Abstract
To simulate the effects of shear stress in regions of the vasculature prone to developing atherosclerosis, we subjected human umbilical vein endothelial cells to reversing shear stress to mimic the hemodynamic conditions at the wall of the carotid sinus, a site of complex, reversing blood flow and commonly observed atherosclerosis. We compared the effects of reversing shear stress (time-average: 1 dyn/cm2, maximum: +11 dyn/cm2, minimum: −11 dyn/cm2, 1 Hz), arterial steady shear stress (15 dyn/cm2), and low steady shear stress (1 dyn/cm2) on gene expression, cell proliferation, and monocyte adhesiveness. Microarray analysis revealed that most differentially expressed genes were similarly regulated by all three shear stress regimens compared with static culture. Comparisons of the three shear stress regimens to each other identified 138 genes regulated by low average shear stress and 22 genes regulated by fluid reversal. Low average shear stress induced increased cell proliferation compared with high shear stress. Only reversing shear stress exposure induced monocyte adhesion. The adhesion of monocytes was partially inhibited by the incubation of endothelial cells with ICAM-1 blocking antibody. Increased heparan sulfate proteoglycan expression was observed on the surface of cells exposed to reversing shear stress. Heparinase III treatment significantly reduced monocyte adhesion. Our results suggest that low steady shear stress is the major impetus for differential gene expression and cell proliferation, whereas reversing flow regulates monocyte adhesion.
Keywords: reversing flow pattern, gene expression
atherosclerosis is typically localized to the carotid artery sinus, the coronary arteries, the abdominal aorta, and the superficial femoral arteries (30). These regions have complex blood flow patterns that can include flow reversal during each cardiac cycle, leading to the hypothesis that disturbed hemodynamic patterns are atherogenic. Such differences in hemodynamics alter the gene expression profile and ultimately the structure and function of endothelial cells (ECs), resulting in the modulation of EC responses to blood-borne factors and EC interactions with underlying smooth muscle cells, thus increasing the likelihood of atherogenesis (4, 8).
Previous studies comparing “antiatherogenic” nonreversing arterial shear stress to “proatherogenic” reversing arterial shear stress modeled the reversing shear stress in the form of a sine wave and used high steady shear stress and static conditions as controls for the comparison to the proatherogenic waveform (13, 17). However, simulations of the wall shear stress of the carotid sinus have shown that the wall shear stress is not harmonic but is a more complex waveform (7, 19).
We developed a reversing flow (RF) system using a parallel plate that accurately recreates the physiological form of the reversing shear stress found at the carotid sinus wall. We compared the effects of this reversing shear profile to the effects of steady arterial shear stress (15 dyn/cm2) and low steady shear stress (LSS; 1 dyn/cm2) on human umbilical vein ECs (HUVECs), a well-characterized model for arterial EC responses (24), in which HUVEC gene expression clusters tightly with other large vessel ECs (7). Based on our previous work (33, 34) and the work of others (15) showing minimal differences between steady shear stress and net forward pulsatile shear stress, we chose a steady shear stress of 15 dyn/cm2 to approximate the nonreversing pulsatile shear stress found in the straight regions of arteries. We included the steady LSS control of 1 dyn/cm2 to distinguish the gene and functional changes that were responses to low shear stress compared with fluid reversal.
Functional analyses confirmed previous findings that reversing shear stress increases cell proliferation (9, 29) and monocyte adhesion (2, 21, 22). Increased cell proliferation depends on low average shear stress, whereas monocyte adhesion depends on flow reversal. Our microarray results indicated that although there are unique sets of genes controlled by both low average shear stress and by RF, more genes were controlled by low average shear stress. We propose that low-time average shear stress acts as the more significant mechanical force on the regulation of EC gene expression while flow reversal regulates monocyte adhesion.
METHODS
The reversing shear stress system.
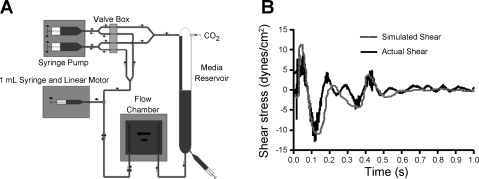
The shear stress profile used in this study (Fig. 1B) was based on a computer simulation of the wall shear stresses at the carotid bifurcation (time-average: 1 dyn/cm2, maximum: +11 dyn/cm2, minimum: −11 dyn/cm2, 1 Hz) (19). A custom flow system was designed to reproduce this waveform in a parallel plate flow system (Fig. 1A). The flow profile was produced by two different waveforms: the steady component of the waveform (−1 dyn/cm2) and the reversing component of the waveform, which had a time-averaged shear stress of 0 dyn/cm2. A standard syringe pump (Harvard Apparatus 33 Twin Syringe Pump) was used to apply the continuous shear stress component. A 1-ml glass syringe (Becton Dickinson) was mounted to a linear motor (MX80L, Parker Motion) regulated by a controller (ACR9000, Parker Motion), which was programmed to deliver the RF component.
Fig. 1.
Reversing flow (RF) system validation. A: fluid oscillations in the RF system are driven by a 1-ml syringe attached to a linear motor. The average shear stress of −1 dyn/cm2 was generated by an alternating dual-syringe pump in which one syringe fills the CO2-perfused media reservoir while the other syringe draws media from the media reservoir and across the flow chamber. B: the RF system produces wall shear stresses (solid line), which closely approximate computer simulations of the wall shear stress at the carotid sinus (shaded line). Wall shear stresses produced by the reversing flow system were obtained from high-speed video microscopy used to track polystyrene beads within the flow chamber.
To validate the RF system, polystyrene beads (6 μm, Bangs Laboratory) were tracked (Metamorph, Molecular Devices) under high-speed video microscopy (1,000 frames/s, Phantom V4.2, Vision Research) to directly measure the flow rate through the chamber. The focal plane of the microscope was selected to be the centermost point of the 180-μm gap height between the glass slide and the surface of the parallel plate chamber. Only the beads in focus were tracked and were therefore assumed to represent the maximum velocity through the chamber. Wall shear stress (τw) was calculated using Eq. 1 as follows:
where Vmax is the maximum velocity, μ is the viscosity of the media, and b is the height of the flow chamber.
Experimental protocol.
HUVECs (passages 3–5, Lonza) were seeded on glass slides coated with 0.5% glutaraldehyde cross-linked gelatin, grown to confluence (6), mounted in a parallel plate flow chamber, and exposed to one of four conditions for 24 h: RF (time average: 1 dyn/cm2, maximum: +11 dyn/cm2, minimum: −11 dyn/cm2), arterial high steady shear stress (HSS; 15 dynes/cm2), LSS (1 dyn/cm2), or static culture conditions. All shear stress experiments were performed on identical parallel plate chambers (12). The steady component of the reversing shear stress system and the total flow in the LSS system (1 dyn/cm2) were driven by a standard syringe pump. The HSS system (15 dyn/cm2) was driven by hydrostatic pressure (12).
RNA isolation and quantitative RT-PCR.
Immediately after cells had been exposed to shear stress, total RNA was extracted using TRIzol (Invitrogen); RNA was further purified with DNase (Qiagen) and RNeasy MinElute Cleanup Kit (Qiagen) according to the manufacturers' instructions. RNA quantity and quality were assessed by loading 1 μl of sample RNA onto a RNA 6000 Nano chip (Agilent) and analyzed with an Agilent 2100 Bioanalyzer to determine the amount and integrity of RNA recovered. Samples having an RNA integrity number of 9.5 or greater were used for microarray experiments.
Total RNA from each sample was reverse transcribed into cDNA using SuperScript II (Invitrogen). Predesigned PCR primers and Quantitect SYBR Green PCR Master Mix were purchased from Qiagen. Primers were added to the master mix at a ratio of 1:5. Each reaction was performed with 8 μl of diluted cDNA and 12 μl of master mix containing diluted primers. The quantitative RT-PCRs were performed on a MyIQ (Bio-Rad) with a 15-min activation step at 95°C, 50 cycles at 94°C for 15 s, 55°C for 30 s, and 72°C for 30 s, and a ramped melting cycle. Samples were normalized based on 18S RNA expression. Fold changes were determined using the ΔΔCt method. Quantitative RT-PCR was used to verify the gene expression from RNA isolated from five independent experiments.
Microarray analysis of gene expression changes.
Microarray hybridization and imaging were performed at the Morehouse School of Medicine Functional Genomics Core Facility. Briefly, RNA was linearly amplified to cRNA using Cy3-labeled CTP and a Low RNA Input Fluorescent Linear Amplification Kit (Agilent) according to the manufacturer's instructions. Cy3-labeled cRNA for each sample was hybridized to a 44k Whole Human Genome Microarray (Agilent) according to the manufacturer's instructions. Slides were dried under nitrogen and scanned on an Agilent DNA Microarray Scanner.
Microarray images were analyzed with Agilent Feature Extraction software according to the manufacturer's instructions. Genespring GX 10 was used to flag genes as either present, marginal, or absent based on default protocols within the software. This software was also used for the analysis of the microarray data with normalization to the 75th percentile of all samples and baseline transformation to the median of all samples for each gene. Genes were sorted for differential expression based on one-way ANOVA assuming equal variances followed by a post hoc Student-Neuman-Keuls test with a false discovery rate of 5%. Differentially expressed genes were identified as those genes having adjusted P values of <0.05 and flags marked as present or marginal in at least 50% of the samples for conditions with fold changes of at least 1.5-fold. The microarray data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) and are accessible through Gene Series accession number GSE16706.
Cell proliferation.
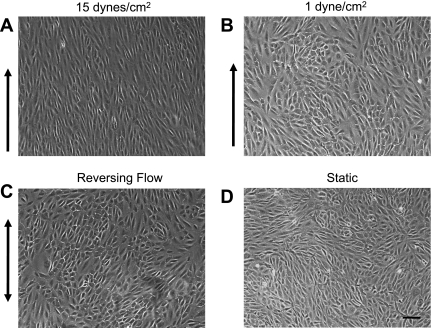
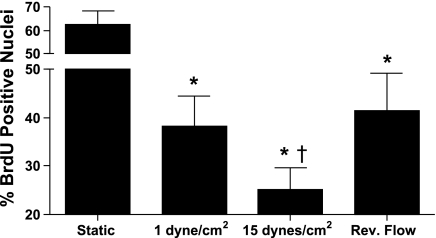
Shear stress experiments were performed in the presence of 10 μM bromodeoxyuridine (BrdU; EMD Biosciences) for 24 h. Cells were fixed in 4% paraformaldehyde, treated with 2 N HCl and 0.1% Triton X-100, and stained using rat anti-BrdU antibody (Abcam) followed by staining using goat anti-rat Alexa fluor 563 (Invitrogen) and Hoechst-33258 (Invitrogen). Standard fluorescent microscopy was used to image the cells. ImageJ (National Institutes of Health) was used to count BrdU-stained cells and Hoechst-33258-stained cells from the same frame. For each experimental condition, eight frames were analyzed for each experiment with a total of five experiments. The percentage of cells that divided was determined by dividing the number of BrdU-positive cells by the total number of cells as measured by Hoechst-33258 staining. Only experiments in which all shear stressed slides were confluent (e.g., Fig. 2) were included in the results.
Fig. 2.
Variations in fluid flow patterns cause altered endothelial cell (EC) morphology. A: phase-contrast images of ECs exposed to 15 dyn/cm2 of shear stress for 24 h produce elongated cells that are aligned with the direction of shear stress. B–D: ECs exposed to 1 dyn/cm2 (B) and ECs exposed to RF (C) are not aligned and are similar to the morphology of cells grown under static conditions (D). Arrows indicate the direction of flow. Scale bar = 100 μm.
Monocyte adhesion assay.
Monocyte adhesion assays were performed on HUVECs pretreated for 24 h with one of the four experimental conditions. Shear stressed slides were moved to a microscope stage, and CellTracker Green 5-chloromethylfluorescein diacetate CMFDA (Invitrogen)-stained THP-1 cells (a human acute monocytic leukemia cell line) in complete media (106 cells/ml) were perfused by a syringe pump at 1 dyn/cm2 for 5 min across the shear stressed HUVECs. Flow was then stopped for 30 s followed by 5 min more perfusion with complete media. Adherent cells were counted immediately in 10 frames in each of 5–6 separate experiments.
In the indicated experiments, immediately after the exposure to 24 h of reversing shear stress, cells were treated with 10 μg/ml functional blocking antibody against either ICAM-1 (clone 15.2, Santa Cruz Biotechnology) or VCAM-1 (clone 1G11, Santa Cruz Biotechnology) or 10 μg/ml mouse IgG as a control (Santa Cruz Biotechnology) for 30 min at 37°C under static conditions. In some experiments, immediately after the exposure to 24 h of reversing shear stress, cells were treated for 2 h with 15 mU heparinase III (Sigma) at 37°C under static culture conditions as previously described (11). Control cells were exposed to 2 h of static culture in complete media without heparinase III.
ICAM-1 and VCAM-1 surface expression.
ECs exposed to shear stress were trypsinized and resuspended in FACS buffer (110 mM NaCl, 10 mM KCl, 1 mM MgCl2, 10 mM glucose, 30 mM HEPES, and 1.5 mM CaCl2) containing 1.0% (vol/vol) human serum albumin (CSL Behring, Kankakee, IL). ECs were labeled with antibodies specific for ICAM-1 (R&D Systems), VCAM-1 (Santa Cruz Biotechnology), and mouse IgG isotype controls (R&D Systems) at 4°C for 1 h in the dark. All samples were analyzed on a BD LSR flow cytometer (Becton-Dickinson, Franklin Lakes, NJ) using BD FACSDiVa software. A total of 10,000 events were collected on a log scale. The data presented are the fold changes of the mean fluorescence intensity with respect to ECs grown under static conditions.
Heparan sulfate proteoglycan staining.
Cells exposed to 24 h of shear stress or static conditions were stained for heparan sulfate proteoglycan (HSPG) as previously described (25). Briefly, immediately after shear stress, cells were labeled with CellTracker Green CMFDA (Invitrogen) for 45 min at 37°C and then rinsed with ice-cold PBS. Cells were then incubated on ice with primary heparan sulfate antibody (US Biological) at 1:100 dilution for 1 h followed by 3 ice-cold PBS rinses and 4% paraformaldehyde fixation. Cells were incubated with secondary antibody (Alexa fluor 568, Invitrogen) at 1:200 dilution for 30 min. Cells were mounted on glass coverslips and imaged using standard fluorescent microscopy.
Statistics.
Statistical analysis for microarrays was performed as described in Microarray analysis of gene expression changes in methods. For all other data, one-way ANOVA assuming equal variances followed by a Newman-Keuls post hoc test was used to determine statistical significance, with P < 0.05. All results are expressed as means ± SE.
RESULTS
High-speed video tracking of polystyrene beads through the parallel plate chamber allowed for measurements of the average flow rate and calculation of the wall shear stress in the chamber. Figure 1B shows a visual comparison of the wall shear stress within the parallel plate flow chamber to the in vivo shear stress at the wall of the carotid sinus as determined by computer simulations performed by Perktold and Rappitsch (19). This comparison shows that our system accurately recreates the reversing shear stress found in vivo at the wall of the carotid sinus. Because this area of the vasculature is particularly prone to atherosclerosis, validation of the ability of our system to model flow within the carotid sinus indicates that the system is appropriate for studying the effects of this altered flow type on ECs.
ECs treated with HSS of 15 dyn/cm2 for 24 h aligned parallel to the flow direction (Fig. 2A). ECs exposed to LSS (1 dyn/cm2) or to reversing shear stress were randomly oriented, similar to ECs under static conditions (Fig. 2, B–D).
A comparison of HUVECs exposed to 24 h of RF, 15 dyn/cm2 (HSS), 1 dyn/cm2 (LSS), or static culture was performed with a whole human genome microarray. One-way ANOVA with a false discovery rate of 5% revealed 4,767 genes with statistically significant differences between at least 2 of the treatment conditions. A post hoc Student-Newman-Keuls test was performed to determine which conditions were significantly different from each other for each gene. Differentially expressed genes were identified as those that passed the Student-Newman-Keuls test and had flags marked as present or marginal in at least 50% of the samples for those conditions that had >1.5-fold changes in expression between at least two of the four HUVEC treatment conditions. A total of 4,017 differentially expressed genes were identified.
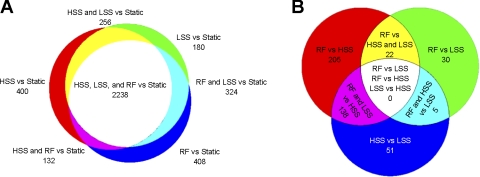
ECs exposed to all shear stress regimens differentially expressed ∼3,000 genes compared with cells in static culture (Table 1). On the other hand, a comparison of gene expression between the three shear stress types revealed much smaller numbers of differentially expressed genes. (See Supplemental Table 1 for lists of gene names and fold changes associated with each of the comparisons shown in Table 1.)1 Numbers of differentially expressed genes in comparisons of the three shear stress conditions to static culture are shown on the proportional Venn diagram shown in Fig. 3A (5). In total, 3,938 genes were found to be differentially expressed compared with static culture after the exposure to HSS, LSS, or RF. Of these, 57% were differentially expressed in comparisons of all three shear stress regimens to static culture (white region, Fig. 3A) Further examination of these genes shows that there was 100% agreement for all of the shear stress conditions in the direction of differential expression compared with static. Thus, for these genes, exposure to shear stress regulates the direction of change in gene expression relative to static culture, while the shear stress magnitude and waveform regulate the amplitude of this change.
Table 1.
Number of differentially expressed genes between conditions
| Static | HSS | LSS | |
|---|---|---|---|
| HSS | 3,026 | ||
| LSS | 2,998 | 194 | |
| RF | 3,102 | 365 | 57 |
HSS, high steady shear stress; LSS, low steady shear stress; RF, reversing flow.
Fig. 3.
Venn diagrams providing insight into the mechanisms regulating differential gene expression. A: differentially expressed genes in a comparison of high shear stress (HSS), low shear stress (LSS), or RF to static conditions. Approximately 57% of the differentially expressed genes are found in the central white area of the diagram, indicating that these genes were differentially expressed by all three flow conditions compared with static conditions. B: differentially expressed genes in a comparison of the three flow conditions to each other. The 138 genes found in the magenta region of the diagram are regulated by LSS. The 22 genes found in the yellow region of the diagram are regulated by RF. The five genes found in the blue-green region of the diagram are regulated by HSS.
In comparisons among the three flow types, the greatest number of differentially expressed genes was found in a comparison of RF and HSS, with 365 genes being differentially expressed (Table 1). A comparison of HSS with LSS showed that 194 genes were differentially expressed, whereas only 57 genes were differentially expressed when RF and LSS were compared. These genes may contribute to the differences in EC phenotype that exist between ECs in the carotid sinus and ECs from other regions of the vasculature, which do not experience blood flow disturbances caused by curvature of the vessels or branching. The Venn diagram shown in Fig. 3B shows the overlap among these three comparisons.
In our study, there were two conditions that have low average shear stress: LSS and RF. Examination of the magenta portion of Fig. 3B showed that when either LSS or RF was compared with HSS, there were 138 genes that were found in both lists of differentially expressed genes. Further examination showed that in all of these genes, both LSS and RF generated changes in the same direction of either up- or downregulation compared with HSS. Based on this similar response to both LSS and RF compared with HSS, these genes can be termed “regulated by low average shear stress” (Supplemental Table 2). RF was also compared with both HSS and LSS; the yellow portion of Fig. 3B shows that there are 22 genes that overlap between these two comparisons (Supplemental Table 3); 100% of the genes showed similar directions of up- or downregulation in response to RF compared with both HSS and LSS. Based on this, these genes can be termed “regulated by RF.” Further examination of Fig. 3B showed that in the blue-green section there were five genes that overlapped (Supplemental Table 4). This portion of the Venn diagram represents genes differentially expressed under LSS with similar expression under RF and HSS. In this case, both HSS and RF have been exposed to high shear stresses for at least some part of each cycle. As with the other genes in overlapping lists, exposure to HSS and RF induced differential expression compared with LSS in the same direction of either up- or downregulation. Thus, these five genes may be regulated by exposure to HSS during a fraction of the cycle despite the fact that the average shear stress applied to RF is low.
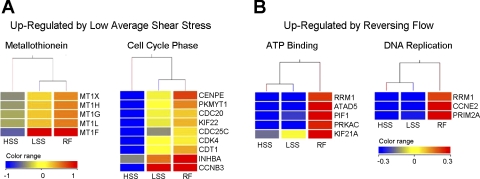
DAVID functional annotation clustering was used to group downregulated and upregulated genes based on function. These functional groups are shown in Fig. 4 and Supplemental Fig. 1 as hierarchical clusters of associated genes and conditions. These hierarchical clusters include downregulation by low average shear stress of genes associated with anatomical structure development, cell communication, response to external stimuli, blood vessel morphogenesis, chemical homeostasis, hydrolase activity, and actin filament organization (Supplemental Fig. 1). Genes upregulated by low average shear stress were associated with metallothioneins and the cell cycle phase, in particular, the mitotic phase (Fig. 4A). Genes differentially expressed by reversing shear stress relate to ATP binding and DNA replication (Fig. 4B).
Fig. 4.
Hierarchical clusters of functional groups. A: functional groups upregulated by LSS. B: functional groups upregulated by RF. Flow conditions and genes associated with functional groups identified by DAVID were clustered according to expression patterns. The color range shown is indicative of the normalized natural log-transformed signal values for each gene according to the normalization procedure described in methods.
The microarray data were confirmed with quantitative RT-PCR for six genes: two genes upregulated by low average shear stress [metallothionein 1F and cyclin B3 (CCNB3)], two genes downregulated by low average shear stress [Kruppel-like factor 2 (KLF2) and natriuretic peptide receptor A], and two genes upregulated by RF [primase polypeptide 2A (PRIM2A) and cyclin E2 (CCNE2)]. Comparisons of quantitative RT-PCR data and microarray data are shown in Supplemental Fig. 2. The four genes regulated by low average shear stress had similar changes in expression as measured by either quantitative RT-PCR or microarray. Of the two genes that were upregulated by RF, only PRIM2A had agreement between quantitative RT-PCR and microarray data. CCNE2 showed no significant change in expression between the three flow conditions when measured by quantitative RT-PCR. Overall, the quantitative RT-PCR analysis confirmed differential expression for five of the six genes analyzed.
Because a number of genes regulated by low average shear stress were associated with regulation of the cell cycle, i.e., CCNB3, cyclin-dependent kinase 4 (CDK4), and cell division cycle 25C (CDC25C) (see Fig. 4 and Supplemental Fig. 2), we investigated changes in EC proliferation (Fig. 5). Cells exposed to either static culture, HSS, LSS, or RF were shear stressed in the presence of BrdU. Although static cultures had the greatest fraction of BrdU-positive cells, comparisons among the shear stress conditions showed that the number of BrdU-positive cells increased more than twofold under reversing shear stress and 1 dyn/cm2 compared with 15 dyn/cm2 (Fig. 5). These data indicate that exposure of ECs to shear stress inhibits cell proliferation. Compared with HSS, both RF and LSS increased cell proliferation, indicating that low time average shear stress is involved in the regulation of cell division.
Fig. 5.
Cell proliferation is inhibited by HSS compared with LSS. ECs were exposed to shear stress in the presence of 10 μM bromodeoxyuridine (BrdU). Cells were fixed and stained for BrdU incorporation using an anti-BrdU antibody and nuclear DNA using Hoechst-33258. Exposure to all three forms of shear stress caused a reduction in BrdU incorporation compared with static culture (*P < 0.001) Exposure to HSS caused the greatest reduction in BrdU incorporation compared with the other two shear stress conditions (†P < 0.05). There were no significant differences between steady LSS and reversing shear stress. The percentage of nuclei that stained positively for BrdU was determined by counting nuclei in 5 experiments with 8 frames/experiment.
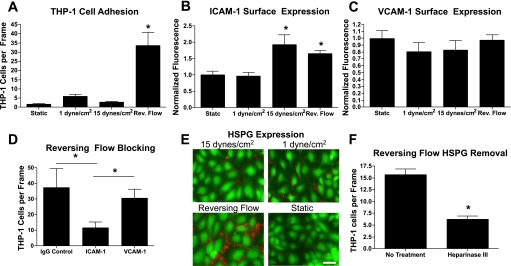
To assess the ability of reversing shear stress to lead to increased inflammation and leukocyte recruitment, a monocyte adhesion assay was performed on HUVECs pretreated with each shear stress condition. During perfusion, monocyte rolling was not observed for any of the EC shear stress regimens; however, when the monocyte perfusion was stopped for 30 s, significantly increased monocyte adhesion was observed on cells pretreated with RF (Fig. 6A). Increases in ICAM-1 surface expression were observed for both HSS and RF (Fig. 6B), but no changes in VCAM-1 surface expression were observed (Fig. 6C). Pretreatment with ICAM-1 blocking antibody, but not VCAM-1 blocking antibody, significantly decreased monocyte adhesion (Fig. 6D). Cells exposed to reversing shear stress had the highest surface HSPG expression (Fig. 6E). To examine the involvement of HSPG in monocyte adhesion, cells exposed to reversing shear stress were treated with heparinase III. Cells treated with heparinase III showed reduced HSPG immunofluorescence (data not shown). Monocyte adhesion was significantly reduced in cells treated with heparinase III (Fig. 6F).
Fig. 6.
THP-1 cells have greater adhesion to ECs exposed to reversing flow. A: THP-1 cells were perfused across ECs pretreated for 24 h with the indicated shear stress conditions. Flow was stopped for 30 s, allowing the THP-1 cells to interact, and, after a 5-min rinse, adherent cells were counted in 10 frames in each of 5–6 separate experiments. *P < 0.001 between RF and all three other conditions. B: ICAM-1 surface expression was significantly upregulated in ECs exposed to 15 dyn/cm2 of shear stress or RF for 24 h compared with ECs exposed to 1 dyn/cm2 of shear stress or static fluid (*P < 0.05). Data represent flow cytometry data of ICAM-1 fluorescent intensities normalized to the average ICAM-1 fluorescent intensity of ECs exposed to static conditions. C: VCAM-1 surface expression was not significantly changed under any shear stress condition. D: functional blocking of ICAM-1 significantly reduced monocyte adhesion in cells pretreated with RF compared with ECs blocked with either mouse control IgG or VCAM-1 antibody (*P < 0.05). E: surface heparan sulfate proteoglycan expression was elevated in cells exposed to reversing shear stress compared with the three other conditions. Scale bar = 50 μm. F: treatment of cells exposed to reversing shear stress with heparinase III significantly attenuated monocyte adhesion (*P < 0.05).
DISCUSSION
We compared the effects of physiological reversing shear stress to HSS and LSS. We investigated the changes in cell morphology, gene expression, cell proliferation, and monocyte adhesion under RF compared with HSS, LSS, and static culture. ECs exposed to reversing shear stress and to 1 dyn/cm2 of steady shear stress resembled the morphology of static cultures (Fig. 2) and were not oriented with the direction of flow, in contrast to ECs exposed to high shear stress, which were elongated and aligned with the flow direction. Similar differences in cell morphology were seen in vivo when porcine ECs found in the descending thoracic aorta (undisturbed unidirectional flow) were compared with porcine ECs found adjacent to a branch of the aorta (separated disturbed flow) (8), providing further evidence that our RF system mimics in vivo fluid flow disturbances found in branching regions of the vasculature.
Fluid flow induced differential gene expression in ∼7% of all genes. This number was similar to, although slightly higher than, those in previous reports, which indicated that shear stress affects between 1% and 6% of genes (1, 3, 13, 16, 17). Differences in the number of genes reported as differentially expressed can be attributed to microarray analysis techniques, microarray platforms, and differences in experimental setup. In our system, a comparison of shear stressed to static cultured cells revealed that 57% of differentially expressed genes were similarly up- or downregulated by HSS, LSS, and RF compared with static culture. This indicates that up- or downregulation of these genes is controlled simply by the presence of flow, while the type of flow determines the magnitude of response rather than the direction of response compared with static culture.
We compared our data with findings from other groups who examined EC gene expression under shear stress and static culture. Our data comparing HSS to static culture confirmed 72% of the genes reported by Ohura et al. (17) to be differentially regulated in HUVECs exposed to 15 dyn/cm2 of laminar shear stress compared with static culture. Our data confirmed differential expression for 47% of the genes reported by McCormick et al. (16) as having fold changes of at least 1.5-fold in a comparison of HUVECs exposed to 25 dyn/cm2 for 24 h versus static culture. This agreement with previously published reports on steady shear stress provides further validation of our microarray results.
The comparison of RF to HSS identified 365 differentially expressed genes between the two conditions (Table 1). The RF profile that we used has a low time-averaged shear stress of ∼1 dyn/cm2. Therefore, further comparisons were performed to determine whether differential gene expression was induced by low average shear stress or flow reversal; 138 genes were identified as being regulated by low average shear stress, whereas only 22 genes were identified as being regulated by flow reversal (Fig. 3B).
Other studies have used parallel plate or cone and plate systems to study the effects of either disturbed or oscillating flow on EC gene expression (1, 2, 7, 13, 17, 22). Some of these studies modeled the reversing shear stress waveform as an oscillating sine wave function. While a sine wave is a simpler shear stress to implement in an in vitro system, the physiological shear stress is more complex and includes much more rapid changes in shear stress direction (Fig. 1B). Also, the time-averaged shear stress in some models was set at zero (22), and, as a result, there was no net movement across the cells, a situation that does not occur in vivo. Another limiting factor of previous studies is the lack of a low steady shear stress control, which would allow for the discrimination of responses due to time-average LSS from those due to fluid shear stress reversal.
The shear stress profile we used was based on computer-simulated data of the shear stress at the wall of the carotid sinus (19). Our use of a parallel plate chamber enabled us to perform a flow-based monocyte adhesion assay on cells pretreated with different shear stress conditions. Although we have used this system to model the reversing shear stress at the carotid sinus, the linear motor can be easily programmed to deliver other equally complex shear stress waveforms.
The RF profile that we studied is similar to the “atheroprone” waveform studied by Dai et al. (7). This group used a cone and plate device to apply either atheroprone RF or atheroprotective pulsatile non-RF to ECs. This group found 159 genes that were differentially regulated in a comparison of ECs exposed to either atheroprone or atheroprotective flow profiles. In our system, the comparison of RF to HSS revealed differential regulation of 13 of these same genes. Our data further revealed that 10 of these 13 genes were regulated by low average shear stress rather than flow reversal [apolipoprotein L3, CD34, CD58, cysteine-rich protein 1, cytochrome P-450 (CYP)1B1, KLF2, MEF2A, parathyroid hormone-like hormone, CDC20, and metallothioneins].
We have recently confirmed the regulation of CYP1B1 by low average shear stress (6). KLF2 has been shown to be attenuated under reversing shear stress compared with nonreversing shear stress (7); however, other published work has shown KLF2 is also equally attenuated under LSS (10, 28), supporting our observation that KLF2 is regulated by low average shear stress. Regulation of metallothioneins was also reported by Ohura et al. (17), who examined the effects of turbulent flow compared with high steady laminar flow; however, in their system, turbulent flow caused downregulation of metallothionein expression, whereas in our system reversing shear stress caused the upregulation of metallothioneins. We found metallothioneins to be one of the few gene groups differentially regulated by nonreversing pulsatile shear stress compared with steady shear stress (33). These differences indicate that metallothionein expression is highly sensitive to variations in shear stress, which may be important for the regulation of downstream signaling events.
Our results indicate that a number of genes that regulate the cell cycle (including CCNB3, CDK4, and CDC25C) are upregulated by low average shear stress compared with high steady shear stress. CCNB3 is a member of the cyclin B family, which is known to regulate transition from the G2 phase to mitosis. Expression of CCNB3 peaks during the S and G2/M phases, with lower expression levels during the G0 and G1 phases (26). CDK4 is activated in the early G1 phase by D-type cyclins, leading to the passage through the G1/S phase restriction point and to S phase initiation (20). CDC25C is a phosphatase that directs dephosphorylation of the cyclin B/CDC2 complex to trigger entry into the M phase (23). Accumulation of these and other components of the cell cycle machinery in ECs exposed to low time-average shear stress is indicative of cell cycle progression. To assess the effects of differential expression of these cell cycle genes on cell proliferation, we analyzed BrdU incorporation (Fig. 5). The increased BrdU incorporation suggests that cell proliferation increased in cells exposed to reversing shear stress compared with cells exposed to HSS. Interestingly, similar results were observed for the LSS control, suggesting that the time-average LSS regulates cell proliferation.
We observed increased monocyte adhesion to ECs after exposure to reversing shear stress (Fig. 6A). Increased monocyte adhesion was not observed on ECs exposed to high or low steady shear stress, indicating that fluid reversal is responsible for the development of a more adhesive and activated EC phenotype. Previous studies of EC exposure to oscillatory flow have shown a similar increase in monocyte adhesion (2, 21, 22). In these studies, monocytes were applied under static conditions for 30–45 min to ECs pretreated with oscillatory shear stress. In our study, monocytes were perfused across ECs at 1 dyn/cm2 during which an assessment of monocyte rolling or firm adhesion revealed no binding between the flowing monocytes and ECs. However, when fluid flow was stopped for 30 s and then returned to 1 dyn/cm2, significant numbers of monocytes remained firmly adherent only to ECs pretreated with RF. These data suggest that flow reversal fails to induce the expression of selectins or other molecules involved in initial leukocyte rolling and binding; however, exposure to RF leads to increased firm adhesion, likely mediated by integrin ligands. Antibody blocking of ICAM-1 (Fig. 6D) resulted in significantly decreased monocyte adhesion, indicating that ICAM-1 is partially mediating the monocyte adhesion. Sorescu et al. (22) also showed ICAM-1-mediated monocyte adhesion in ECs exposed to oscillatory shear stress. Our flow cytometry data indicated that the surface expression of ICAM-1 was not only elevated in ECs exposed to reversing shear stress but also high steady shear stress (Fig. 6B); thus, an additional mechanism beyond surface expression of ICAM-1 protein appears to be involved, since increased monocyte adhesion was not observed under high steady shear stress. Recent work has shown ICAM-1 clustering in response to leukocyte adhesion and transmigration (31); therefore, it is possible that cells exposed to reversing shear stress are more prone to undergo ICAM-1 clustering in response to monocyte interactions resulting in stronger monocyte adhesion.
We also observed that surface HSPG expression was elevated on cells exposed to reversing shear stress (Fig. 6E). To the authors' knowledge, this is the first report showing increased HSPG expression in cells exposed to oscillatory or reversing shear stress. To investigate the role of HSPG in monocyte adhesion, we subjected cells to reversing shear stress and then treated the cells with heparinase III to remove HSPG. Cells treated with heparinase III had reduced monocyte adhesion (Fig. 6F). A previous report (32) showed that HSPG primarily localizes to cell-cell junctions when ECs are exposed to 15 dyn/cm2 of shear stress. We also observed that HSPG localized to cell-cell junctions in all three shear stress conditions (Fig. 6E). Endothelial HSPG has been shown to be both a ligand for L-selectin and to be important in the lumenal presentation of chemokines (14, 27). There are two possibilities to explain the role of increased HSPG expression on monocyte adhesion in ECs subjected to RF: 1) HSPG directly binds monocytes, which then adhere to ECs through ICAM-1-mediated firm adhesion or 2) HSPG directly binds and clusters chemokines on the lumenal surface of ECs, which in turn activate monocytes in close proximity, lending to firm adhesion to ECs. The regulation of surface HSPG expression by fluid shear stress merits additional study.
In this study, we used gelatin, a hydrolyzed form of collagen, as the substrate for the ECs. In vivo, ECs typically have a basement membrane of laminin, collagen, and entactin. However, at areas of disturbed shear, fibronectin deposits are present in the basement membrane, along with fibrinogen at later stages of atherosclerosis. Orr et al. (18) showed significant increases in inflammatory pathway activation under disturbed shear stress when cells were seeded on fibronectin or fibrinogen compared with collagen due to the differences in integrins that bind to these matrixes. It may be that cells seeded on fibronectin or fibrinogen would have an increased inflammatory response under our reversing shear stress waveform.
In summary, we developed a physiological reversing shear stress system that can be used to reproduce the wall shear stress at the carotid sinus. Using a low steady shear stress control, we show that most gene expression changes in cells exposed to reversing shear stress are regulated by low average shear stress and not fluid shear stress reversal. We also show that low average shear stress is the major force responsible for increases in cell proliferation compared with cells exposed to arterial levels of steady shear stress. Interestingly, we showed increased monocyte adhesion only in cells exposed to reversing shear stress. Our findings provide further insight into endothelial responses to mechanical forces and may be important in understanding the mechanisms of atherosclerotic development and localization to regions of disturbed flow.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HL-18672 and HL-70537 and by a National Science Foundation Graduate Research Fellowship (to D. E. Conway). The authors also acknowledge the Morehouse School of Medicine Functional Genomics Core Facility, supported by NIH Grant G12-RR-00303, for performing the microarray hybridization and scanning.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the technical input of Matthew J. Goette, Courtney V. Mitchell, Yumiko Sakurai, Leslie A. Coburn, and Ankit K. Shah. The authors thank Dr. Andrew Yee for contributions to the initial design of the reversing flow system.
Footnotes
Supplemental Material for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Brooks AR, Lelkes PI, Rubanyi GM. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics 9: 27–41, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res 82: 532–539, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Chen BP, Li YS, Zhao Y, Chen KD, Li S, Lao J, Yuan S, Shyy JY, Chien S. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics 7: 55–63, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng 36: 554–562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow S, Rodgers P. Applet for Drawing 3 Set Area-Proportional Venn Diagrams (online). http://www.cs.kent.ac.uk/people/staff/pjr/EulerVennCircles/EulerVennApplet.html [16November2009].
- 6.Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc Res 81: 669–677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA 101: 14871–14876, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies PF. Endothelial transcriptome profiles in vivo in complex arterial flow fields. Ann Biomed Eng 36: 563–570, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Jr, Gimbrone MA., Jr Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci USA 83: 2114–2117, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van Berkel TJ, Pannekoek H, Horrevoets AJ. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol 167: 609–618, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 93: e136–e142, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotech Bioeng 32: 1053–1060, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci USA 98: 4478–4485, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuffre L, Cordey AS, Monai N, Tardy Y, Schapira M, Spertini O. Monocyte adhesion to activated aortic endothelium: role of L-selectin and heparan sulfate proteoglycans. J Cell Biol 136: 945–956, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 38: 1949–1971, 2005 [DOI] [PubMed] [Google Scholar]
- 16.McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, Chittur KK. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci USA 98: 8955–8960, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohura N, Yamamoto K, Ichioka S, Sokabe T, Nakatsuka H, Baba A, Shibata M, Nakatsuka T, Harii K, Wada Y, Kohro T, Kodama T, Ando J. Global analysis of shear stress-responsive genes in vascular endothelial cells. J Atheroscler Thromb 10: 304–313, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-κB activation by flow: a potential role in atherosclerosis. J Cell Biol 169: 191–202, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perktold K, Rappitsch G. Computer simulation of local blood flow and vessel mechanics in a compliant carotid artery bifurcation model. J Biomech 28: 845–856, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 28: 2925–2939, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res 95: 773–779, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem 278: 31128–31135, 2003 [DOI] [PubMed] [Google Scholar]
- 23.St Clair S, Manfredi JJ. The dual specificity phosphatase Cdc25C is a direct target for transcriptional repression by the tumor suppressor p53. Cell Cycle 5: 709–713, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Terramani TT, Eton D, Bui PA, Wang Y, Weaver FA, Yu H. Human macrovascular endothelial cells: optimization of culture conditions. In Vitro Cell Dev Biol Anim 36: 125–132, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci USA 101: 16483–16488, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tschop K, Muller GA, Grosche J, Engeland K. Human cyclin B3. mRNA expression during the cell cycle and identification of three novel nonclassical nuclear localization signals. FEBS J 273: 1681–1695, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol 6: 902–910, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Wang N, Miao H, Li YS, Zhang P, Haga JH, Hu Y, Young A, Yuan S, Nguyen P, Wu CC, Chien S. Shear stress regulation of Kruppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun 341: 1244–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 29.White CR, Haidekker M, Bao X, Frangos JA. Temporal gradients in shear, but not spatial gradients, stimulate endothelial cell proliferation. Circulation 103: 2508–2513, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Wootton DM, Ku DN. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu Rev Biomed Eng 1: 299–329, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Kowalski JR, Yacono P, Bajmoczi M, Shaw SK, Froio RM, Golan DE, Thomas SM, Luscinskas FW. Endothelial cell cortactin coordinates intercellular adhesion molecule-1 clustering and actin cytoskeleton remodeling during polymorphonuclear leukocyte adhesion and transmigration. J Immunol 177: 6440–6449, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Yao Y, Rabodzey A, Dewey CF., Jr Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol 293: H1023–H1030, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Yee A, Bosworth KA, Conway DE, Eskin SG, McIntire LV. Gene expression of endothelial cells under pulsatile non-reversing vs. steady shear stress; comparison of nitric oxide production. Ann Biomed Eng 36: 571–579, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Yee A, Sakurai Y, Eskin SG, McIntire LV. A validated system for simulating common carotid arterial flow in vitro: alteration of endothelial cell response. Ann Biomed Eng 34: 593–604, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.