Abstract
Lung arteriovenous (AV) shunts or malformations cause significant morbidity and mortality in several distinct clinical syndromes. For most patients with lung AV shunts, there is still no optimal treatment. The underlying molecular and cellular etiology for lung AV shunts remains elusive, and currently described animal models have insufficiently addressed this problem. Using a tetracycline-repressible system, we expressed constitutively active Notch4 (Notch4*) specifically in the endothelium of adult mice. More than 90% of mice developed lung hemorrhages and respiratory insufficiency and died by 6–7 wk after gene expression began. Vascular casting and fluorescent microsphere analysis showed evidence of lung AV shunts in affected mice. Cessation of Notch4* expression reversed these pathophysiological effects. Assessment of the vascular morphology revealed enlarged, tortuous vessels in the lungs that resembled arteriovenous malformations. By using whole lung organ culture, we demonstrated the effects of constitutively active Notch4 on the lung vasculature to be a primary lung phenomenon. Together, our results indicate the importance of Notch signaling in maintaining the lung vasculature and offer a new, reliable model with which to study the pathobiology of lung arteriovenous shunts and malformations.
Keywords: vascular, pulmonary, endothelium, Notch
lung arteriovenous (AV) shunting and malformations (AVMs) can occur in a variety of clinical settings, including hepatopulmonary syndrome (32), congenital heart disease (resulting from cavopulmonary anastomosis) (2, 38), hereditary hemorrhagic teleangiectasia (HHT) (8), and as isolated lesions (11). Approximately one-third of patients with HHT (incidence 1 in 10,000) (8) or cirrhosis (incidence 5% of the general population) (12, 34) have been estimated to harbor lung AV shunts or malformations. In addition to pulmonary symptoms such as hypoxia, fatigue, hemoptysis, and hemothorax, patients with lung AV shunts also experience significant central nervous system events such as cerebral abscess formation, stroke, transient ischemic attack, cerebral hemorrhage, and chronic migraines (7). Treatment of lung AV shunts depends on the specific clinical syndrome and may include embolization (6, 17), surgical resection (7), redirection of hepatic-vein flow (35), and liver transplantation (19). Since none of these therapies is both definitive and readily available for most patients, no optimal treatment for lung AV shunts currently exists. Lung AV shunts therefore remain a vexing clinical problem.
Part of the challenge in understanding the biology of lung AV shunts lies in their variable demographic and morphological characteristics. These lung lesions can be acquired or inherited, diffuse or discrete, and can occur in pediatric or adult patient populations. In addition, lesions that include enlargement or aneurysmal dilation of vessels as well as tortuous nests of vessels at the arteriovenous junction are variably described as “shunts,” “fistulae,” and “arteriovenous malformations.” This variety in presentation and nomenclature has made the study of lung AV shunts a challenging field that includes several animal models.
Data from the sheep and rat models of cavopulmonary anastomosis, and the rat model of hepatopulmonary syndrome (common bile duct ligation), have shed some light on the molecular mediators that may regulate lung AV shunts. These studies have demonstrated elevations in local hypoxia-inducible factor (HIF-1α), vascular endothelial growth factor (VEGF) (25, 28), angiotensin-pathway proteins (24, 26), and endothelin-1 receptor B. In addition, these models have indicated a possible association between lung AV shunts and pulmonary endothelial nitric oxide synthase (eNOS) (9, 44) and oxidative stress (25). None of these molecular associations, however, has completely characterized the mechanism underlying the ultimate pathological findings in lung AV shunts. Moreover, the lack of a sound murine model for lung AV shunts has prevented further discoveries using powerful transgenic technology. Development of such a model would be a significant contribution to the field.
More recent data indicate possible links between the aforementioned molecular mediators and Notch signaling in lung vascular homeostasis. For example, Zhang et al. (43) have found that, in the rat model of hepatopulmonary syndrome, activation of lung endothelial VEGF-A, eNOS, and Akt is accompanied by pulmonary angiogenesis. In addition, Notch signaling has emerged as a key mediator of angiogenesis, in particular for its role in arteriovenous specification (16, 42). The transmembrane Notch receptor is critical to proper differentiation of all mammalian tissues studied to date, enabling cell fate decisions through cell-cell communication (18). Ligand binding of the Notch extracellular domain results in a series of cleavage events that releases the intracellular domain (ICD), which translocates to the nucleus and activates transcription of downstream genes (18). Disruptions of Notch signaling, both loss-of-function and gain-of-function, result in abnormal vascular remodeling and arteriovenous shunting, which demonstrate the importance of Notch receptors in proper vascular maintenance (15, 20, 40). Based on previous data that demonstrate an elevation in endothelial Notch4 expression before lung AV shunts develop in sheep (23) and additional studies that show consistent expression of Notch4 in mouse lung endothelium (41), we hypothesized that expression of constitutively active endothelial Notch4 (Notch4*) would induce lung AV shunts (enlarged arteriovenous communications) as a primary phenomenon.
MATERIALS AND METHODS
Mice.
Tie2-tTA:TRE-int3 (henceforth to be called Notch4* mutants) and TRE-LacZ transgenic mice have been described (5). The Tie2-tTA construct consists of a gene encoding the tetracycline transactivator (tTA) that is coupled to the Tie2 promoter, which confers endothelial specificity. The TRE-int3 and TRE-LacZ constructs consist of a tetracycline response element (TRE) coupled to the gene for a constitutively active form of Notch4 (int3) or to the gene encoding β-galactosidase (LacZ). Breeding mice to bear both the Tie2-tTA and TRE-int3 or TRE-LacZ constructs allows for temporally regulatable gene expression. In the presence of tetracycline or doxycycline, the tTA is inactive, whereas upon withdrawal of tetracycline or doxycycline, the tTA can bind the TRE and promote gene expression. For adult mouse experiments, breeding pairs and pups were given a Dox (200 mg/kg, Bio-Serv) diet until postnatal day 21. Adult mice were studied between 6 and 7 wk after withdrawal of the Dox diet. Embryos were genotyped as described previously (3). All animals were treated in accordance with the guidelines of the UCSF Institutional Animal Care and Use Committee, and all protocols were approved by this committee.
Organ culture of whole embryonic lungs.
For experiments using organ culture of whole embryonic lungs, pregnant dams were treated with Tet sucrose solution (0.5 mg/ml tetracycline, 50 mg/ml sucrose, Sigma) in their drinking water from conception until embryonic day (E) 10.5. Embryos were then harvested on E11.5, and lung buds were dissected en bloc in ice-cold HBSS. The lung buds were then placed on a 0.4-μm transwell filter and cultured in DMEM/F12, 10% FBS, ascorbic acid (0.1 mg/ml), and penicillin/streptomycin (50 U/ml) for 48 h in 5% CO2 at 37°C. Lung buds were then fixed with 4% PFA and dehydrated, and whole mounts were stained for the endothelial cell marker CD31 so that blood vessels could be viewed.
Gene expression analysis.
Total RNA was extracted from whole lungs with TRIzol (Invitrogen) and reverse transcribed. Quantitative real-time PCR was performed using the MJ Research (Bio-Rad) DNA Engine Opticon 2 system. Normalized expression was calculated using hypoxanthine guanine phosphoribosyltransferase (HPRT) as a reference. Int3 (Notch4*) cDNA was amplified with transgene-specific primers that do not amplify the endogenous Notch4 gene: 5′-gggtcttccagttcaccaag-3′ and 5′-tttgccccctcca-tataaca-3′. The following primer sequences were used for HPRT: 5′-agctactgtaatgat-cagtcaacg-3′ and 5′-agaggtccttttcaccagca-3′.
Arterial blood gas analysis.
After mice were anesthetized with 2% isoflurane in room air, the femoral artery was cannulated via cutdown with a specially designed catheter inserted to the level of the thoracic aorta. Mice were given 50 μl of heparin (50 mg/ml) subcutaneously. Isoflurane anesthesia was titrated between 2 and 3% to a respiratory rate of ∼100. Two hundred microliters of arterial blood were sampled and kept on ice until analysis with an arterial blood gas analyzer (Chiron/Diagnostics Critical Care Systems).
Lung procurement, inspection, and vascular imaging.
After being fed the Dox diet for either 4 or 6–7 wk (when mice began to exhibit symptoms), mice were anesthetized with 2% isoflurane and given 50 μl of heparin (50 mg/ml) subcutaneously. Mice were then euthanized and exsanguinated by transecting the abdominal aorta. After median sternotomy and chest wall retraction, anterior and posterior surfaces of the lungs were carefully inspected, and the total number of hemorrhages per animal was noted and recorded as the “lung hemorrhage count.” Following left atriotomy and gentle perfusion of the pulmonary artery with 3 ml of PBS, a tracheal tube was placed, and the lungs were inflated with 4% PFA or 3% low-melting point agarose using a 23-cm column. Casting was done using the following specific modifications for lung tissue. First, blood was cleared from the lungs by transecting the carotid artery and then infusing 3 ml of PBS into the inferior vena cava. Microfil casting agent (Flowtech, 1:2 dilution + 3.2% curing agent) was infused into the pulmonary artery via hand injection by an experienced technician. Lungs were then inflated with 10% formalin via tracheal tube, fixed in 10% formalin overnight, and then cleared with ethanol-methylsalicylate per the manufacturer's instructions.
In some mice, fluorescent agarose perfusion was performed to better visualize the complete vascular network. After clearing the blood from the lungs as described for the casting procedure above, left atriotomy was performed, and the pulmonary artery was cannulated. The lungs were perfused first with 1.5 ml of 1% PFA, then with 0.5 ml of fluorescein isothiocyanate-dextran, lysine-fixable (2.5 mg/ml, 70 kDa, anionic, Invitrogen), in 0.7% agarose type VII (Sigma-Aldrich) in PBS at 55°C. Silk ties around the base of the heart and the pulmonary artery trapped the perfusate in the vascular tree. The lungs were then inflated with 4% PFA at 4°C, using a 23-cm column, causing the agarose to solidify. To remove the residual air from the airways, lungs were inflated and deflated three to four times with the head of the mouse slightly elevated. The lungs were excised en bloc, fixed in 4% PFA overnight, and then cleared with a gradient of sucrose solutions, up to 60% sucrose over 2 days, at 4°C. Lungs were then dissected into individual lobes and imaged with a Leica MZ16 FA microscope using Image-Pro Plus software.
Vascular shunting.
After vascular casting with Microfil and clearing, lung blocks were dissected into individual lobes, inspected under a dissecting microscope, and imaged from anterior and posterior perspectives using a Leica MZ16 microscope. Due to the viscosity of the casting agent, the veins of the pulmonary vasculature typically do not fill. Therefore, vein filling seen in captured images was interpreted as an indication of arteriovenous shunting. The number of affected lobes (main lobar pulmonary vein filling) was counted for each animal.
For microsphere experiments, mice were anesthetized with 2–3% inhaled isoflurane, and the right carotid artery was isolated and ligated distally. Next, 1.25 × 105 6-μm-diameter fluorescent microspheres (Molecular Probes) in PBS were injected into the inferior vena cava while the isolated carotid artery was transected proximally. All of the exsanguinated arterial blood was recovered by aspiration, and microspheres were counted manually using a fluorescence microscope and hemacytometer.
Immunohistochemistry and lung bud analysis.
β-galactosidase was detected by activity or with an anti-β-galactosidase antibody as we have described (5). Cultured whole organ lung buds were immunostained to illuminate the blood vessels with anti-CD31 antibody (BD Pharmingen, clone MEC 13.3, 1:500 dilution) using the protocol previously published by Metzger et al. (27). All cultured lung buds were photographed under ×2.5 magnification using a fluorescence microscope. The perpendicular diameter of every blood vessel surrounding an empty air space was quantified using Image-Pro Plus software. Vessels greater than 50 μm in diameter and all air spaces in each specimen (whole organ block of both lungs) were counted by a single observer, blinded to the identity of the specimens.
Statistical analysis.
All data are expressed as means ± SD. Groups were compared using Student's t-tests and analysis of variance with Bonferroni post hoc testing where appropriate. A P value of <0.05 was considered statistically significant.
RESULTS
Notch4* expression is associated with lung hemorrhages and respiratory insufficiency.
In previous studies, we demonstrated endothelial-specific expression of Notch4* and/or lacZ reporter in embryonic and adult tissues such as yolk sac, liver, brain, and uterus (5, 13, 29) in the Tie2-tTA:TRE-int3 (now referred to as Notch4* mutants) and Tie2-tTA:TRE-lacZ mice. Because anti-Notch4-ICD immunostaining cannot distinguish between Notch4* and endogenous Notch4-ICD, and because of the relative abundance of Notch4 specifically in the lung (41), for the current study we chose to measure lacZ reporter expression as a surrogate for Notch4* expression. Colocalizing immunohistochemistry for β-galactosidase and CD31, an endothelial cell marker, showed lacZ reporter expression to be restricted to the endothelium, and histochemical β-galactosidase staining demonstrated robust, diffuse activity of the tTA throughout the lung (Fig. 1). Quantitative real-time PCR of RNA isolated from lung homogenates demonstrated a significant increase in Notch4* (int3) expression when doxycycline was withdrawn from the diet, starting at 1 wk and persisting at 5 wk after doxycycline withdrawal. Reinstitution of doxycycline promptly reversed Notch4* expression (Fig. 1). We have previously shown that Notch4* upregulates endogenous Notch4 by approximately threefold (5), and so it is possible that the subsequently observed effects of the transgene are partially the results of increased levels of endogenous Notch4. However, since endogenous Notch4 is not constitutively active, the effect of the transgene is most likely to be predominated by a direct Notch4* mechanism.
Fig. 1.
Notch4* expression in adult mouse lung endothelium. A–C: immunostaining with anti-β-gal demonstrates colocalization with the endothelial marker CD31 (scale bars, 50 μm). D: when the tetracycline transactivator (tTA) was coupled to the lacZ transgene, β-gal reporter of tTA activity was diffusely detected by frozen section X-gal staining (scale bar, 100 μm). E: quantitative PCR analysis of RNA extracted from whole lung shows increased expression of Notch4* (int3) in mutants (Tie2-tTA/TRE-int3) relative to controls or Dox-treated mutants. Reinstitution of doxycycline results in prompt reversal of tTA activity and repression of Notch4* expression. *ΔΔCt = (Ct,int3 − Ct,HPRT)mouse x - (Ct,int3 − Ct,HPRT)TRE-int3Ave.
By 6 wk after doxycycline was withdrawn, most Notch4* mutants began to show signs of illness, including disheveled fur, increased respiratory rate, decreased activity, and a hunched posture. When mutants were euthanized between 6 and 7 wk after doxycycline was withdrawn, more than 90% of animals had gross and microscopic evidence of lung hemorrhages (Fig. 2). The hemorrhages did not have a predilection for any particular lung lobe and corresponded to the general impression by the investigator of the severity of illness in the animals. Ill mutants were found to have punctate hemorrhages with a variable distribution, whereas moribund mice often were found to have entire lobes that were hemorrhagic. Hemorrhages were rarely seen in littermate controls and were always single and punctate. When a subset of mutants and controls were analyzed 4 wk after doxycycline withdrawal to determine if hemorrhaging preceded the onset of symptoms, no gross or microscopic hemorrhages were observed (data not shown). Arterial blood gas analysis at 6–7 wk after doxycycline withdrawal showed significant impairments in oxygenation and ventilation, as demonstrated by decreased Po2 and increased Pco2, in the mutants (Fig. 3). Another subset of eight mutants had doxycycline reinstituted in their diet at the first signs of sickness (slightly decreased activity, disheveled fur, increased respiratory rate) ∼6 wk after initial doxycycline withdrawal. The majority of these mice (7/8 or 87.5%) recovered, and at necropsy 6 wk after doxycycline reinstitution, the lungs appeared normal with no gross hemorrhages (Supplemental Fig. S1. Supplemental data for this article is available online at the AJP-Lung web site.). Therefore, expression of Notch4* was associated with diffuse lung hemorrhages and significant respiratory insufficiency, and cessation of Notch4* expression reversed these pathophysiological effects.
Fig. 2.
Lung hemorrhages observed with endothelial expression of Notch4*. Preperfusion (A and D) and postperfusion and post-agarose inflation (B and E) lung specimens demonstrate numerous hemorrhages (arrowheads) throughout the mutant lung lobes compared with controls (n = 14/group). C and F: on routine H&E-stained sections, red blood cells can be seen within the alveoli of mutants compared with controls (scale bars, 50 μm). Counts indicate total number of hemorrhages detected in each whole organ block of both lungs and are represented as means ± SD.
Fig. 3.
Notch4* mutants exhibit pulmonary insufficiency. Six to seven weeks after doxycycline was withdrawn from the diet, arterial blood gas analysis demonstrates decreased oxygenation and ventilation in the mutants (gray bars, n = 8), with significantly decreased Po2 and increased Pco2 compared with controls (white bars, n = 9). Error bars represent SD.
Arteriovenous shunting in adult Notch4* lungs.
To further assess the origin of the lung dysfunction seen in Notch4* mutants, we performed vascular casting to view the pulmonary vasculature 6–7 wk after doxycycline withdrawal. Due to the viscosity of the Microfil casting agent and the small caliber of the pulmonary capillaries, the casting material normally fills only the arterial circulation. Consistent with this casting behavior, the casting agent was observed in the venous circulation in only a single lung lobe of the six control mice. In contrast, the casting agent filled at least one main lobar vein in five of the seven Notch4* mutants, with an average of 2.4 ± 2.1 main lobar veins filling (out of a possible 5) per animal (Fig. 4, A–E). Filling of main lobar veins in the Notch4* mutants was evenly distributed over the left, right apical, right middle, right caudal, and right accessory lobes. The capillary network failed to hold the casting material, and no other obvious vascular defects were noted.
Fig. 4.
Arteriovenous shunting in Notch4* mutant adult mouse lungs. A–D: vascular casts of the Notch4* mutants demonstrate consistent filling of the main lobar veins (arrows) as well as the main lobar arteries (arrowheads), whereas the viscous casting agent fails to fill the venous circulation of the controls. Representative lobes from both right and left lungs are shown. E: on average, more than 2 main lobar veins filled per mutant (n = 7) vs. virtually none in the controls (n = 6). F: a large number of 6-μm-diameter fluorescent microspheres passes into the arterial circulation in the mutants (n = 3), whereas significantly fewer were seen in that of controls (n = 5) and mutants that had Dox diet reinstituted (n = 3). Error bars represent SD.
When fluorescent microspheres 6-μm in diameter were injected into the venous circulation, a significant number of microspheres passed through the lungs and were recovered in the arterial blood of the Notch4* mutants 6–7 wk after doxycycline withdrawal, whereas none were recovered in the control mice. A subset of mutants had doxycycline reinstituted in their diet at the first signs of sickness ∼6 wk after initial doxycycline withdrawal. When these mice were analyzed 6 wk after reinstitution of doxycycline, significantly fewer microspheres were recovered in the arterial blood (Fig. 4F). Therefore, expression of Notch4* was associated with development of arteriovenous shunts in a reversible fashion.
AVM-like lesions develop in Notch4* mutant lungs.
Examination of the fluorescent agarose-perfused lungs allowed for visualization of the complete arteriovenous network in littermate control mice, including the capillary network intervening between arterial and venous circulations. In general, the larger-caliber arterial circulation is positioned in a dorsal position, whereas the larger-caliber venous circulation is positioned in a ventral position. A typical arborization pattern of the vasculature was visualized in the lungs of littermate controls (Fig. 5, A–C).
Fig. 5.
Notch4* mutants develop AVM-like lung lesions. A–C: in lungs of control mice, the complete vascular network could be visualized, from the arterial (red arrowheads) to venous (blue arrowheads) circulations, including the intervening capillary network. D–F: in some areas of the Notch4* mutant lungs, the complete vascular network could be seen; however, there were also many areas of poor perfusion. G–L: Notch4* mutants also exhibited many enlarged, tortuous vessels, suggestive of arteriovenous malformations (red arrows). All images are of right cranial lobes. Scale bars, 200 μm (A, D, I, L), 500 μm (B, E, H, K), 2 mm (C, F, G, J).
In the Notch4* mutants, in general, the deeper arterial vasculature, and in many areas, the capillary interface between artery and vein could be visualized (Fig. 5, D–F). However, several areas of the vasculature were obscured, possibly due to hemorrhage that did not clear with the sucrose gradient or other flow abnormalities. In addition, the mutants exhibited areas of enlarged, tortuous vessels, in particular near the hilum of the individual lobes (Fig. 5, G–L). Thus, Notch4* expression resulted in vascular perfusion irregularities and development of AVM-like lesions in the lungs.
Effects of Notch4* expression are a primary lung phenomenon.
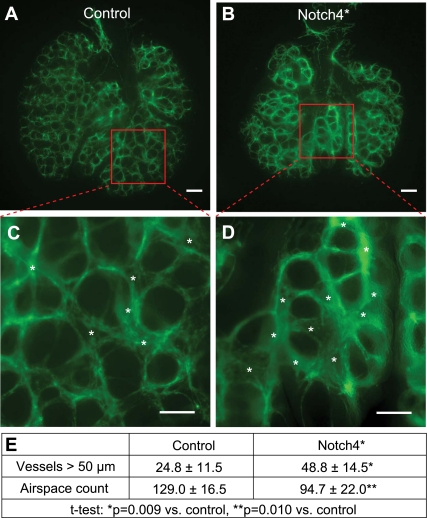
To assess the effects of Notch4* expression outside the influence of systemic effects and blood flow, we cultured embryonic lung bud explants. After tetracycline was removed from the drinking water of pregnant dams at E10.5, lung buds were dissected at E11.5, cultured for 48 h, and then fixed and stained for CD31. We found that when incubation began, the lung buds from mutants and controls were grossly indistinguishable, but that after 48 h of whole lung organ culture, Notch4* mutant lung explants developed a significantly increased number of large (>50 μm diameter) vessels and fewer air spaces (Fig. 6).
Fig. 6.
Enlarged vessels seen in Notch4* mutant embryonic lung bud explants. A–D: mutant lung buds develop more large blood vessels and fewer air spaces than controls. Asterisks mark larger blood vessels. Scale bars, 200 μm (A and B), 100 μm (C and D). E: quantification demonstrates a significantly increased number of large vessels and fewer air spaces in the mutants (n = 5/group, counts indicate number of vessels or air spaces per whole organ block of both lungs and are represented as means ± SD).
DISCUSSION
In this study, we hypothesized that expression of constitutively active endothelial Notch4 would induce enlarged arteriovenous communications (AV shunts) as a primary phenomenon. Previously, we have shown that Notch4* expression in adult mice results in reversible portosystemic shunting and enlarged hepatic arteries (5). In addition, we recently demonstrated upregulation of endothelial Notch signaling in human brain AVM specimens (30). In the current study, our results indicate that sustained Notch4 activity in the lung endothelium results in arteriovenous shunting with development of AVM-like lesions in adult mouse lungs and enlarged embryonic lung vessels in whole organ lung explant cultures. These findings suggest that aberrant Notch4 expression may play a role in disease states that result in lung arteriovenous shunting.
Notch4* expression is independently associated with lung vessel enlargement.
When we first recognized lung abnormalities in the Notch4* mutants, we acknowledged that these abnormalities could also be explained by possible systemic effects of Notch4* expression such as heart failure and effects on other organ systems. These systemic effects occur because our transgenic mouse model lacks site specificity, i.e., the transgene is expressed in the endothelium throughout the organism when doxycycline/tetracycline is withdrawn from the diet. Therefore, to determine if Notch4* expression could have an independent effect on the lung vasculature, we used the explant culture technique for the whole lung, which is well described in the literature. By timing the removal of tetracycline to cause onset of Notch4* expression at the time of embryo dissection and the beginning of lung explant culture, we were able to assess the independent effects of the transgene. Although this way of studying the transgene required working in an embryonic system, the results nevertheless demonstrate a primary effect of Notch4* expression. Thus, our analysis shows that Notch4* expression is independently associated with increased numbers of enlarged lung vessels, and correlates well with other reports of Notch4 gain-of-function causing enlarged blood vessels (5, 13, 21).
Notch4* mutants provide a new model and pathway to explore the biology of lung AV shunts.
Currently, there are few animal models of lung shunts/AVMs, and no reliable adult transgenic mouse models have been described. In one existing model, conditional deletion of the TGF-β receptor Alk1 (also known as Acvrl1) from the lung endothelium resulted in enlarged, tortuous blood vessels, but those mutant mice died by E18.5 and are therefore suboptimal for studying potential therapeutic strategies (31). Another study of adult mice heterozygous for the Alk1 gene found that only 1 out of 47 developed lung lesions at age 11 mo (37). In addition, both the conditional knockout and heterozygous Alk1 mutants are described as models for the human disease HHT, which constitutes only a small fraction of patients with lung AV shunts. Other models, such as the sheep (26) and rat (39) cavopulmonary anastomosis models, can be cumbersome, expensive, and technically challenging. The rat hepatopulmonary syndrome (common bile duct ligation) model (10) has been productive and yielded important insights, but the lack of abundant transgenic rat strains is limiting, and application of this model in mice has not been published. In this study, we have shown that the induction of enlarged arteriovenous communications in the lungs of adult mice can be achieved in a temporally regulatable and reversible fashion. Our Notch4* mouse model, therefore, provides a novel approach for studying adult lung AV shunts by cross-breeding with other transgenic mice or investigating potentially inhibitory pharmacological agents.
Notch signaling may be a common effector in lung AV shunt biology.
The development of reversible arteriovenous shunting in the lungs of Notch4* mutants and the molecules implicated in other lung AV shunt/AVM models suggest that the Notch pathway may play a central mechanistic role. The effects of HIF-1α and VEGF, which are elevated in the sheep and rat lung AVM models (25, 28), have been shown to be mediated through Notch signaling (22, 33). In addition, Akt, which is increased in the rat hepatopulmonary syndrome model, acts via Notch signaling in some systems (1). Finally, Notch has been shown to play a critical role in orchestrating the biological responses to TGF-β signaling (14), such as the lung defects described in the Alk1 mice above.
How Notch signaling leads to AV shunting has not been completely described, but we and others (5, 21) have proposed that Notch-induced inhibition of new vessel sprouts leads to existing vessel enlargement. Our vascular casting and microsphere data demonstrate the presence of enlarged intrapulmonary arteriovenous channels. This process mimics that which has been described for the development of arteriovenous malformations in HHT, in which enlargement of the postcapillary venules is the initial morphological change (4). It is possible, then, that Notch activation is the final common pathway of various lung-AV shunt-inducing disease entities, causing vessel enlargement at the capillary bed interface. This initial vessel enlargement could then lead to changes in blood flow and cause further enlargement and tortuosity (36), resulting in morphological vascular changes that ultimately result in clinically and radiographically apparent lung lesions. In summary, our results, together with the associations of Notch with the HIF-1α, VEGF, Akt, and TGF-β pathways, suggest that Notch signaling may be a key effector in the development of lung AV shunts.
In conclusion, this study shows that expression of constitutively active endothelial Notch4 directly leads to pulmonary arteriovenous shunting in mice. Furthermore, aberrant Notch signaling may play a key role in the development of lung AV shunts, and the relevance of this pathway in the pathobiology of lung AV shunts/AVMs warrants further exploration.
GRANTS
This study was supported by the Foundation for Accelerated Vascular Research (formerly Pacific Vascular Research Foundation), National Heart, Lung, and Blood Institute Grants R01-HL-075033 (to R. A. Wang), F32-HL-974002 (to E. B. Jelin), and K08-HL-092062 (to D. Miniati) and an American Lung Association Biomedical Research Grant and an American Pediatric Surgical Association Foundation Enrichment Grant (to D. Miniati).
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Dean Sheppard, Dr. Ross Metzger, and members of their laboratories for technical assistance in studying mouse lung tissue. We also thank Patrick Murphy and Tyson Kim for technical and strategic assistance and other members of the Laboratory for Accelerated Vascular Research for helpful discussions.
Present addresses of J. Ng: New York Medical College, Valhalla, NY 10595; T. R. Carlson: Leydig, Voit, & Mayer, Two Prudential Plaza, Ste. 4900, 180 N. Stetson Ave., Chicago, IL 60601; X. Wu: Data Virtuoso, 100 Springhouse Dr., Collegeville, PA 19426.
REFERENCES
- 1.Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB. Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest 118: 3660–3670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein HS, Brook MM, Silverman NH, Bristow J. Development of pulmonary arteriovenous fistulae in children after cavopulmonary shunt. Circulation 92: II309–II314, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Braren R, Hu H, Kim YH, Beggs HE, Reichardt LF, Wang R. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol 172: 151–162, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braverman IM, Keh A, Jacobson BS. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J Invest Dermatol 95: 422–427, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Carlson TR, Yan Y, Wu X, Lam MT, Tang GL, Beverly LJ, Messina LM, Capobianco AJ, Werb Z, Wang R. Endothelial expression of constitutively active Notch4 elicits reversible arteriovenous malformations in adult mice. Proc Natl Acad Sci USA 102: 9884–9889, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao HS, Chern MS, Chen YC, Chang SC. Recurrence of pulmonary arteriovenous malformations in a female with hereditary hemorrhagic telangiectasia. Am J Med Sci 327: 294–298, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Cottin V, Chinet T, Lavole A, Corre R, Marchand E, Reynaud-Gaubert M, Plauchu H, Cordier JF. Pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: a series of 126 patients. Medicine 86: 1–17, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cottin V, Dupuis-Girod S, Lesca G, Cordier JF. Pulmonary vascular manifestations of hereditary hemorrhagic telangiectasia (rendu-osler disease). Respiration 74: 361–378, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Fallon MB. Mechanisms of pulmonary vascular complications of liver disease: hepatopulmonary syndrome. J Clin Gastroenterol 39: S138–S142, 2005 [PubMed] [Google Scholar]
- 10.Fallon MB, Abrams GA, McGrath JW, Hou Z, Luo B. Common bile duct ligation in the rat: a model of intrapulmonary vasodilatation and hepatopulmonary syndrome. Am J Physiol Gastrointest Liver Physiol 272: G779–G784, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Freedom RM, Yoo SJ, Perrin D. The biological “scrabble” of pulmonary arteriovenous malformations: considerations in the setting of cavopulmonary surgery. Cardiol Young 14: 417–437, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Graudal N, Leth P, Marbjerg L, Galloe AM. Characteristics of cirrhosis undiagnosed during life: a comparative analysis of 73 undiagnosed cases and 149 diagnosed cases of cirrhosis, detected in 4929 consecutive autopsies. J Intern Med 230: 165–171, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Hu H, Guevara-Gallardo S, Lam MT, Fong SY, Wang RA. Artery and vein size is balanced by Notch and ephrin B2/EphB4 during angiogenesis. Development 135: 3755–3764, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluppel M, Wrana JL. Turning it up a Notch: cross-talk between TGF beta and Notch signaling. Bioessays 27: 115–118, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev 18: 2469–2473, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev 14: 1343–1352, 2000 [PMC free article] [PubMed] [Google Scholar]
- 17.Lacombe P, Lagrange C, Beauchet A, El Hajjam M, Chinet T, Pelage JP. Diffuse pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: long-term results of embolization according to the extent of lung involvement. Chest 31: 31, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Lai EC. Notch signaling: control of cell communication and cell fate. Development 131: 965–973, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Lange PA, Stoller JK. The hepatopulmonary syndrome. Effect of liver transplantation. Clin Chest Med 17: 115–123, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128: 3675–3683, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, Biswas S, Turley H, Heikamp E, Hainfellner JA, Harris AL. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res 67: 11244–11253, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA 104: 3219–3224, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma X, Reddy VM, Riemer RK. Notch signaling is involved in development of pulmonary arteriovenous malformations after cavopulmonary anastomosis (Abstract). Circulation 112: II-10, 2005 [Google Scholar]
- 24.Malhotra SP, Reddy VM, Thelitz S, He YP, Hanley FL, Suleman S, Riemer RK. Cavopulmonary anastomosis induces pulmonary expression of the angiotensin II receptor family. J Thorac Cardiovasc Surg 123: 655–660, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Malhotra SP, Reddy VM, Thelitz S, He YP, McMullan DM, Hanley FL, Riemer RK. The role of oxidative stress in the development of pulmonary arteriovenous malformations after cavopulmonary anastomosis. J Thorac Cardiovasc Surg 124: 479–485, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Malhotra SP, Riemer RK, Thelitz S, He YP, Hanley FL, Reddy VM. Superior cavopulmonary anastomosis suppresses the activity and expression of pulmonary angiotensin-converting enzyme. J Thorac Cardiovasc Surg 122: 464–469, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature 453: 745–750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mumtaz MA, Fraga CH, Nicholls CM, Desai S, Vasilyev N, Joshi R, Mee RB, Duncan BW. Increased expression of vascular endothelial growth factor messenger RNA in lungs of rats after cavopulmonary anastomosis. J Thorac Cardiovasc Surg 129: 209–210, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Murphy PA, Lam MT, Wu X, Kim TN, Vartanian SM, Bollen AW, Carlson TR, Wang RA. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci USA 105: 10901–10906, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy PA, Lu G, Shiah S, Bollen AW, Wang RA. Endothelial Notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab Invest 89: 971–982, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SO, Lee YJ, Seki T, Hong KH, Fliess N, Jiang Z, Park A, Wu X, Kaartinen V, Roman BL, Oh SP. ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood 111: 633–642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome–a liver-induced lung vascular disorder. N Engl J Med 358: 2378–2387, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA 105: 6392–6397, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schenk P, Fuhrmann V, Madl C, Funk G, Lehr S, Kandel O, Muller C. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut 51: 853–859, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah MJ, Rychik J, Fogel MA, Murphy JD, Jacobs ML. Pulmonary AV malformations after superior cavopulmonary connection: resolution after inclusion of hepatic veins in the pulmonary circulation. Ann Thorac Surg 63: 960–963, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Sho E, Nanjo H, Sho M, Kobayashi M, Komatsu M, Kawamura K, Xu C, Zarins CK, Masuda H. Arterial enlargement, tortuosity, and intimal thickening in response to sequential exposure to high and low wall shear stress. J Vasc Surg 39: 601–612, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Srinivasan S, Hanes MA, Dickens T, Porteous ME, Oh SP, Hale LP, Marchuk DA. A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet 12: 473–482, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Srivastava D, Preminger T, Lock JE, Mandell V, Keane JF, Mayer JE, Jr, Kozakewich H, Spevak PJ. Hepatic venous blood and the development of pulmonary arteriovenous malformations in congenital heart disease. Circulation 92: 1217–1222, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Starnes SL, Duncan BW, Fraga CH, Desai SY, Jones TK, Mathur SK, Rosenthal GL, Lupinetti FM. Rat model of pulmonary arteriovenous malformations after right superior cavopulmonary anastomosis. Am J Physiol Heart Circ Physiol 283: H2151–H2156, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Uyttendaele H, Ho J, Rossant J, Kitajewski J. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc Natl Acad Sci USA 98: 5643–5648, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development 122: 2251–2259, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev 108: 161–164, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Luo B, Tang L, Wang Y, Stockard CR, Kadish I, Groen TV, Grizzle WE, Ponnazhagan S, Fallon MB. Pulmonary angiogenesis in rat experimental hepatopulmonary syndrome. Gastroenterology 136: 1070–1080, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M, Luo B, Chen SJ, Abrams GA, Fallon MB. Endothelin-1 stimulation of endothelial nitric oxide synthase in the pathogenesis of hepatopulmonary syndrome. Am J Physiol Gastrointest Liver Physiol 277: G944–G952, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.