Abstract
Despite our understanding that medial smooth muscle hypertrophy is a central feature of vascular remodeling, the molecular pathways underlying this pathology are still not well understood. Work over the past decade has illustrated a potential role for the multifunctional calmodulin-dependent kinase CaMKII in smooth muscle cell contraction, growth, and migration. Here we demonstrate that CaMKII is enriched in vascular smooth muscle (VSM) and that CaMKII inhibition blocks ANG II-dependent VSM cell hypertrophy in vitro and in vivo. Specifically, systemic CaMKII inhibition with KN-93 prevented ANG II-mediated hypertension and medial hypertrophy in vivo. Adenoviral transduction with the CaMKII peptide inhibitor CaMKIIN abrogated ANG II-induced VSM hypertrophy in vitro, which was augmented by overexpression of CaMKII-δ2. Finally, we identify the downstream signaling components critical for ANG II- and CaMKII-mediated VSM hypertrophy. Specifically, we demonstrate that CaMKII induces VSM hypertrophy by regulating histone deacetylase 4 (HDAC4) activity, thereby stimulating activity of the hypertrophic transcription factor MEF2. MEF2 transcription is activated by ANG II in vivo and abrogated by the CaMKII inhibitor KN-93. Together, our studies identify a complete pathway for ANG II-triggered arterial VSM hypertrophy and identify new potential therapeutic targets for chronic human hypertension.
Keywords: remodeling, histone deacetylase, MEF2
smooth muscle cell hypertrophy contributes to vascular dysfunction and maintenance of hypertension. Angiotensin II (ANG II) potently induces vascular smooth muscle (VSM) hypertrophy and hypertension. The multifunctional calcium/calmodulin-dependent protein kinase II (CaMKII) is expressed in VSM cells (VSMC) (30) and activated by ANG II (1). However, the potential role of CaMKII in VSM is poorly understood. In cardiac muscle CaMKII causes hypertrophy by the histone deacetylase (HDAC)/MEF2 pathway (5), but it is unknown whether this mechanism is operative or important in VSM. CaMKII inhibition has been proposed as a novel therapy for arrhythmias and heart failure (34), where hypertrophy is a common early event, but it is unknown whether modulation of CaMKII activity could be beneficial in vascular disease. Previous work in VSM has demonstrated that CaMKII may enhance mechanical responses to KCl-triggered cell membrane depolarization in vitro (14) and that CaMKII inhibition can reduce hypertension (17) and VSM contractile responses of ANG II in diabetes (33). While CaMKII has been implicated in ANG II-induced MEF2 activity in VSMC (21), there are no data that directly support a role of CaMKII in vascular hypertrophy. Furthermore, the upstream and downstream signaling mechanisms for CaMKII in VSM are unknown.
Here we tested the hypothesis that CaMKII mediates VSM hypertrophy, a critical pathological response to ANG II. CaMKII inhibition significantly reduced ANG II-induced VSM hypertrophy in vitro and abrogated ANG II hypertension and medial hypertrophy in vivo. CaMKII overexpression increased nuclear export of HDAC4, leading to MEF2 activation and VSM hypertrophy in vitro and in vivo, while CaMKII inhibition caused opposite effects. The ANG II/CaMKII hypertrophic pathway was critically dependent on the phosphorylation of HDAC4 at the known CaMKII phosphorylation sites S467 and S632 (6). Overexpression of the dominant-negative HDAC4 double mutant S467A,S632A eliminated the hypertrophic action of ANG II in VSM. These findings strongly support the notion that CaMKII mediates VSM hypertrophy and demonstrate a novel role for CaMKII signaling in VSM function and pathological arterial remodeling. Additionally, our findings define the specific molecular pathway that connects CaMKII activation to nuclear transcriptional pathways for ANG II signaling in VSM.
METHODS
Reagents.
The following reagents were used: KN-93 (Alexis Biochemicals), KN-92 (EMD Chemicals), ANG II (Sigma Aldrich), antibodies for S623-phosphorylated HDAC4 (p-HDAC4) (Abcam), antibodies for HDAC4 and β-galactosidase (Millipore), antibodies for smooth muscle-actin, topoisomerase III, GAPDH (Santa Cruz Biotechnology), and MEF2A, -C, and -D and for T312-phosphorylated MEF2A (Abcam), HDAC4 small interfering RNA (siRNA) adenoviruses (AdenoSilence RNAi HDAC4-v1–3 viruses), and the control virus EGFP-v1 (Millipore). The polyclonal antibody for total CaMKII was raised against the COOH terminus of mouse CaMKII-δ2.
Plasmids and adenoviruses.
The cDNA for hemagglutinin (HA)-tagged CaMKIIN, a gift from Dr. Thomas Soderling (Oregon Health and Sciences University, Portland, OR) was inserted into the adenoviral shuttle vector pacAd5 CMV IRES enhanced green fluorescent protein (eGFP) pA with the BamHI and EcoRI restriction sites. A myc tag was added by cloning the cDNA for mouse CaMKII-δ2, the most common CaMKII isoform in proliferating VSM (12), into pCMV-myc (Clontech). myc-Tagged CaMKII-δ2 was inserted into pacAd5 CMV IRES with the BamHI and XhoI restriction sites. The point mutations HDAC4 S467,632A were generated with the QuikChange II Site-Directed Mutagenesis Kit (Stratagene). Adenovirus recombination and amplification was performed by the Gene Transfer Vector Core at the University of Iowa (3). Viral titers were determined with the Adeno-X rapid titer kit (Clontech). Control virus expressing eGFP was generated by recombination and amplification of the shuttle vector.
Cell culture.
Rat aortic smooth muscle cells (RASM) were isolated from male Sprague-Dawley rat (Harlan Sprague-Dawley) thoracic aorta by enzymatic digestion and grown in Dulbecco's modified Eagle's medium (DMEM) with glutamine, essential amino acids, vitamins, 25 mM HEPES, 4.5 g/l glucose, and 10% FCS. Cells at passages 4–10 were used. Cells were incubated in serum-free medium for 24 h before addition of ANG II. All experiments were performed at ∼70% cell confluence.
Immunoblotting.
Twenty-microgram aliquots of whole cell lysate from RASM were resolved by SDS-polyacrylamide electrophoresis, transferred to polyvinylidene difluoride membrane, and immunoblotted as indicated in Figs. 5 and 7–9. The proteins were visualized with the ECL chemiluminescence system (Amersham).
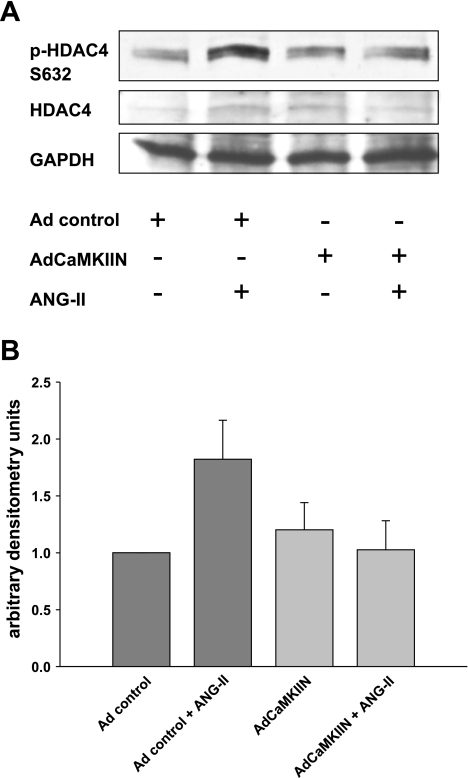
Fig. 5.
ANG II activates HDAC4 in VSM by CaMKII-mediated phosphorylation. A: immunoblotting for p-HDAC4 S632 phosphorylation in response to ANG II in RASM after adenoviral (Ad) overexpression of CaMKIIN; 20 μg of protein was separated and probed for p-HDAC4 S632, HDAC4, and GAPDH. Representative blot is shown. B: densitometry of 3 independent experiments.
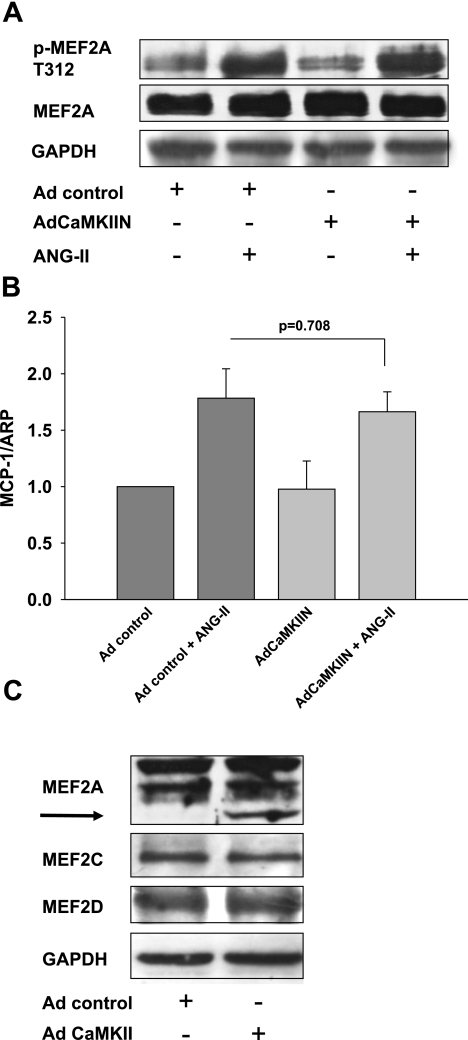
Fig. 7.
CaMKII does not mediate activation of MEF2 by phosphorylation or change MEF protein levels. A: immunoblotting for p-MEF2A. RASM were infected with control virus or adenovirus expressing the CaMKII inhibitor CAMKIIN. RASM were serum starved for 24 h at 70% confluence. Then 100 nM ANG II was added for 10 min. Twenty micrograms of protein was separated and blotted for pMEF2A T312 and MEF2A (anti-MEF2A antibody; Abcam). Representative blot of 3 independent experiments is shown. B: quantitative real-time PCR for monocyte chemoattractant protein-1 (MCP1) was performed after infection of RASM with adenovirus expressing CaMKIIN or control virus. Total RNA was extracted after RASM were serum starved for 24 h at 70% confluence, followed by treatment with 100 nM ANG II for 12 h. Histograms show the relative amount of MCP1 mRNA (n = 4). ARP, acidic ribosomal phosphoprotein. C: immunoblotting for MEF2A, -C, and -D in RASM with adenoviral overexpression of CaMKII and controls. Fifteen micrograms of protein was separated and blotted for MEF2-A (anti-MEF2A antibody; Santa Cruz), -B, and -C and GAPDH. Representative blot of 4 independent experiments is shown.
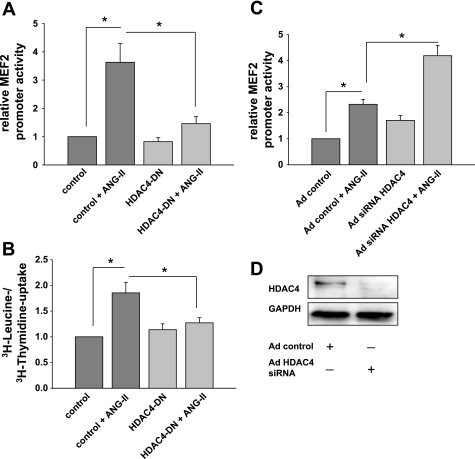
Fig. 8.
ANG II-induced MEF2 promoter activity and smooth muscle hypertrophy are mediated by CaMKII phosphorylation of HDAC4 S467,632. A: MEF2-luciferase promoter assays were performed after cotransfection of MEF2-luciferase and TK Renilla luciferase cDNA with dominant-negative (DN) HDAC4 mutant S467,632A cDNA lacking the CaMKII phosphorylation sites. Control experiments were transfected with empty vector cDNA. Seventy-two hours after infection, 100 nM ANG II was added for 18 h. MEF2 promoter activity was measured and adjusted for TK Renilla activity (n = 7). *P < 0.05. B: overexpression of CaMKII-resistant HDAC4 S467,632A DN constructs prevented VSM hypertrophy. cDNA for pcDNA3.1 HDAC4-S467,632A and pcDNA3.1(+) control vector was delivered by electroporation into RASM at low density. RASM were grown to 70% confluence, serum starved in serum-free medium for 24 h, and then treated with 100 nM ANG II or vehicle for 24 h in serum-free medium. [3H]leucine incorporation was determined and normalized to [3H]thymidine uptake as described in text (n = 4). *P < 0.05. C: MEF2 promoter activity in RASM infected with adenovirus expressing HDAC4 small interfering RNA (siRNA) or control and transfected with MEF2-luciferase and TK Renilla cDNA constructs. RASM were treated with ANG II for 18 h. MEF2-luciferase was adjusted for TK Renilla activity (n = 5). *P < 0.05. D: immunoblotting for HDAC4 in control and HDAC4 siRNA-infected RASM 72 h after infection. Twenty micrograms of protein was separated and probed for HDAC4 and GAPDH. Representative blot of 3 independent experiments is shown.
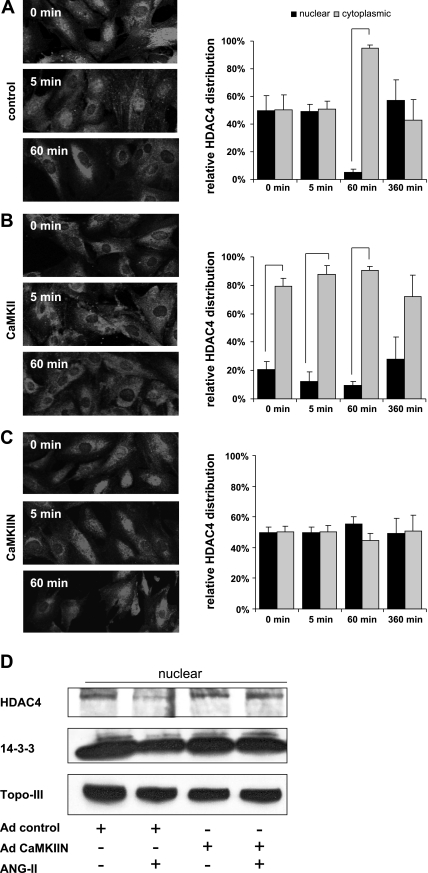
Fig. 9.
Translocation of HDAC4 from the nucleus to the cytoplasm by ANG II requires CaMKII. A–C, left: growth-arrested 80% confluent RASM were treated with 100 nM ANG II for 0–360 min and fixed with paraformaldehyde. The subcellular localization of HDAC4 was determined in VSMC infected with control adenovirus (A), adenovirus overexpressing CaMKII (B), or CaMKIIN (C) by immunofluorescence with an anti-HDAC4 antibody. Representative frames are shown. A–C, right: a minimum of 100 cells in 5 frames were assessed for HDAC localization and summarized as % of nuclear vs. cytoplasmic localization (all bars represent P < 0.05). D: nuclear protein fractions were prepared from VSMC after infection with adenovirus expressing CaMKIIN or control and treatment with 100 nM ANG II for 1 h. Twenty micrograms of protein was separated and immunoblotted for HDAC-4, 14-3-3, and the nuclear protein marker topoisomerase III (Topo-III).
Nuclear fractionation.
Nuclear protein fractions were obtained with a Pierce kit. The fractions were subjected to SDS-PAGE and immunoblotting as described above.
In vitro VSM hypertrophy assays.
To measure hypertrophy of VSMC, cells were plated at low density. After infection with adenoviruses overnight, VSMC were grown to 70% confluence for 2 days in DMEM containing 10% calf serum and then serum starved in serum-free medium for 24 h. Twenty-four hours before harvest, cells were incubated with [3H]leucine (2 μCi/ml) or [3H]thymidine (2 μCi/ml) in the presence or absence of 100 nmol/l ANG II. [3H]leucine and [3H]thymidine incorporation were measured as described previously (11) and expressed as ratios (19). We also determined the [3H]leucine and [3H]thymidine uptake after delivery of the pcDNA3.1 HDAC4-S467,632A and pcDNA3.1(+) control vector by electroporation with Nucleofector II according to the manufacturer's recommendations (Amaxa). Transfected cells were treated as described above.
Morphometry was performed in RASM infected with adenoviruses at a multiplicity of infection (MOI) of 500 (22). The cells were grown to 70% confluence, growth arrested in serum-free medium for 24 h, and treated with 100 nM ANG II for another 24–48 h. The cells were fixed in 10% paraformaldehyde and embedded in Vectashield embedding solution (Vector); digital images were taken under a LSM 510m laser scanning microscope. VSM cell size was determined by measuring the cell area of at least 50 cells for each condition with LSM Image Examiner software (Zeiss).
Immunohistochemistry.
RASM were infected with adenoviruses expressing myc-CaMKII-δ2, HA-CaMKIIN, or control adenovirus at a MOI of 500, growth arrested for 24 h, and treated with 100 nM ANG II as indicated. The cells were fixed in 4% paraformaldehyde for 15 min, permeabilized, blocked in normal goat serum for 1 h, and incubated with primary antibody overnight at 4°C and with Alexa 568-labeled secondary antibody for 1 h. The slides were mounted with Vectashield. Digital images were taken under a LSM 510m laser scanning microscope. The subcellular localization of HDAC4 was determined by assessing the HDAC4 distribution pattern in least 100 cells in 5 or more frames for each condition with LSM Image Examiner software.
Promoter assays.
RASM were infected with adenoviruses overnight and then transfected with MEF2-luciferase reporter (a gift from Dr. Andrew Lassar, Harvard University, Boston, MA) and pRL-TK Renilla (Promega) at a ratio of 20:1 with the use of Effectene at a ratio of 1:10 total DNA to reagent for 8 h or overnight. Alternatively, transfection was performed with MEF2-luciferase reporter or HDAC4 mutant S467,643A in the expression vector pcDNA3.1(+) and pRL-TK Renilla at a ratio of 10:1:1. At 70% confluence, the cells were growth arrested in serum-free medium for 24 h. Seventy-two hours after infection, 100 nM ANG II was added for 18 h. The Dual-Luciferase Reporter Assay System (Promega) was used according to the manufacturer's instructions to determine the promoter activity. The MEF2-luc activity was adjusted for TK Renilla expression.
HDAC deacetylase activity assays.
RASM were plated in 96-well plates and infected with adenoviruses 72 h before each experiment. Cells were incubated for 24 h in serum-free media before exposure to addition of 100 nM ANG II and Fluor de Lys substrate. Untreated cells and cells incubated with 100 nM ANG II in the presence of 1 μM trichostatin A were used as controls. Deacetylase activity was determined in triplicate, as recommended by the manufacturer (Biomol).
Quantitative real-time PCR.
Total RNA was isolated with the RNeasy Kit according to the manufacturer's recommendations. Preparation of the RNA included digestion with proteinase K and DNase I to eliminate possible genomic DNA contamination. cDNA was prepared from 1 μg of total RNA with SuperScript III enzyme (Invitrogen) and random nanomer primers. Message expression was quantified with an iQ Lightcycler instrument (Bio-Rad) and SYBR green dye and normalized to acidic ribosomal phosphoprotein (ARP) rRNA.
Blood pressure and hypertrophy measurements in vivo.
Eight-week-old C57BL/6 mice were implanted with minipumps to administer 1.25 μg·kg−1·min−1 ANG II or normal saline for 10 days. KN-93 (20 μg·kg−1·day−1) was injected intraperitoneally on days 2–10. KN-93 dosing was based on studies in which KN-93 reduced adverse left ventricular remodeling after myocardial infarction.
Blood pressures were monitored daily by tail cuff (Visitech Systems) on days 1–10 after a 7-day training period before implantation of the minipumps. On day 10, aortic tissue was harvested. For the analysis of vascular hypertrophy, all animals were perfused with saline and subsequently pressure fixed at 100 mmHg with paraformaldehyde. Aortas were embedded in paraffin, and 5-μm cross sections were cut starting distal to the takeoff of the subclavian artery and stained with hematoxylin and eosin. Digital images were obtained with a Zeiss Axioskop microscope. To determine cross-sectional wall area, the perimeters of the internal and external elastic laminas were traced. The area inside each respective perimeter was determined, and the difference between these areas was reported. All measurements were completed with the use of NIH Image (version 1.62).
The University of Iowa Institutional Animal Care and Use Committee approved all studies. All procedures were in compliance with the standards for care and use of laboratory animals of the Institute of Laboratory Animal Resources, National Academy of Sciences.
MEF2 reporter mice.
MEF2 reporter mice (a gift from Dr. Eric Olson, University of Texas Southwestern, Dallas, TX) that harbor a lacZ reporter gene under the control of tandem copies of the MEF2 DNA-binding site were given 1.25 μg·kg−1·min−1 ANG II or normal saline for 10 days by minipump. KN-93 (20 μg·kg−1·day−1) was injected twice daily intraperitoneally on days 2–10. We used the inactive congener compound KN-92 at the same concentration in the control group. On day 10, aortic tissue was harvested. Immunohistochemical detection of β-galactosidase was performed in frozen sections of the descending thoracic aorta.
Statistical analysis.
Data are shown as means ± SE unless stated otherwise. Statistical significance was assessed by ANOVA on untransformed data, followed by comparison of group averages by contrast analysis, with the use of SigmaStat 4.0 (Systat). A P value <0.05 was considered statistically significant.
RESULTS
CaMKII inhibition prevents VSM hypertrophy and hypertension by ANG II.
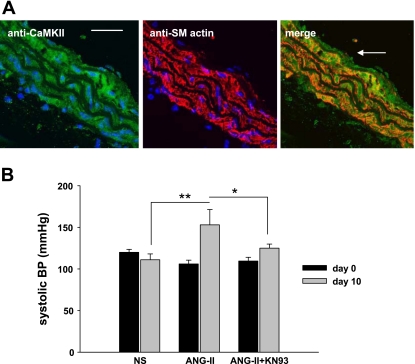
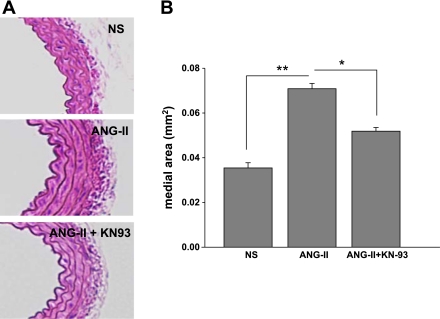
CaMKII is expressed in the aortic media of C57BL/6 mice (Fig. 1A). To investigate the role of CaMKII in VSM hypertrophy in vivo, we tested the effects of CaMKII inhibition on ANG II-induced medial hypertrophy. As expected, systolic blood pressure significantly increased in mice treated with ANG II for 10 days (47 ± 18 mmHg, n = 5, P < 0.01; Fig. 1B). In contrast, mice receiving ANG II infusion together with the CaMKII inhibitor KN-93 displayed a significantly blunted hypertensive response (15 ± 4 mmHg, n = 6, P < 0.05). There was no difference in blood pressure in normal saline-treated mice receiving KN-93 or sham treatment. Mice treated with the CaMKII inhibitor KN-93 exhibited decreased aortic medial hypertrophy compared with littermates treated with ANG II alone (Fig. 2; 0.070 ± 0.002 vs. 0.052 ± 0.002 mm2, P < 0.005). These findings suggest that CaMKII is critical for ANG II-mediated arterial hypertension and medial hypertrophy in vivo.
Fig. 1.
Calcium/calmodulin-dependent protein kinase II (CaMKII) inhibition prevents angiotensin II (ANG II)-induced hypertension. A: CaMKII expression in mouse aorta. Left: immunofluorescent detection of CaMKII in aortic cross sections from C57BL/6 mice (calibration bar, 20 μm). Center: immunostaining for smooth muscle (SM) actin that demarcates the aortic media. Right: merged image from left and center shows that CaMKII is present in vascular smooth muscle (VSM) (arrow luminal side) (×60). B: increase in systolic blood pressure (BP) by ANG II is blunted by CaMKII inhibition. Mice were infused with 1.25 μg·kg−1·min−1 ANG II or normal saline (NS) for 10 days. ANG II-treated mice were either treated with the CaMKII inhibitory drug KN-93 or with the DMSO vehicle. KN-93 (20 μg/kg) or DMSO was injected intraperitoneally twice daily on days 2–10. Data are presented as means ± SD (n = 4–6/group). *P < 0.05, **P < 0.01.
Fig. 2.
CaMKII inhibition prevents ANG II-induced VSM hypertrophy in vivo. A: CaMKII inhibition decreases medial hypertrophy. Aortas were obtained from mice used for studies in Fig. 1B. Five-micrometer cross sections were cut starting distal to the takeoff of the subclavian artery and stained with hematoxylin and eosin. B: summary data for medial descending thoracic aorta thickness from 6 sections for each aorta with n = 6 mice/group. Cross-sectional wall area was determined by tracing the perimeters of the internal and external elastic laminas. The area inside each respective perimeter was determined, and the difference between these areas is reported. All measurements were completed with the use of NIH Image (version 1.62). *P < 0.05, **P < 0.01.
CaMKII is required for hypertrophic signaling by ANG II in vitro.
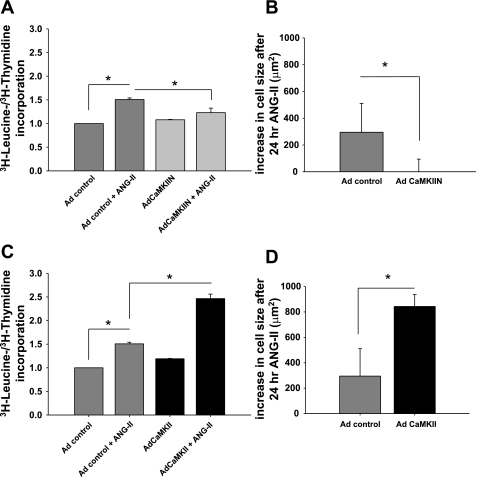
Our in vivo studies in mice suggest that CaMKII is critical for prohypertrophic ANG II signaling effects in vivo but do not address whether CaMKII induces VSM hypertrophy directly, independent of effects on arterial blood pressure. To test whether CaMKII contributes directly to an ANG II-dependent VSM hypertrophic signaling pathway, we next measured ANG II effects on cultured VSMC with CaMKII inhibition. We infected aortic VSM with adenovirus expressing the highly specific CaMKII peptide inhibitor CaMKIIN that inhibits all isoforms of CaMKII (7). CaMKII inhibition by CaMKIIN expression reduced ANG II-induced hypertrophy, as assessed by two independent assays. First, ANG II increased the [3H]leucine uptake by 50% in RASM incubated with 100 nM ANG II for 24 h (Fig. 3A). The increase in [3H]leucine was a reflection of VSM hypertrophy rather than hyperplasia, because [3H]thymidine uptake did not increase after ANG II treatment (data not shown). CaMKII inhibition significantly attenuated the ANG II-induced increase in [3H]leucine uptake, adjusted to [3H]thymidine uptake, to 14% (Fig. 3A; P < 0.05 compared with control virus-infected VSM). Second, CaMKII inhibition abolished ANG II-dependent increases in two-dimensional cell surface area. Treatment with ANG II for 24 h increased the cell area by ∼18% (292 ± 59 μm2) in control cells expressing GFP alone. Overexpression of CaMKIIN almost completely prevented the increase in cell hypertrophy, with an increase of 3 μm2 after 24 h (Fig. 3B; P < 0.05, n = 50) and 28 μm2 after 48-h administration of ANG II (data not shown).
Fig. 3.
CaMKII regulates ANG II-induced VSM cell (VSMC) hypertrophy in vitro. CaMKII is necessary for ANG II-induced VSMC hypertrophy. A: rat aortic smooth muscle cells (RASM) were infected with adenovirus (Ad) expressing the CaMKII inhibitor CaMKIIN, grown to 70% confluence, serum starved in serum-free medium for 24 h, and then treated with 100 nM ANG II or vehicle for 24 h in serum-free medium. [3H]leucine incorporation was determined and normalized to [3H]thymidine uptake. Data are expressed as % increase in [3H]leucine incorporation induced by ANG II over the appropriate control. Each bar represents mean of 7 experiments performed in triplicate. *P < 0.05. B: RASM were infected with adenovirus expressing the CaMKII inhibitor CaMKIIN, grown to 70% confluence, serum starved in serum-free medium for 24 h, and then treated with 100 nM ANG II or vehicle for 24 h in serum-free medium. We identified adenovirus-infected cells by green fluorescent protein (GFP) fluorescence and traced 50–100 cells for each condition. No increase in cell size was noted when CaMKII was inhibited by CaMKIIN. *P < 0.05. C: RASM were infected with adenovirus expressing CaMKII and treated as described in A. [3H]leucine incorporation was determined and normalized to [3H]thymidine uptake in cells treated with 100 nM ANG II or vehicle for 24 h. D: RASM were infected with adenovirus expressing CaMKII and treated as described in B. Increase in cell size after ANG II for 24 h of VSMC infected with adenovirus expressing CaMKII is shown. *P < 0.05.
In contrast, overexpression of CaMKII increased VSM hypertrophy. We only detected a nonsignificant increase of 20% in [3H]leucine uptake at baseline without ANG II. After the administration of ANG II for 24 h, [3H]leucine/[3H]thymidine uptake increased by 107% compared with 50% in control cells (P < 0.05; Fig. 3C). Second, cell area increased by 840 ± 97 μm2 (49%) in CaMKII-overexpressing VSM cells 24 h after ANG II treatment compared with 292 ± 59 μm2 or 18% in control virus-infected cells (Fig. 3D; P < 0.05, n = 50). These results suggest that VSM hypertrophy is positively regulated by CaMKII activity during ANG II stimulation and strongly support our hypothesis that CaMKII is required for ANG II-initiated hypertrophy in VSM, independent of systemic effects on blood pressure.
ANG II increases HDAC4 phosphorylation by activating CaMKII.
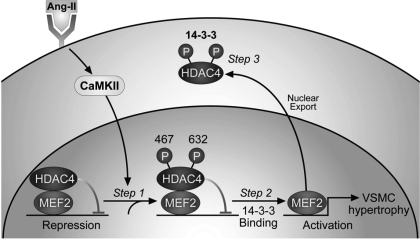
Class II histone deacetylases form a complex with transcription factor MEF2 gene regulatory elements, resulting in repression of gene promoters harboring MEF2-binding sites (16). The class II histone deacetylase HDAC4 is regulated by CaMKII (6). Phosphorylation of S467 and S632 by CaMKII reveals binding site for the chaperone 14-3-3. Binding of 14-3-3 to phosphorylated HDAC4 results in nuclear export of HDAC4 and derepression and subsequent activation of MEF2-dependent gene transcription (Fig. 4) (5). On the basis of our findings that CaMKII was required for ANG II-mediated VSM hypertrophy, we next tested whether MEF2 derepression after phosphorylation of HDAC4 by CaMKII was necessary for VSM hypertrophy.
Fig. 4.
Model depicting the role of CaMKII in histone deacetylase (HDAC)4/MEF2-dependent VSMC hypertrophy. CaMKII is activated by ANG II and specifically phosphorylates HDAC4 S467 and 632. HDAC4 represses MEF2 transcriptional activity at baseline. Phosphorylation of HDAC4 S467 and 632 by CaMKII generates binding sites for the chaperone 14-3-3. With the subsequent export of HDAC4, MEF2 is derepressed and MEF2-dependent gene transcription activated. MEF2-dependent gene transcription contributes to ANG II-induced VSMC hypertrophy.
In VSM treated with ANG II, we detected phosphorylation of the CaMKII phosphorylation site HDAC4 S632. Overexpression of the CaMKII inhibitor CaMKIIN abolished HDAC4 S632 phosphorylation in response to ANG II (Fig. 5). These findings indicate that HDAC4 is phosphorylated in response to ANG II stimulation in VSM and that HDAC4 phosphorylation is mediated by CaMKII.
HDAC4 belongs to the class II histone deacetylases that promote chromatin condensation by deacetylation of conserved lysine residues in histone tails, thereby repressing transcription (9). However, the deacetylase activity and nonenzymatic repression of MEF2 signaling are regulated in separate domains in HDACs, and a possible relationship between these two distinct functions is not well defined. While VSM displayed baseline deacetylation activity, neither CaMKII overexpression nor CaMKII inhibition or administration of ANG II significantly affected deacetylase activity (data not shown). While the assay measures deacetylase activity of all class II HDACs and is not specific for HDAC4, these data suggest that CaMKII activity specifically targets nonenzymatic actions of HDAC, while enzymatic activity of HDAC is CaMKII independent.
CaMKII overexpression results in increased MEF2 DNA binding and promoter activity.
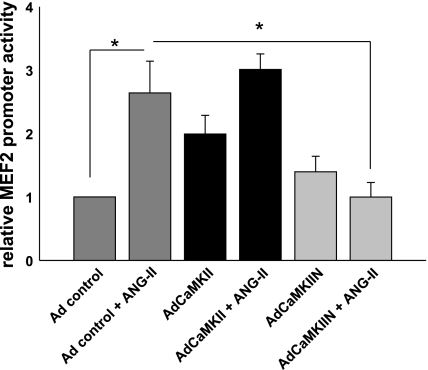
We next investigated the role of CaMKII activation in MEF2-dependent signaling. On the basis of our results so far, we expected to find an increase in MEF2 promoter activity with CaMKII overexpression and ANG II treatment. ANG II treatment increased MEF2 promoter activity 2.6-fold in VSM infected with control virus compared with baseline (Fig. 6; P < 0.05). Overexpression of CaMKII increased ANG II-induced promoter activity at baseline ∼2.0-fold (P < 0.05 compared with control VSM cells). ANG II treatment increased MEF2 promoter activity 3.0-fold in CaMKII-overexpressing VSM cells compared with controls. Expression of the CaMKII inhibitor CaMKIIN did not significantly change the promoter activity at baseline. However, VSM cells with CaMKII inhibition by CaMKIIN overexpression showed no increase in MEF2 promoter activity by ANG II treatment. These data support the concept that CaMKII mediates activation of the prohypertrophic signaling HDAC4/MEF2 pathway in response to ANG II.
Fig. 6.
CaMKII is required for ANG II-induced MEF2 promoter activity. MEF2-luciferase promoter assays after adenoviral (Ad) overexpression of CaMKII or CaMKIIN. VSMC were infected with adenoviruses overnight before transfection with MEF2-luciferase and TK Renilla construct for 8 h. Seventy-two hours after infection, 100 nM ANG II was added for 18 h. MEF2 promoter activity was measured and adjusted for TK Renilla activity (n = 7). *P < 0.05.
CaMKII does not regulate MEF2 activation by p38-dependent phosphorylation.
Several other mechanisms could explain the induction of MEF2-dependent gene transcription by CaMKII. MEF2 transcriptional activity is also increased in response to ANG II by a pathway involving mitogen-activated protein kinase kinase 6 (MKK6) and p38 (20, 28, 35). MKK6 is the upstream activator of p38 and has a putative low-stringency CaMKII phosphorylation site on S135. p38 phosphorylates MEF2A on T312 and T319. ANG II increases the transcription of the chemokine monocyte chemoattractant protein-1 (MCP1) by a p38/MEF-2 dependent pathway (29). We therefore tested the hypothesis that the phosphorylation of MEF2A by p38 is CaMKII dependent. However, we did not detect any change in phosphorylation of MEF2A in response to ANG II treatment under CaMKII or CaMKIIN overexpression (Fig. 7A). In accordance with this, we did not detect a decrease in MCP1 mRNA expression with CaMKII inhibition (Fig. 7B).
MEF2A and -D are the major MEF2 isoforms in vascular smooth muscle (27, 35). ANG II increases MEF2A protein expression in cultured VSMC (28). We tested whether CaMKII overexpression also increases MEF2 protein levels. We consistently detected an additional lower-molecular-weight band in MEF2A Western blots, possibly a splice variant or degradation product of MEF2A (27). The overall expression of MEF2A in CaMKII-overexpressing VSMC was not significantly increased compared with control cells (1.2-fold). We did not detect any differences in protein expression of MEF2C or MEF2D with ANG II treatment or CaMKII overexpression (Fig. 7C).
Activation of MEF2 activity by CaMKII is essential for VSM hypertrophy.
To understand the role of CaMKII on activation of the HDAC4/MEF2 pathway, we studied the effect of a CaMKII phosphorylation-deficient HDAC4 mutant on MEF2 reporter activity and VSM hypertrophy. We performed MEF2 reporter assays in VSM that were cotransfected with the dominant-negative HDAC4 S467,632A mutant that cannot be phosphorylated by CaMKII. We detected a 3.6-fold increase in promoter activity in response to ANG II in control experiments (Fig. 8A). The dominant-negative mutant HDAC4 S467,632A did not significantly change basal MEF2 promoter activity. However, overexpression of HDAC4 S467,632A significantly reduced the MEF2 promoter activity response to ANG II compared with control VSMC (1.4-fold, P < 0.05). These experiments strongly support the hypothesis that CaMKII enables ANG II-induced MEF2 promoter activity.
We next investigated the effect of the CaMKII-phosphorylation deficient HDAC4 mutant on VSM hypertrophy. We performed hypertrophy assays by [3H]leucine and [3H]thymidine uptake in VSMC that were transfected by electroporation with a control construct or a construct for the dominant-negative mutant HDAC4 S467,632A. We detected overexpression of HDAC4 protein after transfection with the dominant-negative mutant HDAC4 by Western blot (data not shown). With ANG II treatment, an increase of [3H]leucine/[3H]thymidine uptake assays of 85 ± 20% was seen in control cells. When the dominant-negative mutant HDAC4 S467,632A was overexpressed, [3H]leucine and [3H]thymidine uptake only increased by 20 ± 10% with ANG II treatment (Fig. 8B). Together, these data demonstrate that ANG II induces activation of CaMKII. CaMKII in turn phosphorylates HDAC4 S467,632 and activates MEF2-dependent gene transcription, leading to VSMC hypertrophy.
HDAC4 is a repressor of MEF2 transcriptional activity. The downregulation of HDAC4 protein by siRNA consequently resulted in an increase in MEF2 promoter activity. In response to ANG II, the promoter activity increased 2.3-fold in control samples. Downregulation of HDAC4 by siRNA resulted in a 1.7-fold increase of promoter activity at baseline without ANG II and a 4.2-fold increase after treatment with ANG II (Fig. 8, C and D). The significant increase under HDAC4 downregulation may be due to additional MEF2 promoter activation by other pathways such as the phosphorylation of MEF2 by p38 in response to ANG II.
CaMKII is required for ANG II-induced nuclear export of HDAC.
CaMKII specifically phosphorylates HDAC4 S467,632, leading to presentation of docking sites for the chaperone 14-3-3 protein. 14-3-3 Protein binding to phosphorylated HDAC4 results in export of HDAC4 from the nucleus to the cytoplasm (16). CaMKII also inhibits the reimport of HDAC4 into the nucleus (6). The net effect of increased nuclear export and reduced nuclear import is an increase in cytoplasmic HDAC4. We therefore measured the effect of CaMKII or CaMKIIN overexpression on the subcellular localization of HDAC4 in response to ANG II treatment. In VSM infected with control virus, ANG II significantly increased cytoplasmic HDAC4 within 60 min (Fig. 9A). Overexpression of CaMKII profoundly changed the HDAC4 distribution pattern to become predominantly cytoplasmic at baseline and with ANG II treatment (Fig. 9B). In contrast, HDAC4 was detected in both nucleus and cytoplasm after overexpression of the CaMKII peptide inhibitor CaMKIIN at baseline. No increase of cytoplasmic HDAC4 was noted after ANG II treatment in CaMKIIN-expressing VSMC (Fig. 9C). We also performed Western blots of nuclear fractions of VSMC expressing CaMKIIN and control cells. After ANG II treatment for 60 min, we detected nuclear export of HDAC4 and 14-3-3 in control cells but not in CaMKIIN-expressing cells (Fig. 9D). These results show that CaMKII activity determines the subcellular distribution of HDAC4, and that increased CaMKII activity favors redistribution of HDAC4 to the cytoplasm.
Activation of MEF2 by ANG II in vivo is regulated by CaMKII.
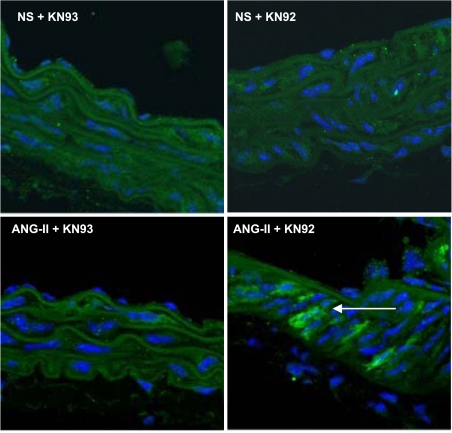
Our data suggested that CaMKII mediates ANG II-induced VSM hypertrophy by activating MEF2-dependent transcription in vitro. We therefore tested whether ANG II activates HDAC-dependent signaling in the aortic media. For this purpose, MEF2 reporter mice were treated with normal saline or ANG II (1.25 μg·kg−1·min−1 for 10 days) (18). ANG II- or normal saline-treated mice received the CaMKII inhibitor KN-93 daily (20 μmol/kg ip) (34) or the inactive congener compound KN-92. As expected, MEF2 activity was strongly activated in the aortic media of mice infused with ANG II (Fig. 10) but not in saline-infused control mice. In contrast, KN-93 (but not KN-92) administration prevented MEF2 activity in the aortic media. These data support the concept that ANG II activates MEF2-dependent transcription in the aortic media. The activation of MEF2-dependent gene transcription in vivo by ANG II is CaMKII dependent because the CaMKII inhibitor KN-93 abolished β-galactosidase expression.
Fig. 10.
Activation of MEF2 by ANG II in vivo is regulated by CaMKII. MEF2 reporter mice that harbor tandem MEF2 consensus DNA-binding sites driving a lacZ reporter gene were infused with 1.25 μg·kg−1·min−1 ANG II or normal saline for 10 days. Reporter mice received either the CaMKII inhibitor KN-93 or the inactive compound KN-92 (20 μg/kg) by intraperitoneal injection twice daily. Immunofluorescence for β-galactosidase and nuclear counterstaining with DAPI is shown (n = 4 mice/group).
DISCUSSION
The major finding of this study is that CaMKII is a mediator of ANG II-mediated smooth muscle hypertrophy. Specifically, we show that CaMKII mediates an ANG II signaling pathway that involves phosphorylation of HDAC4 and activation of MEF2. CaMKII inhibition does not alter the basal vascular phenotype, but on activation with ANG II CaMKII inhibition prevents the hypertensive response and decreases vascular hypertrophy. Our results suggest that CaMKII coordinates ANG II signaling events essential for VSM hypertrophy.
CaMKII-γ and -δ isoforms are strongly expressed in VSM (26); however, to date its role in the vasculature is not well defined. CaMKII phosphorylates type II HDACs (HDAC4, -5, -7, and -9). Under baseline conditions, HDACs repress transcriptional activity by binding to the transcription factor MEF2 and favor condensed DNA. When HDAC is phosphorylated in response to neurohumoral stimuli, it associates with the chaperone 14-3-3 and is exported from the nucleus. Consequently, MEF2 is derepressed, and a hypertrophic program of gene expression is activated. CaMKII signals specifically to HDAC4 by binding to a unique docking site that is absent in other class II HDACs. Phosphorylation of HDAC4 S467 and S632 by CaMKII exposes binding sites for 14-3-3 (6). Depending on its subcellular localization, CaMKII can either induce nuclear export or block reimport from the cytoplasm to the nucleus. Our findings with overexpression of the mainly cytoplasmic isoform CaMKII (CaMKII-δ2) are consistent with a mechanism in which CaMKII phosphorylation causes increased HDAC4 retention in the cytoplasm. The activation of this pathway causes cardiac myocyte hypertrophy in vitro. CaMKII inhibition has been demonstrated to prevent myocardial hypertrophy in vivo (34).
MEF2 activity can also be altered by HDAC5 in vascular smooth muscle (21, 32). HDAC5 is phosphorylated by protein kinase D but not CaMKII (32). The subsequent nuclear export of HDAC5 mediates ANG II-induced MEF2 activation and VSMC hypertrophy. Another study demonstrated that ANG II-mediated MEF2 activation involves phosphorylation of HDAC5 by CaMKII after association with the scaffold protein G protein-coupled receptor (GPCR)-kinase2 interacting protein 1 (GIT1) (21). While both HDAC4 and -5 can be activated by ANG II, recent data suggest a regulatory function for HDAC4 (4). HDAC4 and HDAC5 form heterooligomers. HDAC5 acquires responsiveness to CaMKII after phosphorylation of HDAC4 or transphosphorylation by CaMKII bound to HDAC4. Thus HDAC4 integrates upstream Ca2+-dependent signals via its association with CaMKII and transmits these signals to HDAC5 by protein-protein interactions (4).
The transcription factor MEF2 is present in different isoforms (A–D) and splice variants in VSM and is activated in vascular development (15) and neointima formation after carotid artery balloon injury (8). A recent study identified MEF2 and HDAC4 as mediators underlying the phenotypic switch between activated and quiescent VSMC. MEF2 represses c-Jun expression in quiescent VSMC, whereas platelet-derived growth factor derepresses c-Jun expression through CaMKs (10). The authors noted an upregulation of MEF2A protein levels under treatment with serum. An increase of MEF2A protein under ANG II treatment has been reported in the past (28). We noted an additional lower-molecular-weight band in MEF2A Western blots under CaMKII overexpression. This band could represent a splice variant of MEF2A.
The subcellular localization of HDAC4 is dependent on cell density. During growth conditions, HDAC4 is distributed throughout the cell. In contrast, under serum-free quiescent conditions and once the VSMC have reached confluence, HDAC4 is detected mainly in the nucleus (10). We found a similar distribution pattern that was profoundly affected by CaMKII.
While we demonstrate that ANG II activates MEF2-dependent gene transcription by phosphorylation of HDAC4, MEF2 can be phosphorylated by p38 in response to ANG II and increase the expression of the inflammatory mediator MCP1 (29). We investigated whether the activation of MEF2 by ANG II via a pathway involving MKK6 and p38 was CaMKII dependent, because MKK has a putative CaMKII phosphorylation site. However, we did not detect any decrease in MEF2 phosphorylation under CaMKII inhibition nor any effect of CaMKII inhibition on ANG II-induced MCP-1 mRNA expression. While ANG II can activate MEF2-dependent gene transcription by direct phosphorylation or HDAC phosphorylation and derepression, the regulation by CaMKII is restricted to the latter mechanism. In the study on MCP1 expression by p38 and MEF2, a mutation in the single putative MEF2 binding site in the MCP1 promoter region did not affect ANG II-induced increase of the promoter activity (29). It was concluded that MEF2 transactivates the MCP1 gene via a protein-protein interaction with some other DNA-bound transcription factors without by itself binding to DNA. It is possible that indirect transactivation via a protein-protein interaction does not require HDAC derepression. This could explain why we did not detect a decrease in MCP1 transcription levels under CaMKII inhibition.
CaMKII phosphorylates smooth muscle myosin light chain kinase either directly (14) or through phosphorylation of the MAP kinase ERK1/2 (25) and thereby increases tonic force maintenance in aortic smooth muscle. This mechanism likely explains the antihypertensive effect of the CaMKII inhibitor KN-93 (Fig. 1B). Apart from regulation of smooth muscle contraction, recent studies have reported an involvement of CaMKII in smooth muscle cell growth and migration, but to date these studies lack a detailed dissection of the underlying signaling pathways (12, 13).
Numerous pathways have been implicated in ANG II-induced VSM hypertrophy (31). We detected a significant increase in VSM hypertrophy after ANG II treatment with CaMKII overexpression (Fig. 3). However, after overexpression of CaMKII the MEF2 promoter activity did not increase significantly with ANG II treatment (Fig. 6). These findings suggest that CaMKII induces VSM hypertrophy through other, as yet unidentified, pathways in addition to MEF2 activation. Numerous studies have reported the activation of the MAP kinase ERK1/2 by CaMKII in response to ANG II treatment (2). ERK1/2 has been implicated in ANG II-induced cellular growth and hypertrophy via regulation of PHAS-1 (inhibitor of eukaryotic initiation factor 4E) (24).
In summary, our data establish that CaMKII mediates ANG II-related pathological processes associated with hypertension and smooth muscle hypertrophy. The hypertrophic activity of ANG II is mediated through phosphorylation of HDAC4 S467 and 632 by CaMKII with subsequent induction of MEF2-dependent gene transcription. CaMKII inhibition in vivo also decreased the hypertensive response to ANG II. The inhibition of CaMKII activity may represent a novel approach to prevent deleterious vascular remodeling in hypertension. Our findings in isolated cells do not exclude that the CaMKII inhibitor KN-93 decreases vascular hypertrophy by its hemodynamic effect. However, it is also important to realize that we did not study resistance vessels in this model. Our findings in aortic tissues may not be directly applicable to resistance vessels, and, therefore, future studies will be required. In this study, we achieved inhibition of CaMKII in vivo by administration of the CaMKII inhibitor KN-93. While our data were congruent with previous studies that used KN-93 for CaMKII inhibition, a drawback to currently available small-molecule inhibitors of CaMKII is their imperfect specificity (23). In the future, it will be of interest to develop more specific, smooth muscle-limited in vivo animal models of CaMKII expression and inhibition.
GRANTS
This work was supported in part by funding from the following grants: American Heart Association 0860061Z and 0930086N and a Carver Trust Medical Research Initiative Grant (I. M. Grumbach); National Heart, Lung, and Blood Institute (NHLBI) Grants HL-079031, R01-HL-62494, and R01-HL-70250 (M. E. Anderson); NHLBI Grants R01-HL-084583 and R01-HL-083422 and the Pew Scholars Trust (P. J. Mohler); and a University of Iowa Cardiovascular Center Interdisciplinary Research Fellowship (W. Li).
DISCLOSURES
The authors are not aware of financial conflict(s) with the subject matter or materials discussed in this manuscript with any of the authors, or any of the authors' academic institutions or employers.
ACKNOWLEDGMENTS
The authors acknowledge viral preparation by the University of Iowa Gene Transfer Vector Core.
REFERENCES
- 1.Abraham ST, Benscoter H, Schworer CM, Singer HA. In situ Ca2+ dependence for activation of Ca2+/calmodulin-dependent protein kinase II in vascular smooth muscle cells. J Biol Chem 271: 2506–2513, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Abraham ST, Benscoter HA, Schworer CM, Singer HA. A role for Ca2+/calmodulin-dependent protein kinase II in the mitogen-activated protein kinase signaling cascade of cultured rat aortic vascular smooth muscle cells. Circ Res 81: 575–584, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther 7: 1034–1038, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol 28: 3437–3445, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res 98: 15–24, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest 116: 1853–1864, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci USA 95: 10890–10895, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Firulli AB, Miano JM, Bi W, Johnson AD, Casscells W, Olson EN, Schwarz JJ. Myocyte enhancer binding factor-2 expression and activity in vascular smooth muscle cells. Association with the activated phenotype. Circ Res 78: 196–204, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Fischle W, Kiermer V, Dequiedt F, Verdin E. The emerging role of class II histone deacetylases. Biochem Cell Biol 79: 337–348, 2001 [PubMed] [Google Scholar]
- 10.Gordon JW, Pagiatakis C, Salma J, Du M, Andreucci JJ, Zhao J, Hou G, Perry RL, Dan Q, Courtman D, Bendeck MP, McDermott JC. Protein kinase A-regulated assembly of a MEF2·HDAC4 repressor complex controls c-Jun expression in vascular smooth muscle cells. J Biol Chem 284: 19027–19042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994 [DOI] [PubMed] [Google Scholar]
- 12.House SJ, Ginnan R, Armstrong S, Singer HA. Calcium/calmodulin-dependent protein kinase II-delta isoform regulation of vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol 292: C2276–C2287, 2007 [DOI] [PubMed] [Google Scholar]
- 13.House SJ, Singer HA. CaMKII-δ isoform regulation of neointima formation after vascular injury. Arterioscler Thromb Vasc Biol 28: 441–447, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Kim I, Je HD, Gallant C, Zhan Q, Riper DV, Badwey JA, Singer HA, Morgan KG. Ca2+-calmodulin-dependent protein kinase II-dependent activation of contractility in ferret aorta. J Physiol 526: 367–374, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Q, Lu J, Yanagisawa H, Webb R, Lyons GE, Richardson JA, Olson EN. Requirement of the MADS-box transcription factor MEF2C for vascular development. Development 125: 4565–4574, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Miska EA, Karlsson C, Langley E, Nielsen SJ, Pines J, Kouzarides T. HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J 18: 5099–5107, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muthalif MM, Karzoun NA, Benter IF, Gaber L, Ljuca F, Uddin MR, Khandekar Z, Estes A, Malik KU. Functional significance of activation of calcium/calmodulin-dependent protein kinase II in angiotensin II-induced vascular hyperplasia and hypertension. Hypertension 39: 704–709, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Naya FJ, Wu C, Richardson JA, Overbeek P, Olson EN. Transcriptional activity of MEF2 during mouse embryogenesis monitored with a MEF2-dependent transgene. Development 126: 2045–2052, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Okamoto K, Kato S, Arima N, Fujii T, Morimatsu M, Imaizumi T. Cyclin-dependent kinase inhibitor, p21Waf1, regulates vascular smooth muscle cell hypertrophy. Hypertens Res 27: 283–291, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ornatsky OI, Cox DM, Tangirala P, Andreucci JJ, Quinn ZA, Wrana JL, Prywes R, Yu YT, McDermott JC. Post-translational control of the MEF2A transcriptional regulatory protein. Nucleic Acids Res 27: 2646–2654, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang J, Yan C, Natarajan K, Cavet ME, Massett MP, Yin G, Berk BC. GIT1 mediates HDAC5 activation by angiotensin II in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 28: 892–898, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peiró C, Llergo JL, Angulo J, López-Novoa JM, Rodríguez-López A, Rodríguez-Mañas L, Sánchez-Ferrer CF. Effects of captopril, losartan, and nifedipine on cell hypertrophy of cultured vascular smooth muscle from hypertensive Ren-2 transgenic rats. Br J Pharmacol 121: 1438–1444, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezazadeh S, Claydon TW, Fedida D. KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)amino-N-(4-chlorocinnamyl)-N-methylbenzylamine), a calcium/calmodulin-dependent protein kinase II inhibitor, is a direct extracellular blocker of voltage-gated potassium channels. J Pharmacol Exp Ther 317: 292–299, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Rocic P, Jo H, Lucchesi PA. A role for PYK2 in ANG II-dependent regulation of the PHAS-1-eIF4E complex by multiple signaling cascades in vascular smooth muscle. Am J Physiol Cell Physiol 285: C1437–C1444, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Rokolya A, Singer HA. Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am J Physiol Cell Physiol 278: C537–C545, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Schworer CM, Rothblum LI, Thekkumkara TJ, Singer HA. Identification of novel isoforms of the delta subunit of Ca2+/calmodulin-dependent protein kinase II. Differential expression in rat brain and aorta. J Biol Chem 268: 14443–14449, 1993 [PubMed] [Google Scholar]
- 27.Suzuki E, Guo K, Kolman M, Yu YT, Walsh K. Serum induction of MEF2/RSRF expression in vascular myocytes is mediated at the level of translation. Mol Cell Biol 15: 3415–3423, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki E, Nishimatsu H, Satonaka H, Walsh K, Goto A, Omata M, Fujita T, Nagai R, Hirata Y. Angiotensin II induces myocyte enhancer factor 2- and calcineurin/nuclear factor of activated T cell-dependent transcriptional activation in vascular myocytes. Circ Res 90: 1004–1011, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Suzuki E, Satonaka H, Nishimatsu H, Oba S, Takeda R, Omata M, Fujita T, Nagai R, Hirata Y. Myocyte enhancer factor 2 mediates vascular inflammation via the p38-dependent pathway. Circ Res 95: 42–49, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem 264: 17907–17912, 1989 [PubMed] [Google Scholar]
- 31.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 52: 639–672, 2000 [PubMed] [Google Scholar]
- 32.Xu X, Ha CH, Wong C, Wang W, Hausser A, Pfizenmaier K, Olson EN, McKinsey TA, Jin ZG. Angiotensin II stimulates protein kinase D-dependent histone deacetylase 5 phosphorylation and nuclear export leading to vascular smooth muscle cell hypertrophy. Arterioscler Thromb Vasc Biol 27: 2355–2362, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yousif MH, Akhtar S, Walther T, Benter IF. Role of Ca2+/calmodulin-dependent protein kinase II in development of vascular dysfunction in diabetic rats with hypertension. Cell Biochem Funct 26: 256–263, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med 11: 409–417, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Zhao M, New L, Kravchenko VV, Kato Y, Gram H, di Padova F, Olson EN, Ulevitch RJ, Han J. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol 19: 21–30, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]