Abstract
We have previously reported gender differences in ventricular remodeling and development of heart failure using the aortocaval fistula model of chronic volume overload in rats. In contrast to males, female rats exhibited no adverse ventricular remodeling and less mortality in response to volume overload. This gender-specific cardioprotection was lost following ovariectomy and was partially restored using estrogen replacement. However, it is not known if estrogen treatment would be as effective in males. The purpose of this study was to evaluate the structural and functional effects of estrogen in male rats subjected to chronic volume overload. Four groups of male rats were studied at 3 days and 8 wk postsurgery as follows: fistula and sham-operated controls, with and without estrogen treatment. Biochemical and histological studies were performed at 3 days postsurgery, with chronic structural and functional effects studied at 8 wk. Measurement of systolic and diastolic pressure-volume relationships was obtained using a blood-perfused isolated heart preparation. Both fistula groups developed significant ventricular hypertrophy after 8 wk of volume overload. Untreated rats with fistula exhibited extensive ventricular dilatation, which was coupled with a loss of systolic function. Estrogen attenuated left ventricular dilatation and maintained function in treated rats. Estrogen treatment was also associated with a reduction in oxidative stress and circulating endothelin-1 levels, as well as prevention of matrix metalloproteinase-2 and -9 activation and breakdown of ventricular collagen in the early stage of remodeling. These data demonstrate that estrogen attenuates ventricular remodeling and disease progression in male rats subjected to chronic volume overload.
Keywords: cardiac function, heart failure, hypertrophy, aortocaval fistula, hormone, gender, contractility
we have reported gender differences in the development of heart failure using the aortocaval fistula rat model of chronic volume overload (16). In this previous study, male rats experienced 25% mortality following 8 wk of volume overload associated with marked increases in left ventricular (LV) volume and compliance. In contrast, female rats had only 2% mortality with no dilatation or increase in ventricular compliance. Furthermore, this apparent cardioprotection, which was lost following ovariectomy, was partially restored by estrogen replacement (17). These findings demonstrate that, in contrast to males, intact, cycling female rats are able to successfully compensate for the increased myocardial stress associated with chronic volume overload and that this cardioprotection is largely due to ovarian-derived estrogen.
With regard to the mechanisms contributing to ventricular dilatation in this model of cardiac volume overload, we have demonstrated that cardiac mast cells play a causal role in the progressive adverse ventricular remodeling (8, 22). During the acute phase of volume overload, increased cardiac mast cell density is associated with matrix metalloproteinase (MMP) activation and myocardial collagen degradation. Prevention of these acute cellular and extracellular events using agents that inhibit mast cell degranulation provides protection against chronic ventricular dilatation and dysfunction (10). Blockade of endothelin (ET)-1 or tumor necrosis factor (TNF)-α, factors involved in mast cell signaling, provides similar cardioprotection (23, 31, 32). Furthermore, we found that estrogen supplementation following ovariectomy prevents the degradation of collagen associated with cardiac mast cell activation, suggesting that estrogen alters cardiac mast cell phenotype, thereby preventing activation of MMPs (11). However, it is not known if estrogen treatment of males subjected to chronic volume overload will confer the same degree of cardioprotection as found in ovariectomized females. Accordingly, the purpose of this study was to evaluate the structural and functional effects of estrogen treatment in male rats subjected to chronic volume overload and to investigate the mechanisms involved in the acute phase of remodeling.
METHODS
Studies were performed using 8-wk-old male Sprague-Dawley (Hsd:SD) rats that were housed under standard environmental conditions and maintained on commercial rat chow and tap water ad libitum. All studies conformed to the principles of the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of South Carolina's Institutional Animal Care and Use Committee. Isoflurane was used for anesthesia (4% induction/2.5% maintenance, balance oxygen). Postoperative analgesia was provided by buprenorphine hydrochloride (0.025 mg/kg sc) administered to the rats immediately postsurgery. At the experimental endpoints, animals were anesthetized with pentobarbital sodium (50 mg/kg ip), and hearts were removed under deep anesthesia.
Surgical Procedures
Induction of chronic volume overload.
Infrarenal aortocaval fistula was created in rats as previously described (17). Briefly, a ventral abdominal laparotomy was performed to expose the aorta and caudal vena cava. An 18-gauge needle was inserted in the exposed ventral abdominal aorta below the renal arteries and advanced through the medial wall into the vena cava to create the fistula. The needle was withdrawn, and the ventral aortic puncture site was sealed with cyanoacrylate. Creation of a successful fistula was visually evident by the pulsatile flow of oxygenated blood in the vena cava.
Estrogen treatment.
17β-Estradiol, hereafter referred to as estrogen, was delivered via time release pellets (Innovative Research of America, Sarasota, FL). Pellets, which administered a dose of 0.02 mg·kg−1·day−1, were implanted subcutaneously 3 days before creation of the fistula. This dose produces serum levels of ∼35 pg/ml, a level comparable to the average estrogen level in intact female rats (17).
Experimental Protocol
The protective effects of estrogen on chronic remodeling induced by 8 wk of volume overload were studied in three age-matched groups: sham-operated controls (Sham), fistula-induced chronic volume overload (Fist), and fistula treated with estrogen (Fist + Est). In addition, the effects of estrogen on the response to 3 days of volume overload were studied in similar groups, including an additional group of surgical controls treated with estrogen (Sham + Est). This acute time point was examined based on our previous characterization of this model demonstrating significant increases in cardiac mast cell density and MMP activity following the induction of volume overload. These changes in mast cell density and MMP activity resulted in significant collagen degradation during the first 3–5 days of volume overload in untreated male rats (8, 9).
At the experimental endpoint, each rat was weighed and anesthetized. Following laparotomy, fistula patency was visually confirmed. In the 8-wk groups, a tracheotomy was performed, and rats were attached to a ventilator, and, following thoracotomy to expose the heart, cardiac output was measured using a Doppler flow probe (model 3SB; Transonic Systems, Ithaca, NY). The heart was then isolated for the assessment of LV size and function as described below. After completion of the functional studies, the atria and great vessels were removed, and the LV, including septum and right ventricle (RV), were separated and weighed. Lung wet weight was measured after the esophagus and trachea were trimmed away and the pleural surface was blotted dry.
Assessment of Ventricular Size and Function
LV volume and function were evaluated ex vivo using a blood-perfused isolated heart preparation as previously described (17). Before removal of the heart from the anesthetized rat, the carotid arteries were ligated, and a cannula was inserted into the thoracic aorta and secured with a silk ligature. Retrograde perfusion of the coronary arteries with blood from the perfusion reservoir was begun as soon as the cannula was secured. The heart was then quickly removed from the chest and attached to the apparatus. Arterial blood from the carotid artery of a support rat was pumped to a pressurized reservoir for retrograde perfusion of the isolated heart. A constant coronary perfusion pressure of 100 ± 5 mmHg was maintained. The coronary venous effluent was collected and returned to the support rat through a jugular vein catheter. LV pressure was continuously recorded using a pressure transducer attached to a latex balloon inserted through the mitral valve orifice into the LV chamber. Once the heart developed stable isovolumetric contractions, the balloon volume that produced an LV end diastolic pressure (EDP) of 0 mmHg, V0, was determined. Balloon volume was then increased by 5- to 20-μl increments until an LVEDP of 25 mmHg was attained. The balloon was then emptied, and this procedure was repeated to obtain a minimum of three consistent runs.
Analysis of the Collagen Matrix
Paraffin-embedded blocks were prepared from midventricular sections of LV. Sections (5 μm) were stained with collagen specific picrosirius red. Photomicrographs of 20 random regions, avoiding perivascular collagen, were obtained using a Zeiss Axiovert 200 fluorescent microscope with a ×40 objective. Collagen volume fraction (CVF) was determined from these images using ImageProPlus 4.0.
Oxidative Stress Assay
The extent of lipid peroxidation, a byproduct of oxidative stress, was determined by malondialdehyde (MDA) analysis. As previously described, the reaction of lipid peroxides with thiobarbituric acid is a sensitive assay for lipid peroxidation in tissue (34).
LV Mast Cell Density
Sections of formalin-fixed LV tissue were stained with pinacyanol erythrosinate (PE). PE selectively stains and differentiates mast cells (5). The total mast cell population in the LV section was counted and divided by the tissue area obtained from a digitized image of the section to yield the number of mast cells per square millimeter.
ET-1 and TNF-α Assay
Serum levels of ET-1 and TNF-α were determined using commercially available ELISA immunoassay kits [Alpco Diagnostics (Salem, NH) and R&D Systems (Minneapolis, MN), respectively].
Zymography Assay for MMP Activity
From each sample, 30 μg of proteins were loaded for electrophoresis using nonreducing conditions on an 8% SDS-PAGE containing gelatin (1 mg/ml from porcine skin; Sigma Chemicals, St. Louis, MO). After electrophoresis, the gels were washed two times in 2.5% Triton X-100 for 30 min each at room temperature, rinsed in water, and incubated for 18 h in a substrate buffer at 37°C (50 mmol/l Tris·HCl, 0.15 mol/l NaCl, 5.0 mmol/l CaCl2, and 0.05% Brij 35, pH 7.5). After the incubation period, the gels were stained using 0.5% Coomassie Brilliant Blue R-250 and destained with 40% methanol and 10% acetic acid. Molecular weights were determined using standard prestained molecular weight markers (Bio-Rad). Gelatinolytic bands were measured with an image analyzer (VersaDoc; Bio-Rad), and MMP-2 and MMP-9 activities were determined from the lytic bands corresponding to their activated forms (62 and 82 kDa, respectively). Gels were run in duplicate, and an extract from a single Sham control heart was used as a standard on all gels for comparison between gels. The activity of lytic bands on each gel was normalized to this common control. Once normalized, the activities from each group were averaged, and the Sham group was set to 100%.
Data and Statistical Analyses
The EDP and peak isovolumetric pressure were determined following each increment in balloon volume (ADInstruments Chart version 5). Further processing and statistical analyses were performed using analysis software (Graphpad 5.0; Prism). LV volumes were adjusted to account for the volume displaced by the balloon material. Pressure-volume (P-V) relations generated for the balloon alone indicated that the balloon's contribution to LVEDP was negligible over the experimental LV volume range. Diastolic LV P-V data were fit to a third-order polynomial regression, and V0 for each P-V curve was calculated. Using this regression equation, LV end diastolic volumes were calculated at intervals of 2.5 mmHg LVEDP to graph average P-V relationships for each group. Systolic LV P-V data were fit using linear regression, and the slopes of these relations were used as indexes of intrinsic chamber contractility. Linear relations were statistically compared by analysis of covariance. Grouped data comparisons were made by one-way ANOVA, and comparison of discrete data points on nonlinear relations were analyzed using two-way ANOVA. When a significant F ratio (P < 0.05) was obtained, intergroup comparisons were made using a modified t-test and Bonferroni bounds. Statistical significance was taken to be P < 0.05/k, where k is equal to the number of groups compared (29).
RESULTS
Acute Studies (3 Days of Volume Overload)
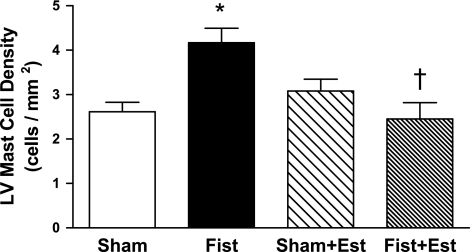
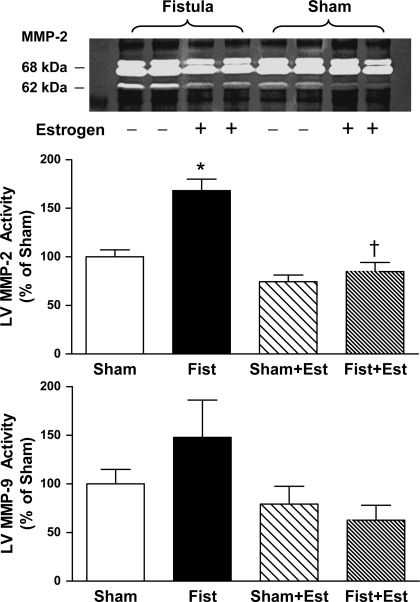
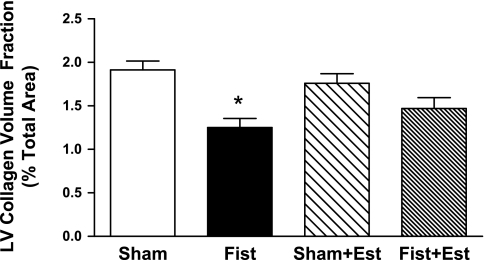
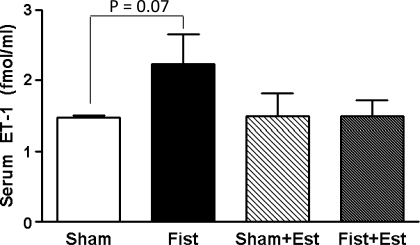
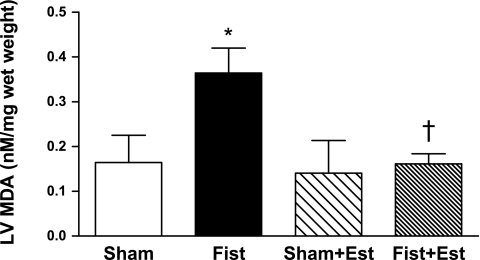
Patent fistulas were visually confirmed in the short-term rats before tissue collection. LV mast cell density was significantly increased by 60% in the untreated Fist group (4.2 ± 0.7 cells/mm2, P < 0.05 vs. Sham; Fig. 1). Estrogen treatment prevented the fistula-induced increase in cardiac mast cell density but did not significantly alter mast cell density in the Sham hearts. The increase in cardiac mast cell density in untreated Fist hearts was associated with a concomitant increase in MMP-2 activity (62 kDa band, 68% increase vs. Sham, P < 0.05; Fig. 2). MMP-2 activation postfistula was prevented by estrogen [Fist + Est vs. Sham, P = not significant (NS)]. Furthermore, estrogen did not significantly alter latent MMP-2 levels in any group (data not shown). MMP-9 activity was more variable within each group, and the increase in the untreated Fist group did not achieve statistical significance. However, a direct comparison of the untreated and treated fistula groups (i.e., Fist vs. Fist + Est) indicates a substantial reduction in MMP-9 activity by estrogen. The increase of MMP activity in the untreated Fist group was associated with a reduction in LV CVF (34% decrease vs. Sham, P < 0.05; Figs. 3 and 4). Estrogen treatment did not affect CVF in Sham hearts but did attenuate the fistula-induced reduction in the treated Fist + Est group (P = NS vs. Sham). In all of the acute study groups, serum levels of TNF-α were not detectible (<5 pg/ml, data not shown). Serum ET-1 levels (Fig. 5) were increased in the untreated Fist group (51% increase vs. Sham, P = 0.07) and unchanged in the Fist + Est group relative to Sham (P = 0.47). Figure 6 depicts the extent of LV lipid peroxidation in each group, as measured by MDA concentration. Volume overload produced a significant degree of cardiac oxidant stress (122% increase vs. Sham, P < 0.05) that was completely prevented by estrogen treatment. Estrogen did not alter oxidative stress levels in treated Shams.
Fig. 1.
Left ventricular (LV) mast cell density at 3 days postsurgery as determined by pinacyanol erythrosinate (PE) staining. Sham, sham-operated control; Fist, fistula; Est, estrogen treated. P < 0.05 vs. Sham (*) and vs. Fist (†).
Fig. 2.
LV matrix metalloproteinase (MMP)-2 and MMP-9 activity at 3 days postsurgery. Note that the pictured zymogram was intentionally overloaded to clearly illustrate the differences in the MMP-2 active band at 62 kDa. The overloaded zymogram was not used for calculation of MMP activity. MMP-9 bands are not pictured. P < 0.05 vs. Sham (*) and vs. Fist (†).
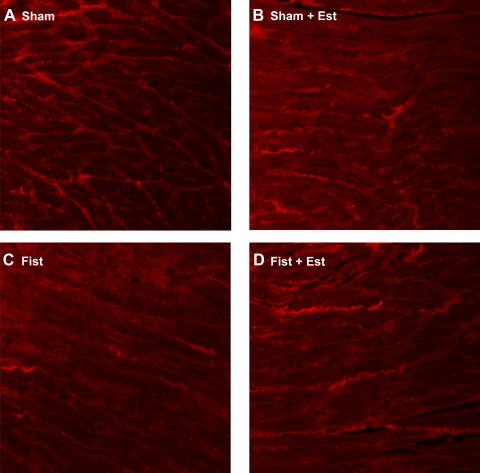
Fig. 3.
Representative picrosirius red (PSR) staining of midventricular sections from each group at 3 days postsurgery. A: Sham; B: Sham + Est; C: Fist; D: Fist + Est.
Fig. 4.
LV collagen volume fraction at 3 days postsurgery as determined by PSR staining of midventricular sections. *P < 0.05 vs. Sham.
Fig. 5.
Serum endothelin-1 (ET-1) levels at 3 days postsurgery as determined by ELISA immunoassay. Differences were not significant, although levels of ET-1 were greatest in the untreated Fist group (P = 0.07 vs. Sham).
Fig. 6.
LV oxidative stress levels at 3 days postsurgery as determined by malondialdehyde (MDA) analysis of tissue extracts. P < 0.05 vs. Sham (*) and vs. Fist (†).
Chronic Studies (8 wk of Volume Overload)
Group-averaged cardiac output, LV, RV, and lung and body weights are listed in Table 1. Cardiac output was significantly increased 4- to 4.5-fold in both of the fistula groups, confirming both the patency of the fistula and equivalent degrees of volume overload. LV weights were significantly increased in both the Fist and Fist + Est groups (105 and 44%, respectively, relative to sham operated, P < 0.05). RV and lung weights were also significantly increased in both fistula groups relative to Sham controls. However, estrogen treatment significantly attenuated the increase in LV, RV, and lung weights postfistula vs. untreated fistula rats (P < 0.05). The estrogen treatment also reduced body weight (P < 0.05 relative to untreated Fist and Sham).
Table 1.
Group comparison of heart, lung, and body weights at 8 wk postsurgery
| Group | Body Wt, g | LV, mg | RV, mg | Lung, mg | CO, ml/min |
|---|---|---|---|---|---|
| Sham (n = 8) | 365 ± 19 | 824 ± 63 | 216 ± 39 | 1,493 ± 102 | 31 ± 3 |
| Fist (n = 9) | 415 ± 47* | 1,695 ± 286* | 558 ± 102* | 2,560 ± 522* | 144 ± 3* |
| Fist + Est (n = 6) | 267 ± 16*† | 1,184 ± 165*† | 342 ± 29*† | 1,656 ± 198† | 133 ± 2* |
Values are means ± SD, except for cardiac output, which are means ± SE; n, no. of rats. LV, left ventricle; RV, right ventricle; CO, cardiac output; Fist, fistula; Est, estradiol.
P < 0.05 vs. Sham (*) and
P < 0.05 vs. Fist (†).
Functional analysis.
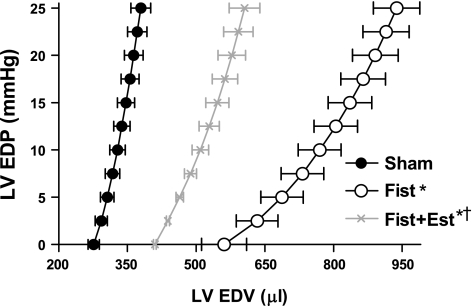
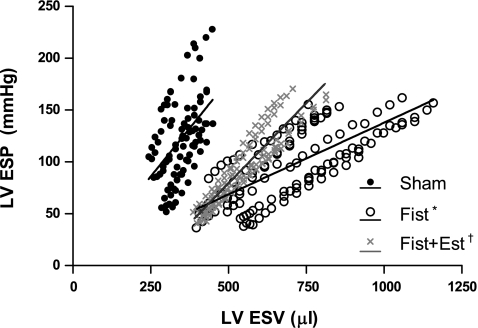
The group-averaged diastolic P-V relations are shown in Fig. 7. As indicated by the rightward shift of the diastolic P-V relation, untreated Fist rats developed significant LV dilatation (203% relative to Sham V0, P < 0.05). Estrogen treatment significantly attenuated this increase in LV volume (149% relative to Sham, P < 0.05 vs. both Sham and untreated Fist). Compliance, as measured by the volume required to increase EDP from 0 to 25 mmHg, was greatest in the hearts of untreated Fist rats (366% relative to Sham, P < 0.05). This increase in compliance was significantly attenuated by estrogen treatment (190% relative to Sham, P < 0.05 vs. Sham and untreated Fist). Systolic P-V relations are depicted in Fig. 8. The intrinsic chamber contractility, indicated by the slope of the systolic P-V relation, was significantly depressed following 8 wk of volume overload (67% reduction, P < 0.01 by analysis of covariance), whereas the estrogen-treated rats with fistula maintained systolic function that was comparable to Sham animals (P = NS) (see Table 2 for compiled parameters).
Fig. 7.
Group-averaged LV end-diastolic pressure-volume curves from blood-perfused isolated heart analyses demonstrating structural remodeling and alterations in compliance at 8 wk postsurgery. Data points are shown as means ± SE. Parameters for each group are listed in Table 2. P < 0.05 vs. Sham (*) and vs. Fist (†).
Fig. 8.
LV systolic pressure-volume data points and group-averaged linear regression from isolated heart analyses at 8 wk postsurgery. Parameters for each group are listed in Table 2. P < 0.05 vs. Sham (*) and vs. Fist (†).
Table 2.
Comparison of diastolic and systolic parameters from isolated heart function
| Diastolic |
Systolic |
||
|---|---|---|---|
| Group | V0, μl | V0–25/25, μl/mmHg | Slope Psys-V, mmHg/μl |
| Sham (n = 6) | 276 ± 12 | 4.12 ± 0.63 | 0.39 ± 0.07 |
| Fist (n = 6) | 561 ± 49* | 15.07 ± 0.79* | 0.13 ± 0.01* |
| Fist + Est (n = 5) | 410 ± 3*† | 7.83 ± 1.41*† | 0.31 ± 0.01† |
Values are means ± SE; n, no. of rats. V0, LV volume at an end diastolic pressure of 0 mmHg; V0–25, volume required to increase end diastolic pressure from 0 to 25 mmHg; Psys-V, peak systolic pressure-volume relation. Data are for functional parameters from blood-perfused isolated heart assessed 8 wk postsurgery.
P < 0.05 vs. Sham (*) and
P < 0.05 vs. Fist (†).
DISCUSSION
Estrogen has been shown to provide cardioprotection in various models of cardiac disease, including the aortocaval fistula model of heart failure (4, 15, 17, 19, 21, 38, 39, 45, 47). We previously demonstrated the cardioprotective effects of estrogen in ovariectomized female rats subjected to chronic volume overload, reflected by attenuation of ventricular hypertrophy and dilatation, maintenance of cardiac function, and prevention of congestive heart failure (CHF) (17). However, because testosterone may interfere with the protective effects of estrogen, it was not known if estrogen treatment would confer similar cardioprotective effects in male rats (26, 48). Accordingly, the present study sought to determine if estrogen treatment would prevent or attenuate the adverse ventricular remodeling and dysfunction that occur in male rats secondary to chronic cardiac volume overload, and to evaluate the acute mechanisms involved.
Effects of Estrogen on the Acute Response to Volume Overload in Male Rats
Previously, we determined that the initial phase (first 2 wk) of remodeling postfistula is critical in determining the eventual outcome of disease progression (22). Of particular importance is the acute activation and increased density of cardiac mast cells that causes an increase in myocardial MMP activity and degradation of extracellular collagen (8). Myocardial extracellular collagen degradation has been shown to lead to ventricular dilatation both in humans and in animal models of heart failure (28, 37, 40, 44). In the current study, untreated males had increased LV mast cell density postfistula that was accompanied by a substantial increase in MMP-2 activity and collagen degradation. These adverse events triggered by volume overload were prevented by estrogen treatment, and, as a result, the structural ventricular dilatation induced postfistula was significantly attenuated. These findings are in agreement with our previous results demonstrating that estrogen prevents the collagen degradation following chemically induced cardiac mast cell degranulation (11). In contrast, chemically induced mast cell degranulation in hearts isolated from ovariectomized rats produced MMP-2 activation and collagen degradation. These effects were prevented by treating the ovariectomized rats with estrogen, suggesting that estrogen alters cardiac mast cell phenotype. This effect of estrogen on mast cells may contribute to the cardioprotection demonstrated by male rats treated with estrogen.
We have previously shown that ET-1 can induce cardiac mast cell degranulation and that antagonism of ET receptors improves the functional outcome of volume overload in male rats (30–32). In the current study, we found that serum ET-1 levels were normal in estrogen-treated rats postfistula but were increased in untreated volume-overloaded rats. It is well known that estrogen inhibits the production of ET-1 in vascular endothelial cells (1–3, 20, 46). Accordingly, our findings together with those of others suggest that estrogenic inhibition of ET-1 production contributes to the prevention of the fistula-induced cardiac mast cell response. Although we have previously shown an increase of myocardial tissue levels of TNF-α in untreated male rats postfistula, we did not find detectable serum levels in any of our study groups (32). However, serum levels may not be indicative of local ventricular tissue levels of TNF-α.
Oxidant stress is identified as a key mechanism contributing to the development of CHF and adverse ventricular remodeling (24, 35, 42). In the untreated male rats, sustained volume overload induced a substantial increase in myocardial oxidative stress, which was completely abrogated by estrogen treatment. Oxidative stress has been shown to cause activation of mast cells, as well as in vitro activation of MMPs (27, 41, 43). Thus the prevention of volume overload-induced oxidant stress by estrogen may contribute to the demonstrated cardioprotective effects of this hormone. A drawback to the MDA method for determining oxidative stress is that the source of the oxidant stress is not identified. Another limitation of this study relates to the many cardiovascular and systemic effects derived from both genomic and nongenomic pathways modulated by estrogen. Functional estrogen receptors have been identified on multiple cardiovascular cell types, including cardiomyocytes, cardiac fibroblasts, vascular smooth muscle, and endothelial cells (18, 25, 36). Accordingly, estrogen likely mediates a number of effects that contribute to the beneficial modulation of myocardial remodeling beyond the specific focus on mast cells and extracellular collagen changes that we have identified herein. For example, estrogen has known effects on endothelial and inducible nitric oxide synthase (eNOS and iNOS) activity that may contribute to the cardioprotective effects observed (6, 21, 33). These estrogenic eNOS and iNOS effects may play a role in blunting the cardiac mast cell response to volume overload, since nitric oxide has been shown to inhibit noncardiac mast cell activation and mast cell-dependent inflammation (13, 14). Although little is known about estrogenic effects on cardiac mast cells, our previous study demonstrated that estrogen treatment prevents MMP activation and collagen degradation following chemically induced mast cell degranulation in isolated rat hearts (11). Furthermore, we have determined that in vivo treatments that prevent this acute mast cell activation following volume overload, including ET receptor antagonism and mast cell stabilization, have a protective effect on the cardiac extracellular collagen matrix and improve long-term outcome (10, 31, 32). As such, the comparable finding that estrogen treatment prevents the acute cardiac mast cell response induced by volume overload is significant and indicative of a relationship between estrogenic modulation of mast cells and chronic improvements in diastolic and systolic cardiac function.
Effects of Estrogen on Structural and Functional Remodeling in Male Rats with Chronic Volume Overload
In agreement with our previous findings, the untreated male rats developed significant biventricular hypertrophy, LV dilatation, and increased chamber compliance in response to volume overload (16). Treatment of males with estrogen significantly attenuated this adverse remodeling to a degree comparable with that reported in ovariectomized rats treated with estrogen (17). Moreover, several studies have demonstrated similar effects of estrogen on pathologic hypertrophy using other models of cardiac disease in ovariectomized females (15, 39, 45, 47). For example, estrogen treatment of ovariectomized mice reduced the amount of hypertrophy induced by both pressure overload and myocardial infarction (15, 45, 47). In a similar fashion, estrogen prevented the onset of hypertension, development of hypertrophy, and heart failure in ovariectomized spontaneously hypertensive heart failure (SHHF) rats (39).
The excessive dilatation and altered compliance in the untreated male fistula hearts was associated with a significant reduction in intrinsic chamber contractility as measured by the slope of the systolic P-V relation. In contrast, the attenuation of both LV structural dilatation and compliance changes achieved by estrogen treatment prevented this depression of myocardial chamber contractility. These findings are consistent with previous observations in ovariectomized female SHHF rats and in our studies using the fistula model, wherein treatment with estrogen is associated with normal cardiac function and prevents the onset of heart failure (17, 39). In the current study, the development of pulmonary edema at 8 wk postfistula was attenuated by estrogen treatment and, when coupled with the maintenance of systolic function, indicate that estrogen prevented the progression to decompensation and heart failure. A possible mechanism associated with the decrease in pulmonary edema in estrogen-treated animals is the positive effect of estrogen on nitric oxide synthase activity, which leads to decreased vascular permeability, particularly in the lung (7, 12). Although the cardioprotective effects of estrogen on chronic remodeling induced by volume overload are significant, it remains unknown if an acute treatment of estrogen is capable of providing long-term benefits or if the adverse remodeling would ensue once treatment is withdrawn. As in our previous studies using ovariectomized rats, estrogen treatment attenuated the adverse remodeling response to chronic volume overload in male rats. The reduced amount of structural dilatation in the estrogen-treated group represents an improved ability of the heart to compensate for the increased myocardial stress of cardiac volume overload, thereby allowing the heart to maintain adequate function, and preventing the progression to heart failure. We believe the results reported herein are the first to demonstrate a functional cardioprotective role of estrogen in male rats with a sustained cardiac volume overload and that this cardioprotective action is coupled to a reduction in LV oxidative stress, blockade of MMP activation, and prevention of collagen degradation. Future studies are warranted to assess the dependence of this cardioprotection on specific estrogen receptor subtypes. Moreover, additional studies are necessary to fully assess the estrogenic modulation of extracellular matrix proteins during remodeling, including matrix-degrading enzymes and their inhibitors.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant 2R01 HL-073990 (J. S. Janicki) and American Heart Association Grant 0435298N (J. D. Gardner).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Akishita M, Kozaki K, Eto M, Yoshizumi M, Ishikawa M, Toba K, Orimo H, Ouchi Y. Estrogen attenuates endothelin-1 production by bovine endothelial cells via estrogen receptor. Biochem Biophys Res Commun 251: 17–21, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Akishita M, Ouchi Y, Miyoshi H, Orimo A, Kozaki K, Eto M, Ishikawa M, Kim S, Toba K, Orimo H. Estrogen inhibits endothelin-1 production and c-fos gene expression in rat aorta. Atherosclerosis 125: 27–38, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Ba ZF, Lu A, Shimizu T, Szalay L, Schwacha MG, Rue LW, III, Bland KI, Chaudry IH. 17beta-Estradiol modulates vasoconstriction induced by endothelin-1 following trauma hemorrhage. Am J Physiol Heart Circ Physiol 292: H245–H250, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bakir S, Mori T, Durand J, Chen YF, Thompson JA, Oparil S. Estrogen-induced vasoprotection is estrogen receptor dependent: evidence from the balloon-injured rat carotid artery model. Circulation 101: 2342–2344, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Bensley SH. Pinacyanol erthrosinate as a stain for mast cells. Stain Technology 27: 269–273, 1952 [DOI] [PubMed] [Google Scholar]
- 6.Binko J, Majewski H. 17β-Estradiol reduces vasoconstriction in endothelium denuded rat aortas through inducible NOS. Am J Physiol Heart Circ Physiol 274: H853–H859, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Breithaupt-Faloppa AC, Vitoretti LB, Coelho FR, dos Santos Franco AL, Domingos HV, Sudo-Hayashi LS, Oliveira-Filho RM, Tavares de LW. Nitric oxide mediates lung vascular permeability and lymph-borne IL-6 after an intestinal ischemic insult. Shock 32: 55–61, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Brower GL, Chancey AL, Thanigaraj S, Matsubara BB, Janicki JS. Cause and effect relationship between myocardial mast cell number and matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol 283: H518–H525, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Brower GL, Henegar JR, Janicki JS. Temporal evaluation of left ventricular remodeling and function in rats with chronic volume overload. Am J Physiol Heart Circ Physiol 271: H2071–H2078, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Brower GL, Janicki JS. Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J Card Fail 11: 548–556, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chancey AL, Gardner JD, Murray DB, Brower GL, Janicki JS. Modulation of cardiac mast cell-mediated extracellular matrix degradation by estrogen. Am J Physiol Heart Circ Physiol 289: H316–H321, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Cho MM, Ziats NP, Pal D, Utian WH, Gorodeski GI. Estrogen modulates paracellular permeability of human endothelial cells by eNOS- and iNOS-related mechanisms. Am J Physiol Cell Physiol 276: C337–C349, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Coleman JW. Nitric oxide: a regulator of mast cell activation and mast cell-mediated inflammation. Clin Exp Immunol 129: 4–10, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis BJ, Flanagan BF, Gilfillan AM, Metcalfe DD, Coleman JW. Nitric oxide inhibits IgE-dependent cytokine production and Fos and Jun activation in mast cells. J Immunol 173: 6914–6920, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Donaldson C, Eder S, Baker C, Aronovitz MJ, Weiss AD, Hall-Porter M, Wang F, Ackerman A, Karas RH, Molkentin JD, Patten RD. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ Res 104: 265–75, 11p, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner JD, Brower GL, Janicki JS. Gender differences in cardiac remodeling secondary to chronic volume overload. J Card Fail 8: 101–107, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Gardner JD, Brower GL, Voloshenyuk TG, Janicki JS. Cardioprotection in female rats subjected to chronic volume overload: synergistic interaction of estrogen and phytoestrogens. Am J Physiol Heart Circ Physiol 294: H198–H204, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Grohé C, Kahlert S, Löbbert K, Stimpel M, Karas RH, Vetter H, Neyses L. Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett 416: 107–112, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Hayashi T, Jayachandran M, Sumi D, Thakur NK, Esaki T, Muto E, Kano H, Asai Y, Iguchi A. Physiological concentration of 17beta-estradiol retards the progression of severe atherosclerosis induced by a high-cholesterol diet plus balloon catheter injury: role of NO. Arterioscler Thromb Vasc Biol 20: 1613–1621, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Hong HJ, Liu JC, Chan P, Juan SH, Loh SH, Lin JG, Cheng TH. 17beta-estradiol downregulates angiotensin-II-induced endothelin-1 gene expression in rat aortic smooth muscle cells. J Biomed Sci 11: 27–36, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Huang A, Sun D, Koller A, Kaley G. 17beta-estradiol restores endothelial nitric oxide release to shear stress in arterioles of male hypertensive rats. Circulation 101: 94–100, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Janicki JS, Brower GL, Gardner JD, Forman MF, Stewart JA, Jr, Murray DB, Chancey AL. Cardiac mast cell regulation of matrix metalloproteinase-related ventricular remodeling in chronic pressure or volume overload. Cardiovasc Res 69: 657–665, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Jobe LJ, Melendez GC, Levick SP, Du Y, Brower GL, Janicki JS. TNF-α inhibition attenuates adverse myocardial remodeling in a rat model of volume overload. Am J Physiol Heart Circ Physiol 297: H1462–H1468, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kameda K, Matsunaga T, Abe N, Hanada H, Ishizaka H, Ono H, Saitoh M, Fukui K, Fukuda I, Osanai T, Okumura K. Correlation of oxidative stress with activity of matrix metalloproteinase in patients with coronary artery disease. Possible role for left ventricular remodelling. Eur Heart J 24: 2180–2185, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Karas RH, Patterson BL, Mendelsohn ME. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation 89: 1943–1950, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Leinwand LA. Sex is a potent modifier of the cardiovascular system. J Clin Invest 112: 302–307, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leon H, Bautista-Lopez N, Sawicka J, Schulz R. Hydrogen peroxide causes cardiac dysfunction independent from its effects on matrix metalloproteinase-2 activation. Can J Physiol Pharmacol 85: 341–348, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res 46: 214–224, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Motulsky HJ. Intuitive Biostatistics. New York, NY: Oxford Univ Press, 1995 [Google Scholar]
- 30.Murray DB, Gardner JD, Brower GL, Janicki JS. Endothelin-1 mediates cardiac mast cell degranulation, matrix metalloproteinase activation, and myocardial remodeling in rats. Am J Physiol Heart Circ Physiol 287: H2295–H2299, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Murray DB, Gardner JD, Brower GL, Janicki JS. Effects of nonselective endothelin-1 receptor antagonism on cardiac mast cell-mediated ventricular remodeling in rats. Am J Physiol Heart Circ Physiol 294: H1251–H1257, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Murray DB, McMillan R, Brower GL, Janicki JS. ETA selective receptor antagonism prevents ventricular remodeling in volume-overloaded rats. Am J Physiol Heart Circ Physiol 297: H109–H116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nuedling S, Kahlert S, Loebbert K, Doevendans PA, Meyer R, Vetter H, Grohe C. 17β-Estradiol stimulates expression of endothelial and inducible NO synthase in rat myocardium in-vitro and in-vivo. Cardiovasc Res 43: 666–674, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358, 1979 [DOI] [PubMed] [Google Scholar]
- 35.Radovanovic S, Krotin M, Simic DV, Mimic-Oka J, Savic-Radojevic A, Pljesa-Ercegovac M, Matic M, Ninkovic N, Ivanovic B, Simic T. Markers of oxidative damage in chronic heart failure: role in disease progression. Redox Rep 13: 109–116, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Russell KS, Haynes MP, Sinha D, Clerisme E, Bender JR. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc Natl Acad Sci USA 97: 5930–5935, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan TD, Rothstein EC, Aban I, Tallaj JA, Husain A, Lucchesi PA, Dell'Italia LJ. Left ventricular eccentric remodeling and matrix loss are mediated by bradykinin and precede cardiomyocyte elongation in rats with volume overload. J Am Coll Cardiol 49: 811–821, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Sbarouni E, Iliodromitis EK, Bofilis E, Kyriakides ZS, Kremastinos DT. Short-term estrogen reduces myocardial infarct size in oophorectomized female rabbits in a dose dependent manner. Cardiovasc Drugs Ther 12: 457–462, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Sharkey LC, Holycross BJ, Park S, Shiry LJ, Hoepf TM, McCune SA, Radin MJ. Effect of ovariectomy and estrogen replacement on cardiovascular disease in heart failure prone SHHF/Mcc- fa cp rats. J Mol Cell Cardiol 31: 1527–1537, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 102: 1944–1949, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Sudoh N, Toba K, Akishita M, Ako J, Hashimoto M, Iijima K, Kim S, Liang YQ, Ohike Y, Watanabe T, Yamazaki I, Yoshizumi M, Eto M, Ouchi Y. Estrogen prevents oxidative stress-induced endothelial cell apoptosis in rats. Circulation 103: 724–729, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Sun Y. Oxidative stress and cardiac repair/remodeling following infarction. Am J Med Sci 334: 197–205, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Suzuki Y, Yoshimaru T, Inoue T, Niide O, Ra C. Role of oxidants in mast cell activation. Chem Immunol Allergy 87: 32–42, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Tyagi SC, Kumar S, Voelker DJ, Reddy HK, Janicki JS, Curtis JJ. Differential gene expression of extracellular matrix components in dilated cardiomyopathy. J Cell Biochem 63: 185–198, 1996 [DOI] [PubMed] [Google Scholar]
- 45.van EM, Grohe C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 17Beta-estradiol attenuates the development of pressure-overload hypertrophy. Circulation 104: 1419–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Wingrove CS, Stevenson JC. 17 beta-Oestradiol inhibits stimulated endothelin release in human vascular endothelial cells. Eur J Endocrinol 137: 205–208, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Zhan E, Keimig T, Xu J, Peterson E, Ding J, Wang F, Yang XP. Dose-dependent cardiac effect of oestrogen replacement in mice post-myocardial infarction. Exp Physiol 93: 982–993, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou J, Ng S, Adesanya-Famuiya O, Anderson K, Bondy CA. Testosterone inhibits estrogen-induced mammary epithelial proliferation and suppresses estrogen receptor expression. FASEB J 14: 1725–1730, 2000 [DOI] [PubMed] [Google Scholar]