracha and Bloedel, 1996; Bracha et al., 2001). We speculated that all nodes that receive the information about the CS and US, including sites within the cerebellum, could be sites of plasticity underlying learning (Fig. 2, sites labeled with a star). This position appears to be supported by the available data: (1) consolidation experiments suggesting cerebellar cortical involvement; (2) the dependency of CR acquisition on the synthesis of new proteins in the IN suggesting an important role for cerebellar nuclei; and (3) combined infusions of GABA and glutamate receptor ligands in the IN indicating that CRs are supported at least partially by extra-cerebellar substrates. These data mandate that future research should focus on examining the role of both cerebellar and extra-cerebellar sites in the classical conditioning of the eyeblink reflex. The continued application of novel approaches will lead to the resolution of these questions in the relatively near future.
Acknowledgments
This work was supported by NIH Grant R01NS036210
References
- Aksenov D, Serdyukova N, Irwin K, Bracha V. GABA neurotransmission in the cerebellar interposed nuclei: involvement in classically conditioned eyeblinks and neuronal activity. J Neurophysiol. 2004;91:719–727. doi: 10.1152/jn.00859.2003. [DOI] [PubMed] [Google Scholar]
- Aksenov D, Serdyukova N, Bloedel JR, Bracha V. 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2004. The role of tonic activity and phasic modulation of interposed cerebellar nuclear cells in the expression of conditioned eyeblinks. 136.6. [Google Scholar]
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002a;34:1011–1020. doi: 10.1016/s0896-6273(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Ivarsson M, Millar L, Yeo CH. Cerebellar mechanisms in eyeblink conditioning. Ann N Y Acad Sci. 2002b;978:79–92. doi: 10.1111/j.1749-6632.2002.tb07557.x. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Rahman S, Yeo CH. Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI. J Neurosci. 2001;21:5715–5722. doi: 10.1523/JNEUROSCI.21-15-05715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chen L, Kim JJ, Thompson RF. Cerebellar cortical inhibition and classical eyeblink conditioning. Proc Natl Acad Sci U S A. 2002;99:1592–1597. doi: 10.1073/pnas.032655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batini C, Billard JM, Daniel H. Long-term modification of cerebellar inhibition after inferior olive degeneration. Exp Brain Res. 1985;59:404–409. doi: 10.1007/BF00230921. [DOI] [PubMed] [Google Scholar]
- Bengtsson F, Svensson P, Hesslow G. Feedback control of Purkinje cell activity by the cerebello-olivary pathway. Eur J Neurosci. 2004;20:2999–3005. doi: 10.1111/j.1460-9568.2004.03789.x. [DOI] [PubMed] [Google Scholar]
- Berthier NE, Moore JW. Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response. Exp Brain Res. 1986;63:341–350. doi: 10.1007/BF00236851. [DOI] [PubMed] [Google Scholar]
- Berthier NE, Moore JW. Activity of deep cerebellar nuclear cells during classical conditioning of nictitating membrane extension in rabbits. Exp Brain Res. 1990;83:44–54. doi: 10.1007/BF00232192. [DOI] [PubMed] [Google Scholar]
- Billard JM, Batini C, Daniel H. The red nucleus activity in rats deprived of the inferior olivary complex. Behav Brain Res. 1988;28:127–130. doi: 10.1016/0166-4328(88)90088-5. [DOI] [PubMed] [Google Scholar]
- Bloedel JR, Bracha V. On the cerebellum, cutaneomuscular reflexes, movement control and the elusive engrams of memory. Behav Brain Res. 1995;68:1–44. doi: 10.1016/0166-4328(94)00171-b. [DOI] [PubMed] [Google Scholar]
- Bracha V, Bloedel JR. The multiple pathway model of circuits subserving the classical conditioning of withdrawal reflexes. In: Bloedel JR, Ebner TJ, Wise SP, editors. Acquisition of Motor Behavior in Vertebrates. Cambridge: MIT Press; 1996. pp. 175–204. [Google Scholar]
- Bracha V, Irwin KB, Webster ML, Wunderlich DA, Stachowiak MK, Bloedel JR. Microinjections of anisomycin into the intermediate cerebellum during learning affect the acquisition of classically conditioned responses in the rabbit. Brain Res. 1998;788:169–178. doi: 10.1016/s0006-8993(97)01535-7. [DOI] [PubMed] [Google Scholar]
- Bracha V, Kolb FP, Irwin KB, Bloedel JR. Inactivation of interposed nuclei in the cat: classically conditioned withdrawal reflexes, voluntary limb movements and the action primitive hypothesis. Exp Brain Res. 1999;126:77–92. doi: 10.1007/s002210050718. [DOI] [PubMed] [Google Scholar]
- Bracha V, Zhao L, Irwin K, Bloedel JR. Intermediate cerebellum and conditioned eyeblinks. Parallel involvement in eyeblinks and tonic eyelid closure. Exp Brain Res. 2001;136:41–49. doi: 10.1007/s002210000563. [DOI] [PubMed] [Google Scholar]
- Carrea RME, Reissig M, Mettler FA. The climbing fibers of the simian and feline cerebellum; experimental inquiry into their origin by lesions of the inferior olives and deep cerebellar nuclei. J Comp Neurol. 1947;87:371–365. doi: 10.1002/cne.900870304. [DOI] [PubMed] [Google Scholar]
- Cartford MC, Gohl EB, Singson M, Lavond DG. The effects of reversible inactivation of the red nucleus on learning-related and auditory-evoked unit activity in the pontine nuclei of classically conditioned rabbits. Learning and Memory. 1997;3:519–531. doi: 10.1101/lm.3.6.519. [DOI] [PubMed] [Google Scholar]
- Chen L, Bao SW, Lockard JM, Kim JJ, Thompson RF. Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice. J Neurosci. 1996;16:2829–2838. doi: 10.1523/JNEUROSCI.16-08-02829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Clark GA, McCormick DA, Lavond DG, Thompson RF. Effects of lesions of cerebellar nuclei on conditioned behavioral and hippocampal neuronal responses. Brain Res. 1984;291:125–136. doi: 10.1016/0006-8993(84)90658-9. [DOI] [PubMed] [Google Scholar]
- Clark RE, Lavond DG. Reversible lesions of the red nucleus during acquisition and retention of a classically conditioned behavior in rabbits. Behav Neurosci. 1993;107:264–270. doi: 10.1037//0735-7044.107.2.264. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Curr Opin Neurobiol. 2005;15:667–674. doi: 10.1016/j.conb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Dow RS, Moruzzi G. The physiology and pathology of the cerebellum. Minneapolis: University of Minnesota Press; 1958. [Google Scholar]
- Hardiman MJ, Ramnani N, Yeo CH. Reversible inactivations of the cerebellum with muscimol prevent the acquisition and extinction of conditioned nictitating membrane responses in the rabbit. Exp Brain Res. 1996;110:235–247. doi: 10.1007/BF00228555. [DOI] [PubMed] [Google Scholar]
- Hausser M, Raman IM, Otis T, Smith SL, Nelson A, Du Lac S, Loewenstein Y, Mahon S, Pennartz C, Cohen I, Yarom Y. The beat goes on: Spontaneous firing in mammalian neuronal microcircuits. Journal of Neuroscience. 2004;24:9215–9219. doi: 10.1523/JNEUROSCI.3375-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Krupa DJ, Thompson RF. Inhibitory cerebello-olivary projections and blocking effect in classical conditioning. Science. 1998;279:570–573. doi: 10.1126/science.279.5350.570. [DOI] [PubMed] [Google Scholar]
- Kolb FP, Irwin KB, Bloedel JR, Bracha V. Conditioned and unconditioned forelimb reflex systems in the cat: involvement of the intermediate cerebellum. Experimental Brain Research. 1997;114:255–270. doi: 10.1007/pl00005634. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Lang EJ. GABAergic and glutamatergic modulation of spontaneous and motor-cortex-evoked complex spike activity. J Neurophysiol. 2002;87:1993–2008. doi: 10.1152/jn.00477.2001. [DOI] [PubMed] [Google Scholar]
- Lye RH, O’Boyle DJ, Ramsden RT, Schady W. Effects of the unilateral cerebellar lesion on the acquisition of the eye-blink conditioning in man. J Physiol (Lond ) 1988;403:58. [Google Scholar]
- MacKay WA, Murphy JT. Cerebellar modulation of reflex gain. Progress in Neurobiology. 1979;13:361–417. doi: 10.1016/0301-0082(79)90004-2. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Steinmetz JE, Thompson RF. Lesions of the inferior olivary complex cause extinction of the classically conditioned eyeblink response. Brain Res. 1985;359:120–130. doi: 10.1016/0006-8993(85)91419-2. [DOI] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Mauk MD. A mechanism for savings in the cerebellum. J Neurosci. 2001;21:4081–4089. doi: 10.1523/JNEUROSCI.21-11-04081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Nores WL, Mauk MD. Inhibition of climbing fibres is a signal for the extinction of conditioned eyelid responses. Nature. 2002;416:330–333. doi: 10.1038/416330a. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Palestini M, Strata P. The inhibitory effect of the olivocerebellar input on the cerebellar Purkinje cells in the rat. J Physiol. 1982;332:187–202. doi: 10.1113/jphysiol.1982.sp014409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilaweera WU, Irwin KB, Zenitsky GD, Aksenov DP, Bracha V. Are feedback loops involved in the formulation of cerebellar neuronal correlates of conditioned eyeblinks? Soc Neurosci Abstr. 2002:79.6. [Google Scholar]
- Nilaweera WU, Zenitsky GD, Bracha V. Inactivation of cerebellar output axons impairs acquisition of conditioned eyeblinks. Brain Res. 2006;1122:143–153. doi: 10.1016/j.brainres.2006.08.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Medina JF, Nores WL, Mauk MD. Trying to understand the cerebellum well enough to build one. Ann N Y Acad Sci. 2002;978:425–438. doi: 10.1111/j.1749-6632.2002.tb07585.x. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Medina JF, Riusech FA, Mauk MD. Learning-induced plasticity in deep cerebellar nucleus. Journal of Neuroscience. 2006;26:12656–12663. doi: 10.1523/JNEUROSCI.4023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton RW. Bilateral cerebellar lesions disrupt conditioned eyelid responses in unrestrained rats. Behav Neurosci. 1988;102:586–590. doi: 10.1037//0735-7044.102.4.586. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Stowe GT, Pendlebury WW. Disrupted eyelid conditioning in a patient with damage to cerebellar afferents. Behav Neurosci. 1989;103:898–902. doi: 10.1037//0735-7044.103.4.898. [DOI] [PubMed] [Google Scholar]
- Svensson P, Bengtsson F, Hesslow G. Cerebellar inhibition of inferior olivary transmission in the decerebrate ferret. Exp Brain Res. 2005:1–13. doi: 10.1007/s00221-005-0086-y. [DOI] [PubMed] [Google Scholar]
- Thompson RF. The neurobiology of learning and memory. Science. 1986;233:941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Timmann D, Baier PC, Diener HC, Kolb FP. Classically conditioned withdrawal reflex in cerebellar patients. 1. Impaired conditioned responses. Exp Brain Res. 2000;130:453–470. doi: 10.1007/s002219900225. [DOI] [PubMed] [Google Scholar]
- Voneida TJ, Christie D, Bogdanski R, Chopko B. Changes in instrumentally and classically conditioned limb-flexion responses following inferior olivary lesions and olivocerebellar tractotomy in the cat. Journal of Neuroscience. 1990;10:3583–3593. doi: 10.1523/JNEUROSCI.10-11-03583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JP, Harvey JA. Acute inactivation of the inferior olive blocks associative learning. Eur J Neurosci. 1998;10:3321–3332. doi: 10.1046/j.1460-9568.1998.00400.x. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. I. Lesions of the cerebellar nuclei. Exp Brain Res. 1985;60:87–98. doi: 10.1007/BF00237022. [DOI] [PubMed] [Google Scholar]
- Zbarska S, Bloedel JR, Bracha V. Cerebellar dysfunction explains the extinction-like abolition of conditioned eyeblinks after NBQX injections in the inferior olive. Journal of Neuroscience. 2008;28:10–20. doi: 10.1523/JNEUROSCI.3403-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbarska S, Holland EA, Bloedel JR, Bracha V. Inferior olivary inactivation abolishes conditioned eyeblinks: extinction or cerebellar malfunction? Behav Brain Res. 2007;178:128–138. doi: 10.1016/j.bbr.2006.12.012. [DOI] [PubMed] [Google Scholar]