Abstract
NF-κB-mediated proinflammatory response to cigarette smoke (CS) plays a pivotal role in the pathogenesis of chronic obstructive pulmonary disease (COPD). The heterodimer of RelA/p65-p50 (subunits of NF-κB) is involved in transactivation of NF-κB-dependent genes, but interestingly p50 has no transactivation domain. The endogenous role of p50 subunit, particularly in regulation of CS-mediated inflammation in vivo, is not known. We therefore hypothesized that p50 subunit plays a regulatory role on RelA/p65, and genetic ablation of p50 (p50−/−) leads to increased lung inflammation and lung destruction in response to CS exposure in mouse. To test this hypothesis, p50-knockout and wild-type (WT) mice were exposed to CS for 3 days to 6 mo, and inflammatory responses as well as air space enlargement were assessed. Lungs of p50-deficient mice showed augmented proinflammatory response to acute and chronic CS exposures as evidenced by increased inflammatory cell influx and proinflammatory mediators release such as monocyte chemoattractant protein-1 (MCP-1) and interferon-inducible protein-10 (IP-10) compared with WT mice. IKK2 inhibitor (IMD-0354), which reduces the nuclear translocation of RelA/p65, attenuated CS-mediated neutrophil influx in bronchoalveolar lavage fluid and cytokine (MCP-1 and IP-10) levels in lungs of WT but not in p50-deficient mice. Importantly, p50 deficiency resulted in increased phosphorylation (Ser276 and Ser536), acetylation (Lys310), and DNA binding activity of RelA/p65 in mouse lung, associated with increased chromatin remodeling evidenced by specific phosphoacetylation of histone H3 (Ser10/Lys9) and acetylation of H4 (Lys12) in response to CS exposure. Surprisingly, p50-null mice showed spontaneous air space enlargement, which was further increased after CS exposure compared with WT mice. Thus our data showed that p50 endogenously regulates the activity of RelA/p65 by decreasing its phosphoacetylation and DNA binding activity and specific histone modifications and that genetic ablation of p50 leads to air space enlargement in mouse.
Keywords: nuclear factor-κB, oxidants, histone modification, IκB kinase 2, chronic obstructive pulmonary disease
chronic obstructive pulmonary disease (COPD) is a heterogeneous disease characterized by chronic inflammation in the small airways (bronchiolitis) and destruction of lung parenchyma (emphysema). The major risk factor for the development of COPD is inhalation of noxious particles and gases, mostly cigarette smoke (CS) that contains high levels of reactive oxygen species (approximately 1015 to 1017 oxidants/free radicals per puff), reactive aldehydes, and quinones (12, 49). Very little is known about the exact molecular mechanism of CS-induced abnormal lung inflammation that is involved in pathogenesis of COPD. It is well-known that CS-mediated activation of NF-κB plays an important role in sustained proinflammatory response seen in COPD (3, 13, 39, 40). NF-κB regulates gene expression of proinflammatory cytokines, chemokines and matrix metalloproteinases (MMPs). The members of NF-κB family include RelA/p65, RelB, c-Rel, p105/p50 (NF-κB1), and p100/p52 (NF-κB2), which function as a homodimer and heterodimer. The most abundant form of NF-κB is the RelA/p65-p50 heterodimer, which is localized predominately in the cytoplasm bound to IκBα. In response to proinflammatory and oxidative stimuli, such as CS exposure, IκBα is phosphorylated by IKK and degraded, which, in turn, allows RelA/p65-p50 to translocate into the nucleus and activate transcription of a variety of proinflammatory genes (39, 41, 46). However, the endogenous role of p50 subunit particularly in regulation of CS-mediated inflammation in vivo is not known. It is possible that p50 regulates RelA/p65 and hence proinflammatory gene transcription.
The p50 subunit of NF-κB is transcriptionally inactive because of the absence of transactivation domain. The p50 homodimers cannot recruit coactivator complex such as cAMP response element binding protein (CREB)-binding protein (CBP)/p300 and p300/CBP-associated factor (PCAF) to initiate gene transcription, whereas RelA/p65-p50 heterodimers recruit coactivators and mediate NF-κB-dependent gene expression (32, 44, 57). Furthermore, p50 homodimer is reported to repress NF-κB-dependent transcription of proinflammatory genes by competing with other transcriptionally active dimers, such as RelA/p65-p50 for binding to NF-κB motif (19, 26, 38, 41), inducing anti-inflammatory and antiapoptotic genes (26, 53), or recruiting histone deacetylases (HDACs 1–3) to bind to DNA (56, 61). Under normal conditions, a small amount of p50 is present in the nucleus (24, 47), and binding of p50 to the proinflammatory gene (TNF-α) promoter is reported to decrease gene expression (1). We therefore hypothesized that p50 is critical in regulating NF-κB (RelA/p65)-mediated proinflammatory response in the lung by CS exposure. In this study, the function of p50 in CS-induced lung inflammation and emphysema was determined by using mice homozygous for null mutations in NF-κB1 genes, which lack p50 subunit of NF-κB (p50−/−). We also studied whether genetic ablation of p50 has any impact on RelA/p65 activation and histone acetylation in response to CS exposure in mouse lung.
MATERIALS AND METHODS
Materials.
Unless otherwise stated, all biochemical reagents used in this study were purchased from Sigma (St. Louis, MO). Antibodies against phospho-RelA/p65 (Ser276; cat. no. 3037S), phospho-RelA/p65 (Ser536; cat. no. 3036S), acetylated RelA/p65 (Lys310; cat. no. 3035S), acetylated and phosphorylated histone H3 (Lys9/Ser10; cat. no. 9711S), histone H3 (cat. no. 9715), acetylated histone H4 (Lys12; cat. no. 2591S), and histone H4 (cat. no. 2592) were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against IκBα (sc-847), NF-κB RelA/p65 (sc-372), MMP-9 (sc-10737), MMP-12 (sc-30072), and lamin B (sc-6216) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-NF-κB p105/p50 (ab32360) and anti-β-actin (CP01) antibodies were purchased from Abcam (Cambridge, MA), and Calbiochem (La Jolla, CA), respectively.
Mice.
The p50 knockout mice (p50−/−), which had targeted disruption of NF-κB1 gene (42), and wild-type (WT) mice of similar genetic background (C57BL/6J × 129/SvJ and 129/SvJ) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were bred and maintained under specific pathogen-free condition in the Vivarium Facility of the University of Rochester. Targeted disruption of NF-κB1 gene was verified by genotyping, and only homozygous knockout mice were used in the study. WT and p50−/− mice were 8–10 wk of age at the beginning of experiments (air/CS exposure). All procedures were performed in accordance with the standards established by the United States Animal Welfare Acts, as set forth by National Institutes of Health (NIH) guidelines, and the research protocol for these studies was approved by the University Committee on Animal Research.
CS exposure.
Mice were exposed to acute (3 days), subchronic (8 wk), and chronic (4 or 6 mo) CS using Baumgartner-Jaeger CSM2082i cigarette smoking machine (CH Technologies, Westwood, NJ) in the Inhalation Core Facility at the University of Rochester (28, 40, 58–60). In brief, mice were placed in individual compartments of a wire cage, which was placed inside a closed plastic box connected to the smoke source. The smoke was generated from 2R4F research cigarettes containing 11.7 mg of total particulate matter (TPM), 9.7 mg of tar, and 0.76 mg of nicotine per cigarette (University of Kentucky, Lexington, KY). Mice received two 1-h exposures per day, 1 h apart, according to the Federal Trade Commission protocol (1 puff/min of 2-s duration and 35-ml volume) for 3 days to 6 mo. Mainstream CS was diluted with filtered air and directed into the exposure chamber. The smoke exposure (TPM per cubic meter of air) was monitored in real-time with a MicroDust Pro aerosol monitor (Casella CEL, Bedford, United Kingdom) and verified daily by gravimetric sampling. The smoke concentration was set at a nominal value of ∼300 mg/m3 TPM by adjusting the number of cigarettes used to produce smoke and the flow rate of the dilution air. Control mice were exposed to filtered air in an identical manner for the same duration of time. Carbon monoxide concentration in the chamber was 290–300 parts per million (ppm).
Drug administration.
IKK2 inhibitor IMD-0354 was purchased from Sigma. Vehicle (0.5% carboxymethylcellulose) or IMD-0354 was administered to mice (30 mg/kg) by intraperitoneal injection (22, 23) at 2 h before CS exposure daily for 3 days.
Differential cell count in BAL fluid.
Mice were killed at 4 or 24 h after the last exposure by an intraperitoneal injection of pentobarbital sodium (100 mg/kg; Abbott Laboratories, Abbott Park, IL) followed by exsanguination. The lungs were lavaged three times with 0.7 ml of saline via a cannula inserted into the trachea. The aliquots were combined and centrifuged, and the bronchoalveolar lavage (BAL) inflammatory cell pellet was resuspended in saline. The total cell number was determined with a hemocytometer, and cytospin slides (Thermo Shandon, Pittsburgh, PA) were prepared using 50,000 cells per slide. Differential cell counts (∼500 cells per slide) were performed on cytospin-prepared slides stained with Diff-Quik (Dade Behring, Newark, DE).
Hematoxylin and eosin staining and mean linear intercept analysis.
Mouse lungs (which had not been lavaged) were inflated by 1% low-melting agarose at a pressure of 25 cmH2O and then fixed with neutral buffered formalin. Tissues were embedded in paraffin, sectioned (4 μm), and stained with hematoxylin and eosin (H&E). Alveolar size was estimated from the mean linear intercept (Lm) of the air space, which is a measure of air space enlargement/emphysema. Lm was calculated for each sample based on 10 random fields observed at a magnification of ×200 using a cross-line as described previously (16, 25).
Immunohistochemical staining for macrophages.
Lung sections were deparaffinized and hydrated by passage through a series of xylene and graded alcohol. Endogenous peroxidase activity was quenched by exposure to 3% H2O2 in methanol for 30 min. Nonspecific binding of antibodies to the tissues was blocked by incubation of the tissue with 10% normal goat serum (Invitrogen, Carlsbad, CA) for 30 min. For detection of macrophages, the tissues were incubated with anti-mouse Mac-3 monoclonal antibody (BD Pharmingen, Franklin Lakes, NJ) overnight at 4°C at a titer of 1:100. After they were washed, the tissues were incubated with secondary antibody at a titer of 1:1,000 for 30 min. 3,3′-Diaminobenzidine (Vector Laboratories, Burlingame, CA) was used as peroxidase substrate. In each instance, sections from different groups were processed together with equal time for color development. Tissue slides were counterstained with hematoxylin. The number of Mac-3-positive cells in lung sections (5 random microscopic fields per lung section in 3 different sections) was counted manually at ×200 magnification and averaged (7, 16, 57, 59, 60).
Isolation of nuclear extract and acid extraction of histones.
One lobe of the lung was mechanically homogenized in 0.5 ml of buffer A [10 mM HEPES (pH 7.8), 10 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.1 M EDTA, 0.2 mM NaF, 0.2 mM sodium orthovanadate, 1% (vol/vol) Nonidet P-40, 0.4 mM phenylmethylsulfonyl fluoride, and 1 μg/ml leupeptin] on ice. The homogenate was centrifuged at 2,000 rpm for 30 s at 4°C to remove cellular debris. The supernatant was then transferred to a 1.7-ml ice-cold Eppendorf tube and further centrifuged for 30 s at 13,000 rpm at 4°C. The supernatant was collected as a cytoplasmic extract. The pellet was resuspended in 200 μl of buffer C [50 mM HEPES (pH 7.8), 50 mM KCl, 300 mM NaCl, 0.1 M EDTA, 1 mM DTT, 10% (vol/vol) glycerol, 0.2 mM NaF, 0.2 mM sodium orthovanadate, and 0.6 mM phenylmethylsulfonyl fluoride] and placed on the rotator in the cold room for 30 min. After centrifugation at 13,000 rpm for 5 min, the supernatant was collected as the nuclear extract and kept frozen at −80°C for immunoblotting. For extraction of histone protein, pellets from the nuclear extraction were resuspended in 150 μl of deionized water containing 0.2 N HCl and 0.36 N H2SO4. The histone proteins were precipitated from the supernatant, agitated overnight at 4°C, and then centrifuged at 13,000 rpm for 10 min, and the supernatant was transferred into a fresh tube. Ice-cold acetone precipitation samples were incubated overnight at −80°C and centrifuged, and the air-dried pellets were resuspended in 50 μl of deionized water.
Protein assay.
Protein levels were measured with a BCA kit as per the manufacturer's instructions (Thermo Scientific, Rockford, IL). Protein standards were obtained by diluting a stock solution of BSA. Linear regression was used to determine the actual protein concentration of the samples.
Cytokine analysis.
The level of proinflammatory mediators such as monocyte chemoattractant protein-1 (MCP-1) and interferon-inducible protein-10 (IP-10) in lung homogenates were measured by ELISA using respective duo-antibody kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The results were expressed in the samples as picograms per milligram protein.
Western blotting.
Proteins (20 μg) from lung tissue homogenates, including cytoplasmic and nuclear fractions, and histone extracts were separated on a 7.5–14% SDS-polyacrylamide gel. Separated proteins were electroblotted onto nitrocellulose membranes (Amersham, Arlington Heights, IL) and blocked for 1 h at room temperature with 5% BSA. The membranes were then probed with a specific primary antibody (1:1,000 dilution in 5% BSA in PBS containing 0.1% Tween 20) at 4°C overnight. After three washing steps (10 min each), the levels of protein were detected by probing with secondary anti-rabbit, anti-mouse, or anti-goat antibody (1:10,000 dilution in 5% BSA in PBS containing 0.1% Tween 20) linked to horseradish peroxidase for 1 h, and bound complexes were detected using the enhanced chemiluminescence method (PerkinElmer, Waltham, MA). Equivalent loading of the gel was determined by quantitation of protein as well as by reprobing membranes for actin, lamin B, histone H3, or histone H4. The levels of MMP-9 and MMP-12 were estimated in lungs of acute (3 days) and chronic (6 mo) air- or CS-exposed mice by immunoblotting.
EMSA.
NF-κB DNA binding was determined using the EMSA detection kit (Promega, Madison, WI) in the nuclear proteins of mouse lungs. The sequence of NF-κB consensus oligonucleotide was 5′-AGT TGA GGG GAC TTT CCC AGG C-3′ and 3′-TCA ACT CCC CTG AAA GGG TCC G-5′. Synthetic double-stranded oligonucleotides were labeled with [γ-32P]ATP using T4 polynucleotide kinase as recommended by the manufacturer. A binding mixture containing 5 μg of nuclear extract, 2.5 μl of 5× binding buffer [50 mM Tris·HCl (pH 7.5), 250 mM NaCl, 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 20% glycerol, and 0.25 mg/ml poly(dI-dC)·poly(dI-dC)], and 1 μl of γ-32P-labeled double-stranded probe was used for the DNA-binding reaction at room temperature for 30 min. To ensure that the detected bands were specific for NF-κB, a 100-fold excess of unlabeled competitor (NF-κB consensus oligonucleotide) and noncompetitor (activator protein-2 consensus oligonucleotide) was added to the reaction mixture before addition of the probe. Gel tracking dye [250 mM Tris·HCl (pH 7.5), 0.2% bromophenol blue, and 40% glycerol] was loaded into lane 1, and the DNA-protein complexes were loaded into the remaining wells without tracking dye on the 6% nondenaturing polyacrylamide gel. After electrophoresis, the gel was dried and subjected to autoradiography.
Statistical analysis.
Data were presented as means ± SE for a minimum of 3 animals per group. Statistical analysis of significance was calculated using one-way ANOVA followed by Tukey post hoc test for multigroup comparisons using StatView software. P < 0.05 was considered as significant.
RESULTS
Knockout of p50 augmented the inflammatory cell influx in response to CS exposure in mouse lungs.
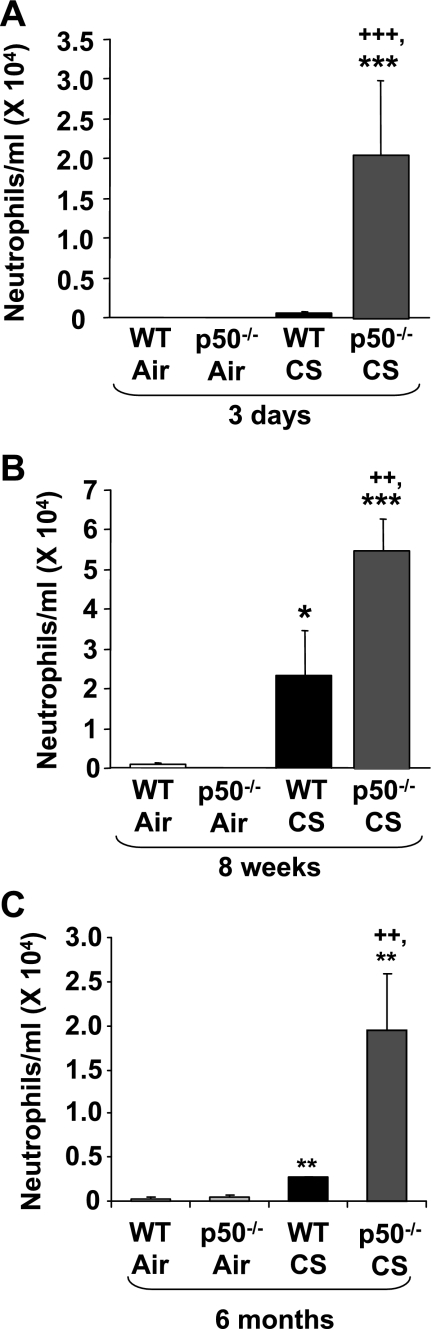
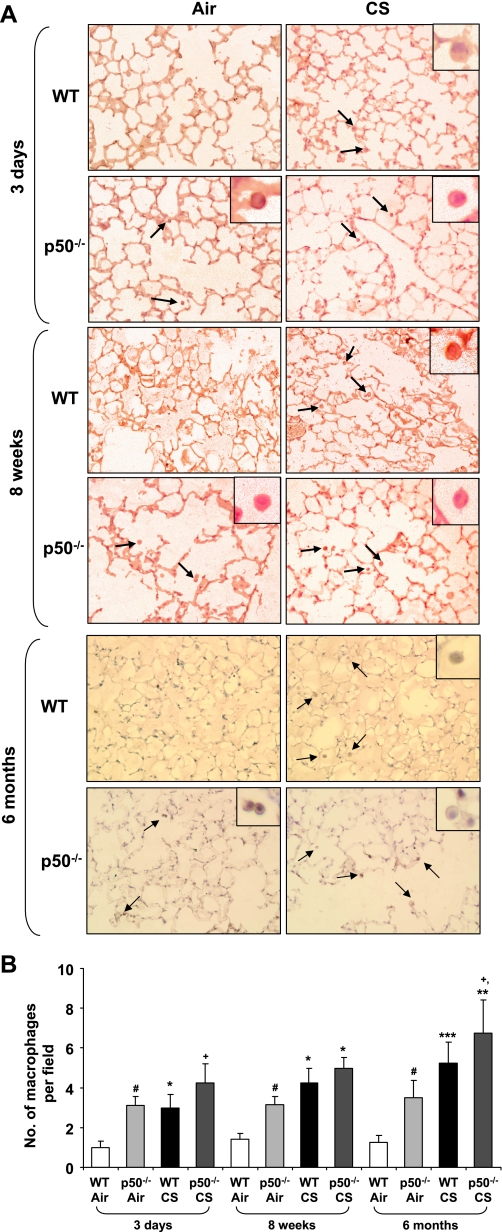
To determine the role of p50 subunit of NF-κB on CS-induced lung inflammation, p50−/− and WT mice were exposed to CS, and inflammatory cell influx into BAL fluid and lung tissue was assessed by Diff-Quik and Mac-3 immunohistochemical staining, respectively. WT mice showed significant neutrophil influx in BAL fluid only at 8 wk, but not at 3 days, of CS exposure, whereas p50−/− mice showed significantly higher neutrophil influx compared with WT mice both at 3 days and 8 wk of CS exposure (Fig. 1). The increased inflammatory cell influx in lung of p50−/− mice was persistent until 6 mo of CS exposure (Figs. 1C and 2). The number of total cells and macrophages was not significantly altered in BAL fluid from 3 days to 6 mo of CS exposure (data not shown). However, macrophage infiltration in lungs of p50−/− mice was significantly increased compared with WT mice in response to acute and subchronic CS exposure (Fig. 2). Thus p50−/− mice showed increased inflammatory cell influx in lung in response to CS exposure.
Fig. 1.
Increased neutrophil influx in bronchoalveolar lavage (BAL) fluid of p50−/− mouse in response to cigarette smoke (CS) exposure. Neutrophils were analyzed by Diff-Quik staining in the cytospin slides, which were prepared using BAL fluid collected by lung lavage in air- or CS-exposed wild-type (WT) and p50−/− mice. Data are shown as means ± SE (n = 3–4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective air-exposed mice. ++P < 0.01, +++P < 0.001, significant compared with CS-exposed WT mice.
Fig. 2.
Increased macrophage influx into the lungs of p50−/− mouse in response to CS exposure. Lung sections of air- or CS-exposed WT and p50−/− mice were stained with anti-mouse Mac-3 antibody. Mac-3-positive cells (dark brown) were identified by immunohistochemical staining, which were indicated by arrows in the picture (A; original magnification: ×200). The assessment of stained macrophages was performed (see insets) (B). Histograms are means of ≥3 experiments ± SE. *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective air-exposed mice. #P < 0.05, significant compared with air-exposed WT mice. +P < 0.05, significant compared with CS-exposed WT mice.
Knockout of p50 increased the proinflammatory mediator levels in lung in response to CS exposure.
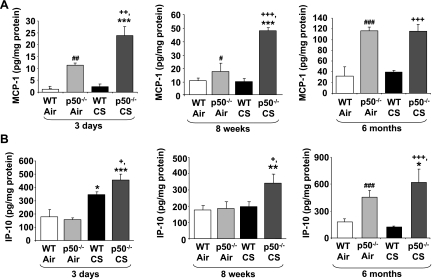
CS-mediated release of proinflammatory mediators recruit macrophages and neutrophils into the lung tissue (2, 43, 59, 60). Therefore, to confirm the increased inflammatory response in p50−/− mice exposed to CS, the levels of proinflammatory mediators (MCP-1 and IP-10) were measured in the lung homogenate using commercially available ELISA kits. Lungs of p50−/− mice showed increased basal level MCP-1 compared with WT mice (Fig. 3A). CS exposure significantly (P < 0.05) increased the level of IP-10 in WT mice at 3 days but did not alter the levels of MCP-1 compared with air-exposed group (Fig. 3). Furthermore, p50−/− mice showed increased levels of MCP-1 and IP-10 in response to 3 days to 6 mo of CS exposure compared with WT mice (Fig. 3). These results confirm that lungs of p50−/− mice are more susceptible than WT mice to CS-mediated inflammation.
Fig. 3.
Increased levels of proinflammatory mediators in lungs of p50−/− mouse in response to CS exposure. The level of proinflammatory mediators such as monocyte chemoattractant protein-1 (MCP-1; A) and interferon-inducible protein-10 (IP-10; B) were measured by ELISA in lung (homogenate) of 3 days to 6 mo air- or CS-exposed WT and p50−/− mice. Data are shown as means ± SE (n = 3–4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective air-exposed mice. #P < 0.05, ##P < 0.01, ###P < 0.001, significant compared with air-exposed WT mice. +P < 0.05, ++P < 0.01, +++P < 0.001, significant compared with CS-exposed WT mice.
Knockout of p50 led to increased air space enlargement in mouse lungs.
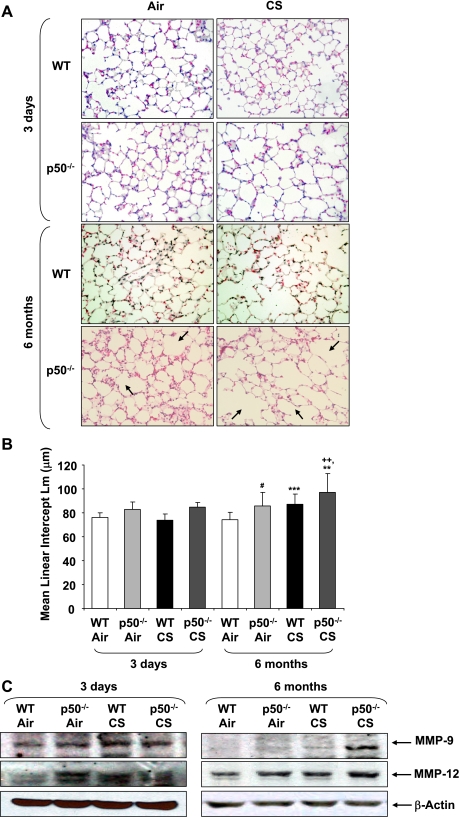
In addition to sustained inflammatory response, emphysema is also associated with CS exposure in humans (21). To assess the role of p50 in CS-mediated air space enlargement/emphysema, Lm of p50−/− mice exposed to air or CS for 3 days to 6 mo was measured. Acute CS exposure did not change the index of alveolar destruction, as assessed by Lm, in either WT or p50−/− mice. The alveolar size was quantitatively greater in air-exposed p50−/− mice at 6 mo compared with 3 days air-exposed WT and p50−/− mice (Fig. 4, A and B). In addition, the spontaneous emphysema (alveolar destruction and air space enlargement) seen in p50−/− mice was further increased by 6 mo of CS exposure. Chronic (6 mo) air- and CS-exposed mice also showed a similar trend of air space enlargement (Fig. 4), however, there was no significant difference between 4 and 6 mo of air- or CS-exposed p50−/− mice (data not shown). MMPs, particularly increased levels of MMP-9 and MMP-12, are involved in CS-induced alveolar wall destruction (59). The levels of MMPs (MMP-9 and MMP-12; Fig. 4C) were increased in lungs of p50-knockout mice in response to CS and air exposures compared with WT group. Taken together, these data suggested that p50 plays an important role in maintaining lung integrity.
Fig. 4.
CS exposure increased air space enlargement in chronic CS-exposed p50−/− mice. Figures shown are hematoxylin and eosin (H&E)-stained lung sections from air- or CS-exposed WT and p50−/− mice. Arrows indicate the air space enlargement (A; original magnification: ×200). Mean linear intercept (Lm) was calculated in H&E-stained slides (B). The levels of matrix metalloproteinases MMP-9 and MMP-12 were estimated in the lung of acute (3 days) and chronic (6 mo) air- or CS-exposed mice by immunoblotting (C). **P < 0.01, ***P < 0.001, significant compared with respective air-exposed mice. #P < 0.05, significant compared with air-exposed WT mice. ++P < 0.01, significant compared with CS-exposed WT mice.
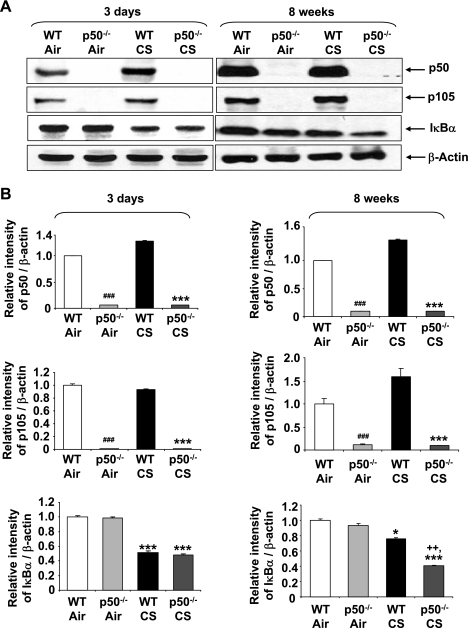
Targeted disruption of p50 increased IκBα degradation only in response to 8 wk, but not 3 days, of CS exposure.
Targeted disruption of p50 was verified by Western blotting. As shown in Fig. 5, both the p105 precursor and processed p50 protein were undetectable by anti-p105/p50 antibody in p50−/− mice. In light of our data showing increased NF-κB-dependent proinflammatory mediators release in lungs of p50−/− mice exposed to CS (Fig. 3), the nuclear translocation of RelA/p65 was determined indirectly by measuring IκBα degradation. Immunoblot analysis showed that IκBα level in lung cytosolic fraction of WT and p50−/− mice was decreased in response to CS exposure. Interestingly, lungs of p50−/− mice showed significant difference in IκBα degradation compared with WT only in response to 8 wk, but not to 3 days, of CS exposure (Fig. 5). These results suggest that p50−/− mice have increased proinflammatory response to acute CS even if the nuclear translocation of NF-κB (IκBα degradation) is the same in WT and p50−/− mice.
Fig. 5.
Increased IκBα degradation in lungs of CS-exposed WT and p50−/− mice. Knockout of NF-κB1 (p105/p50) in lungs of p50−/− mice was confirmed by immunoblotting. IκBα levels were measured in the lung cytosolic fractions of WT and p50−/− mice in response to air or CS exposures. Gel pictures shown are representative of ≥3 separate experiments (A). After densitometric analysis, the values of p50, p105, and IκBα were normalized against β-actin, respectively (B). Data are shown as means ± SE (n = 3–4 per group). *P < 0.05, ***P < 0.001, significant compared with respective air-exposed mice. ###P < 0.001, significant compared with air-exposed WT mice. ++P < 0.01, significant compared with CS-exposed WT mice.
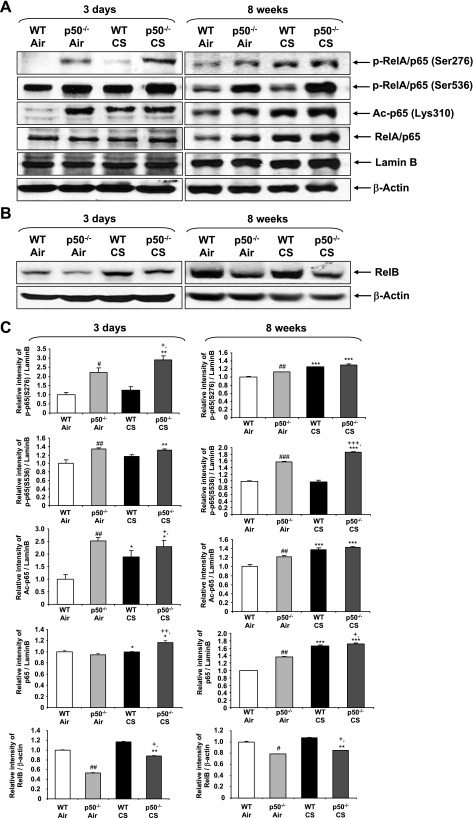
Genetic ablation of p50 led to increased activation of RelA/p65 and decreased levels of RelB in mouse lungs.
Apart from the nuclear translocation of RelA/p65, posttranslational modifications such as site-specific phosphorylation and acetylation play a key role in the activation of NF-κB and CS-mediated lung inflammation (10, 11, 50, 57, 58). We assessed the activation of nuclear NF-κB in mice lungs by measuring phosphorylation (Ser276 and Ser536) and acetylation (Lys310) of RelA/p65 subunit in response to CS exposure. The level of nuclear RelA/p65 was increased in lungs of CS-exposed WT and p50−/− mice compared with respective air-exposed mice. CS exposure increased the phosphorylation of nuclear RelA/p65 on Ser276 and Ser536 residues and acetylation at Lys310 in lungs of WT mice, which were further increased in p50−/− mice (Fig. 6). Furthermore, the basal levels of phosphorylation and acetylation of RelA/p65 were also increased in p50−/− mice compared with WT mice. The level of RelB was reduced, suggesting that anti-inflammatory effect mediated by RelB is lost in p50-deficient mice. Together, these data indicated that increased phosphorylation and acetylation (and thereby activation) of RelA/p65 are the important contributing factors in CS-mediated NF-κB-dependent proinflammatory mediator release in lungs of p50−/− mice.
Fig. 6.
Genetic ablation of p50 led to increased posttranslational modifications of RelA/p65 and decreased RelB levels in mouse lungs. Posttranslational modification or activation of RelA/p65 was assessed by measuring phosphorylation (at Ser276 and Ser536) and acetylation (at Lys310) of RelA/p65 in nuclear fractions of the lung by immunoblotting (A). RelB levels were measured in lung homogenates of WT and p50−/− mice in response to air or CS exposures. Gel pictures shown are representative of ≥3 separate experiments (B). After densitometric analysis, the values of phosphorylated (p-) p65, acetylated (Ac-) p65, p65, and RelB were normalized against lamin B and β-actin, respectively (C). Data are shown as means ± SE (n = 3–4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective air-exposed mice. #P < 0.05, ##P < 0.01, ###P < 0.001, significant compared with air-exposed WT mice. +P < 0.05, ++P < 0.01, +++P < 0.001, significant compared with CS-exposed WT mice. S, serine.
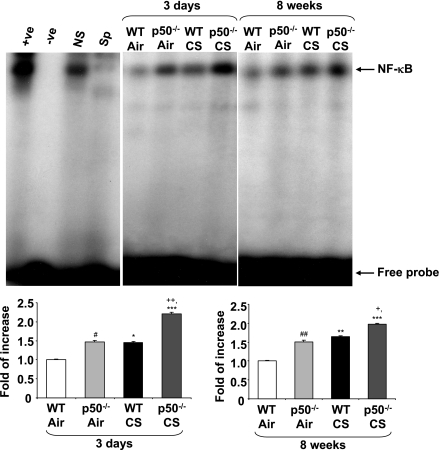
Knockout of p50 increased the DNA binding activity of RelA/p65 in mouse lungs.
An increase in RelA/p65 DNA binding is reported to occur in response to RelA/p65 acetylation, which results in increased transactivation of proinflammatory genes (10, 28, 32). To further investigate the proinflammatory response seen in the lungs of p50−/− mice, which showed increased RelA/p65 acetylation at Lys310, NF-κB DNA binding was determined in mouse lungs in response to CS by EMSA. NF-κB DNA binding activity was increased in the nuclear fractions of WT mouse lungs in response to 3 days and 8 wk of CS exposures. Lungs of p50−/− mice showed increased basal and CS-induced NF-κB DNA binding compared with corresponding WT mice (Fig. 7). These results corroborated the above observations showing the proinflammatory response of p50−/− mice was associated with increased activation of NF-κB in response to CS.
Fig. 7.
NF-κB-DNA binding activity was increased in lungs of p50−/− mice in response to CS exposure. Nuclear NF-κB-DNA binding activity in the lung of air-/CS-exposed WT and p50−/− mice was measured by EMSA using radiolabeled NF-κB oligonucleotide. The specificity of NF-κB signals was determined by specific and nonspecific competitor reactions by adding excess of nonlabeled NF-κB (specific) and activator protein-1 (nonspecific) oligonucleotides to HeLa extract. Positive and negative control reactions were also performed by using HeLa nuclear extracts and water, respectively. Gel pictures are representative of 3 separate experiments. Sp, specific; NS, nonspecific; +ve, positive control; -ve, negative control. After densitometric analysis, each value was normalized against air-exposed WT mice. Data are shown as means ± SE (n = 3–4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with respective air-exposed mice. #P < 0.05, ##P < 0.01, significant compared with air-exposed WT mice. +P < 0.05, ++P < 0.01, significant compared with CS-exposed WT mice.
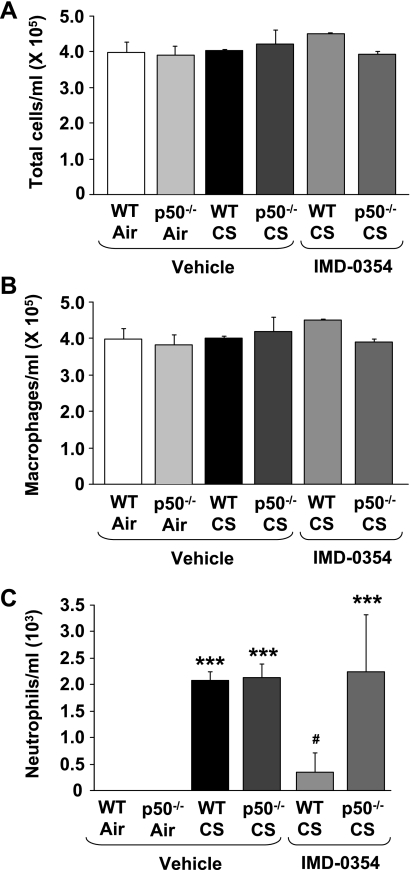
IKK2 inhibitor attenuated CS-mediated neutrophil influx in WT but not in p50−/− mice.
To gain insight into the mechanism of NF-κB activation in p50−/− mice, we investigated whether IKK2 inhibitor (30 mg/kg ip IMD-0354) could prevent the CS-mediated proinflammatory response in p50−/− mice by reducing the nuclear entry of RelA/p65. Acute CS exposure (3 days) did not change the number of total cells and macrophages in BAL fluid (Fig. 8, A and B). WT and p50−/− mice showed increased neutrophil influx in BAL fluid in response to CS at 4 h of postlast exposure (Fig. 8C). Moreover, there was no significant difference between WT and p50−/− mice as observed at 24 h of postlast exposure to CS (Fig. 1). Pretreatment of IKK2 inhibitor decreased CS-mediated neutrophil influx in WT mice compared with corresponding CS-exposed mice. Surprisingly, there was no difference in neutrophil influx in IKK2 inhibitor-administered p50−/− mice compared with corresponding vehicle group in response to CS (Fig. 8C). These results demonstrated that IKK2 inhibitor decreased proinflammatory cell influx only in WT but not in p50−/− mice.
Fig. 8.
IKK2 inhibitor attenuated neutrophil influx in BAL fluid of WT, but not in p50−/− mice, in response to CS exposure. Total (A), macrophage (B), and neutrophil (C) numbers were counted in Diff-Quik-stained cytospin slides, which were prepared using BAL fluid collected from mouse lungs at 4 h of postlast CS exposure. Administration of IKK2 inhibitor (30 mg/kg ip for 3 days) to mice at 2 h before CS exposure was indicated as IMD-0354. Data are shown as means ± SE (n = 3–4 per group). ***P < 0.001, significant compared with respective air-exposed mice. #P < 0.05, significant compared with vehicle-administered CS-exposed WT mice.
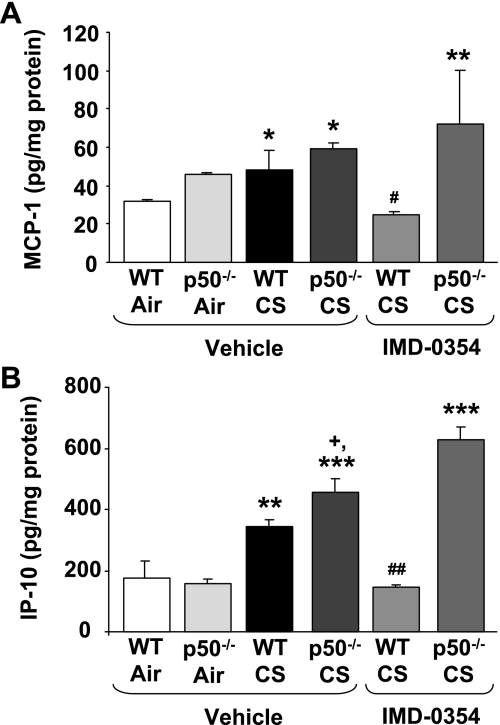
IKK2 inhibitor decreased the proinflammatory cytokine levels in response to CS in WT but not in p50−/− mice.
To confirm the effect of IKK2 inhibitor on CS-mediated lung inflammation in p50−/− mice, the levels of proinflammatory cytokines such as MCP-1 and IP-10 were measured in lung homogenates. CS exposure increased the levels of MCP-1 and IP-10 in lungs of WT and p50−/− mice compared with respective air-exposed WT/p50−/− mice (Fig. 9). In addition, lungs of p50−/− mice showed significantly (P < 0.05) increased levels of IP-10 compared with CS-exposed WT mice (Fig. 9B). IKK2 inhibitor pretreatment significantly decreased the levels of MCP-1 and IP-10 in WT mice compared with vehicle-treated group in response to CS exposure. However, p50−/− mice did not show any change in CS-mediated increase in MCP-1 and IP-10 in response to IKK2 inhibitor pretreatment. These results confirmed that the posttranslational modifications (phosphorylation and acetylation) of NF-κB, rather than nuclear entry, are the key in mediating hyperinflammatory response seen in p50−/− mice.
Fig. 9.
IKK2 inhibitor decreased proinflammatory cytokine levels in lungs of WT, but not p50−/−, mice in response to CS exposure. Lung levels of proinflammatory mediators such as MCP-1 (A) and IP-10 (B) were measured in vehicle or IMD-0354-administered mice, which were killed at 4 h of postlast exposure to CS. Data are shown as means ± SE (n = 3–4 per group). *P < 0.05, **P < 0.01, ***P < 0.001, significant compared with air-exposed WT mice. +P < 0.05, significant compared with CS-exposed WT mice. #P < 0.05, ##P < 0.01, significant compared with vehicle-administered CS-exposed WT mice.
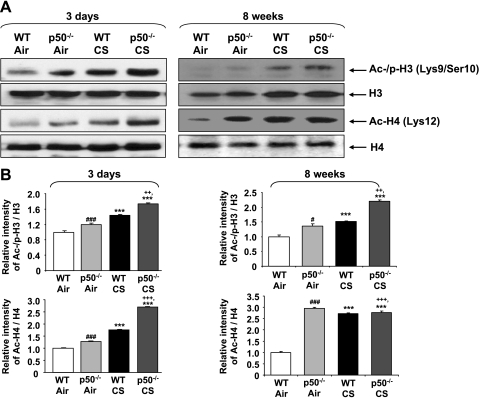
Targeted disruption of p50 altered CS-mediated chromatin remodeling.
It has been shown that phosphorylated RelA/p65 facilitates the recruitment of CBP/p300 and other coactivators to chromatin and thereby induces chromatin remodeling (9, 32, 39). Our data showed increased phosphorylation, acetylation, and DNA binding of nuclear RelA/p65 in p50−/− mice. Chromatin modifications (phosphoacetylation of histone H3 and acetylation of histone H4) were measured in WT and p50−/− mice in response to CS exposure using acid-extracted histone proteins by Western blotting. Phosphoacetylation of histone H3 (Ser10/Lys9) and acetylation of H4 (Lys12) were increased in acute CS-exposed WT mouse lungs (Fig. 10). Targeted disruption of p50 further increased the degree of histone modifications compared with WT mice in response to CS exposure. These results suggested that the proinflammatory response seen in p50−/− mice is associated with increased chromatin modifications, which allow the binding of transcription factors, such as activated RelA/p65, on various proinflammatory gene promoters.
Fig. 10.
Knockout of p50 led to increased chromatin remodeling in mouse lungs in response to CS exposure. Chromatin modification was assessed by measuring phosphorylation (at Ser10) and acetylation (at Lys9) of histone H3 and acetylation of histone H4 (at Lys12) in the lung by immunoblotting using acid-extracted nuclear histone fraction. Gel pictures shown are representative of ≥3 separate experiments (A). After densitometric analysis, the values of Ac-/p-He and Ac-H4 were normalized against total H3 and H4, respectively (B). Data are shown as means ± SE (n = 3–4 per group). ***P < 0.001, significant compared with respective air-exposed mice. #P < 0.05, ###P < 0.001, significant compared with air-exposed WT mice. ++P < 0.01, +++P < 0.001, significant compared with CS-exposed WT mice.
DISCUSSION
It is known that NF-κB (subunit RelA/p65) is involved in CS-mediated lung proinflammatory response in rodents, smokers, and patients with COPD (39, 49, 57). However, the role of p50, the heterodimerization partner of RelA/p65, in regulation of NF-κB-mediated proinflammatory response, particularly by CS, is unknown. We used p50-knockout mice to understand the role in p50 in lung inflammation and lung physiology in response to acute, subchronic, and chronic CS exposures. CS-induced proinflammatory response, especially the release of proinflammatory cytokines/chemokines, is mediated by recruiting macrophages and neutrophils into the lung tissues (2, 43, 59, 60). Therefore, we exposed p50-knockout and WT mice to CS from 3 days (acute) up to 6 mo (chronic) and measured the inflammatory cell influx in BAL fluid and lung tissue. This hypothesis is supported by the observation that p50 is capable of upregulating the anti-inflammatory gene (IL-10) promoter, whereas other NF-κB subunits do not have this property (6). No differences in neutrophil influx in BAL fluid were apparent between WT and p50−/− mice when exposed to air, whereas in response to CS, p50−/− mice showed increased neutrophil influx in BAL fluid compared with WT mice. There was no significant difference in macrophage counts in BAL fluid between WT and p50−/− mice in response to CS, but the influx of macrophages into the lung tissue was increased in p50−/− mice compared with WT mice, which was augmented in response to CS exposure. The susceptibility of p50−/− mice to CS-mediated inflammation is confirmed by the increased levels of NF-κB-dependent proinflammatory mediators MCP-1 and IP-10 in lungs of p50−/− mice compared with WT mice. The increased inflammatory cell influx in lung tissue of p50−/− mice could be responsible for increased proinflammatory cytokine release. These findings are corroborated with the previous observations that p50−/− mice showed an augmented proinflammatory response to LPS (17, 31), IL-1 (5), Escherichia coli pneumonia (30), and myocardial infarction (48), which was associated with increased NF-κB activity and NF-κB-dependent proinflammatory cytokine release (19). However, p50−/− mice showed reduced inflammatory response to ovalbumin (55), IL-1/methylated BSA (4), IL-13 (8), and ozone (14). Moreover, p50 inhibitor andrographolide prevented NF-κB activation in inflammation and deep vein thrombosis (27, 54). The reason for this discrepancy is not known. However, NF-κB is activated in response to various proinflammatory stimuli through different signaling pathways, which might be a reason for the different inflammatory response of the p50−/− mice to various stimuli.
In addition to the increased proinflammatory response to CS exposure as discussed above, p50−/− mice showed spontaneous emphysema after 4 mo of air exposure. This spontaneous air space enlargement was evidenced by a significant increase in Lm, which represents the average size of alveoli, which was further increased in response to chronic CS exposure (4 mo). A similar trend was observed after 6 mo of air and CS exposure to p50−/− mouse lung. The increase in air space enlargement may be due to the increased levels of MMPs in the lungs of p50−/− mice, such as MMP-9 and MMP-12, which play a key role in pathogenesis of emphysema formation (15, 20, 48). It suggests that genetic ablation of p50 in mice affects basal activity thus leading to activation or induction of MMPs, which may be key players involved in air space enlargement in p50-deficient mice.
The transcription of proinflammatory cytokines, chemokines, and MMPs are regulated by the transcription factor NF-κB, especially RelA/65-p50 heterodimers (36, 51). NF-κB activity is mainly controlled by its association with the IκB proteins, which prevents NF-κB entering into the nucleus. CS exposure increased the degradation of IκBα in WT and p50−/− mice lungs, which was associated with increased nuclear levels of RelA/p65. Interestingly, there was a significant difference in IκBα degradation and nuclear RelA/p65 levels between WT and p50−/− mice only in response to subchronic, but not to acute, CS exposure. Our data further show that the nuclear translocation of NF-κB was similar in WT and p50−/− mice, although p50−/− mice showed increased proinflammatory response to acute CS exposure. However, during chronic CS exposure condition, sustained proinflammatory cell influx might cause increased release of proinflammatory mediators and reactive oxygen species, which lead to increased IκBα degradation (via IKK2 activation) and nuclear translocation of RelA/p65 as seen in lungs of p50−/− mice compared with those of WT.
The increased NF-κB-dependent cytokine release in p50−/− mice might be due to increased activation/posttranslational modifications of RelA/p65 as well as due to the absence of its dimerization partner, p50. To prove this contention, we tested the phosphorylation and acetylation status of RelA/p65 in the nuclear fractions of lungs of p50−/− in response to CS. We observed that p50 deficiency leads to increased phosphorylation of RelA/p65 on Ser276 and Ser536. CS-mediated increase in RelA/p65 phosphorylation was further increased in lungs of p50−/− mice compared with WT. Phosphorylation of RelA/p65 at Ser276 promotes the interaction with coactivating acetylase CBP/p300 (32, 33, 56, 57, 61), whereas phosphorylation at Ser536 is a prerequisite for acetylation of RelA/p65 at Lys310 (11). Acetylation of RelA/p65, especially at Lys310, is required for full transactivation function of RelA/p65 (10, 11). CS exposure induced the acetylation of RelA/p65 at Lys310 in WT mouse lungs as reported earlier in vivo and in vitro (40, 59), which was further increased in p50−/− mice. Furthermore, the basal level of acetylation was also increased in lungs of p50−/− mice compared with air-exposed WT. Genetic ablation of p50 indicates that the p50 subunit plays a vital role in regulating modifications in RelA/p65 leading to increased phosphorylation and acetylation (activation) even in the absence of proinflammatory stimuli. Activation of RelA/p65 was associated with reduced levels of RelB in p50-knockout mice exposed to CS. RelB is known to act as an anti-inflammatory transcription factor, and it has been shown that p50 compensates in control of proinflammatory response in RelB-deficient mice (52). Loss of p50-RelB would lead to severe inflammatory response in mouse lung as shown by our data. Overall, these results indicate that p50 subunit plays a regulatory role in controlling the posttranslational modifications of RelA/p65 and thereby limits NF-κB activity.
Posttranslational modifications such as phosphorylation and acetylation regulate the DNA binding properties of RelA/p65 and recruitment of transcriptional coactivators (9). Previous reports showed that CS-mediated phosphorylation and acetylation of RelA/p65 is associated with increased NF-κB DNA binding and histone modifications, which result in proinflammatory cytokine release (28, 32, 59). Genetic ablation of p50 leads to increased DNA binding ability of RelA/p65 in basal condition as well as in response to CS exposure. Binding of activated NF-κB to DNA leads to recruitment of transcriptional coactivators to the chromatin and thereby causes chromatin remodeling. We observed the increased phosphorylation of histone H3 at Ser10, an early step in chromatin remodeling that facilitates its interaction with CBP (37, 45). CS-mediated acetylation of histone H3 at Lys9 and histone H4 at Lys12 is also increased in lungs of p50−/− mice compared with WT. Acetylation of histones unwind the chromatin for the binding of transcription factors to various proinflammatory gene promoters, which is, at least in part, responsible for increased proinflammatory response seen in p50−/− mice. Overall, our data suggest that p50 regulates RelA/p65 modifications, DNA binding activity, and chromatin remodeling.
In NF-κB pathway, phosphorylation-mediated degradation of IκBα by IKK, especially by IKK2, is essential in the nuclear translocation of NF-κB-RelA/p65 and its activation (9, 39). A selective IKK2 inhibitor, IMD-0354, is reported to reduce nuclear translocation and activation of NF-κB by suppressing IKK2 and IκBα degradation (22, 34, 35). Administration of IKK2 inhibitor showed anti-inflammatory effect against CS in WT mice by significantly reducing neutrophil influx into BAL fluid and the levels of proinflammatory mediators such as MCP-1 and IP-10 in mouse lung. Interestingly, IKK2 inhibitor-administered p50−/− mice did not show any reduction in neutrophil influx or in lung cytokine levels compared with vehicle-administered p50−/− mice in response to CS. This might be due to the increased posttranslational modification/activation of RelA/p65 seen in p50−/− mice, which mediates the NF-κB-dependent proinflammatory response despite reduction in the nuclear translocation of RelA/p65. These results further support the role of increased posttranslational modifications (phosphorylation and acetylation and hence sustained binding of RelA/p65 on proinflammatory promoters) in CS-mediated proinflammatory response seen in p50−/− mice rather than the change in the nuclear translocation of RelA/p65.
In conclusion, targeted disruption of p50 resulted in increased lung inflammatory response to CS exposure, which was associated with increased posttranslational modifications such as phosphorylation and acetylation of RelA/p65, increased RelA/p65-DNA binding activity, and histone modifications. Increased NF-κB activation and MMPs levels in p50−/− mice may have an effect in CS-induced air space enlargement in p50 null mice, suggesting that p50 plays a regulatory role by masking the proinflammatory effects of RelA/p65 (subunit of NF-κB) by reducing its phosphorylation, acetylation, and DNA binding. These data demonstrate the role of p50 in NF-κB regulation and in lung pathophysiology.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-085613 and 1-R01-HL-097751-01, Institute for Science and Health, and National Institute of Environmental Health Sciences Grant ES-01247.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Jae-woong Hwang, Dr. Gnanapragasam Arunachalam, Samuel Caito, and Suzanne E. Cook for their assistance during animal experiments.
REFERENCES
- 1.Baer M, Dillner A, Schwartz RC, Sedon C, Nedospasov S, Johnson PF. Tumor necrosis factor alpha transcription in macrophages is attenuated by an autocrine factor that preferentially induces NF-kappaB p50. Mol Cell Biol 18: 5678–5689, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 22: 672–688, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Brown V, Elborn JS, Bradley J, Ennis M. Dysregulated apoptosis and NFkappaB expression in COPD subjects. Respir Res 10: 24–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell IK, Gerondakis S, O'Donnell K, Wicks IP. Distinct roles for the NF-kappaB1 (p50) and c-Rel transcription factors in inflammatory arthritis. J Clin Invest 105: 1799–1806, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell SJ, Anthony DC, Oakley F, Carlsen H, Elsharkawy AM, Blomhoff R, Mann DA. Hepatic nuclear factor kappa B regulates neutrophil recruitment to the injured brain. J Neuropathol Exp Neurol 67: 223–230, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Cao S, Zhang X, Edwards JP, Mosser DM. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J Biol Chem 281: 26041–26050, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro P, Legora-Machado A, Cardilo-Reis L, Valenca S, Porto LC, Walker C, Zuany-Amorim C, Koatz VL. Inhibition of interleukin-1beta reduces mouse lung inflammation induced by exposure to cigarette smoke. Eur J Pharmacol 498: 279–286, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Chapoval SP, Al-Garawi A, Lora JM, Strickland I, Ma B, Lee PJ, Homer RJ, Ghosh S, Coyle AJ, Elias JA. Inhibition of NF-kappaB activation reduces the tissue effects of transgenic IL-13. J Immunol 179: 7030–7041, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 5: 392–401, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J 21: 6539–6548, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol 25: 7966–7975, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 64: 111–126, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Stefano A, Caramori G, Oates T, Capelli A, Lusuardi M, Gnemmi I, Ioli F, Chung KF, Donner CF, Barnes PJ, Adcock IM. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J 20: 556–563, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Fakhrzadeh L, Laskin JD, Laskin DL. Ozone-induced production of nitric oxide and TNF-α and tissue injury are dependent on NF-κB p50. Am J Physiol Lung Cell Mol Physiol 287: L279–L285, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Finlay GA, O‘Driscoll LR, Russell KJ, D'Arcy EM, Masterson JB, FitzGerald MX, O'Connor CM. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am J Respir Crit Care Med 156: 240–247, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Foronjy RF, Mercer BA, Maxfield MW, Powell CA, D'Armiento J, Okada Y. Structural emphysema does not correlate with lung compliance: lessons from the mouse smoking model. Exp Lung Res 31: 547–562, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Gadjeva M, Tomczak MF, Zhang M, Wang YY, Dull K, Rogers AB, Erdman SE, Fox JG, Carroll M, Horwitz BH. A role for NF-kappa B subunits p50 and p65 in the inhibition of lipopolysaccharide-induced shock. J Immunol 173: 5786–5793, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, Cosio MG. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med 170: 974–980, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Han W, Joo M, Everhart MB, Christman JW, Yull FE, Blackwell TS. Myeloid cells control termination of lung inflammation through the NF-κB pathway. Am J Physiol Lung Cell Mol Physiol 296: L320–L327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277: 2002–2004, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 364: 709–721, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Inayama M, Nishioka Y, Azuma M, Muto S, Aono Y, Makino H, Tani K, Uehara H, Izumi K, Itai A, Sone S. A novel IkappaB kinase-beta inhibitor ameliorates bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 173: 1016–1022, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kamon J, Yamauchi T, Muto S, Takekawa S, Ito Y, Hada Y, Ogawa W, Itai A, Kasuga M, Tobe K, Kadowaki T. A novel IKKbeta inhibitor stimulates adiponectin levels and ameliorates obesity-linked insulin resistance. Biochem Biophys Res Commun 323: 242–248, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kang SM, Tran AC, Grilli M, Lenardo MJ. NF-kappa B subunit regulation in nontransformed CD4+ T lymphocytes. Science 256: 1452–1456, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Kawakami M, Paul JL, Thurlbeck WM. The effect of age on lung structure in male BALB/cNNia inbred mice. Am J Anat 170: 1–21, 1984 [DOI] [PubMed] [Google Scholar]
- 26.Kurland JF, Kodym R, Story MD, Spurgers KB, McDonnell TJ, Meyn RE. NF-kappaB1 (p50) homodimers contribute to transcription of the bcl-2 oncogene. J Biol Chem 276: 45380–45386, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Li YD, Ye BQ, Zheng SX, Wang JT, Wang JG, Chen M, Liu JG, Pei XH, Wang LJ, Lin ZX, Gupta K, Mackman N, Slungaard A, Key NS, Geng JG. NF-κB transcription factor p50 critically regulates tissue factor in deep vein thrombosis. J Biol Chem 284: 4473–4483, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, Macnee W, Rahman I. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol 31: 633–642, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Massaro D, Massaro GD. Apoetm1Unc mice have impaired alveologenesis, low lung function, and rapid loss of lung function. Am J Physiol Lung Cell Mol Physiol 294: L991–L997, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Mizgerd JP, Lupa MM, Kogan MS, Warren HB, Kobzik L, Topulos GP. Nuclear factor-kappaB p50 limits inflammation and prevents lung injury during Escherichia coli pneumonia. Am J Respir Crit Care Med 168: 810–817, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Mizgerd JP, Lupa MM, Spieker MS. NF-kappaB p50 facilitates neutrophil accumulation during LPS-induced pulmonary inflammation. BMC Immunol 5: 10, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J 18: 1897–1899, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Okazaki T, Sakon S, Sasazuki T, Sakurai H, Doi T, Yagita H, Okumura K, Nakano H. Phosphorylation of serine 276 is essential for p65 NF-kappaB subunit-dependent cellular responses. Biochem Biophys Res Commun 300: 807–812, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Onai Y, Suzuki J, Kakuta T, Maejima Y, Haraguchi G, Fukasawa H, Muto S, Itai A, Isobe M. Inhibition of IkappaB phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovasc Res 63: 51–59, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Onai Y, Suzuki J, Maejima Y, Haraguchi G, Muto S, Itai A, Isobe M. Inhibition of NF-κB improves left ventricular remodeling and cardiac dysfunction after myocardial infarction. Am J Physiol Heart Circ Physiol 292: H530–H538, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18: 6853–6866, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Park GY, Wang X, Hu N, Pedchenko TV, Blackwell TS, Christman JW. NIK is involved in nucleosomal regulation by enhancing histone H3 phosphorylation by IKKalpha. J Biol Chem 281: 18684–18690, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plaksin D, Baeuerle PA, Eisenbach L. KBF1 (p50 NF-kappa B homodimer) acts as a repressor of H-2Kb gene expression in metastatic tumor cells. J Exp Med 177: 1651–1662, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajendrasozhan S, Yang SR, Edirisinghe I, Yao H, Adenuga D, Rahman I. Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxid Redox Signal 10: 799–811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177: 861–870, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitz ML, Baeuerle PA. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J 10: 3805–3817, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 80: 321–330, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Shapiro SD. The macrophage in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 160: S29–S32, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Sheppard KA, Rose DW, Haque ZK, Kurokawa R, McInerney E, Westin S, Thanos D, Rosenfeld MG, Glass CK, Collins T. Transcriptional activation by NF-kappaB requires multiple coactivators. Mol Cell Biol 19: 6367–6378, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strahl BD, Allis CD. The language of covalent histone modifications. Nature 403: 41–45, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem 278: 24233–24241, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Ten RM, Paya CV, Israel N, Le Bail O, Mattei MG, Virelizier JL, Kourilsky P, Israel A. The characterization of the promoter of the gene encoding the p50 subunit of NF-kappa B indicates that it participates in its own regulation. EMBO J 11: 195–203, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Timmers L, van Keulen JK, Hoefer IE, Meijs MF, van Middelaar B, den Ouden K, van Echteld CJ, Pasterkamp G, de Kleijn DP. Targeted deletion of nuclear factor kappaB p50 enhances cardiac remodeling and dysfunction following myocardial infarction. Circ Res 104: 699–706, 2009 [DOI] [PubMed] [Google Scholar]
- 49.van Eeden SF, Sin DD. Chronic obstructive pulmonary disease: a chronic systemic inflammatory disease. Respiration 75: 224–238, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Vermeulen L, De Wilde G, Notebaert S, Vanden Berghe W, Haegeman G. Regulation of the transcriptional activity of the nuclear factor-kappaB p65 subunit. Biochem Pharmacol 64: 963–970, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Vlahos R, Bozinovski S, Jones JE, Powell J, Gras J, Lilja A, Hansen MJ, Gualano RC, Irving L, Anderson GP. Differential protease, innate immunity, and NF-κB induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. Am J Physiol Lung Cell Mol Physiol 290: L931–L945, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Weih F, Durham SK, Barton DS, Sha WC, Baltimore D, Bravo R. p50-NF-kappaB complexes partially compensate for the absence of RelB: severely increased pathology in p50(−/−)relB(−/−) double-knockout mice. J Exp Med 185: 1359–1370, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wessells J, Baer M, Young HA, Claudio E, Brown K, Siebenlist U, Johnson PF. BCL-3 and NF-kappaB p50 attenuate lipopolysaccharide-induced inflammatory responses in macrophages. J Biol Chem 279: 49995–50003, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Xia YF, Ye BQ, Li YD, Wang JG, He XJ, Lin X, Yao X, Ma D, Slungaard A, Hebbel RP, Key NS, Geng JG. Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50. J Immunol 173: 4207–4217, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. J Exp Med 188: 1739–1750, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-κB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol 291: L46–L57, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Yang SR, Valvo S, Yao H, Kode A, Rajendrasozhan S, Edirisinghe I, Caito S, Adenuga D, Henry R, Fromm G, Maggirwar S, Li JD, Bulger M, Rahman I. IKK alpha causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol 38: 689–698, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol 292: L567–L576, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 294: L1174–L1186, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Yao H, Edirisinghe I, Yang SR, Rajendrasozhan S, Kode A, Caito S, Adenuga D, Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol 172: 1222–1237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell 9: 625–636, 2002 [DOI] [PubMed] [Google Scholar]