Abstract
Hypoxia plays an important role in development, cellular homeostasis, and pathological conditions, such as cancer and stroke. There is also growing evidence that hypoxia is an important modulator of the inflammatory process. Hypoxia-inducible factors (HIFs) are a family of proteins that regulate the cellular response to oxygen deficit, and loss of HIFs impairs inflammatory cell function. There is little known, however, about the role of epithelial-derived HIF signaling in modulating inflammation. Cobalt is capable of eliciting an allergic response and promoting HIF signaling. To characterize the inflammatory function of epithelial-derived HIF in response to inhaled cobalt, a conditional lung-specific HIF1α, the most ubiquitously expressed HIF, deletion mouse, was created. Control mice showed classic signs of metal-induced injury following cobalt exposure, including fibrosis and neutrophil infiltration. In contrast, HIF1α-deficient mice displayed a Th2 response that resembled asthma, including increased eosinophilic infiltration, mucus cell metaplasia, and chitinase-like protein expression. The results suggest that epithelial-derived HIF signaling has a critical role in establishing a tissue's inflammatory response, and compromised HIF1α signaling biases the tissue towards a Th2-mediated reaction.
Keywords: deletion mouse
lung diseases, including chronic obstructive pulmonary disease and asthma, involve a large inflammatory component. The lung's response to allergens involves a complex interplay between resident inflammatory and epithelial cells, cytokine signaling, and the environmental conditions within the tissue. One of the critical environmental features that can impact the inflammatory process is hypoxia.
Hypoxia, a decrease in available oxygen reaching the tissues of the body, has profound cellular and metabolic consequences. The cellular response to hypoxia is regulated by a family of transcription factors called the hypoxia-inducible factors (HIFs) (3). HIFs are primarily regulated at the level of protein stability by a family of prolyl hydroxylases. These prolyl hydroxylase domain (PHD) proteins are members of a broader family of non-heme, iron-, and 2-oxoglutarate-dependent dioxygenases (7). Cobalt has been shown to inhibit PHDs, and this inhibition causes very similar transcriptional outputs to that of hypoxia (24, 28). Recent research using human peripheral blood mononuclear cells has shown that this transcriptional overlap applies to tungsten carbide-cobalt particles, linking hard metal lung disease to hypoxia signaling (17).
HIF1α is the most ubiquitously expressed and widely studied HIF isoform. HIF1α heterodimerizes with the aryl hydrocarbon receptor nuclear translocator (ARNT, also known as HIF1β) forming the functional transcription factor HIF1. HIF1 regulates the expression of more than 100 genes, including genes for glycolytic enzymes, sugar transporters, and proangiogenic and inflammatory factors (8, 13, 18, 25). Moreover, HIF1α has also been shown to modulate inflammation indirectly by influencing the NF-κB signaling pathway (13, 20). Given the relationship between cobalt, HIF1α, and inflammation, it seems likely that HIF1α will impact cobalt-induced injury in vivo. More specifically, it is hypothesized that cobalt-induced HIF1-mediated transcription will impact cobalt-related asthma and/or hard metal lung disease (17).
Cobalt (or hard metal) asthma is one of three occupational respiratory diseases associated with exposure to the transition metal. The other two are hypersensitivity pneumonitis and interstitial lung disease with fibrosis. These diseases are caused by the inhalation of hard metal particles and are characterized by airway constriction, alveolitis, fibrosis, and associated giant cell interstitial pneumonitis (15). Asthma associated with cobalt exposure most likely involves an allergic response and has variable latency periods following initial sensitization (9, 26, 27). Cobalt-specific immunoglobulin isotype E (IgE) has been characterized in workers with signs of cobalt asthma, and their symptoms can be relieved upon removal from the contaminated environment (27). Besides acting as a pro-oxidant and sensitizer in the lung and skin, cobalt has also been characterized as a hypoxia mimic (28).
To characterize the role of HIF1α in cobalt-induced lung injury, a lung-specific HIF1α knockout mouse model was created. In utero deletion of HIF1α led to lethality due to respiratory distress upon parturition (23). In the present study, postnatal deletion of HIF1α from type II and Clara cells had no observable pathology. To elucidate the role of epithelial-derived HIF1α signaling in cobalt-induced lung injury, these mice were exposed to cobalt chloride via oropharyngeal aspiration. Compared with control mice, mice that were HIF1α deficient in their lungs (HIF1αΔ/Δ) exhibited airway infiltration of eosinophils associated with airway epithelial changes, including mucus cell metaplasia and increased levels of the chitinase-like proteins YM1 and YM2. Mice deficient in HIF1α also showed a drastic change in cytokine profiles in their lavage fluid compared with their control. These results suggest that loss of HIF1α from alveolar type II epithelial and Clara cells of the lungs leads to cellular and molecular processes that are associated with asthma following cobalt exposure and that airway epithelial-derived HIF1α plays a critical role in modulating the inflammatory response of the lung.
MATERIALS AND METHODS
Description of mice.
Triple transgenic mice were created by mating HIF1αflox/flox (a generous gift of Randall Johnson, Univ. California San Diego) and SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg transgenic mice (a generous gift of Jeffrey A. Whitsett, Cincinnati Children's Hospital Medical Center) (16, 19, 21, 22). The generated mice, SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg/HIF1αflox/flox, are capable of respiratory epithelium-specific conditional recombination in the floxed HIF1α gene upon exposure to doxycycline (19). In addition to the triple transgenic controls, four additional genotype controls were employed to rule out effects of any one locus in the presence and absence of doxycycline. These include SP-C-rtTA−/tg/(tetO)7-CMV-Cre−/−/HIF1α+/+ (sTH), SP-C-rtTA−/−/(tetO)7-CMV-Cretg/tg/HIF1α+/+ (StH), SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg/HIF1α+/+ (stH), and SP-C-rtTA−/−/(tetO)7-CMV-Cre−/−/HIF1αflox/flox (STh). The HIF1αflox/flox were originally maintained in a C57BL/6 background, whereas the SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg were generated in an FVB/N genetic background. These parental strains were carefully mated to acquire the necessary genotypes for the described experiments, and all of the mice used in this study have been maintained in this mixed C57BL/6 and FVB/N background. Genotyping of the mice was performed by PCR for all three loci as previously described (23).
Doxycycline treatment and animal husbandry.
In utero exposure to doxycycline in the triple transgenic mice led to lethality upon parturition (23). Postnatal recombination was carried out by exposing lactating dams to doxycycline-containing feed (625 mg doxycycline/kg; Harlan Teklad, Madison, WI) and drinking water (0.8 mg/ml, Sigma Chemical) until weaning. Triple transgenic mice were then maintained on the same doxycycline-containing food and water until they were ∼7 wk of age. Doxycycline treatment was terminated 7–10 days before first exposure to metals. These mice will be referred to as HIF1α deficient or HIF1αΔ/Δ throughout the paper. Control animals used in the study were triple transgenic [SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg/HIF1αflox/flox] mice that were maintained on normal food and water ad libitum. All the remaining genotype control lines described above were exposed to doxycycline for the same 7-wk paradigm when appropriate. Mice used in this study were kept at the animal housing facility under the strict hygienic and pathogen-free conditions approved by the university laboratory animal resource (ULAR) regulatory unit. All the animal handling and necropsy protocols were approved by the ULAR regulatory unit of Michigan State University.
Cobalt exposure, tissue harvesting, and processing.
Eighteen mice from each genotype were randomly assigned to one of three groups. Mice were treated with saline and 5 or 10 mM cobalt chloride in 25 μl volume by oropharyngeal aspiration. These cobalt concentrations correspond to 30 and 60 μg daily exposure, respectively. Mice were treated for 5 days on, 2 days off, 5 days on, and then killed 72 h after the last exposure. Mice were assessed for total body weight before first treatment and assessed again before euthanization. Following exposure, mice were anesthetized with pentobarbital sodium (50 mg/ml), and a midline laparotomy was performed. The trachea was exposed and cannulated. The lung and heart were removed en bloc, and the lungs were lavaged with two successive 1-ml volumes of sterile saline. These fractions of bronchoalveolar lavage fluid (BALF) were combined, and total cell counts were performed using a hemocytometer. Differential cell counts were performed in cytospin samples using Diff-Quik reagent (Baxter, FL). The remaining BALF was frozen for cytokine profiling. The right lung lobe was removed and stored in RNAlater RNA stabilizing reagent (Qiagen, Valencia, CA) for RNA isolation. The left lobe was perfusion inflated and fixed in 10% neutral buffered formalin for histopathological analysis.
Histopathology and immunohistochemistry.
At least four to six mice from each genotype and treatment group were analyzed for histopathological changes. Formalin-fixed left lung lobe tissues were paraffin embedded, and 5-μm-thick sections were mounted on glass slides and stained with hematoxylin and eosin or immunostained with HIF1α (1:500 dilution, NB100-479; Novus Biologicals, Littleton, CO), major basic protein (1:500 dilution, Mayo Clinic, AZ), or YM1 (1:100 dilution, AB24608; Abcam, Cambridge, MA) as previously described (23). Other lung sections were histochemically treated with picrosirius red solution for interstitial collagen staining to identify areas of pulmonary fibrosis or with Alcian Blue (pH 2.5)/periodic acid-Schiff (AB-PAS) stain to identify mucosubstances in mucous cells.
RNA isolation and quantitative real-time PCR analysis.
Lung tissue (10 mg) stored in RNAlater RNA Stabilization Reagent was homogenized in RLT buffer (RNeasy RNA isolation Kit, Qiagen) using a Retsch MM200 bead beater system (Retsch, Haan, Germany). Total RNA quantification was performed spectrophotometrically (NanoDrop ND-1000 UV-Vis Spectrophotometer). Total RNA (1 μg) was reverse transcribed using Superscript II reverse transcriptase kit (Invitrogen). cDNAs from each experimental group were pooled, and quantitative real-time PCR array analysis reactions were carried out in duplicate on an ABI Prism 7900HT 384-well block using TaqMan assays (Applied Biosytems, Foster City, CA). The complete table of the genes analyzed is listed in Supplemental Table S1 (Supplemental data for this article is available online at the AJP-Lung web site.). Changes in gene expression were calculated using the 2−ΔΔCt method. An average of the number of cycles of three housekeeping genes, GAPDH, actin-β, and 18S, was used to normalize the expression between samples. Genes that had greater than a twofold change in expression level compared with the control group were further analyzed by qRT-PCR using individual samples run in duplicate to confirm the PCR array results. Samples exhibiting Ct values greater than 40 were deemed undetermined and removed from subsequent analysis. Outliers were removed by Grubbs test and averages compared. The fold changes for these genes are listed in Supplemental Table S2.
Determination of cytokine levels by bead array.
BALF was analyzed for IL-1β, eotaxin, KC, IL-6, IL-10, IL-12, IL-4, IL-5, IL-13, IL-2, Rantes, GM-CSF, INFγ, TNFα, MCP-1, VEGF, MIP-2, and MIP-1α using a Bio-Plex 200 system and reagents (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Briefly, cytokine-specific, antibody-coupled, color-coded beads were allowed to react with the cytokines present in the sample. Following extensive washing, a biotinylated detection antibody was added to the cytokine-bound beads. The sandwich complex formation was detected by the addition of streptavidin-phycoerythrin. Cytokines were identified and quantitated based on bead color and fluorescence. Cytokine levels were calculated by system-specific software (Bio-Plex Manager) using a standard curve derived from a recombinant cytokine standard. A multiplex assay was performed by mixing beads specific to each of the above-listed cytokines and incubating them with 50 μl of undiluted BALF in the provided 96-well filter plate. Following washing, all the respective detection antibodies were added to wells followed by addition of streptavidin-phycoerythrin. Values from blank wells for each cytokine were subtracted from the corresponding cytokine values in each sample. Negative values were set to zero. Outliers were removed by Grubbs test, averages were calculated, and significance was determined by ANOVA followed by the Tukey HSD test.
Quantitative analysis.
All cell counts and cytokine and gene expression data were analyzed by ANOVA followed by the Tukey HSD test. All data from the study were presented as SE. Statistical difference of P value less than 0.05 was considered as significant.
RESULTS
Postnatal deletion of HIF1α.
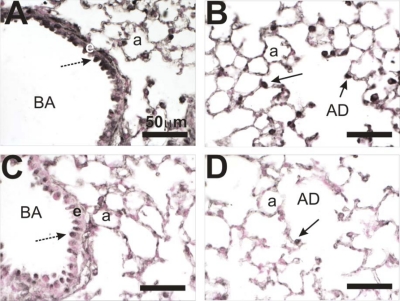
The initial description of the lung-specific cre recombinase model had very little data regarding postnatal deletion; however, it can be assumed that doxy-induced cre expression will be restricted to Clara and type II cells since SP-C expression is restricted to these cell types in the adult animal (19). To determine if prolonged exposure to doxycyline (Dox) could induce recombination within the conditional HIF1α locus, mice were exposed to Dox for ∼7 wk starting on postnatal day 4 (PN4). Initially, they received the drug through their mothers' milk, and then through their food and water. These mice are referred to as HIF1α deficient or HIF1αΔ/Δ. The mice referred to as controls throughout the studies are triple transgenic [SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg/HIF1αflox/flox] mice that were maintained on regular feed and water. The lungs of control and HIF1αΔ/Δ mice were then removed and analyzed for HIF1α expression via immunohistochemistry (IHC). Control mice showed pronounced HIF1α expression in the Clara cells lining the bronchiole airway and type II cells of the alveoli (Fig. 1, A and B). In contrast, the HIF1αΔ/Δ mice showed a marked decrease in expression in these cells, with only minor staining visible in the bronchiole airway lining cells and little or no staining in the alveoli (Fig. 1, C and D). The results suggest that postnatal exposure to Dox can induce significant recombination of the HIF1α locus and that the triple transgenic mouse is a viable model to test the role of HIF1α in cobalt-induced lung injury.
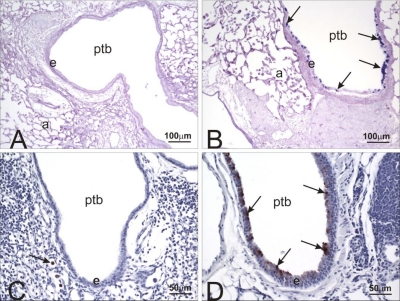
Fig. 1.
Hypoxia-inducible factor 1α (HIF1α) immunohistochemistry of lungs from control and doxycycline-treated mice. Lung tissue sections from control (A and B) and mice that were HIF1α deficient in their lungs (HIF1αΔ/Δ) (C and D) were analyzed by immunohistochemistry using a HIF1α-specific antibody. Control (A and B) and doxycycline (C and D)-treated mice were compared. HIF1α staining is prominent in the epithelial cell (e) lining the bronchiolar airway (BA) (dashed arrow) and type II cells (solid arrow) in the alveolar duct (AD) and alveolus (a). Staining is greatly reduced in the postnatally doxycycline-treated animals (C and D).
Body weight change and cell counts in lavage fluid.
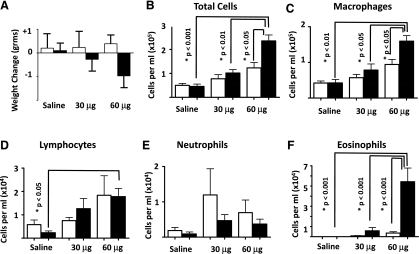
To characterize the role of HIF1α in cobalt-induced lung injury, control and HIF1αΔ/Δ mice were randomly assigned to three groups. Individual groups received sterile saline and 30 or 60 μg of cobalt chloride by oropharyngeal aspiration per day using a 2-wk protocol, 5 days on, 2 days off, 5 days on, and 2 days off. Total body weight of some of the control mice rose slightly during the course of the exposure; however, the body weight gain remained largely unchanged. In contrast, the HIF1αΔ/Δ mice lost weight in a dose-dependent fashion (Fig. 2A). Although this decrease was not significant, it was the first indication that the HIF1α-deficient mice responded differently to cobalt challenge. After the final 2-day incubation, mice were killed, BALF was collected, and lung tissue was processed for light microscopic examination, IHC, and RNA isolation. HIF1α-deficient mice treated with 60 μg of cobalt chloride showed significant increase in total BALF cells compared with 60 μg of cobalt chloride-treated control mice, suggesting that loss of HIF1α in the Clara and type II cells made the mice more susceptible to injury (Fig. 2B). Specific cellularity within the BALF was also different between the two genotypes. Cobalt-treated control and HIF1αΔ/Δ mice showed dose-dependent increases in lymphocytes and macrophages; however, only HIF1αΔ/Δ mice treated with 60 μg of cobalt was significant compared with saline (macrophages and lymphocytes) and 30-μg (macrophages only)-treated HIF1αΔ/Δ mice (Fig. 2, C and D). Moreover, the 60-μg-treated HIF1αΔ/Δ mice displayed a significant increase in macrophages compared with control mice treated with 60 μg of cobalt. Lymphocytes and neutrophils displayed no significant changes when control and HIF1αΔ/Δ mice were compared within treatment groups. Control mice displayed a nonsignificant increase in neutrophils compared with HIF1α-deficient mice (Fig. 2E). In contrast, the HIF1αΔ/Δ mice following cobalt (60 μg) exposure displayed a significant increase in eosinophils compared with all other groups, suggesting that HIF1α plays a role in modulating the lung's inflammatory response to metals (Fig. 2F). These changes were specific to the loss of HIF1α and not due to Dox treatment or the SPC-rtTA or tet-Cre transgenes, as different doxycycline-treated monotransgenic and bitransgenic [SP-C-rtTA−/tg/(tetO)7-CMV-Cre−/−/HIF1α+/+ (sTH), SP-C-rtTA−/−/(tetO)7-CMV-Cretg/tg/HIF1α+/+ (StH), SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg/HIF1α+/+ (stH), and SP-C-rtTA−/−/(tetO)7-CMV-Cre−/−/HIF1αflox/flox (STh)] mice behaved similar to control mice [SP-C-rtTA−/tg/(tetO)7-CMV-Cretg/tg/HIF1αflox/flox (sth), Supplemental Fig. 1].
Fig. 2.
Weight change and cell counts from cobalt-challenged control and HIF1αΔ/Δ mice. Mice were exposed to saline and 30 μg (5 mM) or 60 μg (10 mM) of CoCl2 for 2 wk, 5 days/wk paradigm. Total animal weight change (A) in control (white bars) and HIF1αΔ/Δ (black bars) was calculated by subtracting animal weight on the day of death from weight at the start of exposure. Total cell counts from BALF (B) were assessed from all mice and averaged. Cell differential counts were performed for macrophages (C), lymphocytes (D), neutrophils (E), and eosinophils (F). N > 5 mice/group. *Significance (P values noted).
Histopathology of cobalt-induced injury.
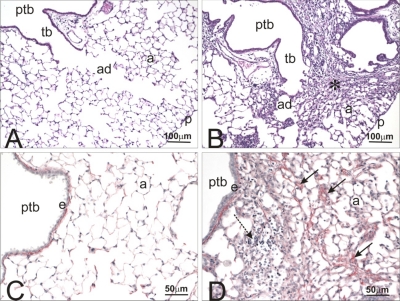
Histologically, no pulmonary lesions were found in control or HIF1αΔ/Δ mice that were instilled with saline alone. In contrast, control mice repeatedly instilled with cobalt had a mild-moderate chronic bronchopneumonia that was histologically characterized by a mononuclear cell infiltrate (mainly small and large lymphocytes, monocytes, and occasional plasma cells) admixed with lesser numbers of neutrophils and eosinophils in the interstitial tissues surrounding large- and small-diameter conducting airways (i.e., axial, preterminal, and terminal bronchioles) and extending into the centriacinar regions of the lung (alveolar ducts and adjacent alveoli) (Fig. 3, A and B). Interstitial fibrosis was a prominent remodeling feature of the alveolar septa in affected parenchymal regions along with minimal to mild hyperplasia of alveolar type II cells and accumulation of mildly hypertrophic macrophages and varying numbers of inflammatory cells (lymphocytes and neutrophils) in alveolar air spaces (Fig. 3, C and D). These cobalt-induced airway and alveolar changes were dose dependent and were more consistently found in the hilar rather than the distal aspects of the lung lobe.
Fig. 3.
Histopathology and picrosirius staining control and cobalt-treated control mice. Light photomicrographs of the lungs of control mice instilled with saline (A and C) or cobalt (B and D). Lung sections in A and B were stained with hematoxylin and eosin, whereas sections in C and D were histochemically treated with picrosirius red solution that stains interstitial collagen (red chromagen in interstitial tissues in the alveolar septa and around bronchiolar airways). In cobalt-treated lungs (B and D), there is marked inflammation and interstitial fibrosis (* in B) around preterminal bronchioles (ptb) and terminal bronchioles (tb) and extending distally into alveolar ducts (ad) and adjacent alveoli (a). There is increased picrosirius red-stained collagen (solid arrows) in the alveolar septa of the cobalt-treated mouse (D) compared with that in the saline-treated control mouse (C). p, Pulmonary pleura; e, bronchiolar epithelium; dashed arrow, mixed inflammatory cell infiltrate.
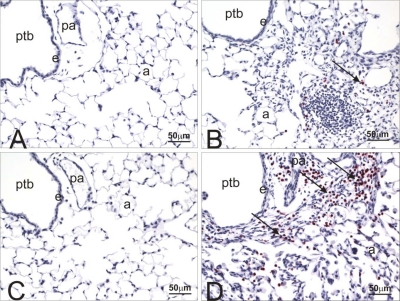
A similar mild-moderate chronic bronchopneumonia with peribronchiolar lymphoplasmacytic inflammation and variable amounts of interstitial alveolar fibrosis airway was present in the lung lobes of cobalt-exposed HIF1αΔ/Δ mice (data not shown). There were, however, marked differences in the character of the inflammatory, airway epithelial, and alveolar macrophage changes in the lungs of these transgenic mice compared with their control counterparts. Along with the lymphocytes and plasma cells, there were markedly more eosinophils in the peribronchiolar and alveolar mixed inflammatory cell influxes in HIF1αΔ/Δ mice compared with those of control mice (Fig. 4D). Another distinctive change found only in HIF1αΔ/Δ mice was mucous cell metaplasia in the airway epithelium lining axial and preterminal bronchioles. This airway epithelial lesion in large-diameter bronchioles consisted of numerous AB/PAS-stained mucous goblet cells in bronchiolar epithelium that is normally devoid of these mucus-secreting cells in mice (Fig. 5, A and B). These areas of epithelial mucous cell metaplasia in cobalt-treated HIF1αΔ/Δ mice were also immunohistochemically positive for YM1/2 chitinase-like proteins (Fig. 5, C and D).
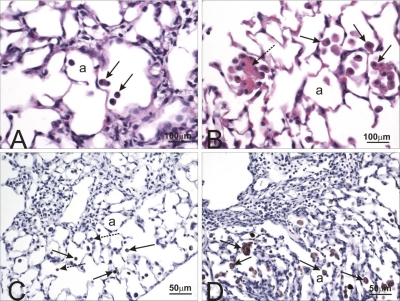
Fig. 4.
Major basic protein staining in lungs from control and HIF1αΔ/Δ mice. Light photomicrographs of the lungs of saline- (A and C) or cobalt-instilled (B and D) control (A and B) and HIF1αΔ/Δ (C and D) mice. All lung sections were immunohistochemically stained for major basic protein to identify infiltrating eosinophils (red chromagen; arrows) and counterstained with hematoxylin. A mixed inflammatory infiltrate consisting of mononuclear leukocytes, eosinophils, and lesser numbers of neutrophils are restricted to the lungs in cobalt-treated mice (B and D). Markedly more eosinophils are present in the peribronchiolar and alveolar regions of the cobalt-treated HIF1αΔ/Δ mouse (D) compared with that of the cobalt-treated control mouse (B). pa, Pulmonary arteriole; a, alveolar air space; e, bronchiolar epithelium.
Fig. 5.
Alcian Blue/periodic acid-Schiff (AB/PAS) stain and YM1/2 immunohistochemistry (IHC). Light photomicrographs of ptb of cobalt-instilled control (A and C) and HIF1αΔ/Δ (B and D) mice. Tissues were stained with AB (pH 2.5)/PAS (A and B) to identify acidic and neutral mucosubstances (magenta stain; arrows) in mucous cells within the bronchiolar epithelium (e). Numerous AB/PAS-stained mucous cells are present only in the airway epithelium lining the preterminal bronchiole in the cobalt-treated HIF1αΔ/Δ mouse (B). Tissues in C and D were immunohistochemically stained for YM1/2 protein (brown chromagen; arrows) and counterstained with hematoxylin. YM1/2 proteins were present only in the bronchiolar epithelium of the cobalt-treated HIF1αΔ/Δ mouse (D). Arrow in C identifies a few alveolar macrophages that were positive for YM1/2 proteins.
Characterization of eosinophils and YM1/2 expression.
In addition to these unique eosinophil and epithelial responses, alveolar macrophages accumulating in alveolar air spaces of cobalt-exposed mice were larger and more eosinophilic in the HIF1αΔ/Δ mice compared with those in similarly exposed control mice (Fig. 6, A and B). Immunohistochemically, the cytoplasm of these phenotypically distinctive macrophages stained positive for YM1/2 protein similar to the mucous goblet cells in the metaplastic bronchiolar epithelium of HIF1αΔ/Δ mice (Fig. 5, C and D). Variably sized needle-shaped or rectangular refractile eosinophilic crystals were also present within these alveolar macrophages or free in the alveolar air spaces (Fig. 6B). These crystals also stained positive for YM1/2 (Fig. 6D). Multinucleated giant cells were also more frequently observed in the cobalt-induced alveolitis of HIF1αΔ/Δ mice compared with those of control mice.
Fig. 6.
Hematoxylin and eosin (H&E) staining and YM1/2 IHC of cobalt-treated control and HIF1αΔ/Δ mice. Light photomicrographs of alveolar macrophages in cobalt-instilled control (A and C) and HIF1αΔ/Δ (B and D) mice. Lung tissues were histochemically stained with H&E (A and B) or immunohistochemically for YM1/2 proteins (C and D). H&E-stained alveolar macrophages in the cobalt-instilled HIF1αΔ/Δ mouse (B) are larger (hypertrophic) and more eosinophilic than the similarly stained lung section from the cobalt-instilled control mouse (A). In B, a group of macrophages is surrounding an extracellular aggregate of eosinophilic crystals (dashed arrow). In addition, more alveolar macrophages immunohistochemically staining for YM1/2 proteins (solid arrows) are present in lung section from the cobalt-instilled HIF1αΔ/Δ mouse (D) compared with that of the cobalt-instilled control mouse (C). Dashed arrows in C, alveolar macrophage with no detectable YM1/2 proteins.
Cobalt-induced gene expression changes.
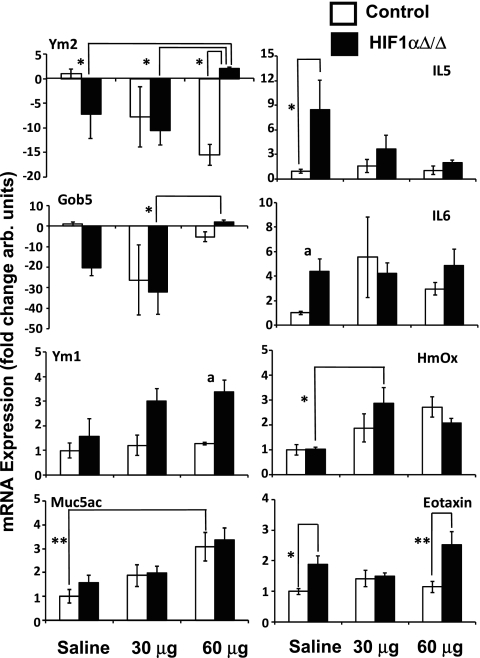
The differences in cobalt-induced pulmonary pathology between the control and HIF1αΔ/Δ suggested an alteration in the stress response upon loss of HIF1α. To begin characterizing this difference, the expression of 63 key genes involved in immunity, inflammation, oxidative stress, and other stress pathways, was assessed by quantitative real-time PCR (Supplemental Table S1). Initially, samples from each treatment group and genotype were pooled and screened. Those genes that showed a difference compared with untreated controls within a genotype or between genotypes were characterized as individual samples (Supplemental Table S2). Six of these genes showed significant changes in expression when compared between genotypes and within treatment or within genotype and between treatments (Fig. 7). Two other genes, Ym1 and IL6, were near significance (0.069 and 0.055, respectively). The expression of Ym2 in the HIF1α-deficient mice was significantly increased following challenge with 60 μg of cobalt compared with the saline-treated HIF1αΔ/Δ mice and the 60-μg cobalt-treated control animals. Gob5 expression followed a similar pattern of expression as that of Ym2; however, its expression was only significantly changed when the 60-μg cobalt-treated HIF1αΔ/Δ animals were compared with the 30-μg-treated ones. Muc5ac and IL5 showed dose-dependent changes in expression within genotypes, and, in the case of IL5, expression was significantly elevated in saline-treated HIF1αΔ/Δ mice compared with saline-treated controls. Eotaxin was also significantly elevated in the HIF1αΔ/Δ mice when the saline-treated mice were compared. Moreover, eotaxin was also significantly different between the genotypes in the 60-μg cobalt-treated mice.
Fig. 7.
Gene expression results. The expression of 63 genes was analyzed by qRT-PCR. Those genes that showed a difference as pooled samples were further analyzed as independent replicates. Expression levels were normalized to the saline-treated control animal and expressed as fold change. aP < 0.07, *P < 0.05, **P < 0.01.
Cytokine profiling.
The pathology of the lung and the gene expression patterns suggested a change in the inflammatory response upon loss of HIF1α from type II and Clara cells. To determine if the changes in cytokine gene expression led to changes in the chemoattractants found in the BALF, profiling of 18 cytokines was performed using a bead array (BioRad). Of the 18 characterized, 10 showed significant difference when compared across genotype or within treatment groups (Table 1). Interestingly, several of these were different when the saline-treated groups of the control and HIF1αΔ/Δ mice were compared [i.e., IL-1β, IL-5, IL-12, GM-CSF, RANTES, TNFα, and VEGF (Table 1)]. This suggests that loss of HIF1α from type II and Clara cells alters the tissue's native cytokine profile. Moreover, cobalt exposure caused a more pronounced phenotype in the HIF1αΔ/Δ animals with respect to cytokine changes.
Table 1.
Cytokine profiles
| Control |
HIF1αΔ/Δ |
|||||
|---|---|---|---|---|---|---|
| Saline | 30 μg | 60 μg | Saline | 30 μg | 60 μg | |
| IL-1β | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.40 ± 0.29 | 7.60 ± 3.91† | 0.00 ± 0.00‡ | 0.00 ± 0.00‡ |
| IL-2 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| IL-4 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.71 ± 1.44‡** | 0.00 ± 0.00 |
| IL-5 | 0.00 ± 0.00 | 2.33 ± 1.67 | 0.20 ± 0.20 | 18.30 ± 10.61† | 3.00 ± 1.21‡ | 0.00 ± 0.00‡ |
| IL-6 | 1.80 ± 0.81 | 1.95 ± 0.97 | 5.00 ± 2.53 | 3.81 ± 2.38 | 3.04 ± 2.06 | 5.75 ± 2.18 |
| IL-10 | 0.00 ± 0.00 | 0.10 ± 0.06 | 0.10 ± 0.06 | 0.00 ± 0.00 | 0.39 ± 0.22 | 0.13 ± 0.06 |
| IL-12 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 3.10 ± 1.12† | 1.86 ± 0.83 | 1.58 ± 0.76 |
| IL-13 | 1.00 ± 0.22 | 0.33 ± 0.11 | 1.17 ± 0.21 | 1.00 ± 0.32 | 0.79 ± 0.18 | 1.00 ± 0.22 |
| Eotaxin | 3.75 ± 0.55 | 3.13 ± 0.80 | 2.13 ± 0.52 | 4.25 ± 0.29 | 2.96 ± 1.04 | 2.96 ± 0.88 |
| GM-CSF | 0.00 ± 0.00 | 1.92 ± 0.99 | 2.33 ± 1.02 | 5.40 ± 3.48† | 2.29 ± 0.52 | 1.17 ± 0.65 |
| IFNγ | 0.85 ± 0.40 | 1.80 ± 0.63 | 2.13 ± 0.58 | 2.50 ± 1.31 | 1.71 ± 0.53 | 1.50 ± 0.36 |
| KC | 31.10 ± 8.40 | 46.10 ± 13.80 | 42.58 ± 8.59 | 59.38 ± 6.02 | 37.58 ± 7.53 | 118.75 ± 33.82‡** |
| MCP1 | 0.00 ± 0.00 | 1.30 ± 0.58 | 2.83 ± 1.49 | 2.00 ± 0.71 | 0.83 ± 0.31 | 2.83 ± 1.08 |
| RANTES | 3.00 ± 1.08 | 25.25 ± 7.61 | 47.75 ± 21.39 | 8.50 ± 3.07† | 17.07 ± 6.31 | 18.08 ± 3.04 |
| TNFα | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 24.05 ± 12.47† | 0.29 ± 0.29‡ | 0.00 ± 0.00‡ |
| MIP2 | 45.40 ± 9.72 | 65.67 ± 13.96 | 75.17 ± 27.54 | 82.63 ± 28.57 | 30.83 ± 5.53 | 90.67 ± 29.76 |
| VEGF | 370.20 ± 16.36 | 383.50 ± 56.75 | 349.20 ± 16.56 | 624.00 ± 138.97† | 399.93 ± 44.04‡ | 538.25 ± 43.06** |
| MIP1a | 3.40 ± 1.98 | 5.00 ± 1.75 | 1.30 ± 0.72 | 5.50 ± 1.59 | 1.92 ± 1.18 | 8.83 ± 2.39** |
Cytokine levels in BALF from cobalt-challenged control and HIF1αΔ/Δ mice. †Significance at 0.05 when compared with saline control between genotypes. ‡Significance at 0.05 when compared with saline-treated mice within genotype. **Significance at 0.05 when compared between genotypes and within treatment group. Control and HIF1α Δ/Δ mice were exposed to saline or 30 or 60 μg of CoCl2 for 2 wk, 5 days/wk paradigm. The levels of cytokines in BALF were assessed using the Bio-Rad Bio-Plex cytokine suspension array system. N >5 mice/group. † P < 0.05, when HIF1αΔ/Δ saline is compared with control saline. ‡ P < 0.05 compared with genotype-matched control. ** P < 0.05 when similar treatments are compared between genotypes.
DISCUSSION
The research describes a model for the postnatal deletion of HIF1α from Clara and type II cells of the lung with the intention of determining the role of the transcription factor in cobalt-induced injury. Control mice repeatedly exposed to cobalt via aspiration had classic signs of metal-induced injury, including increased neutrophils, peribronchial inflammation, and fibrotic lesions. In contrast, the mice deficient in HIF1α in their Clara and type II cells exhibited a Th2-mediated response in response to cobalt challenge. In fact, these HIF1αΔ/Δ mice displayed a pathology that can be described as asthma-like, including increased eosinophilic infiltration, increased chitinase-like protein expression, and mucus cell metaplasia. In addition, these HIF1α-deficient mice expressed an altered panel of cytokines in the absence and presence of cobalt. These results suggest that alveolar epithelium-derived HIF1α plays a critical role in determining the inflammatory response to cobalt challenge, and loss of this critical transcription factor can alter the lung's ability to cope with allergic stress.
Attempts to study cobalt-induced injury in animal models have successfully mimicked some facets of hard metal lung disease. For example, rabbits exposed to low doses of cobalt chloride (0.4 and 2 mg/m3) for 14–16 wk (6 h/day) displayed increased macrophages and lysozyme activity in BALF, interstitial inflammation, and the presence of large vacuolated macrophages (10–12). Guinea pigs exposed to 2.4 mg/m3 of cobalt chloride for 2 wk (6 h/day) had a higher rate of BALF neutrophilic and eosinophilic infiltration when presensitized to the metal (4). The National Toxicology Program exposed rats and mice to cobalt sulfate heptahydrate at doses ranging from 0.3 to 30 mg/m3 for 13 wk (6 h/day) and described interstitial fibrosis, epithelial hyperplasia, and lesions in the upper airways that were more pronounced in the rats (1, 2). Finally, hamster instilled with cobalt chloride (1–1,000 μg/kg) displayed signs of oxidative stress, including alterations in the glutathione ratio (14). To the best of our knowledge, none of these models described chronic eosinophilic infiltration, mucous cell metaplasia, or the increase in chitinase-like proteins as seen in the HIF1αΔ/Δ mice. It has been suggested that an animal model is needed that combines the toxic properties of cobalt with its allergenic potential (15). The HIF1αΔ/Δ mice might be this model. In the presence of cobalt, HIF1αΔ/Δ mice respond with an asthma-like phenotype (i.e., eosinophil infiltration and mucus cell metaplasia) and increased expression of the chitinase-like proteins Ym1 and Ym2.
Chitins are biopolymers not found in mammalian systems, and chitinase and chitinase-like proteins are believed to function as protection against chitin-containing organisms. Ym1 and Ym2 are chitinase-like proteins that share considerable protein homology to the human protein YKL-40 (also known as human cartilage glycoprotein 39 and chitinase 3-like 1). YKL-40 has recently been identified in the lungs and circulation of asthmatics (5). Not only was there a correlation with the presence of YKL-40 and asthma, the levels also correlated with the severity of the disease and the thickening of the subepithelial basement membrane (5). The biological relationship between these enzymes and the etiology of asthma has not been characterized. What is known, however, is that chitinase and chitinase-like proteins are upregulated in asthma models, and, in the case of chitinase proteins, this induction is dependent on IL13. IL13 gene expression is slightly elevated in the cobalt-treated HIF1αΔ/Δ mice, and its lack of significance is most likely due to the timing of analysis (i.e., 14 days after the start of exposure). Increased chitinase expression is required for the subsequent eosinophilia and lymphocytic infiltration (29). Ym2 was significantly increased in the 60-μg-treated HIF1αΔ/Δ mice compared with the 60-μg-treated control mice. Moreover, Ym1 expression was approaching significance (P < 0.07) when a similar comparison was made. In contrast, Ym2 shows a cobalt-induced decrease in expression in the control animals, and Ym1 is unchanged by cobalt treatment in these control mice. These results suggest that cobalt exposure in the HIF1αΔ/Δ mice induces a series of events within the lungs that resembles the progression of asthma in other asthma models, including increased IL13, subsequent increases in Ym1 and Ym2 expression, and the recruitment of eosinophils.
The results suggest that the ability of type II and Clara cells to respond to hypoxia is necessary for the proper lung inflammatory response to metal-induced stress. It is important to point out that this response is not due to loss of HIF1α in resident macrophages. HIF1α expression is still strong in these cells following postnatal doxycycline exposure and confirms that the SP-C promoter confines the expression of cre recombinase to type II and Clara cells (data not shown). In addition, it implies that the observed inflammatory response is not due to the previously established role of HIF1α in myeloid cell-mediated inflammation (6). Together, the results suggest a model for the inflammatory differences between the control and HIF1α-deficient mice following metal exposure. Initially, the cobalt challenge leads to damage within the lung, either at type I or II cells. This damage is communicated to the remaining viable type II cells of the parenchyma directly or through resident macrophages. In control lungs, HIF1α will regulate the expression of classic hypoxia target genes as well as others involved in the inflammatory response. This HIF1-regulated transcription will dictate the lung's inflammatory response to the metal challenge. The modest increases in cytokines, such as IFNγ, and the resulting pathology suggest a Th1-mediated response. Loss of HIF1α in the remaining viable type II cells of the HIF1αΔ/Δ mice would lead to an alteration in the cell's ability to respond to the incoming signals from the cobalt-damaged cells. The decreased levels of HIF1α would compromise the lung's ability to respond to cobalt as a toxicant and putative allergen. Ultimately, this difference in epithelium-derived HIF1α alters the pattern of released cytokines and presumably leads to the differential expression of Th2 chemokines such as IL4, IL5, IL10, and IL13 (Fig. 7, Table 1, and Supplemental Table S2). These changes in cytokines will promote the expression of the chitinase and chitinase-like proteins, such as Ym2, and this would lead to the eosinophilic infiltration seen in the HIF1αΔ/Δ mice following cobalt challenge (Figs. 4–7). This proposed model explains the difference in the inflammatory response between the control and HIF1αΔ/Δ mice; however, it is based on collected data at the end of cobalt exposure. Validation of the model will require a detailed dose and time course response to cobalt challenge.
The striking differences observed following cobalt exposure in the two mice suggest that they will be a powerful tool to understand the relationship between allergy-induced asthma, hypoxia, and inflammation. More importantly, direct comparison of the responses of the control and HIF1αΔ/Δ mice in other asthma models (e.g., ovalbumin challenge) and to other inflammatory inhalants (e.g., ozone) will correlate this relationship to specific cytokines. The research also raises several important questions: Does the loss of HIF1α alter an organism's susceptibility to asthma using other allergens? What role does HIF1α derived from other cell types (e.g., infiltrating inflammatory cells and type I cells) play in modulating this inflammatory response? Does postnatal deletion of HIF1α alter HIF2α, and, if so, what impact does this have on the inflammatory response (23)? Most importantly, does a decrease in HIF1α functionality lead to an increased susceptibility to asthma in humans? More specifically, is it possible that loss or decrease in HIF1α function biases the lung towards a Th2 immune polarization and this increases the susceptibility of individuals towards extrinsic asthma? Even with these unanswered questions, the HIF1αΔ/Δ mice offer an important step forward in understanding the role of the HIF1α signaling cascade in allergy-induced asthma. Finally, it establishes epithelial-derived HIF1α as a major regulator of the lung's response to allergenic compounds and creates a new tool to help understand the relationship between inflammation and asthma progression.
GRANTS
This work was supported by National Institute of Environmental Health Sciences Grants R01-ES-12186 and P42-ES-04911-17.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We appreciate the gift of the conditional HIF1α mice from Dr. Randall Johnson (Univ. of California San Diego) and the SP-C-rtTA/(tetO)7-CMV-Cre transgenic mice from Dr. Jeffrey A. Whitsett (Cincinnati Children's Hospital Medical Center).
REFERENCES
- 1.Bucher JR, Elwell MR, Thompson MB, Chou BJ, Renne R, Ragan HA. Inhalation toxicity studies of cobalt sulfate in F344/N rats and B6C3F1 mice. Fundam Appl Toxicol 15: 357–372, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Bucher JR, Hailey JR, Roycroft JR, Haseman JK, Sills RC, Grumbein SL, Mellick PW, Chou BJ. Inhalation toxicity and carcinogenicity studies of cobalt sulfate. Toxicol Sci 49: 56–67, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Phys Rev 76: 839–885, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Camner P, Boman A, Johansson A, Lundborg M, Wahlberg JE. Inhalation of cobalt by sensitised guinea pigs: effects on the lungs. Br J Ind Med 50: 753–757, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret MC, Aubier M, Pretolani M, Elias JA. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med 357: 2016–2027, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112: 645–657, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604–4613, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gheysens B, Auwerx J, Van den Eeckhout A, Demedts M. Cobalt-induced bronchial asthma in diamond polishers. Chest 88: 740–744, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Johansson A, Camner P, Jarstrand C, Wiernik A. Rabbit alveolar macrophages after inhalation of soluble cadmium, cobalt, and copper: a comparison with the effects of soluble nickel. Environ Res 31: 340–354, 1983 [DOI] [PubMed] [Google Scholar]
- 11.Johansson A, Lundborg M, Wiernik A, Jarstrand C, Camner P. Rabbit alveolar macrophages after long-term inhalation of soluble cobalt. Environ Res 41: 488–496, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Johansson A, Robertson B, Camner P. Nodular accumulation of type II cells and inflammatory lesions caused by inhalation of low cobalt concentrations. Environ Res 43: 227–243, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Jung Y, Isaacs JS, Lee S, Trepel J, Liu ZG, Neckers L. Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor kappa B activation. Biochem J 370: 1011–1017, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis CP, Demedts M, Nemery B. Indices of oxidative stress in hamster lung following exposure to cobalt(II) ions: in vivo and in vitro studies. Am J Respir Cell Mol Biol 5: 163–169, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Lison D, Lauwerys R, Demedts M, Nemery B. Experimental research into the pathogenesis of cobalt/hard metal lung disease. Eur Respir J 9: 1024–1028, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol 208: 281–292, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Lombaert N, Lison D, Van Hummelen P, Kirsch-Volders M. In vitro expression of hard metal dust (WC-Co)–responsive genes in human peripheral blood mononucleated cells. Toxicol Appl Pharmacol 227: 299–312, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflamm 4: 12, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA 99: 10482–10487, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453: 807–811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 17: 3005–3015, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res 60: 4010–4015, 2000 [PubMed] [Google Scholar]
- 23.Saini Y, Harkema JR, LaPres JJ. HIF1α is essential for normal intrauterine differentiation of alveolar epithelium and surfactant production in the newborn lung of mice. J Biol Chem 283: 33650–33657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salnikow K, Donald SP, Bruick RK, Zhitkovich A, Phang JM, Kasprzak KS. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J Biol Chem 279: 40337–40344, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 269: 23757–23763, 1994 [PubMed] [Google Scholar]
- 26.Shirakawa T, Kusaka Y, Fujimura N, Goto S, Kato M, Heki S, Morimoto K. Occupational asthma from cobalt sensitivity in workers exposed to hard metal dust. Chest 95: 29–37, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Shirakawa T, Kusaka Y, Fujimura N, Goto S, Morimoto K. The existence of specific antibodies to cobalt in hard metal asthma. Clin Allergy 18: 451–460, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Vengellur A, Phillips JM, Hogenesch JB, LaPres JJ. Gene expression profiling of hypoxia signaling in human hepatocellular carcinoma cells. Physiol Genomics 22: 308–318, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 304: 1678–1682, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.