Abstract
The transient receptor potential vallinoid type 4 (TRPV4) channel has been implicated in the endothelial shear response and flow-mediated dilation, although the precise functions of this channel remain poorly understood. In the present study, we investigated the role of TRPV4 in shear stress-induced endothelial Ca2+ entry and the potential link between this signaling response and relaxation of small resistance arteries. Using immunohistochemical analysis and RT-PCR, we detected strong expression of TRPV4 protein and mRNA in the endothelium in situ and endothelial cells freshly isolated from mouse small mesenteric arteries. The selective TRPV4 agonist GSK1016790A increased endothelial Ca2+ and induced potent relaxation of small mesenteric arteries from wild-type (WT) but not TRPV4−/− mice. Luminal flow elicited endothelium-dependent relaxations that involved both nitric oxide and EDHFs. Both nitric oxide and EDHF components of flow-mediated relaxation were markedly reduced in TRPV4−/− mice compared with WT controls. Using a fura-2/Mn2+ quenching assay, shear was observed to produce rapid Ca2+ influx in endothelial cells, which was markedly inhibited by the TRPV4 channel blocker ruthenium red and TRPV4-specific short interfering RNA. Flow elicited a similar TRPV4-mediated Ca2+ entry in HEK-293 cells transfected with TRPV4 channels but not in nontransfected cells. Collectively, these data indicate that TRPV4 may be a potential candidate of mechanosensitive channels in endothelial cells through which the shear stimulus is transduced into Ca2+ signaling, leading to the release of endothelial relaxing factors and flow-mediated dilation of small resistance arteries.
Keywords: transient receptor potential, endothelium, endothelium-derived factors, calcium signaling
shear stress generated by blood flow is an important physiological stimulus for vascular endothelial cells (ECs). In most vascular beds, flow induces the release of endothelial vasodilator factors that subsequently cause relaxation of underlying smooth muscles, a phenomenon known as flow-mediated dilation (6). Ca2+ signaling has been thought to be involved in endothelial mechanotransduction and flow-mediated dilation (9). In ECs, an increase in the intracellular Ca2+ concentration ([Ca2+]i) triggers the synthesis of each of the three major endothelium-derived vasodilators: nitric oxide (NO), prostacyclin, and EDHF(s) (14). The application of shear stress increases [Ca2+]i in ECs from a variety of vascular beds (18, 22, 23, 34, 35). The molecular identity of the potential Ca2+-permeable channel(s) involved in shear signal transduction remain largely uncharacterized.
Recent studies have indicated that the transient receptor potential (TRP) vanilloid 4 (TRPV4) channel, a Ca2+-permeable cation channel of the TRP channel superfamily, is expressed in vascular ECs of several species (13, 21, 24, 27, 29, 33, 43, 46, 49). TRPV4 is activated by both chemical and physical stimuli, including the synthetic phorbol derivative 4α-phorbol-12,13-didecanoate (4α-PDD) (40), arachidonic acid and its metabolites [such as epoxyeicosatrienoic acids (EETs)] (39, 41), moderate warmth (>27°C) (19, 42), hypotonic cell swelling (26, 36), pressure (37), membrane stretch (38), and shear stress (16). Additionally, TPRV4 channels have been shown to participate in the regulation of vascular tone, including flow-mediated dilation (13, 21, 24, 27, 29, 33, 43, 49). In particular, genetic deletion of TRPV4 channels results in blunted flow-mediated dilation of mouse carotid arteries (21, 27).
It has yet to be determined whether TRPV4 channel deletion affects the flow-induced dilation of small resistance arteries. This is important since tissue perfusion is regulated at the level of small arteries and arterioles and, therefore, flow-induced dilation is most significant in those vascular beds. Furthermore, although shear-induced Ca2+ responses have been reported in TRPV4-overexpressing human embryonic kidney (HEK)-293 cells (16), it remains to be determined whether shear induces TRPV4 activation and Ca2+ entry in native vascular ECs. In the present study, we examined the expression of TRPV4 and the effects of channel activation on endothelial [Ca2+]i and the release of endothelial relaxing factors in mouse small mesenteric arteries. We also investigated the effects of genetic deletion or blockade of TRPV4 on flow-induced NO- and EDHF-dependent dilation of small mesenteric arteries and on flow-induced Ca2+ signaling in ECs. Finally, we further confirmed flow-induced Ca2+ entry through TRPV4 channels in HEK-293 cells overexpressing these channels.
METHODS
Animals.
Male TRPV4 knockout (TRPV4−/−; n = 33) (37) and male wild-type (WT) C57BL/6J mice (n = 44) were used in this study. TRPV4−/− mice were kindly provided by Dr. Suzuki (Jichi Medical University). Animals were maintained in our animal facility and used at 2–4 mo of age. This study was approved by the Institutional Review Committee. All experiments were conducted in accordance with Institutional Animal Care and Use Committee guidelines. Genotyping of WT and TRPV4−/− animals was performed by PCR with the following primers: TRPV4, forward 5′-TGT TCG GGG TGG TTT GGC CAG GAT AT-3′ and reverse 5′-GCT GAA CCA AAG GAC ACT TGC ATA G-3′ (a 796-bp product in WT animals and no signal in TRPV4−/− animals); and knockout neomycin cassette, forward 5′-GCT GCA TAC GCT TGA TCC GGC TAC-3′ and reverse 5′-TAA AGC ACG AGG AAG CGG TCA GCC-3′ (a 366-bp product in TRPV4−/− animals).
Cell culture.
Human coronary artery ECs (HCAECs) were obtained from Lonza (Walkersville, MD) and cultured in full growth medium (EGM-2 MV, Lonza) at 37°C in a humidified incubator with 5% CO2. ECs were subcultured when the cells reached 70–80% confluence, and cells between passages 4 and 6 were used for experiments. HEK-293 cells were provided by Dr. David Wilcox (Medical College of Wisconsin) and grown in DMEM supplemented with 10% FBS, 100 U/ml penicillin G, and 100 μg/ml streptomycin at 37°C and 5% CO2. HEK-293 cells were used for the present study between passages 6 and 10.
Vascular cell dissociation.
Mice were euthanized with an intraperitoneal injection of pentobarbital sodium (150 mg/kg), and mesenteric tissue was removed and placed in cold HEPES buffer containing (in mM) 138 NaCl, 4.0 KCl, 1.6 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 5.5 glucose, and 10 HEPES (pH 7.4). First- or second-order branches (100–200 μm) from the superior mesenteric artery were quickly dissected, cleaned of connective tissue, and placed in cold HEPES solution. Vascular ECs and smooth muscle cells were enzymatically dissociated from arteries as previously described (48). In brief, artery segments were cut into small rings and incubated for 10 min in a low-Ca2+ dissociation buffer consisting of (in mM) 145 NaCl, 4.0 KCl, 0.1 CaCl2, 1.0 MgSO4, 10 glucose, and 10 HEPES with 0.1% BSA (pH 7.4). The buffer was carefully removed, followed by sequential incubation at 37°C with papain (1.0 mg/ml) and DTT (0.5 mg/ml) in dissociation buffer for 15 min and then collagenase (Sigma blend H, 2.5 mg/ml), trypsin inhibitor (1 mg/ml), and elastase (0.5 mg/ml) for 15–20 min. All enzymes and chemicals were purchased from Sigma. Artery segments were gently triturated to release ECs and smooth muscle cells. Cells were washed with dissociated solution and stored on ice for RT-PCR analysis as described below.
Isometric tension recording.
Mouse small mesenteric arteries (100–200 μm) from WT and TRPV4−/− mice were dissected as described above and mounted in a four-chamber wire myograph (model 610M, Danish Myo Technology) as previously described (48). Artery segments were maintained at 37°C in Krebs physiological saline solution (Krebs-PSS) of the following composition (in mM): 123 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 21 NaHCO3, 1.2 KH2PO4, 0.026 Na2EDTA, and 11 glucose. Krebs-PSS was prewarmed at 37°C and gassed with 21% O2-5% CO2 to maintain a pH of 7.4. The resting tension was set at 1 mN. Arteries were stimulated two times with a high-K+ Krebs solution (K-PSS), in which NaCl was substituted with KCl of equal molar concentration, for 3 min each at 10-min intervals. Vessels were then constricted to 50–75% of maximum KCl responses with the thromboxane mimetic U-46619 (50–300 nM). After the contraction reached steady state, relaxation responses to the selective TRPV4 agonists GSK1016790A (100 nM) and 4α-PDD (1 μM) were determined as paired rings in the absence or presence of following inhibitors: ruthenium red (10 μM, a TRPV4 blocker) or NG-nitro-l-arginine methyl ester (l-NAME; 100 μM, a NO synthase inhibitor). To examine the role of smooth muscle hyperpolarization in GSK1016790A-induced relaxations, arteries were preconstricted with KCl (60 mM, equal molar substitution of NaCl). Where indicated, the endothelium was removed by gently rubbing the intimal surface of the artery with a wire or human hair. The endothelium was considered intact if acetylcholine (10 μM) caused >80% relaxation of U-46619-precontracted arteries and effectively denuded if acetylcholine induced <10% relaxation. Vasodilator responses are expressed as percent maximal relaxation relative to U-46619 preconstriction, with 100% representing full relaxation to basal tension.
Videomicroscopy.
Mesenteric arteries (100–200 μm) from WT and TRPV4−/− mice were cannulated with two glass micropipettes for continuous videomicroscopic measurements of diameter as previously described (31, 50). Vessels were pressurized to an intramural pressure of 60 mmHg under no-flow conditions and equilibrated at 37°C in Krebs-PSS gassed with 21% O2-5% CO2. After 1 h of equilibration, arteries were preconstricted with U-46619 (10–100 nM) to ≈50% of the baseline internal diameter. Flow was produced from a pressure gradient by changing the heights of two pressure reservoirs in equal and opposite directions, as previously described by Kuo et al. (25). Flow-mediated dilations (5–40 cmH2O) were examined on vessels before and after treatment with l-NAME (100 μM) and/or indomethacin (10 μM, a cyclooxygenase inhibitor) for 30 min. Some vessels were contracted with KCl (60 mM) to examine the contribution of smooth muscle hyperpolarization to relaxation responses. Vasodilator responses to papaverine (10−4 M) were determined at the end of experiments. Vascular responses are expressed as percent maximal relaxation relative to U-46619 preconstriction, with 100% representing the passive baseline diameter.
Laminar flow chamber.
A parallel plate chamber, as originally described by Frangos et al. (15), was used to acutely shear ECs. Cells were grown to confluent in 35-mm glass bottom petri dishes and attached to a Vacu-Cell flow device (C&L Instruments) to form a rectangular flow chamber. The flow chamber was placed on the stage of an inverted microscope and assayed for changes in intracellular Ca2+ as described below. Fluid flow through the chamber was driven by a syringe pump (Harvard Apparatus), and fluid flow rates were adjusted to generate a shear stress of 1–20 dyn/cm2. Fluid shear stress (in dyn/cm2) was calculated using the equation: fluid shear stress = 6ηQ/wh2, where η is viscosity (0.007 Poise), Q is flow rate (in ml/s), w is the width of flow chamber (0.716 cm), and h is the height of chamber (0.025 cm).
Measurement of [Ca2+]i.
A fura-2 assay was used to measure [Ca2+]i as we have previously described (47, 50). In brief, cells were grown in 35-mm glass bottom petri dishes and loaded with fura-2 AM (5 μM) at room temperature for 30–60 min in modified HBSS that contained (in mM) 137 NaCl, 5.4 KCl, 1.3 CaCl2, 0.5 MgCl2, 0.4 MgSO4, 4.2 NaHCO3, 0.3 Na2HPO4, 0.4 KH2PO4, 5.5 glucose, and 20 HEPES (pH 7.4). Cells were washed and incubated in fresh HBSS for an additional 15–30 min to allow complete deesterization of the dye. Fluorescence images were captured and analyzed using an image system consisting of an inverted epifluorescence microscope (Nikon TE200) with a ×20 fluor objective, a high-speed wavelength switcher (Lambda DG-4 from Sutter Instruments), a PC-controlled digital charge-coupled device camera (Hamamatsu C4742-95), and Metafluor software (Universal Imaging). Fura-2 was excited alternatively at three wavelengths of 340, 360, and 380 nm. The emitted light was collected through an emission filter of 510 nm. Fura-2 fluorescence was measured every 3 s. Results are presented as the ratio of the fluorescence intensity at 340- versus 380-nm excitation (F340/F380). Endothelial [Ca2+]i was measured in situ in freshly isolated mesenteric arteries using a modified method we have previously described (50). An arterial segment was cut open along its longitudinal axis and pinned onto a Sylgard-coated dish with lumen side upward. Vessels were incubated with fura-2 AM (10 μM) at room temperature for 60 min in Krebs-PSS, followed by the Ca2+ assay as described above. The experiments were performed at room temperature for cells and at 37°C for isolated vessels unless otherwise indicated.
Ca2+ influx assay.
The Mn2+ quenching technique (1, 20) was used to assess Ca2+ influx into cells. Briefly, cells were loaded with fura-2 (5 μM) as described above. The extracellular solution contained (in mM) 145 NaCl, 4 KCl, 1.6 MnCl2 (instead of CaCl2), 1.0 MgCl2, 10 glucose, and 10 HEPES (pH 7.4). The uptake of Mn2+, a Ca2+ surrogate for Ca2+ channels, was monitored by quenching of fura-2 fluorescence excited at 360 nm, an isosbestic wavelength not sensitive changes in [Ca2+]i. Since basal Mn2+ influx differed between experiments, the basal rate for 2–3 min was determined at the beginning of each experiment. The fluorescence intensity at 360 nm was normalized to the baseline, and the slope of Mn2+ quenching measured by fitting a linear regression to the values over 1–2 min and reflected the rate of Mn2+ influx. In some experiments, we performed simultaneous measurements of Ca2+ influx and Ca2+ release from intracellular stores. Ca2+ release was measured as F340/F380, which is not modified by Mn2+ quenching as long as the total Mn2+ concentration does not exceed that of fura-2 (1). Since there was no external Ca2+ in the extracellular solution, an increase in [Ca2+]i reflected only the release of Ca2+ from intracellular stores.
Small interfering RNA transfection.
For RNA inhibition experiments, knockdown of human TRPV4 was performed using On-Target plus SMART pool short interfering (si)RNA (Dharmacon). A nontargeting On-Target plus siControl (Dharmacon) was used as a control. Cells were transfected with siControl or TRPV4 siRNA (50–100 nM) complexed with Lipofectamine 2000 (0.5 μg/ml, Invitrogen) for 4–6 h according to the manufacturer's instructions. Cells were cultured for an additional 48–72 h in fresh media at 37°C, and mRNA and proteins were extracted for RT-PCR and Western blot analysis. Cells were examined for TRPV4 agonist- or flow-induced Ca2+ responses 48–72 h after transfection.
Transient expression of TRPV4.
The full-length human TRPV4 cDNA clone was obtained from Origene and subcloned into pCMV6 mammalian expression vectors as a fusion protein with the COOH-terminus tagged with turbo green fluorescent protein (GFP). This enhanced GFP has an excitation maximum of 482 nm, and therefore [Ca2+]i can be simultaneously measured with fura-2 in cells expressing GFP. TRPV4-GFP coding plasmids were purified by an endotoxin-free plasmid kit (Qiagen) for subsequent transfection. The nucleic sequence of TRPV4-GFP constructs was verified by direct DNA sequencing. The TRPV4-GFP coding plasmids were transiently transfected into HEK-293 cells using the lipid-mediated Lipofectamine 2000 kit (Invitrogen) according to the manufacturer's protocols. In brief, cells were plated in 35-mm glass bottom petri dishes 24 h before transfection. Cells were transiently transfected with 1 μg plasmid DNA/35-mm dish. Ca2+ measurements were performed 24–48 h after transfection. With transient transfection for 24–48 h, 50–80% of cells were found to express TRPV4-GFP fusion proteins based on fluorescence microscopic analysis. The expression of fusion proteins was also confirmed by Western blot analysis using both anti-TRPV4 and anti-GFP antibodies. The localization of fusion proteins in HEK-293 cells was also examined by confocal fluorescence microscope.
Immunofluorescence.
Small segments of mouse mesenteric arteries were freshly dissected, embedded and frozen in OTC compound, and cut into 10-μm sections. Some tissue sections were stained with hematoxylin and eosin to confirm the intact vascular structure before further processing for immunofluorescence staining. Tissue sections were washed in PBS and blocked with 5% normal goat serum in PBS containing 0.3% Triton X-100. Sections were incubated with a polyclonal antibody against TRPV4 (1:100 dilution, Alomone Labs) for 30 min at room temperature, followed by goat anti-rabbit IgG conjugated with Alexa fluor 568 for 1 h. After several washes with PBS, images were immediately captured using a regular fluorescence microscope.
Immunoblot analysis.
ECs or HEK-293 cells were homogenized in ice-cold lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% deoxycholic acid, 0.1% SDS, and 0.5% Nonidet P-40] supplemented with a protease inhibitor cocktail (Roche) and centrifuged at 12,000 g for 10 min at 4°C. Protein samples (10–20 μg) were subjected to 10% SDS-PAGE and transferred to polyvinylidene difluoride or nitrocellulose membranes. Membranes were blotted with a polyclonal antibody against TRPV4 (1:1,000 dilution, MBL), a polyclonal antibody against GFP (1:15,000 dilution, Evrogen), or a monoclonal antibody against β-actin (1:10,000 dilution, Abcam AC-15 clone), followed by horseradish peroxidase-conjugated secondary antibodies. After being washed with Tris-buffered saline-Tween 20 (0.1%), immunoreactive complexes were visualized using the ECL chemiluminescence detection system (Amersham).
RNA extraction and RT-PCR.
RT-PCR was performed on freshly isolated vascular cells as previously reported by Gauthier et al. (17). In brief, ECs or smooth muscle cells were selectively aspirated into a large-bore glass pipette (10- to 20-μm tip). The pipette (containing 10–20 cells) was then placed tip down in a sterile 0.5-ml PCR tube, and the pipette tip broken by gentle pressure to release the cell-containing solution. The tubes were snap frozen in liquid N2. The complete first-strand cDNA synthesis solution (Bio-Rad) except reverse transcriptase was added to each tube, followed by two rapid freeze-thaw cycles to rupture cells and allow access to cellular RNA. Reverse transcriptase was then added, and samples were incubated at 42°C for 1 h. The cDNA was divided into three to four aliquots and subjected to PCR amplification using a 45-cycle touch-down protocol with the following gene-specific primers: TRPV4, forward 5′-CCA AGG ATG AGG GAG GCT-3′ and reverse 5′-GTC GGA TGA TGT GCT GAA AG-3′ [for a 351-bp fragment (NM_022017)]; PECAM-1, forward 5′-GCA AGA AGC AGG AAG GAC AG-3′ and reverse 5′-TGA CAA CCA CCG CAA TGA G-3′ [for a 138-bp fragment (NM_001032378)]; and β-actin, forward 5′-TCC GTA AAG ACC TCT ATG CC-3′ and reverse 5′-TAC TCC TGC TTG CTG ATC C-3′ [for a 220-bp fragment (NM_007393)]. The primers used for RT-PCR in HCAECs were as follows: TRPV4, forward 5′-GAG CAA TGG CCG CAA CGA-3′ and reverse 5′-GCC GTG TGT CCT CAT CCG TC-3′ [for a 596-bp fragment (NM_021625)]; and PECAM-1, forward 5′-AGA CAA CCC CAC TGA AGA C-3′ and reverse 5′-TCC AGA CTC CAC CAC CTT AC-3′ [for a 154-bp fragment (NM_000442)].
Materials and solutions.
U-46619 was obtained from Cayman Chemical, and ruthenium red was obtained from Calbiochem. GSK1016790A (43) was kindly provided by GlaxoSmithKline Pharmaceuticals. All other chemicals were purchased from Sigma. Stock solutions were made in distilled water except for U46619 (ethanol), GSK1016790A and 4α-PDD (DMSO), and indomethacin (0.2 M Na2CO3).
Data analysis.
Data are presented as means ± SE. Significant differences between mean values were evaluated by Student t-test or ANOVA followed by the Student-Newman-Keuls multiple-comparison test. P values of <0.05 were considered statistically significant.
RESULTS
Expression of TRPV4 channels in ECs.
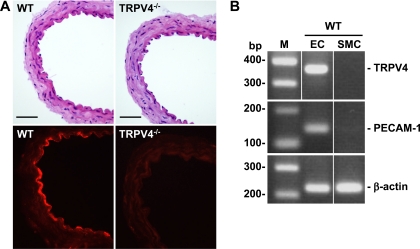
Using immunohistochemical analysis, we detected strong staining for TRPV4 protein in the endothelial layer of mesenteric arteries from WT but not TRPV4−/− animals (Fig. 1A). A weak immunofluorescence was found in the underlying smooth muscles and adventitial layer of mesenteric arteries from both WT and TRPV4−/− animals, which was probably derived from the nonspecific binding of the polyclonal antibodies used in this study. An intact vascular structure was confirmed by hemotoxylin and eosin staining. To further confirm the cell type-specific expression of TRPV4 channels, we examined the expression of TRPV4 mRNA in freshly isolated vascular cells from mouse mesenteric arteries. Consistent with the immunohistochemistry results, TRPV4 transcripts were also detected in ECs but not in smooth muscle cells freshly isolated from WT mesenteric arteries (Fig. 1B). As parallel controls, the endothelial marker PECAM-1 was present only in ECs, whereas the more ubiquitous housekeeping protein β-actin was expressed in both ECs and smooth muscle cells.
Fig. 1.
Transient receptor potential vanilloid 4 (TRPV4) expression in endothelial cells (ECs) of mouse mesenteric arteries. A, top: hematoxylin and eosin staining of wild-type (WT) and TRPV4 knockout (TRPV4−/−) mesenteric arteries. Bottom, immunohistochemical staining of TRPV4 was seen in the endothelium of mesenteric arteries from WT but not TRPV4−/− mice. Scale bar = 5 μm. B: RT-PCR analysis indicated that TRPV4 mRNA was expressed in ECs but not smooth muscle cells (SMCs) freshly isolated from mesenteric arteries of WT animals. The same samples were amplified for the expression of PECAM-1, an endothelial marker, and β-actin, a housekeeping gene present in both ECs and SMCs. M, DNA marker. Results are representative of 3 independent experiments.
TRPV4 agonist-induced Ca2+ response and vasodilator release.
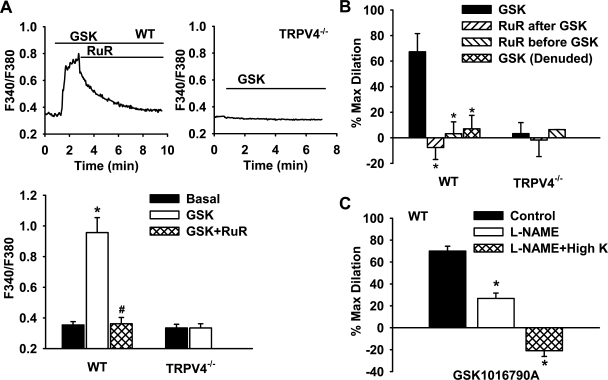
The TRPV4-mediated Ca2+ response was determined in the endothelium of freshly isolated small mesenteric arteries from WT and TRPV4−/− mice (Fig. 2A). Treatment of mesenteric arteries with GSK1016790A (100 nM), a recently developed selective TRPV4 channel agonist (43), induced a robust increase in [Ca2+]i in ECs of WT mice (F340/F380: 0.96 ± 0.10 vs. 0.35 ± 0.02 under basal conditions). Addition of the nonspecific TRPV4 channel blocker ruthenium red (10 μM) rapidly reversed the response to GSK1016790A (0.36 ± 0.04). GSK1016790A had no effect on endothelial Ca2+ in TRPV4−/− animals (0.33 ± 0.03 vs. 0.33 ± 0.02 under basal conditions).
Fig. 2.
TRPV4-mediated endothelial Ca2+ responses and vasodilation in mouse mesenteric arteries. A: addition of the TRPV4-specific agonist GSK1016790A (GSK; 100 nM) caused a rapid increase in the intracellular Ca2+ concentration ([Ca2+]i) in WT, which was blocked by ruthenium red (RuR; 10 μM), a nonselective TRPV4 channel blocker. GSK had no effect in TRPV4−/− mice. Top, representative traces; bottom, summarized data. n = 3–5 mice (20–30 ECs/vessel). *P < 0.05 vs. basal; #P < 0.05 vs. GSK. B: GSK (100 nM) induced potent endothelium-dependent relaxation of mesenteric arteries in WT but not TRPV4−/− animals. This dilation was largely abolished by RuR (10 μM) given before or after GSK. n = 3–6 mice. *P < 0.05 vs. GSK. C: GSK-induced vasodilation was reduced by NG-nitro-l-arginine methyl ester (l-NAME; 100 μM) and abolished by l-NAME plus high K+. n = 3 mice. *P < 0.05 vs. control.
Consistent with its stimulatory effect on endothelial Ca2+, GSK1016790A (100 nM) elicited a robust endothelium-dependent dilation of mesenteric arteries from WT mice (percent maximal dilation: 67.3 ± 14.2% in intact vessels vs. 7.0 ± 10.5% in denuded vessels; Fig. 2B). The vasodilator response was abolished by ruthenium red given either before or after GSK1016790A (−7.6 ± 9.3% and 3.2 ± 9.3%, respectively). No vasodilation response to GSK1016790A was observed in TRPV4−/− animals. In WT mice, GSK1016790A-induced relaxations were markedly inhibited by l-NAME (100 μM), a NO synthase inhibitor (26.7 ± 4.9%), and subsequently abolished by high K+ combined with l-NAME (−20.9 ± 5.2%; Fig. 2C). 4α-PDD (1 μM), another specific and chemically distinct TRPV4 agonist, also dilated mesenteric arteries from WT animals in an endothelium-dependent manner (22.9 ± 5.1% in intact vessels vs. 6.4 ± 1.8% in denuded vessels, n = 4–5). 4α-PDD did not elicit significant vasodilation in TRPV4−/− animals (−0.3 ± 0.2%, n = 4). Together, these data indicate that functional TRPV4 channels are present in ECs of small mesenteric arteries and that activation of these channels leads to endothelial Ca2+ increases and the release of vasodilator factors. Furthermore, no functional response to TRPV4 activation was observed in denuded small arteries.
TRPV4 mediates flow-induced vasodilation.
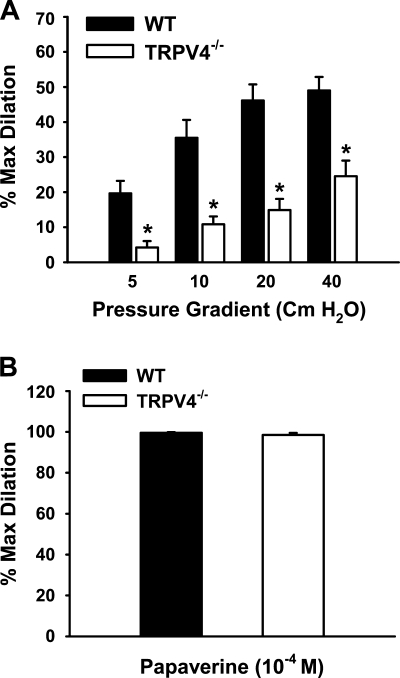
In WT animals, fluid flow elicited flow rate-dependent relaxations (percent maximal dilation: 49.0 ± 3.8%; Fig. 3A). Flow-induced vasodilation was significantly reduced in TRPV4−/− mice (24.5 ± 4.5%). In contrast, responses to the endothelium-independent vasodilator papaverine were similar in WT and TRPV4−/− mice, with maximal dilations of 99.6 ± 0.3% and 98.5 ± 1.0%, respectively (Fig. 3B). Furthermore, there were no differences in contractile responses to U-46619 or high K+ between those animals, as previously reported (49).
Fig. 3.
Role of TRPV4 in flow-induced dilation of mouse mesenteric arteries. A: mesenteric arteries were cannulated and pressurized, and fluid flow was generated by an increasing pressure gradient ranging from 5 to 40 cmH2O. Flow-induced dilations were reduced in TRPV4−/− compared with WT mice. n = 6 mice. *P < 0.05 vs. WT. B: endothelium-independent relaxations in response to papaverine (100 μM) were similar between WT and TRPV4−/− mice. n = 6 mice.
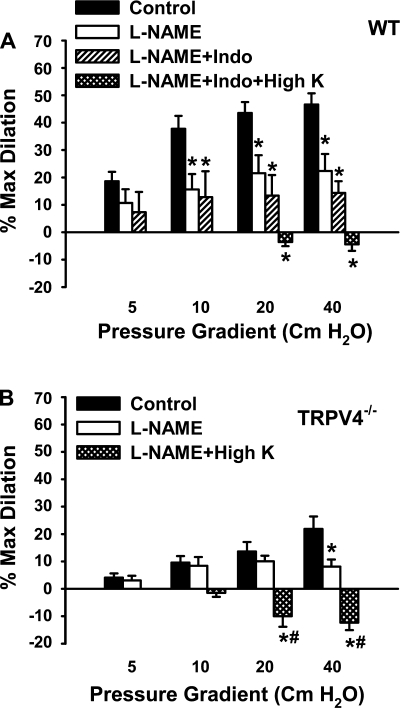
In WT mesenteric arteries, l-NAME markedly inhibited the dilator responses to flow (22.4 ± 6.2%). The addition of indomethacin, a cyclooxygenase inhibitor, did not produce further inhibition (14.4 ± 4.3%). The residual dilation in the presence of l-NAME and indomethacin was abolished by high K+ (−4.4 ± 2.4%; Fig. 4A). These results indicate that both NO and EDHF contribute to the flow-induced relaxation of mouse mesenteric arteries. Compared with WT controls, both the l-NAME- and high K+-sensitive components of relaxation responses to flow were reduced in TRPV4−/− mesenteric arteries (Fig. 4B).
Fig. 4.
Effects of l-NAME, indomethacin (Indo), and high K+ on flow-induced dilation of mouse mesenteric arteries. A: in WT animals, l-NAME (100 μM) inhibited flow-mediated dilations, and the addition of Indo had no further effect. The relaxations were blocked by l-NAME plus Indo and high K+. n = 6 mice. *P < 0.05 vs. control. B: in TRPV4−/− mice, flow-induced dilations were inhibited by l-NAME and converted to constrictions by l-NAME plus high K+. n = 6 mice. *P < 0.05 vs. control. #P < 0.05 vs. l-NAME.
We also examined the effects of TRPV4 inhibition on flow-mediated dilation of mesenteric arteries. Ruthenium red (10 μM) inhibited flow-mediated dilation in WT animals (12.9 ± 1.6% vs. 45.0 ± 5.2% of controls, P < 0.05, n = 6) but not in TRPV4−/− animals (20.6 ± 7.3% vs. 26.7 ± 7.0% of controls, n = 5).
TRPV4 mediates flow-induced Ca2+ influx in ECs.
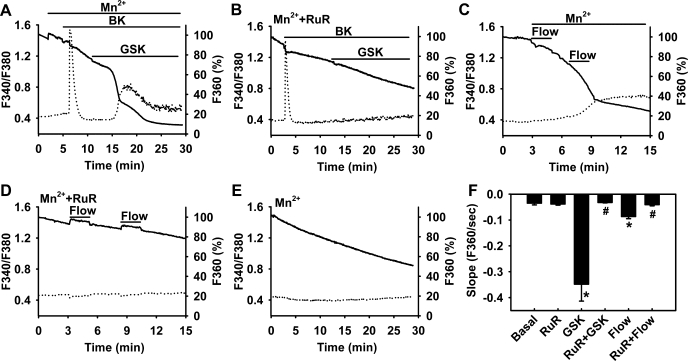
We directly measured TRPV4-mediated Ca2+ responses in HCAECs using the fura-2/Mn2+ quenching assay. By removing Ca2+ in the extracellular buffer, this assay allows simultaneous measurements of Ca2+ entry from the extracellular space and Ca2+ release from intracellular stores. As shown in Fig. 5A, GSK1016790A (5 nM) induced a robust Ca2+ influx in ECs (rate of Mn2+ quench: 0.348 ± 0.065 vs. 0.035 ± 0.007 under basal conditions). Preincubation of cells with ruthenium red (2 μM) eliminated GSK1016790A-induced Ca2+ influx (0.032 ± 0.001; Fig. 5B). Ruthenium red had no significant effect on either basal Ca2+ influx (0.038 ± 0.004) or the Ca2+ release from the endoplasmic reticulum in response to bradykinin (increase in F340/F380 above baseline: 0.53 ± 0.13 vs. 0.49 ± 0.07 of controls). Using a parallel-plate flow chamber, we examined the Ca2+ response to shear stress in HCAECs. Similar to TRPV4 agonists, fluid shear stress (10 dyn/cm2) induced a rapid Ca2+ influx (0.087 ± 0.008; Fig. 5C) that was largely abolished by ruthenium red (0.040 ± 0.004; Fig. 5D). TRPV4 agonists and flow also induced a Ca2+-release transient as indicated by an increase in F340/F380. However, the mechanisms for this Ca2+ release remain unclear because the onset of this transient was much slower than that activated by typical chemical agonists like bradykinin.
Fig. 5.
Role of TRPV4 in flow-induced Ca2+ entry in ECs. Human coronary artery ECs (HCAECs) were loaded with fura-2, and the Mn2+ quenching technique was used to measure Ca2+ influx [fluorescence at 360-nm excitation (F360); solid line] along with Ca2+ release [ratio of fluorescence at 340- to 380-nm excitation (F340/F380); dotted line]. GSK (5 nM) induced marked Ca2+ influx (A), and this response was inhibited by RuR (2 μM; B). In contrast, bradykinin (BK; 1 μM) elicited a rapid Ca2+ release that was accompanied with smaller Ca2+ influx. Similar to GSK, fluid flow (10 dyn/cm2) induced rapid Ca2+ influx (C), which was inhibited by the TRPV4 blocker RuR (D). E: baseline Ca2+ influx. F: summarized data. n = 3–6 independent experiments (20–30 ECs/assay). *P < 0.05 vs. basal; #P < 0.05 vs. GSK or flow.
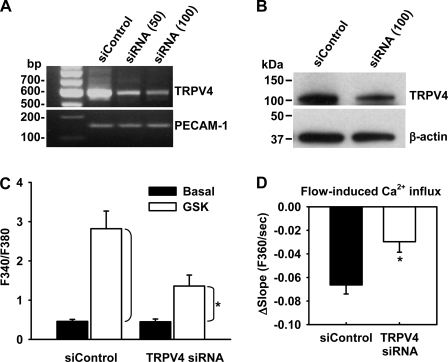
To further confirm that TRPV4 channels are involved in shear-induced Ca2+ entry in ECs, we used an RNA inhibition approach to more specifically target TRPV4 channel function. TRPV4-specific siRNA knocked down mRNA and protein expressions of TRPV4 in HCAECs by ≈80% and 60–70%, respectively, compared with nontargeting control siRNA (Fig. 6, A and B). TRPV4 siRNA did not affect the expression of PECAM-1 or β-actin. The functional knockdown of TRPV4 channels in ECs was confirmed with the fura-2 Ca2+ assay (Fig. 6C). Compared with siControl, TRPV4 siRNA markedly inhibited [Ca2+]i increases in HCAECs in response to the TRPV4 agonist GSK1016790A (5 nM, changes of F340/F380 over baseline: 0.90 ± 0.25 vs. 2.36 ± 0.45 of controls). Importantly, shear stress (10 dyn/cm2)-induced Ca2+ influx in these ECs was also significantly inhibited by TRPV4 siRNA (changes of the slope of fura-2/Mn2+ quenching over baseline: 0.030 ± 0.009 vs. 0.066 ± 0.008 of controls; Fig. 6D).
Fig. 6.
Effect of TRPV4 short interfering (si)RNA on flow-induced Ca2+ entry in ECs. A: RT-PCR analysis of TRPV4 mRNA levels in HCAECs treated with nontargeting siControl (100 nM) or TRPV4-specific siRNA (50–100 nM). B: Western blot analysis of TRPV4 protein expression in HCAECs treated with siControl (100 nM) or TRPV4 siRNA (100 nM). PECAM-1 and β-actin were used as loading controls for RT-PCR and Western blot analysis, respectively. Data are representative of 3–4 independent experiments. C: TRPV4 siRNA reduced cytosolic Ca2+ increases in response to the TRPV4 agonist GSK (5 nM) in fura-2-loaded HCAECs. n = 6. *P < 0.05 vs. siControl. D: TRPV4 siRNA inhibited flow (10 dyn/cm2)-induced Ca2+ influx in HCAECs as indicated by the fura-2/Mn2+ quenching assay. Data are presented as changes of the slope of fura-2/Mn2+ quenching over baseline; n = 4. *P < 0.05 vs. siControl.
We could not use fura-2/Mn2+ quenching assay to study flow-induced Ca2+ responses in ECs in situ of small mesenteric arteries, since these arteries exhibited significant Ca2+ influx under basal conditions.
Flow-induced Ca2+ response in HEK-293 cells overexpressing TRPV4 channels.
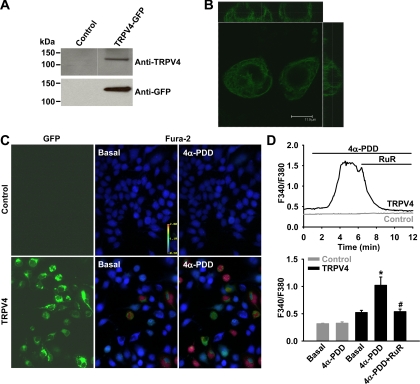
To further confirm the role of TRPV4 channels in flow-induced Ca2+ signaling, HEK-293 cells were transiently transfected with a human TRPV4 transgene tagged with GFP. The expression of TRPV4 proteins in transfected cells was confirmed by Western blot analysis (Fig. 7A). Confocal microscopic imaging analysis revealed plasma membrane localization of TRPV4 channels in transfected cells (Fig. 7B). The TRPV4-mediated Ca2+ response was studied in control and transfected HEK-293 cells (Fig. 7, C and D). Treatment of cells with 4α-PDD (5 μM), a TRPV4 channel opener, caused an increase in [Ca2+]i in TRPV4-transfected cells (F340/F380: 1.02 ± 0.15 vs. 0.52 ± 0.04 under basal conditions). This Ca2+ response was elicited in ∼50–80% of GFP-positive transfected cells. The 4α-PDD response was reversed by the addition of ruthenium red (2 μM, 0.54 ± 0.05). 4α-PDD had no significant effect on [Ca2+]i in nontransfected cells (0.33 ± 0.03 vs. 0.32 ± 0.01 under basal conditions).
Fig. 7.
Expression of functional TRPV4 channels in human embryonice kidney (HEK)-293 cells. Cells were transiently transfected with a mammalian expression vector encoding human TRPV4 with a COOH-terminal green fluorescent protein (GFP) tag. Expression of TRPV4-GFP fusion proteins was confirmed by Western blot analysis with antibodies against both TRPV4 and GFP (A) and by confocal fluorescence microscopy (B). In transfected cells, the TRPV4 agonist 4α-phorbol-12,13-didecanoate (4α-PDD; 5 μM) induced a marked increase in [Ca2+]i, whereas 4α-PDD had no significant effect in nontransfected cells (C). D: representative traces of Ca2+ responses showing that the 4α-PDD-induced Ca2+ increase was subsequently reversed by RuR (2 μM) in cells expressing TRPV4-GFP fusion proteins (top). Bottom, summarized data of Ca2+ responses to 4α-PDD. n = 3 independent experiments (20–30 cells/assay). *P < 0.05 vs. basal; #P < 0.05 vs. 4α-PDD.
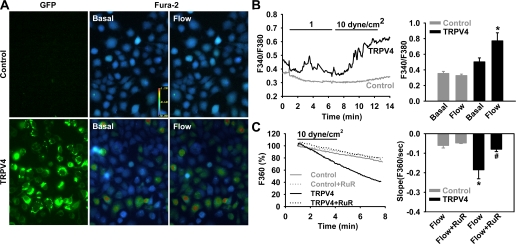
To determine whether flow induces the opening of TRPV4, we examined Ca2+ responses in HEK-293 cells exposed to flow (Fig. 8, A and B). Control HEK-293 cells showed no increase in [Ca2+]i when exposed to flow. However, flow elicited a shear rate-dependent increase in [Ca2+]i in TRPV4-transfected cells, with F340/F380 increasing from 0.50 ± 0.05 to 0.77 ± 0.10 at a shear stress of 10 dyn/cm2. To determined whether the increase in [Ca2+]i was due to an increase in Ca2+ influx, the Mn2+ quenching technique was used. Compared with control HEK-293 cells, transfected cells exhibited a marked increase in shear stress-induced Ca2+ entry (rate of Mn2+ quench: 0.19 ± 0.04 vs. 0.06 ± 0.01 in nontransfected cells), which was blocked by ruthenium red (0.08 ± 0.01; Fig. 8C), confirming a transmembrane flux of Ca2+ into the cell. TRPV4-transfected HEK-293 cells exhibited a similar time course of flow-induced Ca2+ response as that of native ECs (Fig. 5C).
Fig. 8.
Flow-induced Ca2+ response in HEK-293 cells expressing TRPV4 channels. A: fluid flow (10 dyn/cm2) elicited a rapid increase in [Ca2+]i in TRPV4-transfected cells but not in nontransfected controls. B, left: representative traces of Ca2+ responses to flow. Right, summarized data. n = 6 independent experiments (20–30 cells/assay). *P < 0.05 vs. basal. C: compared with nontransfected cells, HEK-293 cells expressing TRPV4 channels exhibited a marked increase in Ca2+ influx in response to flow. This increase was blocked by RuR (2 μM). n = 3–5 independent experiments (20–30 cells/assay). *P < 0.05 vs. control; #P < 0.05 vs. flow.
DISCUSSION
This study provides several new findings regarding the role of TRPV4 channels in shear stress-induced endothelial Ca2+ entry and the potential link between this signaling response and the relaxation of small resistance arteries. First, TRPV4 channels are predominantly expressed in vascular ECs of mouse small mesenteric arteries, and TRPV4 activation induces endothelial Ca2+ entry and vasodilation. Second, genetic deletion of TRPV4 channels inhibits NO- and EDHF-dependent relaxation of small mesenteric arteries in response to flow. Third, blockade of TRPV4 channels by pharmacological inhibitors or gene-specific siRNA markedly inhibits shear stress-induced Ca2+ influx in ECs. Finally, in HEK-293 cells, which are normally unresponsive to flow, transfection of TRPV4 channels induces shear stress-dependent Ca2+ entry with a time course similar to that of ECs. Taken together, these data suggest that the TRPV4 channel is a potential candidate of mechanosensitive channels responsible for flow-induced Ca2+ entry in ECs. The activation of this TRPV4-mediated Ca2+ signaling may ultimately lead to the release of endothelial relaxing factors and subsequent vasodilation.
Ca2+ entry has been thought to be involved in endothelial signal transduction in response to shear stress (9). However, the molecular identity of the specific channel(s) involved in shear signaling remains largely unknown. In the present study, we found that shear stress induced rapid Ca2+ influx in ECs, which was inhibited by the TRPV4 channel blocker and TRPV4-specific siRNA. TRPV4 opening also resulted in robust Ca2+ entry in ECs and the release of endothelial relaxing factors. In addition, flow-induced Ca2+ entry was demonstrated in HEK-293 cells transiently transfected with TRPV4 channels but not in control untransfected cells. The time course of Ca2+ responses to shear was similar in ECs and TRPV4-transfected HEK-293 cells. These results indicate that the TRPV4 channel may serve as shear sensor/transducer through which shear stress induces intracellular Ca2+ signaling and the subsequent release of vasodilator factors in ECs.
Previous studies (10, 30, 49) have indicate that in mouse mesenteric arteries, both NO- and EDHF-dependent mechanisms contribute to the relaxation responses induced by chemical agonists. Typically, dilation is initiated by an increase in intracellular Ca2+ in ECs. In accordance with these findings, we found that flow-induced dilation is also mediated by NO- and EDHF-dependent mechanisms in these arteries. Compared with WT mice, both l-NAME- and K+-sensitive components of relaxation responses were reduced in TRPV4−/− mice. This suggests that the TRPV4 channel, by regulating Ca2+ entry into the cell, mediates a portion of the flow-induced relaxation responses that involves both NO and EDHF components. This notion is also supported by the observation that the TRPV4 agonist GSK1016790A induced a potent endothelium-dependent relaxation of small mesenteric arteries that was reduced by l-NAME and abolished by the combination of l-NAME and high K+. Activation of TRPV4 channels has also been reported to induce NO- and/or EDHF-dependent vasodilation in rat carotid and gracilis arteries and in mouse carotid arteries (21, 24). The specific EDHF(s) involved in flow-induced dilation of mouse mesenteric arteries remains to be identified in future studies. In this vascular bed, H2O2 has been proposed as an EDHF for chemical agonist-induced dilation (30).
In mesenteric arteries, we observed a moderate vasoconstriction (∼20%) to the TRPV4 agonist in the presence of l-NAME and high K+. The constriction to the TRPV4 agonist was absent in denuded vessels, indicating that the response is endothelial dependent. It is therefore possible that some vasoconstrictor factor(s) are released from ECs, but their vasomotor effects are evident only in the absence of endothelial vasodilator factors.
Immunohistochemical analysis has indicated that TRPV4 channels are mainly expressed in the endothelium of mouse mesenteric arteries, similar to those previously reported in conduit vessels such as mouse carotid arteries (27, 43, 49). This expression pattern is consistent with the results of the RT-PCR analysis showing the expression of TRPV4 mRNA in ECs but not in smooth muscle cells. Additionally, no significant vasomotor responses to TRPV4 agonists were observed in denuded mouse small mesenteric arteries, suggesting that the potential contribution of smooth muscle TRPV4 to the overall vascular response is minimal in this vascular bed. Earley et al. (13) recently described a TRPV4-like current activated by 11,12-EET in mesenteric artery myocytes from WT but not TRPV4−/− mice. The reasons for this discrepancy remain unclear. The expression of TRPV4 at either the protein or transcript level in those myocytes and channel activity in response to TRPV4 agonists/blockers have not been examined in that study, and therefore the molecular identities of these TRPV4-like currents may require further characterization. It is of note, however, that the expression of TRPV4 channels has been reported in vascular smooth muscle cells of several vascular beds such as cerebral and pulmonary arteries (12, 29, 45).
The shear-induced Ca2+ response (18, 22, 23, 34, 35) and Ca2+-dependent vasodilation (5, 8, 21) have not been observed in all endothelial cultures and/or vascular beds (2, 11, 32). In particular, Muller et al. (32) reported that in rabbit coronary arterioles, shear stress induces only a small increase in ECs and that clamping of EC [Ca2+]i using BAPTA-AM inhibits dilations to acetylcholine and substance P but does not significantly affect shear stress-induced vasodilation, indicating that a substantial component of the shear stress-induced response occurs through a Ca2+-insensitive pathway. The reasons for these differences regarding the Ca2+ dependency of endothelial shear responses remain unresolved but may involve methodological or species differences. For example, fura-2 has often been used to measure the endothelial Ca2+ response to shear in previous studies, but this probe is less sensitive to localized Ca2+ influx, which most likely occurs during shear. Therefore, a more sensitive method (e.g., Mn2+ quenching assay) (4) may be needed to detect this physiological important Ca2+ influx. Additionally, BAPTA-AM at commonly used concentrations (10–20 μM) seems less effective in buffering near membrane Ca2+ entry from the extracellular space compared with Ca2+ release from intracellular stores (3, 7), although the precise mechanisms are unknown. BAPTA-AM at higher concentrations could be used to examine the role of localized Ca2+ in endothelial shear responses, but higher concentrations are often associated with greater cell toxicity by disrupting intracellular Ca2+ homeostasis. Finally, TRPV4-mediated Ca2+ entry may play a more important role in those smaller arteries such as mouse small mesenteric arteries where flow-mediated dilation involves a significant EDHF-mediated component. It has been reported that the [Ca2+]i threshold is higher for EDHF-dependent dilation than for NO-dependent responses (28). Interestingly, there is still ≈50% of flow-induced dilation in TRPV4−/− mice. The remaining dilation may be mediated by some Ca2+-independent mechanisms such as protein phosphorylation, as previously reported (2).
The mechanisms by which shear stress activates the TRPV4 channel in ECs remain unclear. As previously described, arachidonic acid and its derivatives, such as EETs, have been suggested to act as endogenous ligands of TRPV4 channels (39, 41). In mouse carotid arteries, flow-induced dilation is attenuated by the inhibitors of phospholipase A2 and P-450 epoxygenase, two key enzymes in arachidonic acid metabolism and signaling (24, 27). Alternatively, TRPV4 activation and Ca2+ entry may lead to the production of arachidonic acid metabolites. Shear-induced activation of TRPV4 channels might involve tyrosine phosphorylation, as has been suggested for the channel activation in response to hypotonic stress (44). Further indepth analysis of shear-induced Ca2+ entry and currents in native ECs and/or heterologous overexpression systems might provide valuable insights into the mechanisms of TRPV4 activation by shear stress or other mechanical stimuli.
In summary, the present study provides evidence that TRPV4 channels are involved in shear stress-sensitive Ca2+ influx in ECs and flow-mediated dilation of small resistance arteries. Given the predominant expression of these channels in ECs and the potent stimulation of endothelial relaxing factor release in response to channel activation in both resistance and conduit arteries from a variety of species, TRPV4-mediated signaling may serve as a conserved mechanism contributing to the regulation of vascular tone and other endothelial functions and, therefore, may have important implications for vascular health as well as in diseases such as hypertension and atherosclerosis.
GRANTS
This work was supported in part by American Heart Association Grant 0830042N (to D. X. Zhang) and by National Heart, Lung, and Blood Institute Grants R01-HL080704 and R01-HL094971 (to D. D. Gutterman) and R01-HL68138 (to D. A.Wilcox).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank GlaxoSmithKline Pharmaceuticals for generously providing us with GSK1016790A.
REFERENCES
- 1.Alonso MT, Sanchez A, Garcia-Sancho J. Arachidonic acid-induced calcium influx in human platelets. Comparison with the effect of thrombin. Biochem J 272: 435–443, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayajiki K, Kindermann M, Hecker M, Fleming I, Busse R. Intracellular pH and tyrosine phosphorylation but not calcium determine shear stress induced nitric oxide production in native endothelial cells. Circ Res 78: 750–758, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Basset O, Boittin FX, Dorchies OM, Chatton JY, van Breemen C, Ruegg UT. Involvement of inositol 1,4,5-trisphosphate in nicotinic calcium responses in dystrophic myotubes assessed by near-plasma membrane calcium measurement. J Biol Chem 279: 47092–47100, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, Sturek M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol 294: H2489–H2496, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension 17: 187–193, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Busse R, Fleming I. Regulation of endothelium-derived vasoactive autacoid production by hemodynamic forces. Trends Pharmacol Sci 24: 24–29, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Church JE, Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem 281: 1477–1488, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Cooke JP, Rossitch E, Jr, Andon NA, Loscalzo J, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest 88: 1663–1671, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev 75: 519–560, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding H, Kubes P, Triggle C. Potassium- and acetylcholine-induced vasorelaxation in mice lacking endothelial nitric oxide synthase. Br J Pharmacol 129: 1194–1200, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dull RO, Davies PF. Flow modulation of agonist (ATP)-response (Ca2+) coupling in vascular endothelial cells. Am J Physiol Heart Circ Physiol 261: H149–H154, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97:1270–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol 297: H1096–H1102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 26: 1215–1225, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Frangos JA, McIntire LV, Eskin SG. Shear induced stimulation of mammalian cell metabolism. Biotechnol Bioeng 32: 1053–1060, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Gao X, Wu L, O'Neil RG. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem 278: 27129–27137, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Gauthier KM, Liu C, Popovic A, Albarwani S, Rusch NJ. Freshly isolated bovine coronary endothelial cells do not express the BKCa channel gene. J Physiol 545: 829–836, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geiger RV, Berk BC, Alexander RW, Nerem RM. Flow-induced calcium transients in single endothelial cells: spatial and temporal analysis. Am J Physiol Cell Physiol 262: C1411–C1417, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22: 6408–6414, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallam TJ, Jacob R, Merritt JE. Influx of bivalent cations can be independent of receptor stimulation in human endothelial cells. Biochem J 259: 125–129, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Köhler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS ONE 2: e827, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helmlinger G, Berk BC, Nerem RM. Pulsatile and steady flow-induced calcium oscillations in single cultured endothelial cells. J Vasc Res 33: 360–369, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Hong D, Jaron D, Buerk DG, Barbee KA. Heterogeneous response of microvascular endothelial cells to shear stress. Am J Physiol Heart Circ Physiol 290: H2498–H2508, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Köhler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 26: 1495–1502, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol 259: H1063–H1070, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res 80: 445–452, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Marrelli SP. Mechanisms of endothelial P2Y1- and P2Y2-mediated vasodilatation involve differential [Ca2+]i responses. Am J Physiol Heart Circ Physiol 281: H1759–H1766, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Marrelli SP, O'Neil RG, Brown RC, Bryan RM., Jr PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol 292: H1390–H1397, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106: 1521–1530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+ channels. Circulation 103: 1992–1998, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Muller JM, Davis MJ, Kuo L, Chilian WM. Changes in coronary endothelial cell Ca2+ concentration during shear stress- and agonist-induced vasodilation. Am J Physiol Heart Circ Physiol 276: H1706–H1714, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117: 1065–1074, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Schwarz G, Callewaert G, Droogmans G, Nilius B. Shear stress-induced calcium transients in endothelial cells from human umbilical cord veins. J Physiol 458: 527–538, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen J, Luscinskas FW, Connolly A, Dewey CF, Jr, Gimbrone MA., Jr Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. Am J Physiol Cell Physiol 262: C384–C390, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2: 695–702, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278: 22664–22668, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res 104: 1123–1130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2+ permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res 97: 908–915, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277: 13569–13577, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277: 47044–47051, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis R, Xu X. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: part 2. J Pharmacol Exp Ther 326: 443–452, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Xu H, Zhao H, Tian W, Yoshida K, Roullet JB, Cohen DM. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J Biol Chem 278: 11520–11527, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L1267–L1276, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res 97: 853–863, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Yu JZ, Zhang DX, Zou AP, Campbell WB, Li PL. Nitric oxide inhibits Ca2+ mobilization through cADP-ribose signaling in coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 279: H873–H881, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Zhang DX, Gauthier KM, Chawengsub Y, Campbell WB. ACh-induced relaxations of rabbit small mesenteric arteries: role of arachidonic acid metabolites and K+. Am J Physiol Heart Circ Physiol 293: H152–H159, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 53: 532–538, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang DX, Yi FX, Zou AP, Li PL. Role of ceramide in TNF-α-induced impairment of endothelium-dependent vasorelaxation in coronary arteries. Am J Physiol Heart Circ Physiol 283: H1785–H1794, 2002 [DOI] [PubMed] [Google Scholar]