Abstract
Reactive oxygen and nitrogen species (ROS and RNS, respectively) generate nitrotyrosine and activate latent resident myocardial matrix metalloproteinases (MMPs). Although in chronic heart failure (CHF) there is robust increase in ROS, RNS, and MMP activation, recent data suggest that hydrogen sulfide (H2S, a strong antioxidant gas) is cardioprotective. However, the role of H2S in mitigating oxidative and proteolytic stresses in cardiac remodeling/apoptosis in CHF was unclear. To test the hypothesis that H2S ameliorated cardiac apoptosis and fibrosis by decreasing oxidative and proteolytic stresses, arteriovenous fistula (AVF) was created in wild-type (C57BL/6J) mice. The hearts were analyzed at 0, 2, and 6 wk after AVF. To reverse the remodeling, AVF mice were treated with NaHS (an H2S donor, 30 μmol/l in drinking water) at 8 and 10 wk. The levels of MMPs were measured by gelatin-gel zymography. The levels of nitrotyrosine, tissue inhibitors of metalloproteinase (TIMPs), β1-integrin, and a disintegrin and metalloproteinase-12 (ADAM-12) were analyzed by Western blots. The levels of pericapillary and interstitial fibrosis were identified by Masson trichrome stains. The levels of apoptosis were measured by identifying the TdT-mediated dUTP nick end labeling (TUNEL)-positive cells and caspase-3 levels. The results suggested robust nitrotyrosine and MMP activation at 2 and 6 wk of AVF. The treatment with H2S donor mitigated nitrotyrosine generation and MMP activation (i.e., oxidative and proteolytic stresses). The levels of TIMP-1 and TIMP-3 were increased and TIMP-4 decreased in AVF hearts. The treatment with H2S donor reversed this change in TIMPs levels. The levels of ADAM-12, apoptosis, and fibrosis were robust and integrin were decreased in AVF hearts. The treatment with H2S donor attenuated the fibrosis, apoptosis, and decrease in integrin.
Keywords: fibrosis, extracellular matrix, ADAM, integrin, matrix metalloproteinase, tissue inhibitor of metalloproteinase, left ventricle hypertrophy, collagen, TUNEL, apoptosis, homocysteine
although in gastrointestinal and nervous systems hydrogen sulfide (H2S) plays a crucial role as a signaling molecule (12, 24), recent studies established that H2S is a cardioprotective gas (4, 21, 27). However, its role in regulating the ECM remodeling and apoptosis is still nebulous. Considering the tremendous therapeutic potential, H2S gas has attracted the attention of biomedical research (2, 5, 9, 10, 12). It is a strong antioxidant and is generated endogenously by two important enzymes involved in sulfur-containing amino acid [cysteine and homocysteine (Hcy)] metabolism, namely cystathionine β-synthase (CBS) and cystathionine γ-lyase (CGL) (26). Paradoxically, it is interesting that hyperhomocysteinemia (HHcy), a precursor of H2S, can be cardioprotective. The CGL is ubiquitous; the CBS is not present in the vascular tissues. Therefore, under normal condition, only half of Hcy can be converted to H2S. However, there is strong potential for gene therapy of CBS to vascular tissue that can mitigate the detrimental effects of Hcy by converting to H2S. This scenario is possible if we can increase the activities of both enzymes, CBS and CGL, in every tissue by gene therapy.
Several biochemical changes took place in the heart during stress condition and to maintain homeostasis, including the levels of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) changes. MMPs are a family of structurally and functionally related zinc-dependent endoproteinases that play a pivotal role in ECM remodeling through its proteolytic effect (19). MMP-2 and MMP-9 have collagen binding fibronectin type II inserts in their catalytic domain, making those indispensible for ECM remodeling in the heart, where elastin-to-collagen ratio determines the extent of cardiac dysfunction in chronic heart failure (CHF) (13). MMPs are in a latent state in the heart, maintaining normal elastin-to-collagen ratio through matrix synthesis and degradation. However, when reactive oxygen and nitrogen species (ROS and RNS) engender oxidative stress, MMPs get activated, leading to deposition of excess matrix protein (collagen), which, in turn, remodels ECM by changing the elastin-to-collagen ratio, causing fibrosis that ultimately leads to CHF (14, 15). To modulate MMP activity and suppress ECM turnover, there are endogenous inhibitors of MMPs called TIMPs (23). TIMP-4 prevents the activation of MMP-2 and MMP-9 and is predominantly present in cardiac tissue, hence it is also called cardiac-specific inhibitor of metalloproteinase (CIMP) (13). On the other hand, TIMP-1 and TIMP-3 increase with oxidative stress. TIMP-1 has been implicated in cardiac fibrosis (11), whereas TIMP-3 induces apoptosis in vascular smooth muscle cells (1).
Oxidative stress generated by ROS and RNS alarms not only MMPs and TIMPs, but also the downstream β1-integrin and a disintegrin and metalloproteinase-12 (ADAM-12). To simulate the condition of oxidative stress, arteriovenous fistula (AVF) was created in mice, and the change in the levels of MMPs, TIMPs, β1-integrin, and ADAM-12 were assessed to confirm that the heart was under oxidative and proteolytic stress. Then these AVF mice were treated with H2S, and the change in the levels of MMPs, TIMPs, β1-integrin, and ADAM-12 were recorded. Interestingly, we found that H2S has mitigated the oxidative and proteolytic stresses in AVF mice. In this way, we attempted to figure out how H2S inhibits fibrosis and apoptosis (that leads to CHF) by regulating MMPs, TIMPs, β1-integrin, and ADAM-12. Furthermore, we proposed a model for H2S-dependent ECM remodeling resulting in cardioprotection.
METHODS
Animal model.
Male C57BL/6J wild-type mice (8–10 wk old) were obtained from The Jackson Laboratory (Bar Harbor, ME) and kept in the animal care facility of University of Louisville where ambient environmental conditions (12:12-h light-dark cycle, 22–24°C) were maintained. The animals were fed standard chow and water ad libitum. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University Of Louisville School of Medicine in accord with animal care and use program guidelines of the National Institutes of Health.
H2S measurements.
Blood (0.5 ml) was collected, and plasma was separated from each mice. The plasma levels of H2S were estimated by colorimetric titration as previously described (20). The sulfide measurement was a critical, and very tricky, parameter to measure. Also, recent work has shown that levels of H2S in blood were far lower than 30 μM, and exogenous sulfide was very rapidly taken up or oxidized in the blood. There has been recent controversy over sulfide quantification techniques, especially those that used strong acid that could free acid-labile sulfide from the sample and artificially increase detected amounts of hydrogen sulfide. The H2S values could be inflated as an artifact of the technique. Nonetheless, we (20) previously measured sulfide production from left ventricle (LV) homogenates. We adapted a similar method to measure the plasma levels of H2S.
AVF mouse model of CHF.
To create chronic volume overload stress on the heart, aorta-to-vena cava shunt was created using 22-gauge needles as previously described (7). Mice were anesthetized with tribromoethanol (100 mg/kg ip), which has minimal effects on cardiovascular function in mice (17). The AVF was created in 30 mice. In this protocol, time 0 was considered as control (6 mice). The mice were monitored and examined at 2 wk (6 mice) and 6 wk (6 mice) of AVF. The rest of the mice were administered with H2S donor, NaHS (30 μmol/l; Sigma Chemical), in the drinking water, and these mice were killed at 8 and 10 wk (n = 6 in each group). The sham controls at 2, 6, 8, and 10 with or without NaHS were used to compare with AVF and AVF + H2S-treated mice.
The amount of NaHS (30 μmol/l) was soluble in aqueous conditions and was based on the fact that the normal physiological concentration of H2S in the blood was in the range of 10–100 μM (18). We infused NaHS at 30 μmol/l in drinking water (as donor of H2S). After collecting blood, the hearts were removed. The left and right ventricle were separated. The tissue extracts were prepared as previously described (7).
Western blot analysis of nitrotyrosine, TIMP-1, TIMP-3, β1-integrin, ADAM-12, and caspase-3.
Western blot analyses were performed on LV tissue homogenates using 10% SDS-PAGE. Bradford method was used to estimate total protein, and 25 μg of protein was loaded in each well of electrophoresis gels. After electrophoresis, proteins were transferred to polyvinylidene difluoride membrane, blocked with 5% fat-free milk, and blotted with respective primary monoclonal antibodies: anti-nitrotyrosine, TIMP-1, TIMP-3, β1-integrin, ADAM-12, and caspase-3 (Chemicon). As a loading control, actin blots were used. The bands were normalized with actin controls.
Zymography.
Gelatin-gel zymography was performed on LV tissue homogenates using 1.5% gelatin gel, a substrate for MMP-2 and MMP-9 (7). Freshly dissected tissue samples were quickly homogenized in an ice-cold extraction buffer (1:3 wt/vol) containing 10 mM cacodylic acid, 20 mM ZnCl2, 1.5 mM NaN3, and 0.01% Triton X-100, pH 5.0, and centrifuged at 800 g for 10 min, keeping the temperature 4°C. Supernatant was collected for protein estimation using Bradford assay, and 100 μg of protein samples was loaded in 7.5% SDS-PAGE containing 1.5% gelatin as MMP substrate under nonreducing conditions. After complete electrophoresis, gel was washed in 2.5% Triton X-100 with rocking for 30 min with one change of Triton after 15-min rinse in distilled water for 10 min and then incubated overnight in substrate buffer (50 mM Tris·HCl, 5 mM CaCl2, and 0.02% NaN3, pH 7.5) at 37°C with gentle shaking. After incubation, gels were stained for 15–30 min in 0.05% Coomassie blue R-250 in acetic acid-isopropyl alcohol-water (1:3:6 by vol), distained in water, and observed under bright white light. Gels were then scanned for lysis band intensity, photographed, and dried for permanent record. The lysis band intensity was used to estimate the weight of active collagenase/weight of tissue.
All gels were run from the same samples. In addition, total concentration of protein was measured and kept the same in all samples. Therefore, the actin loading control blot in every figure appeared to be the same even if different gels (zymography vs. Western) were used.
Histological analysis of collagen fibrosis.
To determine the levels of fibrosis and role of H2S in mitigation of fibrosis in CHF, LV tissue sections (10 μm) were stained with Masson trichrome blue for histology from hearts of mice of 0- and 6-wk AVF and 8- and 10-wk H2S treatment. We focused on identifying the pericapillary and interstitial fibrosis by histology. The levels of collagen were measured by total hydroxy proline estimation as previously described (15).
TUNEL labeling and apoptotic cell measurements.
TdT-mediated dUTP nick end labeling (TUNEL) assay was used to determine the number of apoptotic cells in the LV of 0 and 6 wk of AVF mice and 8 and 10 wk of H2S-treated mice. LV tissues were snap-frozen following the procedure of Ovechkin et al. (16) and labeled with TUNEL (Upstate Cell Signaling Solution, Lake Placid, NY) as per the instructions of the company. Cells were counted and scored from five randomly selected areas of each slide, and quantitative analysis was performed by employing Image-Pro Plus (Media Cybernetics, Silver Spring, MD).
Statistical analysis.
Values are given as averages ± SD from n = 6 in each group. Differences between groups were evaluated by using a two-way ANOVA followed by the Bonferroni post hoc test (25). The results of the four comparisons by Bonferroni test are reported as 0, 6, 8, and 10 wk without the treatments. P < 0.05 was considered significant.
RESULTS
H2S levels.
Endogenous H2S production was reduced in AVF hearts. However, the treatment with NaHS normalized the levels of H2S generation in the hearts of AVF mice (Table 1). The sham groups ± NaHS at all time points were used for comparison with AVF ± NaHS treatments. We compared the data from AVF alone at 8 and 10 wk and with H2S-treated groups. The results suggested that the changes in the levels of nitrotyrosine, MMP, TIMP-1, -3, and -4, β1-integrin, and ADAM-12 at 8 and 10 wk AVF were mitigated after H2S treatment.
Table 1.
Gravimetric parameters of mice after 0, 2, 6, 8, and 10 wk of AVF and 8 and 10 wk of AVF + NaHS treatment (parentheses)
| NaHS Treatment | |||||
|---|---|---|---|---|---|
| 0 wk | 2 wk | 6 wk | 8 wk | 10 wk | |
| Body wt, g | 22 ± 1 | 23 ± 1 | 22 ± 2 | 22 ± 1 (23 + 2) | 23 ± 2 (22 ± 1) |
| HW, g | 0.18 ± 0.1 | 0.21 ± 0.02 | 0.26 ± 0.02 | 0.31 ± 0.03 (0.23 ± 0.02)* | 0.38 ± 0.04 (0.21 ± 0.02)* |
| H2S, μmol/l | 35 ± 1 | 21 ± 2 | 9 ± 1 | 6 ± 1 (25 ± 3)* | 4 ± 1 (38 ± 3)* |
| Col, μg/mg | 2.5 ± 0.1 | 3.2 ± 0.2 | 4.5 ± 0.3 | 5.6 ± 0.7 (3.5 ± 0.3)* | 7.6 ± 0.8 (2.8 ± 0.3)* |
Values are averages ± SD.
P < 0.05 compared with untreated. Comparisons were made between sham ± H2S vs. arteriovenous fistula (AVF) ± H2S at each time point. Heart weight (HW) and collagen (Col) were estimated.
There were three ways to address the mechanism of remodeling: 1) prevention of remodeling; 2) attenuation of remodeling; and 3) reversal of remodeling. Here, we studied the reversal of remodeling. Therefore, it was important to compare the samples in a longitudinal fashion and compare with 0, 2, and 6 wk and treatment with 8 and 10 wk.
H2S mitigated nitrotyrosine formation and oxidative stress in CHF.
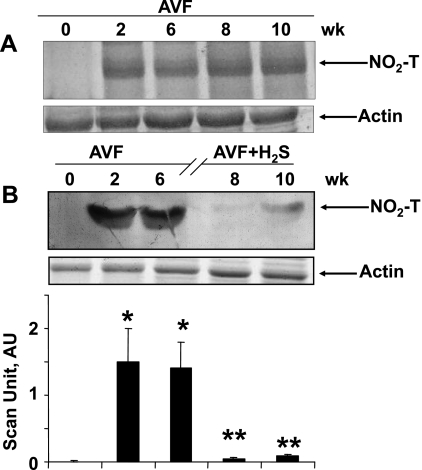
The major nitrated band is shown in Fig. 1. The entire gel bands were scanned and are represented by bar graphs (Fig. 1B). There was no nitrotyrosine generation in the hearts at the beginning of AVF. The temporal data from AVF at all the points, i.e., 0, 2, 6, 8, and 10 wk, are shown in Fig. 1A. The results suggested that the levels of nitrotyrosine were increased at 2, 6, 8, and 10 wk of AVF (Fig. 1A). The data from sham groups mimic the data at 0 wk in all groups. Interestingly, the treatment with H2S reversed the nitrotyrosine formation in AVF hearts (Fig. 1B).
Fig. 1.
Western blot analysis of nitrotyrosine (NO2-T). A: left ventricle (LV) tissue homogenates were analyzed at 0, 2, 6, 8, and 10 wk after arteriovenous fistula (AVF). Corresponding actin bands are shown. B: LV tissue at 0, 2, and 6 wk after AVF and 8 and 10 wk after AVF treated with NaHS (H2S donor). Corresponding actin bands are shown. Bar graphs represent the scan arbitrary unit (AU) normalized with actin. Each bar is an average ± SD from n = 6 in each group. *P < 0.001 compared with 0 wk. **P < 0.001 compared with 6 wk.
Since the mechanism of remodeling and MMP activation preceded the nitrotyrosine formation (22), to reverse the remodeling, NaHS (H2S donor) was administered at 6 wk after AVF. The data are presented in Figs. 1B–6.
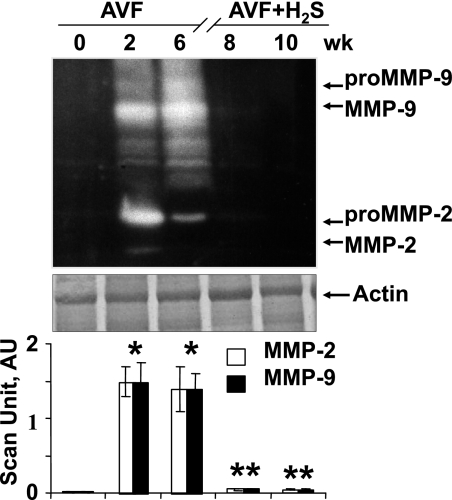
Fig. 2.
Gelatin-gel zymographic analysis of matrix metalloproteinases (MMPs) at 0, 2, and 6 wk after AVF and 8 and 10 wk after AVF treated with NaHS (H2S donor). Corresponding actin bands are shown. Bar graphs represent the total MMP activity scan AU normalized with actin. Each bar is an average ± SD from n = 6 in each group. *P < 0.001 compared with 0 wk. **P < 0.001 compared with 6 wk.
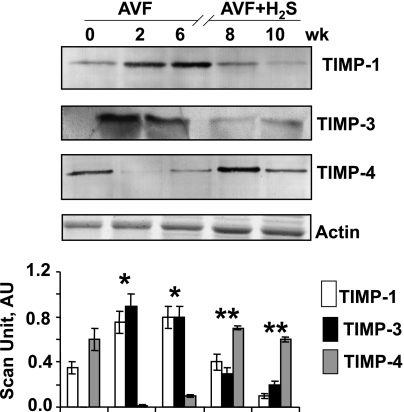
Fig. 3.
Western blot of tissue inhibitors of metalloproteinase (TIMPs) at 0, 2, and 6 wk after AVF and 8 and 10 wk after AVF treated with NaHS (H2S donor). Corresponding actin bands are shown. Bar graphs represent the scan AU normalized with actin. Each bar is an average ± SD from n = 6 in each group. *P < 0.001 compared with 0 wk. **P < 0.001 compared with 6 wk.
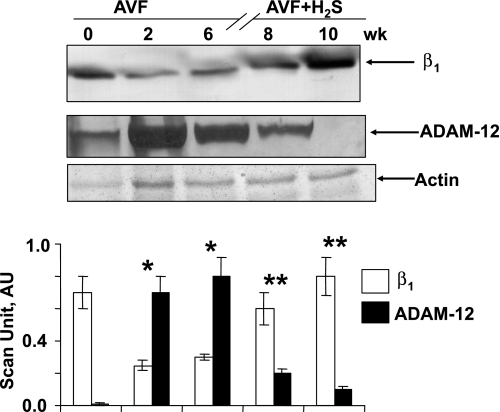
Fig. 4.
Western blot of β1-integrin (β1) and a disintegrin and metalloproteinase-12 (ADAM-12) at 0, 2, and 6 wk after AVF and 8 and 10 wk after AVF treated with NaHS (H2S donor). Corresponding actin bands are shown. Bar graphs represent the scan AU normalized with actin. Each bar is an average ± SD from n = 6 in each group. *P < 0.001 compared with 0 wk. **P < 0.001 compared with 6 wk.
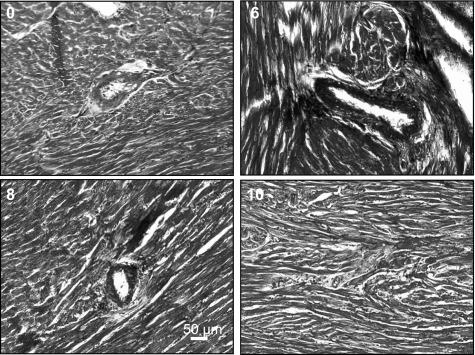
Fig. 5.
Ten-micrometer LV tissue sections were stained with Masson trichrome blue for collagen, focusing on pericapillary and interstitial fibrosis. Histographic analysis of collagen fibrosis at 0 and 6 wk after AVF and 8 and 10 wk after AVF treated with NaHS (H2S donor) was performed. All panels were recorded at ×10 magnification; bar = 50 μm.
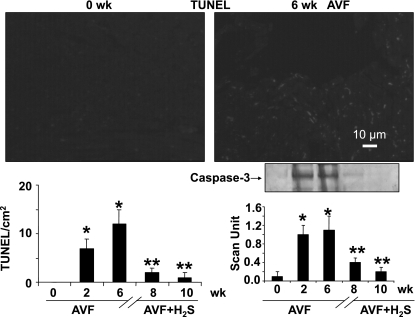
Fig. 6.
TdT-mediated dUTP nick end labeling (TUNEL) analysis of mice apoptotic cells at 0 and 6 wk after AVF (bar = 10 μm). The bar graphs represent the number of TUNEL-positive cells in a cm2 area at 0, 2, and 6 wk after AVF and 8 and 10 wk after AVF treated with NaHS (H2S donor). The gel insert shows caspase-3 Western blot analysis at 0, 2, and 6 wk after AVF and 8 and 10 wk after AVF treated with NaHS (H2S donor). The bar graphs represent scanned values of caspase-3. Identical amount of total protein was loaded onto each lane in the gel. Each bar is an average ± SD from n = 6 in each group. *P < 0.001 compared with 0 wk. **P < 0.001 compared with 6 wk.
Role of H2S in MMP inactivation.
MMP-9 and MMP-2 remained in latent form in normal hearts (Fig. 2). However, when AVF was created, which caused stress, MMP-9 and MMP-2 became active, and their activity was enhanced in a time-dependent manner that was apparent when its level in 2- and 6-wk AVF mice were compared (Fig. 2). Interestingly, the active MMPs turned inactive in 8- and 10-wk mice (as its levels declined) after they were treated with H2S (Fig. 2), suggesting H2S mitigated oxidative and proteolytic stresses in AVF. Zymography gels routinely showed both latent and active MMP-2/9 bands. Therefore, it was not surprising that various bands for MMP-2 and MMP-9 were observed. Interestingly, in control and AVF + H2S lanes, we did not see activity for both latent and active. This suggested that the H2S treatment mitigated both latent and active MMP expressions.
Role of different TIMPs during heart failure.
In control mice, TIMP-4 was present in adequate amount, but TIMP-1 and TIMP-3 were almost absent. In volume overload stressed condition, TIMP-1 and TIMP-3 increased, but TIMP-4 decreased as evidenced in AVF mice (Fig. 3). However, when AVF mice were treated with H2S, TIMP-4 level were increased, whereas TIMP-1 and TIMP-3 levels were decreased (Fig. 3), suggesting that H2S normalized the TIMP levels in AVF mice.
β1-Integrin and ADAM-12.
β1-Integrin and ADAMs are membrane-anchored proteins involved in cell-matrix interactions. In 2- and 6-wk AVF mice, β1-integrin levels were decreased more than in the 0-day mice, but ADAM-12 levels were enhanced, indicating high fibrosis in ECM (Fig. 4). However, after 6 wk, when mice were treated with H2S donor molecule (NaHS), they regained their normal levels (Fig. 4), suggesting remodeling of ECM to its normal condition. This observation further supports H2S mitigated recovery from fibrosis.
Interstitial and pericapillary fibrosis.
The 0-day histological sections of LV tissue reflected the normal condition of interstitial and pericapillary tissue (Table 1 and Fig. 5). However, 6-wk AVF mice showed high level of fibrosis around the capillary as well as in interstitial regions (Fig. 5). These pathological conditions attenuated in 8- and 10-wk mice when mice at 6 wk were treated with H2S (Table 1 and Fig. 5).
Apoptosis.
TUNEL assay for apoptosis in 0- and 6-wk AVF mice as well as 8- and 10-wk H2S-treated mice (Fig. 6) showed that the numbers of apoptotic cells were increased in AVF mice, but once it was treated with H2S, the number of apoptotic cells significantly decreased. Although the TUNEL staining may not show clear nuclei, which can show apoptosis, the TUNEL is in the nuclei. Moreover, we supported the TUNEL data with Western blot analysis of caspase-3 [an enzyme that cleaves poly(ADP-ribose) polymerase-1 (PARP); Fig. 6]. These complementary results improved the reliability of TUNEL measurements. Therefore, H2S did not merely revitalize the interstitial tissues, but rather decreased apoptosis and maintained the normal homeostasis of ECM.
DISCUSSION
The study demonstrated, mechanistically, that the cardiac fibrosis and apoptosis in CHF were reversed by administration of H2S. Therefore, it is suggested that H2S played a significant role in attenuation of cardiac structure and function abnormalities in volume overload-induced CHF. In this study, we reported that chromic volume overload-induced CHF model caused pericapillary and interstitial fibroses and apoptosis, leading to structural and functional abnormalities. The treatment with H2S mitigated these abnormalities. However, it is possible that the changes in nitrotyrosine, MMP-2 and -9, TIMP-1 and -4, ADAM-12, TUNEL staining, and fibrosis were maintained at the same level or greater or were reduced up to 10 wk following AVF. In addition, some of the decreases in these measured parameters may occur independently of the NaHS treatment. However, we have performed the experiments with controls in each group. We concluded that the NaHS had significant effects on oxidative remodeling.
Chronic volume overload caused the robust increase in oxidative stress that decreased the availability of NO that, in turn, increased nitrotyrosine generation. In this way, the equilibrium shifted toward nitrotyrosine and MMP activation (24). High nitrotyrosine (Fig. 1) and MMP-2 and MMP-9 (Fig. 2) in 2 and 6 wk of AVF mice supported these findings. TIMP-1 and TIMP-3 increased in AVF (Fig. 3), suggesting their putative role in fibrosis and apoptosis. However, TIMP-4 decreased in AVF (Fig. 3), pointing to its role as cardioprotective metalloproteinase. Interestingly, when AVF mice that showed high oxidative and proteolytic levels were treated with H2S, they attained normal levels of nitrotyrosine, MMP-2, MMP-9, and TIMP-1, -3, and -4 (Figs. 1–3), revealing the cardioprotective role of H2S in mitigating oxidative and proteolytic stresses. These findings extended concrete support to other findings that H2S is a cardioprotective gas (3, 4, 8).
β1-Integrin was a surface signal molecule involved in matrix remodeling leading to CHF. It was documented that MMP-9 induced shedding of β1-integrin (6). There was a drastic decrease in β1-integrin level in 2- and 6-wk AVF mice, which was normalized after H2S treatment in 8- and 10-wk (Fig. 4). From this, we inferred that H2S was indulged in mitigating proteolytic effect of MMPs caused by oxidative stress. ADAM-12 was a signal molecule downstream to proteinase-activated receptor-1 (PAR-1) that was involved in matrix remodeling (16). In AVF mice, ADAM-12 level increased but normalized after H2S treatment (Fig. 4), supporting the role of H2S as antioxidant. The role of H2S as antioxidant and antiproteolytic agent was further confirmed at histological level (Fig. 5). High oxidative stress induced apoptosis, causing cell death. To assess the role of H2S in mitigating apoptosis, the number of apoptotic cells in AVF mice and H2S-treated AVF mice were scored. There was a significant decrease in apoptotic cells in H2S-treated mice, which further supported that H2S was an antiapoptotic gas (21).
Limitations.
In Fig. 2, we labeled pro-MMP and MMP bands distinctly. It was a paradox that sometimes (depending on the unfolding of the tertiary structure of MMP by the detergents in the sample buffer) the activity of pro-MMP can be seen in zymographic gels. However, this was not always the case. We observed marginal expression of MMPs in AVF at 0 wk. The decrease in activity in AVF + H2S 8- and 10-wk groups may be multifactorial (i.e., direct inhibition of MMP active site by H2S and/or the regression of MMP expression). Although MMP-2 was constitutively expressed in the normal myocardium, MMP-9 was inducible.
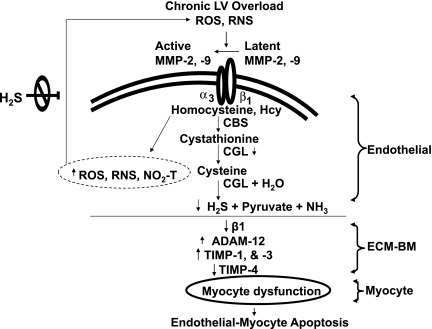
It is widely known that the amount of NaHS decays/evaporates very fast, within minutes or hours depending on the type of buffer or medium being used. Therefore, we controlled the concentration of NaHS in the drinking water by changing the drinking water every day. The findings of this study attempt to propose that H2S regulated MMPs in endothelium and thereby controls all the downstream pathways leading to CHF (Fig. 7). Finer dissection of biochemical reactions involved in the whole pathway will shed further light on this model and provide concrete support. H2S ameliorated oxidative stress by decreasing ROS and RNS in chronic volume overload-induced heart failure. It acted at two levels, first on ROS/RNS that convert latent MMPs into active MMPs outside the endothelium, and second on ROS/RNS presented inside endothelium that lead to fibrosis in ECM basement membrane (Fig. 7). Volume overload led to LV hypertrophy, causing endothelial myocyte apoptosis (Fig. 7). H2S controlled apoptosis of myocytes by regulating MMPs and TIMPs.
Fig. 7.
Schematic presentation of activation of latent MMPs by reactive oxygen and nitrogen species (ROS and RNS) causing integrin shedding and activation, leading to decreased H2S production, interendothelial myocyte fibrosis, and capillary endothelial myocyte death. The treatment with H2S mitigates ROS and RNS stress and endothelial myocyte apoptosis/dysfunction. CBS, cystathionine β-synthase; CGL, cystathionine γ-lyase; ECM-BM, ECM basement membrane.
GRANTS
This study was supported, in part, by National Institutes of Health Grants HL-71010, HL-74185, and HL-88012.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 over expression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest 101: 1478–1487, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia M. Hydrogen sulfide as a vasodilator. IUBMB Life 57: 603–606, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res 105: 365–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang L, Geng B, Yu F, Zhao J, Jiang H, Du J, Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids 34: 573–585, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Chen CQ, Xin H, Zhu YZ. Hydrogen sulfide: third gaseous transmitter, but with great pharmacological potential. Acta Pharmacol Sin 28: 1709–1716, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Cox MJ, Hawkins UA, Hoit BD, Tyagi SC. Attenuation of oxidative stress and remodeling by cardiac inhibitor of metalloproteinase protein transfer. Circulation 109: 2123–2128, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Cox MJ, Sood HS, Hunt MJ, Chandler D, Henegar JR, Aru GM, Tyagi SC. Apoptosis in the left ventricle of chronic volume overload causes endocardial endothelial dysfunction in rats. Am J Physiol Heart Circ Physiol 282: H1197–H1205, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104: 15560–15565, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal 8: 661–670, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18: 1165–1167, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Lindsay MM, Maxwell P, Dunn FG. TIMP-1: a marker of left ventricular diastolic dysfunction and fibrosis in hypertension. Hypertension 40: 136–141, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Lowicka E, Beltowski J. Hydrogen sulfide (H2S) - the third gas of interest for pharmacologists. Pharmacol Rep 59: 4–24, 2007 [PubMed] [Google Scholar]
- 13.Moshal KS, Tyagi N, Moss V, Henderson B, Steed M, Ovechkin A, Aru GM, Tyagi SC. Early induction of matrix metalloproteinase-9 transduces signaling in human heart end stage failure. J Cell Mol Med 9: 704–713, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mujumdar VS, Smiley LM, Tyagi SC. Activation of matrix metalloproteinase dilates and decreases cardiac tensile strength. Int J Cardiol 79: 277–286, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Mujumdar VS, Tyagi SC. Temporal regulation of extracellular matrix components in transition from compensatory hypertrophy to decompensatory heart failure. J Hypertens 17: 261–270, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Ovechkin AV, Tyagi N, Rodriguez WE, Hayden MR, Moshal KS, Tyagi SC. Role of matrix metalloproteinase-9 in endothelial apoptosis in chronic heart failure in mice. J Appl Physiol 99: 2398–2405, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Papaioannou VE, Fox JG. Efficacy of tribromoethanol anesthesia in mice. Lab Anim Sci 43: 189–192, 1993 [PubMed] [Google Scholar]
- 18.Patacchini R, Santicioli P, Giuliani S, Maggi CA. Hydrogen sulfide (H2S) stimulates capsaicin-sensitive primary afferent neurons in the rat urinary bladder. Br J Pharmacol 142: 31–34, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 75: 346–359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sen U, Vacek T, Kumar M, Moshal KS, Hayden MR, Tyagi SC. Cardioprotective role of sodium thiosulfate on chronic heart failure by modulating endogenous H2S generation. Pharmacology 82: 201–213, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Sivarajah A, Collino M, Yasin M, Benetti E, Gallicchio M, Mazzon E, Cuzzocrea S, Fantozzi R, Thiemermann C. Anti apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock 31: 267–274, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Sood HS, Cox MJ, Tyagi SC. Generation of nitrotyrosine precedes activation of metalloproteinase in myocardium of hyperhomocysteinemic rats. Antioxid Redox Signal 4: 799–804, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal 1: re6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Tarone RE. A modified Bonferroni method for discrete data. Biometrics 46: 515–522, 1990 [PubMed] [Google Scholar]
- 26.Teng X, Isbell TS, Crawford JH, Bosworth CA, Giles GI, Koenitzer JR, Lancaster JR, Doeller JE, Kraus DW, Patel RP. Novel method for measuring S-nitrosothiols using hydrogen sulfide. Methods Enzymol 441: 161–172, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Huang H, Liu P, Tang C, Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening K ATP channels. Can J Physiol Pharmacol 85: 1248–1253, 2007 [DOI] [PubMed] [Google Scholar]