Abstract
Inflammatory bowel disease and arthritis are associated with contact activation that results in cleavage of kininogen to form high molecular weight kininogen (HKa) and bradykinin. We have previously demonstrated that HKa can stimulate inflammatory cytokine and chemokine secretion from human monocytes. We now show that HKa can upregulate tissue factor antigen and procoagulant activity on human monocytes as a function of time (1–4 h) and HKa concentration (75–900 nM). The amino acid sequence responsible to block HKa effects is G440–H455. The HKa receptor macrophage-1 (Mac-1; CD11b18) is the binding site as shown by inhibition by a monoclonal antibody to CD11b/18. Chemical inhibitors of JNK, ERK, and p38 signaling pathways block cell signaling, as does an inhibitor to the transcription factor NF-κB. A combination of monoclonal antibodies to TNF-α and IL-1β but neither alone inhibited the HKa induction of tissue factor. These results suggest that HKa mimics LPS by triggering a paracrine pathway in monocytes that depends on TNF-α and IL-1β. Antibodies to kininogen or peptidomimetics might be a useful and safe therapy in inflammatory diseases or sepsis involving cytokines.
Keywords: cytokines, chemokines
the plasma kallikrein-kinin system has been related to inflammation from the time it was recognized (29). Initially, it was thought that bradykinin was the only effector molecule involved. However, when kininogen is cleaved by kallikrein to release bradykinin, cleaved high molecular weight kininogen (HKa) was found to have potent antiangiogenic activity (3) because of conformational changes (32), which expose previously buried peptide regions. The responsible domain is D5 (11.27 kDa), a component of the light chain of HKa (13). HKa and D5 can stimulate the synthesis and release inflammatory cytokines from human monocytes (15) including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 as well as inflammatory chemokines IL-8 and monocyte chemoattractant protein-1 (MCP-1). This observation has provided a direct link to inflammatory diseases such as rheumatoid arthritis (28) and inflammatory bowel disease (11). Receptors for HKa were first identified on neutrophils to include macrophage-1 (Mac-1; CD11b18) (9) and on endothelial cells to include urokinase plasminogen activator receptor (uPAR) (4), gClqR (12), and cytokeratin 1 (10). Leukocyte function-associated antigen-1 (LFA-1; CD11a18) on monocytes is also a binding site for HKa.
Tissue factor (TF) initiates blood coagulation by serving as a cofactor for the autoactivation of plasma factor VII in the presence of anionic phospholipids in the cell membranes. Normally, TF is only detectable in the adventitial cells within the vessel wall (6). However, TF can be induced by endotoxin on the cell surface of the endothelial cell or the monocyte and could be rate limiting (26). The activation of these cells leads to a reversal of the normal encryption of TF in these cells.
There has been intense interest in the relationship between inflammation and coagulation recently. Prekallikrein is cleaved to an active enzyme, which can cleave kininogen on the cell surface. HKa, a major product of the kininogen proteolysis, is a potent stimulator of the formation of inflammatory cytokines and chemokines (15). These small effector molecules can enhance the synthesis and secretion of TF from the same cell—the monocyte. Thus a powerful paracrine mechanism linking coagulation to inflammation is revealed. We postulate that HKa could induce TF by transcriptional regulation of its synthesis. Intracellular signaling pathways involved in TF synthesis include JNK-c-Jun, mitogen-activated protein (MAP) kinases such as ERK and p38, and the transcription factor NF-κB, which result in changes in mRNA.
Several lines of evidence suggest that uncleaved one-chain kininogen (HK) may also play an antithrombotic role in vivo in addition to its role in inflammation. A knockout of the kininogen-1 gene leads to increased time to thrombosis in mice after arterial damage by Rose Bengal administration and laser-induced arterial injury (23). Rats with a mutation leading to decreased kininogen secretion and kininogen deficiency occlude the aorta after endothelial injury six times more rapidly than rats with normal kininogen levels (5). Kininogen binds to GPIb on platelets (1). Platelets from patients with GPIb deficiency (Bernard-Soulier disease) aggregate to lower concentrations of thrombin, probably because of the increased binding of that enzyme in the absence of kininogen (27).
Cleavage of HK that occurs in inflammation and sepsis would lead to increased plasma HKa levels, which releases cytokines and/or chemokines from monocytes. Since cytokines such as TNF-α and IL-1β are known to stimulate both TF expression and procoagulant activity (PCA), we hypothesized that HKa might upregulate TF expression and PCA on monocytes. We have explored which amino acid sequences on D5 were required for the inhibition of TF expression. We have tested which of the four HKa receptors on monocytes are responsible and which of the signaling pathways are responsible. Finally, we have determined which of the cytokines and/or chemokines are playing the predominant role on the activity of HKa in stimulating monocyte TF.
MATERIALS AND METHODS
Proteins and antibodies.
Purified HKa was purchased from Enzyme Research (South Bend, IN). HK was >95% a single band of 110 kDa on both nonreduced and reduced SDS electrophoresis. HKa was composed of two bands: 62-kDa, amino acids (aa) 1–362, and 46 kDa, (aa) 420–626, representing the heavy chain and light chain, respectively (29). A monoclonal antibody (MAb) against uPAR was purchased from American Diagnostica (Stamford, CT); MAb to Mac-1 (αM-subunit), 2LPM19c, from DAKO (Carpentaria, CA); and MAb against LFA-1 (αL-subunit) from Antigenix America (Huntington Station, NY). The MAb 74.5.2, which recognizes the HK binding site on gC1qR, was prepared and was a gift from Dr. Berhane Ghebrehiwet (SUNY at Stoney Brook). Polyclonal antibodies against peptide Gly440–His455 and peptide Gly486–Lys502 of HKa were raised in rabbits by standard methods. Neutralizing MAbs against TNF-α, IL-1β, IL-8, and MCP-1 were obtained from eBioscience (San Diego, CA). The neutralizing rabbit antibody to human TF (No. 4502) was purchased from American Diagnostica (Stamford, CT). Two conjugated AF-647 (mouse monoclonal IgG and anti-TF mouse MAb) were a gift to our laboratory (BD Bioscience, San Jose, CA).
Control of LPS contamination.
Sterile and pyrogen-free working conditions were maintained to minimize any contamination by LPS. Endotoxin assayed using QCL-1000 Chromogenic Limulus Amebocyte lysate kit from BioWhittaker (Walkersville, MD) indicated that all proteins and reagents had <0.01 EU/ml endotoxin. Peripheral blood mononuclear cells were isolated from normal subjects on a Histopaque gradient (Sigma Chemical, St. Louis, MO). The cells were suspended in Hanks' balanced salt solution, 0.1% BSA (HBSSA).
Immunofluorescence staining and flow cytometric analysis.
HKa (600 nM)/buffer (control) were incubated for 180 min at 37°C with 2 × 106/ml mononuclear cells suspended in HBSSA. Following this incubation, immunofluorescence staining and flow cytometric analysis were done as described previously (18). After modification, in brief, each cell suspension was washed twice (centrifuged at 800 g for 5 min) in cold PBS and resuspended in 1 ml PBS. After 1 ml of cold 1% formaldehyde buffer was added and immediate stirring, the cells were fixed for at least 30 min and then were washed twice. The fixed cells were aliquoted into polypropylene tubes and incubated for 30 min at 4°C either with an anti-TF MAb-Alexa Fluor 647 for the detection of TF expression or with IgG-Alexa Fluor 647 as a control. Unlabeled and labeled samples with the isotype controls were also prepared. The stained cells were washed twice, resuspended in PBS, and analyzed by flow cytometry using BD LSRII, a 4 laser (355 nm UV, 405 nm violet, 488 nm blue, and 633 nm red HeNe laser) and a 42 parameter (12 color) benchtop flow cytometer.
TF assays: PCA.
To confirm that the TF expressed on HKa stimulation to mononuclear cells is active, mononuclear cells were preincubated with the neutralizing MAb against TF (10 μg/ml) or the isotype control IgG (10 μg/ml) for 10 min before stimulation with HKa (600 nM) or LPS (10 μg/ml) for another 180 min at 37°C. Following this incubation, the cell suspension was centrifuged at 13,000 g for 5 min, and after being washed twice, the pellets were used for TF PCA by the two-stage assay as described previously (14). In brief, 5 nmol/l of recombinant FVIIa, 250 nmol/l of human coagulation factor X, and 8.3 mmol/l of human prothrombin were mixed together (final volume was 120 μl) and incubated at 37°C for 30 min. Bovine plasma (100 μl) containing PS 30:PC 70 [12.5 mmol/l and 100 μl of CaCl2 (25 mmol/l)] was added, and the clotting time was recorded in an Anelung coagulometer (AC-1a, Burladingen, Germany).
TF expression on human peripheral blood mononuclear cells: time course and concentration response to HKa.
LPS-free HKa (600 nM) was incubated for 0, 60, 120, 180, and 240 min at 37°C with 2 × 106/ml mononuclear cells suspended in HBSSA. Separately, LPS-free HKa (0, 75, 150, 300, 600, and 900 nM) was incubated for 180 min at 37°C with 2 × 106/ml mononuclear cells suspended in HBSSA. Following this incubation, each cell suspension was washed twice with HBSSA, resuspended in 1% Triton X-100 in 0.05 M Tris and 0.1 M NaCl (pH 8.5), and stirred for 12 h at 4°C. The suspension was centrifuged at 13,000 g for 15 min to separate cell debris. TF antigen levels were measured in supernatant using the Imubind Tissue Factor ELISA kit (American Diagnostica, Greenwich, CT) (16). For TF PCA following incubation, the cell suspension was washed twice with HBSSA and the pellet was frozen and kept at −80°C until the day of the assay.
Determination of critical amino acids on polypeptides derived from HKa responsible for expression of TF on human peripheral blood mononuclear cells.
Mononuclear cells were preincubated with the polyclonal antibodies (10 μM) against peptide Gly440–His455 and peptide Gly486–Lys502 for 30 min at 37°C before stimulation with HKa or D5 (600 nM) for another 180 min at 37°C. Following this incubation, the cell suspension was centrifuged at 13,000 g for 5 min and samples were assayed for TF by ELISA and PCA.
Receptors involved in the expression of TF from human peripheral blood mononuclear cells by HKa.
Mononuclear cells were preincubated with the MAbs (0.1, 0.5, or 5.0 μg/ml) against uPAR, Mac-1, LFA-1, and gC1qR for 30 min at 37°C before stimulation with HKa (600 nM) for another 180 min at 37°C. Following this incubation, the cell suspension was centrifuged at 13,000 g for 5 min and samples were assayed for TF by ELISA.
Signaling pathway inhibition experiments.
For pathway inhibition, 1, 10, and 100 μM of carbobenzoxyl-l-leucyl-l-leucinal (Z-LLL-CHO; MG-132) was used as a selective inhibitor of NF-κB; anthra [1,9-cd]pyrazol-6(2H)-one (SP-600125) as a selective inhibitor of JNK and SB-202190; 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)1H-imidazole(FHPI) as a selective p38 inhibitor; and 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio) butadiene (U-0126) as a selective inhibitor of ERK activation. All inhibitors were obtained from Calbiochem (La Jolla, CA), dissolved in DMSO (0.1–0.5%), and diluted into HBSSA. Mononuclear cells were preincubated with the respective inhibitors for 60 min before stimulation with HKa (600 nM) for another 120 min at 37°C. Following this incubation, the cell suspension was centrifuged at 13,000 g for 5 min and the pellet was washed and used for the assay of TF by ELISA.
TF mRNA formation by HKa.
The detection of TF mRNA from mononuclear cells was performed by reverse transcriptase-polymerase chain reaction (RT-PCR) using sequence-specific primers. Briefly, total RNA was prepared using TRIzol reagent (Invitrogen). Specific primers for human full-length TF were designed to anneal to sequences in exons on both sides of one intron of the mRNA to exclude amplification of potential contaminating genomic DNA. The following primer pairs were used: TF forward, 5′-ACAGACCTTCCAGGAGAATG-3′; and TF reverse, 5′-GCAGTTCAGTGATCGTACAG-3′. RNA (100 ng) was used as template in a one-step RT-PCR reaction (SuperScript One-Step RT-PCR with Platinum Taq, Invitrogen). RT for cDNA synthesis was accomplished in a 30-min incubation at 50°C, which was followed by PCR cycling as follows: initial denaturation at 94°C for 3 min, followed by 25 cycles of 30 s at 94°C, 30 s of annealing at 55°C, and 1 min of extension at 72°C using 0.2 μM primers. The RT-PCR reactions should yield a product of 588 bp, reflecting full-length TF.
TF expression on mononuclear cell by HKa: effects of neutralizing of IL-1β, TNF-α, IL-8, or MCP-1.
Mononuclear cells were preincubated with the neutralizing MAbs against IL-1β, TNF-α, IL-8, or MCP-1 (1.8 μM) either individually or in various combinations for 10 min before stimulation with HKa (600 nM) for another 180 min at 37°C. Following this incubation, the cell suspension was centrifuged at 13,000 g for 5 min and the pellet was used for the assay of TF by ELISA.
Experimental design and data analysis.
All experiments were performed in three to five different donors. Each condition in each donor was repeated three times (triplicates) except in Fig. 1A, which was done from one donor in duplicate. All results are expressed as means ± SE. All results were analyzed by unpaired Student's t-test (P < 0.05, P < 0.001, and P < 0.001 as indicated). Because of the variability of monocytes response in different donors, all studies have their own positive standard (endotoxin) and negative controls (cell alone with buffer). Where appropriate, paired Student's t-tests were used. The term significant in the results indicates P < 0.001. Analysis of variance was performed using PRISM in Figs. 2 and Fig. 4.
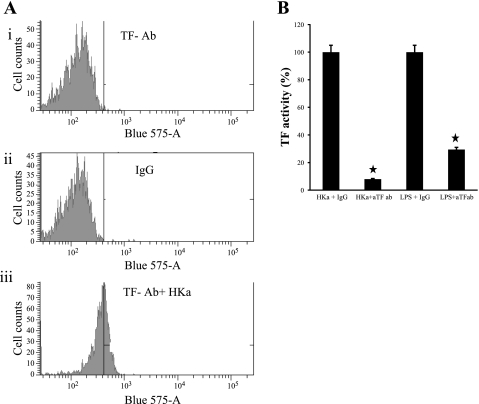
Fig. 1.
Effect of high molecular weight kininogen (HKa) on monocyte tissue factor (TF) expression. A: immunofluorescence analysis of human mononuclear cells incubated with HKa for 3 h. The cells were stained with anti-TF MAb-Alexa Fluor 647 (i) or IgG-Alexa Fluor 647 (ii) as a control. Another aliquot of cells was stained with IgG-PE (iii) as a control. B: the antibody to TF inhibited TF activity. Mononuclear cells were preincubated 10 min with or without the anti-TF antibody. The cells were treated for 3 h with or without HKa (600 nM) or LPS. TF activity was measured by TF procoagulant assay. ★P < 0.05.
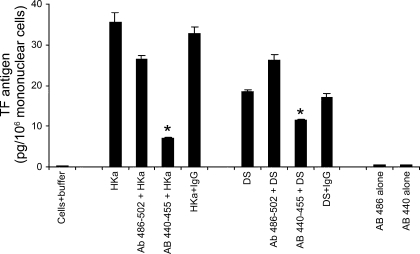
Fig. 2.
Effects of antibodies to G486–K502 and G440–H455 peptides of HKa on TF expression. Mononuclear cells were preincubated with anti-peptide antibodies at a concentration of 1.8 μM [3 times higher than uncleaved one-chain kininogen (HK) plasma concentration 600 nM]. Anti-peptide antibodies to G486–K502 or G440–H455 were each incubated for 30 min and then challenged with HKa (600 nM) or D5 (600 nM) for 3 h. Data are plotted as means ± SE. *P < 0.05. Experiments were performed in triplicate.
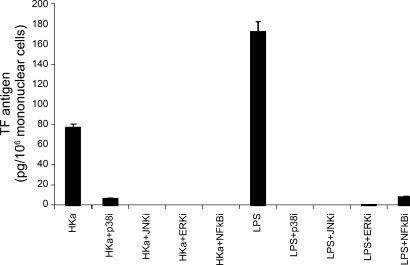
Fig. 4.
Tissue factor expression was greatly attenuated by selective inhibitors of p38, JNK, ERK, and NF-κB. Mononuclear cells were preincubated 1 h without or with 10 μM SB-202190 (a p38 inhibitor), 10 μM SP-600125 (a JNK inhibitor), 10 μM U-0126 (an ERK inhibitor), or 10 μM MG-132 (an NF-κB inhibitor). The stock concentration was 10 mM in DMSO vehicle for all inhibitors, except the JNK inhibitor was 25 mM. The dilution was 1:1,000 in all cases. Cells incubated with DMSO alone showed no inhibition of TF at 1:1,000 dilution. The cultures were then incubated for 3 h without or with HKa (600 nM). Bars represent means of TF expression ± SE (n = 3 experiments).
RESULTS
HKa upregulated TF expression on mononuclear cells in a concentration and time-dependent manner.
TF initiates the clotting cascade. It binds to factor VII and promotes the formation of factor VIIa, which converts factor X to factor Xa, which cleaves prothrombin to thrombin. In this study, we hypothesized that HKa can upregulate TF expression in monocytes. We used an ELISA to measure TF antigen on mononuclear cells. HKa in a concentration-dependent manner upregulated TF expression to 4.78 ± 0.68, 25.5 ± 1.11, 58.5 ± 2.28, 91.4 ± 2.21, and 105.0 ± 3.51 pg/106 cells at 75, 150, 300, 600, and 900 nM, respectively. At 300 nM, intact HK did not stimulate TF expression. As a positive control, LPS (10 ng/ml) increased TF expression to 117.00 ± 6.12 pg/106 cells. In mononuclear cells, TF expression stimulated by HKa (600 nM) was first detected at 2 h (21.4 ± 2.43 pg/106 mononuclear cells) and reached a plateau at 3 to 4 h (155 ± 5.49 and 164 ± 4.38 pg/106 mononuclear cells, respectively).
HKa stimulates TF antigen on the monocyte surface.
The above studies show that HKa stimulated TF expression by monocytes measured as antigen as a function of concentration and time. However, it is also important to show that TF is on the surface of the monocyte where it can interact with plasma coagulation factors such as factor VII and factor X. To demonstrate this, we first used flow cytometry (18). Fig. 1A,i shows that in the absence of HKa, TF antigen measured by binding of fluorescent-labeled TF antibody did not increase over the levels of fluorescent-labeled IgG (Fig. 1A,ii). When HKa was added, a threefold increase in TF antigen was demonstrated (Fig. 1A,iii) compared with the level of TF antigen in the absence of HKa.
Mononuclear cells stimulated by HKa expressed active TF.
Anti-TF antibody significantly inhibited TF activity from 100% to 8.04% and from 100% to 30% for HKa and LPS-stimulated mononuclear cells, respectively (Fig. 1B).
Effects of antibodies to G486–K502 and G440–H455 peptides of HKa on TF expression.
We showed that synthetic D5-peptides, G486–K502, H475–H485, and G440–H455, had different potency on migration or proliferation (3). As shown in Fig. 2, the antibody to G486–K502 region modestly inhibited HKa-induced TF expression from 35.6 ± 2.18 to 26.4 ± 1.01 pg/106 mononuclear cells, whereas the antibody to G440–H455 region significantly (P < 0.05) inhibited HKa-induced TF expression to 6.91 ± 0.22 pg/106 mononuclear cells. Similarly, the antibody to G486–K502 region did not inhibit D5-induced TF expression, whereas the antibody to G440–H455 region significantly inhibited D5-induced TF expression from 18.4 ± 0.51 to 11.5 ± 0.28 pg/106 mononuclear cells. Neither of the antibodies to G486–K502 or G440–H455 region in the absence of D5 had any effect on TF expression.
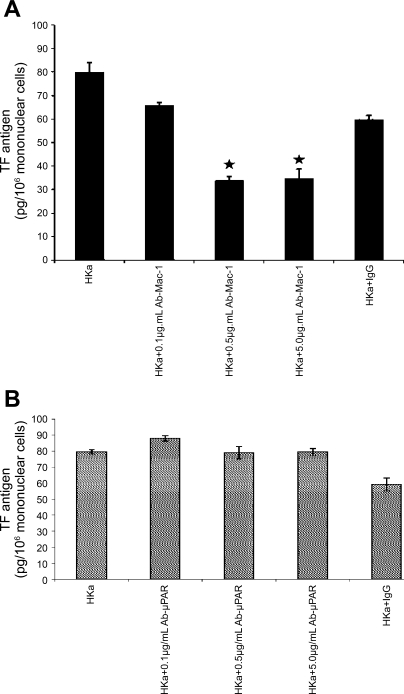
Antibodies to kininogen receptors Mac-1 (CD11b/18) but not LFA-1 (CD11a/18); uPAR or gC1qR inhibited TF expression on mononuclear cells.
Antibody to human Mac-1 (0.1, 0.5, and 5.0 μg/ml) significantly inhibited [from 79.7 ± 2.15 to 65.53 ± 4.05 (P < 0.05) to 33.43 ± 1.58 (P = 0.0001) and to 34.57 ± 2.0 (P = 0.001)] pg TF/106 mononuclear cells when stimulated by HKa (600 nM), respectively (n = 3 experiments) (Fig. 3A). Anti-uPAR antibody (0.1, 0.5, and 5.0 μg/ml) did not significantly inhibit TF expression on mononuclear cells (Fig. 3B) at any concentration. Anti-LFA-1 antibody (CD11a/18) or anti-gC1qR antibody and IgG (isotype matched) also did not inhibit TF expression from mononuclear cells (data not shown).
Fig. 3.
Effects of antibodies to macrophage-1 (Mac-1; CD11b18), urokinase plasminogen activator receptor (uPAR), leukocyte function-associated antigen-1 (CD11a18), and gC1qR on TF expression. A: the antibody to Mac-1 inhibited TF expression induced by HKa. Mononuclear cells were preincubated with the MAb (0.1, 0.5, or 5.0 μg/ml) against Mac-1 for 30 min before stimulation with HKa (600 nM). Data are represented as means ± SE (n = 3 experiments). Comparisons were made with the control. ★P < 0.05. B: antibody to uPAR had no effects on TF expression. Mononuclear cells were preincubated without or with a variety of concentration of the MAbs (0.1, 0.5, or 5.0 μg/ml) against uPAR for 30 min at 37°C and then treated with HKa (600 nM) for additional 180 min at 37°C. Data are presented as in A. None of the differences is significant.
TF expression was greatly attenuated by selective inhibitors of p38, ERK, JNK, and NF-κB.
Signaling through the different MAP kinase families is known to regulate the expression of TF in a stimulus and cell-context dependent manner (7, 8, 22, 33). To determine which signaling pathways were responsible for HKa-induced TF expression, we used selective pharmacological inhibitors for each of the families of MAP kinases and NF-κB. When mononuclear cells were pretreated with SB-202190 (p38 inhibitor), SP-600125 (JNK inhibitor), U-0126 (ERK inhibitor), and MG-132 (NF-κB inhibitor), each at 10 μM and then exposed to HKa, TF expression was significantly inhibited (Fig. 4) (P < 0.0001). The inhibition of p38, JNK, ERK, and NF-κB inhibitors was >90–95%, indicating that HKa activated p38, JNK, ERK, and NF-κB signaling pathways that are responsible for TF expression. The LPS-induced TF expression was also blocked by each of p38, JNK, ERK, or NF-κB inhibitors by about 90–95%.
HKa-induced TF expression was blocked by the combination of antibodies to IL-1β and TNF-α.
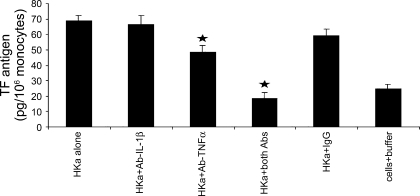
TNF-α and IL-1β induce TF expression in a variety of cell types. We found that HKa (600 nM) released 130 pg/ml TNF-α and 30 pg/ml IL-1β at 180 min (15), but significantly, HKa induced TF expression by indirectly releasing TNF-α and IL-1β. Thus we used the anti-TNF-α and anti-IL-1β antibodies to neutralize the released TNF-α and IL-1β. As shown in Fig. 5, the antibody to TNF-α modestly but significantly attenuated TF expression (56.7 ± 4.97 pg/106 mononuclear cells). In contrast the antibody to IL-1β alone did not inhibit TF expression (74.6 ± 6.18) from its control IgG level (74.9 ± 4.64 pg/106 mononuclear cells). To our surprise, antibodies to IL-1β and to TNF-α synergistically decreased TF expression to 26.4 ± 3.68, which is close to the baseline level (30.5 ± 2.91 pg/106 mononuclear cells), suggesting that TNF-α and IL-1β are the major mediators responsible for TF expression. We reported that HKa-stimulated mononuclear cells secrete the chemokines IL-8 and MCP-1 (15). We investigated other chemokine effects on TF expression by using antibodies to IL-8 or MCP-1 in the presence of HKa. Neither of the antibodies to IL-8 and MCP-1 alone or in combination inhibited TF expression (data not shown).
Fig. 5.
Combination of antibodies to IL-1β and TNF-α completely blocked TF expression. Mononuclear cells were preincubated with the neutralizing MAb against IL-1β, TNF-α, or IL-1β plus TNF-α for 10 min before stimulation with HKa (600 nM) for an additional 180 min at 37°C. Data are plotted as means ± SE. Comparisons were made vs. control. ★P < 0.05.
Detection of monocyte TF mRNA synthesis induced by HKa.
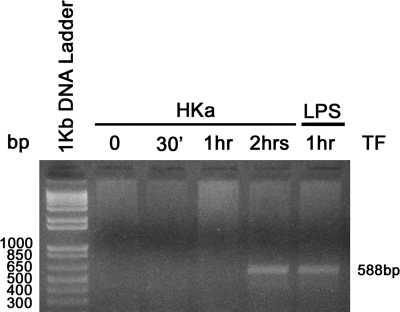
TF can be synthesized by monocytes when activated by lipopolysaccharides, cytokines, or immune complexes (25), and it can be triggered in the presence of activated platelets (2). We therefore investigated whether HKa could induce human monocyte synthesis of TF mRNA. Synthesis was detected using a sensitive reverse transcription-polymerase chain reaction (RT-PCR) using human TF-specific primers (31). As shown in Fig. 6, HKa induced the TF mRNA synthesis at 2 h, whereas LPS induced the TF mRNA synthesis at 1 h.
Fig. 6.
HK stimulated mRNA synthesis of TF in mononuclear cells. Mononuclear cells were incubated with HKa or LPS for the indicated time. Total RNA of the cells was isolated by TRIzol reagent. RT-PCR was carried out as described in materials and methods. PCR products were separated in 1% agarose gel. The detection of the 588-bp DNA bands indicated TF mRNA synthesis from corresponding background (0 time point).
DISCUSSION
The results of this study demonstrate that TF antigen and PCA are stimulated by HKa in a range of concentration from 75 to 900 nM. These ranges of concentration indicate that as little as 10% of HKa, corresponding to a realized decrease in HK, which is seen in inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease, is sufficient to stimulate TF expression and activity. The time course shows no increase at 1 h, a minimal increase at 2 h, and a maximum increase at 3 to 4 h. This pattern of responses is similar to the synthesis of TF observed with bacterial lipopolysaccharide (endotoxin) over time. Two sequences of HKa have been found to have biological activity: G440–H455 stimulates endothelial proliferation, whereas H486–K502 enhances endothelial migration and inhibits adhesion (3). A polyclonal antibody to the former sequence inhibits TF expression but not to the latter. G484–L491 inhibited cancer metastasis in vivo and invasion in vitro (13), G440–H455 is a Zn2+ binding site for HKa, and Zn2+ is required for cells to bind HKa.
Monocytes and neutrophils have at least four receptors for HKa: Mac-1 (CD11b/CD18), LFA-1 (CD11a/CD18), uPAR (CD87), and gC1qR. Each of them plays different roles in response to a variety of stimuli. Different roles of Mac-1 and LFA-1 have been described by Orlova et al. (24). They demonstrated that high-mobility group box 1-mediated neutrophil recruitment was inhibited in mice deficient in the β2-integrin Mac-1 but not in those deficient in LFA-1. McGilvray et al. (21) revealed that cross-linking of the LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) integrins on the surface of THP-1 monocytic cells induced the accumulation of tyrosine phosphoproteins and an increase in ERK-2 activity. β2-Integrin cross-linking also led to a marked increase TF measured as in 4 h PCA in THP-1 cells and purified human monocytes. Selective ERK inhibition with PD-98059 (10 μM) was able to block the integrin-dependent induction of PCA in both THP-1 cells and human monocytes, which is essential for the subsequent expression of TF. In agreement with those observations, the antibody to Mac-1 but not LFA-1, uPAR, and gC1qR inhibits TF expression on mononuclear cells stimulated by HKa, indicating that Mac-1 plays critical roles in chemokine secretion and TF expression.
TF is critically important for initiating the activation of coagulation zymogens leading to the generation of thrombin, which can be seen in many pathophysiological conditions, such as septic shock (17), diabetes, deep venous thrombosis (34), and inflammatory bowel disease (30). Many stimuli including cytokines and coagulation proteases can elicit TF synthesis. TNF-α clusters and activates TNF receptors, which recruit a distinctive set of adapter proteins (TRADD, RIP, and TRAF2) to mediate TNF signaling (19, 20), which activates MAP kinases and NF-κB. The MAP kinases important for signaling the increased expression of the procoagulatory and inflammatory protein TF in monocytes are the stress-activated kinases p38 and JNK. The signaling pathways were probed using low molecular weight inhibitors. SB-202190 (p38 inhibitor), SP600125 (JNK inhibitor), as well as MG-132 (NF-κB inhibitor) completely blocked HKa-induced TF expression. These pathways resemble those of LPS and include NF-κB, p38, and JNK. However, unlike LPS, an inhibitor of ERK can prevent stimulation of TF activity by HKa. Although TF can initiate signaling pathways, it is distinctly possible that the upregulation of TF by HKa is due to the effect of cytokines on chemokines alone or in combination rather than a direct effect on TF transcription. The only antibody to a cytokine that displayed significant inhibition is that to TNF-α, which is modestly effective. An antibody to IL-1β is not an effective inhibitor of HKa-stimulated TF, but the combination of antibodies to TF and IL-1α produces almost complete inhibition, suggesting that IL-1β potentiated TNF-α initiated TF expression.
In this study, we clearly demonstrated that HKa can trigger TF synthesis by monocytes in a time- and concentration-dependent manner. We also reveal that Mac-1 is the most critical receptor for HKa-induced TF expression. Both MAP kinases and NF-κB participate in TF expression by HKa. Finally, we also demonstrated that HKa induced TF expression through IL-1β and TNF-β. Thus HKa should be added to the list of inflammatory mediators in monocytes.
GRANTS
This work was supported by National Institutes of Health Grants R01-CA-83121, R01-AR-051713, and T32-HL-07777 (to R. W. Colman).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Bradford HN, Dela Cadena RA, Kunapuli SP, Dong JF, Lopez JA, Colman RW. Human kininogens regulate thrombin binding to platelets through the glycoprotein Ib-IX-V complex. Blood 90: 1508–1515, 1997 [PubMed] [Google Scholar]
- 2.Celi A, Pellegrini G, Lorenzet R, De Blasi A, Ready N, Furie BC, Furie B. P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci USA 91: 8767–8771, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colman RW, Jameson BA, Lin Y, Johnson D, Mousa SA. Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood 95: 543–550, 2000 [PubMed] [Google Scholar]
- 4.Colman RW, Pixley RA, Najamunnisa S, Yan W, Wang J, Mazar A, McCrae KR. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2 and 3 of the urokinase receptor. J Clin Invest 100: 1481–1487, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colman RW, White JV, Scovell S, Stadnicki A, Sartor RB. Kininogens are antithrombotic proteins in vivo. Arterioscler Thromb Vasc Biol 19: 2245–2250, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Drake TA, Cheng J, Chang A, Taylor FB., Jr Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol 142: 1458–1470, 1993 [PMC free article] [PubMed] [Google Scholar]
- 7.Eto M, Kozai T, Cosentino F, Joch H, Luscher TF. Statin prevents tissue factor expression in human endothelial cells: role of Rho/Rho-kinase and Akt pathways. Circulation 105: 1756–1759, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem 277: 32124–32132, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Gustafson EJ, Schmaier AH, Wachtfogel YT, Kaufman N, Kucich U, Colman RW. Human neutrophils contain and bind high molecular weight kininogen. J Clin Invest 84: 28–35, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasan AA, Zisman T, Schmaier AH. Identification of cytokeratin 1 as a binding protein and presentation receptor for kininogens on endothelial cells. Proc Natl Acad Sci USA 95: 3615–3620, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isordia-Salas I, Pixley RA, Li F, Sainz I, Sartor RB, Adam A, Colman RW. Kininogen deficiency modulates chronic intestinal inflammation in genetically susceptible rats. Am J Physiol Gastrointest Liver Physiol 283: G180–G186, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Joseph K, Ghebrehiwet B, Peerschke EI, Reid KB, Kaplan AP. Identification of the zinc-dependent endothelial cell binding protein for high molecular weight kininogen and factor XII: identity with the receptor that binds to the globular “heads” of C1q (gC1q-R). Proc Natl Acad Sci USA 93: 8552–8557, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawasaki M, Maeda T, Hanasawa K, Ohkubo I, Tani T. Effect of His-Gly-Lys motif derived from domain 5 of high molecular weight kininogen on suppression of cancer metastasis both in vitro and in vivo. J Biol Chem 278: 49301–49307, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Key NS, Slungaard A, Dandelet L, Nelson SC, Moertel C, Styles LA, Kuypers FA, Bach RR. Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood 91: 4216–4223, 1998 [PubMed] [Google Scholar]
- 15.Khan MM, Bradford HN, Isordia-Salas I, Liu Y, Wu Y, Espinola RG, Ghebrehiwet B, Colman RW. High-molecular-weight kininogen fragments stimulate the secretion of cytokines and chemokines through uPAR, Mac-1, and gC1qR in monocytes. Arterioscler Thromb Vasc Biol 26: 2260–2266, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MM, Hattori T, Niewiarowski S, Edmunds LH, Jr, Colman RW. Truncated and microparticle-free soluble tissue factor bound to peripheral monocytes preferentially activate factor VII. Thromb Haemost 95: 462–468, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Li A, Chang AC, Peer GT, Hinshaw LB, Taylor FB., Jr Comparison of the capacity of rhTNF-alpha and Escherichia coli to induce procoagulant activity by baboon mononuclear cells in vivo and in vitro. Shock 5: 274–279, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Luther T, Flossel C, Hietschhold V, Koslowski R, Müller M. Flow cytometric analysis of tissue factor (TF) expression on stimulated monocytes—comparison to procoagulant activity of mononuclear blood cells. Blut 61: 375–378, 1990 [DOI] [PubMed] [Google Scholar]
- 19.MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal 14: 477–492, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Madge LA, Pober JS. TNF signaling in vascular endothelial cells. Exp Mol Pathol 70: 317–325, 2001 [DOI] [PubMed] [Google Scholar]
- 21.McGilvray ID, Lu Z, Wei AC, Rotstein OD. MAP-kinase dependent induction of monocytic procoagulant activity by beta2-integrins. J Surg Res 80: 272–279, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Mechtcheriakova D, Schabbauer G, Lucerna M, Clauss M, De Martin R, Binder BR, Hofer E. Specificity, diversity, and convergence in VEGF and TNF-alpha signaling events leading to tissue factor up-regulation via EGR-1 in endothelial cells. FASEB J 15: 230–242, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Merkulov S, Zhang WM, Komar AA, Schmaier AH, Barnes E, Zhou Y, Lu X, Iwaki T, Castellino FJ, Luo G, McCrae KR. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood 111: 1274–1281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, Chavakis T. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J 26: 1129–1139, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osterud B. Cellular interactions in tissue factor expression by blood monocytes. Blood Coagul Fibrinolysis 6, Suppl 1: S20–S25, 1995 [PubMed] [Google Scholar]
- 26.Pawlinski R, Mackman N. Tissue factor, coagulation proteases, and protease-activated receptors in endotoxemia and sepsis. Crit Care Med 32: S293–S297, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Puri RN, Zhou F, Hu CJ, Colman RF, Colman RW. High molecular weight kininogen inhibits thrombin-induced platelet aggregation and cleavage of aggregin by inhibiting binding of thrombin to platelets. Blood 77: 500–507, 1991 [PubMed] [Google Scholar]
- 28.Sainz IM, Isordia-Salas I, Castaneda JL, Agelan A, Liu B, DeLa Cadena RA, Pixley RA, Adam A, Sartor RB, Colman RW. Modulation of inflammation by kininogen deficiency in a rat model of inflammatory arthritis. Arthritis Rheum 52: 2549–2552, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Sainz IM, Pixley RA, Colman RW. Fifty years of research on the plasma kallikrein-kinin system: from protein structure and function to cell biology and in-vivo pathophysiology. Thromb Haemost 98: 77–83, 2007 [PubMed] [Google Scholar]
- 30.Srirajaskanthan R, Winter M, Muller AF. Venous thrombosis in inflammatory bowel disease. Eur J Gastroenterol Hepatol 17: 697–700, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Stephens AC, Ranlall NF, Rivers RP. Suppression of HUVEC tissue factor synthesis by antisense oligodeoxynucleotide. Thromb Res 122: 99–107, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Weisel JW, Nagaswami C, Woodhead JL, DeLa Cadena RA, Page JD, Colman RW. The shape of high molecular weight kininogen. Organization into structural domains, changes with activation, and interactions with prekallikrein, as determined by electron microscopy. J Biol Chem 269: 10100–10106, 1994 [PubMed] [Google Scholar]
- 33.Xuereb JM, Sie P, Boneu B, Constans J. Inhibition of tissue factor synthesis by disruption of ERK kinases and PKC signaling pathways in human vascular SMCs. Thromb Haemost 84: 129–136, 2000 [PubMed] [Google Scholar]
- 34.Yoshitaka T, Abe N, Minagawa H, Date H, Sakoma Y, Nishida K, Ozaki T. Disease-specific screening for deep venous thrombosis and pulmonary thromboembolism using plasma D-dimer values after total knee arthroplasty. Mod Rheumatol 18: 359–365, 2008 [DOI] [PubMed] [Google Scholar]