Abstract
The augmentation index predicts cardiovascular mortality and is usually explained as a distally reflected wave adding to the forward wave generated by systole. We propose that the capacitative properties of the aorta (the arterial reservoir) also contribute significantly to the augmentation index and have calculated the contribution of the arterial reservoir, independently of wave reflection, and assessed how these contributions change with aging. In 15 subjects (aged 53 ± 10 yr), we measured pressure and Doppler velocity simultaneously in the proximal aorta using intra-arterial wires. We calculated the components of augmentation pressure in two ways: 1) into forward and backward (reflected) components by established separation methods, and 2) using an approach that accounts for an additional reservoir component. When the reservoir was ignored, augmentation pressure (22.7 ± 13.9 mmHg) comprised a small forward wave (peak pressure = 6.5 ± 9.4 mmHg) and a larger backward wave (peak pressure = 16.2 ± 7.6 mmHg). After we took account of the reservoir, the contribution to augmentation pressure of the backward wave was reduced by 64% to 5.8 ± 4.4 mmHg (P < 0.001), forward pressure was negligible, and reservoir pressure was the largest component (peak pressure = 19.8 ± 9.3 mmHg). With age, reservoir pressure increased progressively (9.9 mmHg/decade, r = 0.69, P < 0.001). In conclusion, the augmentation index is principally determined by aortic reservoir function and other elastic arteries and only to a minor extent by reflected waves. Reservoir function rather than wave reflection changes markedly with aging, which accounts for the age-related changes in the aortic pressure waveform.
Keywords: arteries, blood pressure, wave reflection
a greater understanding of the mechanisms responsible for the morphology of the aortic pressure waveform may further our understanding of studies such as Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) and Second Australian National Blood Pressure Trial (ANBP2), where there were lower event rates with newer antihypertensive regimes (e.g., angiotensin-converting enzyme- and calcium channel blocker-based therapy) than older regimes (β-blocker- and diuretic-based therapy), despite an equivalent brachial blood pressure (8, 9, 34). The augmentation index (AIx) is a measure of the pressure increment from the shoulder of the systolic waveform, normalized to pulse pressure, and is considered a proxy measure of wave reflection (21, 23, 26). Aortic (or central) AIx can be readily estimated from the pulse waveform measured directly at the carotid or radial arteries with (3, 4) or without (5) the use of a transfer function. However, studies examining the relationship between the AIx and cardiovascular events or mortality have produced disparate conclusions (6, 7, 10, 18, 31).
One explanation of these contradictory findings is that the AIx depends on both the timing and magnitude of reflected waves and that differential changes in each of these factors (e.g., with age) may account for inconsistencies. An alternative explanation is that the AIx is not simply or even largely a result of discrete reflected waves (33). Indeed, a metaanalysis of the published literature has failed to identify the changes in reflection timing on which the current hypotheses for pressure augmentation are based (1).
Recent studies have proposed that the capacitive or reservoir function of the aorta and large elastic arteries plays a major (and neglected) role in determining the morphology of the pulse waveform (30) and that the pressure waveform can be explained in terms of a reservoir pressure related to arterial compliance and an “excess” or wave-related pressure because of traveling waves, as first proposed by Lighthill (17). In anesthetized dogs, reflected waves were negligible in the proximal aorta after allowance for reservoir pressure, implying as originally suggested by Womersley that the design of the arterial tree minimizes backward wave reflections (36). Therefore, we hypothesized that once reservoir pressure is taken into account, the magnitude of reflections would be minimal in the human aorta.
We tested this hypothesis by using a “wave reservoir” model, which calculates reservoir pressure on the basis of the pressure and flow velocity waveforms. This approach was used to examine the relative contributions of longitudinal waves and the reservoir to the morphology of the aortic pressure waveform and to the magnitude of the AIx. Furthermore, we go on to explore how the reservoir and wave pressure components change with aging to determine the shape of the arterial pressure waveform.
METHODS
Subjects.
Eighteen subjects in whom the probability of coronary artery disease was considered relatively low were recruited from a group of patients scheduled for coronary angiography. All subjects rested in bed for 1 h before angiography. Exclusion criteria included previous coronary intervention, valvular pathology, regional wall motion abnormality, and non-sinus rhythm. All subjects had good left ventricular function with ejection fraction exceeding 55%. All subjects were required to refrain from coffee and alcohol for at least 12 h and fasted for at least 9 h before the beginning of the study. Subjects who smoked were asked to refrain from smoking for at least 24 h before the study. All subjects received heparin (5,000 units intravenously) before the hemodynamic measurement phase; no other drugs were administered. Each subject gave written informed consent for participation in this study, which was approved by our local ethics committee. All studies were performed in accordance with our institution's guidelines. Of the 18 subjects screened, 15 were found to have no significant coronary artery disease on angiography (characteristics of these subjects are shown in Table 1), and these individuals proceeded to have measurements of aortic flow velocity and pressure. A 0.014-in.-diameter pressure wire (Wavewire) and a Doppler-flow wire (Flowwire, Volcano Therapeutics, formerly Jomed) were positioned 5 cm from the aortic valve using fluoroscopy. Each wire was carefully positioned to ensure that its sensor tip was aligned and that there was a stable signal. Concurrent analog output feeds were taken from an electrocardiogram and Wavewire and Flowwire consoles into a National Instruments DAQ-Card AI-16E-4 and acquired at 1 kHz using Labview. The recorded data were analyzed off-line using custom-written Matlab software (Mathworks, Natick, MA). The blood pressure and Doppler velocity recordings were filtered using a least-squares (Savitzky-Golay) polynomial smoothing filter (25) and ensemble-averaged using the ECG R wave as a fiducial point.
Table 1.
Data for continuous variables or categories
| Variable | Value |
|---|---|
| Subjects recruited | 18 |
| Age, yr | 54 ± 10.3 |
| Female sex | 13 (68) |
| Height, cm | 164 ± 8.9 |
| Weight, kg | 75 ± 15 |
| Blood pressure, mmHg | |
| Systolic | 151 ± 22 |
| Diastolic | 82 ± 12 |
| Total cholestrol, mmol/l | 5.02 ± 1.1 |
| HDL cholestrol, mmol/l | 1.6 ± 0.9 |
| Triglycerides, mmol/l | 1.5 ± 0.9 |
| History of diabetes mellitus, n (%) | 0 (0) |
| History of ischemic heart disease, n (%) | 4 (21) |
| History of hypertension, n (%) | 11 (58) |
| History of smoking, n (%) | 9 (47) |
| Drugs | |
| Asprin, n (%) | 9 (47) |
| Statin, n (%) | 8 (42) |
| Calcium antagonist, n (%) | 2 (11) |
| β-Blocker, n (%) | 4 (21) |
| Angiotensin II blocker, n (%) | 1 (5) |
| α-Blocker, n (%) | 1 (5) |
Data are means ± SD or n (%).
AIx and augmentation pressure calculations.
We used a conventional approach that defines the beginning of augmentation as the shoulder or inflection point (using the fourth-derivative method). At that instant in time, a reference value was taken for each of the two or three pressure components (forward wave pressure, backward wave pressure, and when included, reservoir pressure, Fig. 3). The highest subsequent value of that pressure component minus its reference value at the time of inflection is defined as the pressure augmentation by that component. The AIx was calculated as previously described (21).
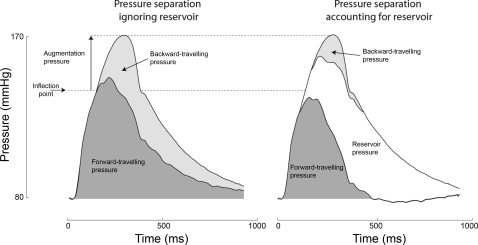
Fig. 3.
Calculation of the components of augmentation pressure ignoring (left) and accounting for (right) the aortic reservoir pressure. Pressure was separated first using conventional separation technique, which ignores the reservoir pressure, and then using the wave reservoir technique, which accounts for aortic reservoir pressure. Augmentation pressure was calculated as the rise in pressure between the inflection point and peak pressure. With the use of the wave-only analysis (left; which ignores the aortic reservoir), the augmentation pressure is composed predominantly from backward-traveling pressure with a small contribution from the ongoing forward-traveling pressure. When the reservoir is accounted for (right), pressure augmentation primarily arises from reservoir pressure, with a far smaller contribution arising from backward-traveling pressure. The forward-traveling pressure was found to no longer contribute.
As separated pressure is potentially sensitive to the imputed timings, we also calculated the area under the curve (or the pressure integral) over the complete cardiac cycle for each component, as a more time-independent measure.
Calculation of the arterial reservoir pressure and pressure separation.
During left ventricular contraction, blood enters the aorta faster than it can leave; this increasing volume distends the aorta, increasing the reservoir pressure (12). The reservoir pressure generated is therefore determined by the instantaneous difference between inflow and outflow and the arterial compliance. The relationship between reservoir pressure and flow into the reservoir can also be viewed as the transverse impedance (19). During diastole, there is no inflow through the aortic valve and reservoir pressure declines quasiexponentially as blood leaves the aorta. We calculated the reservoir pressure using pressure and flow velocity as described by Wang et al. (30). Following the calculation of the reservoir pressure (Fig. 1, label 1), the pressure attributable to longitudinal waves was calculated by subtracting the reservoir pressure from the measured pressure (Fig. 1, label 2). Forward (Eq. 1) and backward (Eq. 2) pressures were then calculated using wave intensity analysis. This time domain approach gives essentially identical results to wave separation performed using frequency domain (impedance)-based approaches (28). An analysis was undertaken for wave-only and wave-reservoir models (Fig. 1, label 3), where dP is the incremental change in the measured or wave pressure, and dU the incremental change in blood velocity. ρ is the density of blood (taken as 1,050 kg/m3), and c is the wave speed calculated using the single-point equation (11).
| (1) |
| (2) |
The reflection coefficient (calculated as peak Pbackward/peak Pforward), separated pressures, augmentation pressure, and AIx were calculated by both ignoring and accounting for the arterial reservoir.
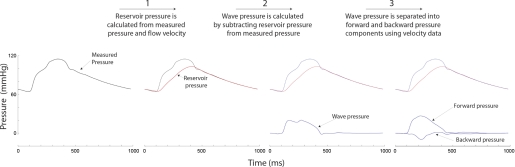
Fig. 1.
Illustration of the 3-stage process used to separate measured pressure into its reservoir, forward, and backward pressure components. Reservoir pressure is calculated from measured pressure and velocity. Wave pressure is calculated by subtracting reservoir pressure from measured pressure. Wave pressure is separated into forward and backward pressure components using velocity data.
Reproducibility.
The reproducibility of hemodynamic measurements was assessed by examining separate 30-s recordings of blood pressure and velocity for each patient. The standard deviation of the difference between these replicate recordings in the aorta was ±5.9 mmHg for mean blood pressure (5% within subject coefficient of variation) and ±0.31 ms−1 for mean Doppler velocity (16% within subject coefficient of variation).
Statistical analysis.
StatView 5.0 (SAS Institute, Cary, NC) was used for statistical analyses. Continuous variables are reported as means ± SE and categorical variables as n (in %). Comparisons were made using Student's t-test for continuous data, and a χ2 test was used for categorical variables. Associations were examined using linear regression. P < 0.05 was taken as statistically significant.
RESULTS
The effect of the reservoir pressure on the calculated separated pressures.
An example of a pressure waveform separated into forward and backward components, with and without inclusion of the reservoir pressure, is shown in Fig. 2. Accounting for the reservoir pressure markedly reduced peak backward (reflected) pressure by 88% (P < 0.001), peak forward pressure by 33% (P < 0.001), and the reflection coefficient by 81% (P < 0.001, Table 2).
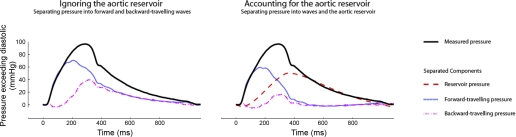
Fig. 2.
Impact of accounting for the arterial reservoir pressure on the calculated forward- and backward-traveling pressure waves. The pressure wave is separated into its constituent components ignoring (left) and then accounting for (right) the aortic reservoir. When the aortic reservoir is ignored, both the forward and backward peak pressures are substantial and during diastole, they are equal and decrease slowly and continuously. When the reservoir pressure is taken into account, the backward-traveling (reflected) wave is significantly reduced and forward and backward wave pressures are absent following closure of the aortic valve.
Table 2.
Constituents of pulse pressure, systolic pressure, and augmentation pressure (peak) with and without accounting for the reservoir
| Variable | Ignoring Reservoir | Accounting for Reservoir | P |
|---|---|---|---|
| Pulse pressure, mmHg | |||
| Forward | 45.0 ± 3.1 | 30.0 ± 2.3 | <0.001 |
| Backward | 20.1 ± 1.3 | 2.6 ± 0.5 | <0.001 |
| Reservoir | — | 44.3 ± 3.5 | — |
| Reflection coefficient, % | 45 | 13 | <0.001 |
| Systolic pressure, mmHg | |||
| Forward | 101.7 ± 3.8 | 57.4 ± 7.4 | <0.001 |
| Backward | 45.6 ± 1.8 | 5.0 ± 1.7 | <0.001 |
| Reservoir | — | 84.8 ± 11.3 | |
| Augmentation pressure, mmHg | |||
| Forward | 6.5 ± 2.2 | — | — |
| Backward | 16.2 ± 1.8 | 5.8 ± 4.4 | <0.001 |
| Reservoir | — | 19.8 ± 9.3 |
Values are means ± SE. Pulse pressure, systolic pressure, and augmentation pressure were separated into their respective forward and backward ± reservoir pressure. The pulse pressure components were calculated after subtraction of diastolic pressure. After accounting for the reservoir pressure, both forward and backward pressure was significantly reduced. Statistical comparisons were made using a paired Student's t-test.
Calculation of constituent components of the augmentation pressure.
Augmentation pressure (the rise in pressure from the inflection point to peak systolic pressure) was 22.7 ± 13.9 mmHg, and the AIx was 33 ± 10%.
When the pressure constituents of the AIx were calculated ignoring the arterial reservoir, the peak backward (reflected) pressure component of the augmentation pressure was larger than the peak forward pressure component (16.2 ± 7.6 vs. 6.5 ± 9.4 mmHg, P < 0.001, Table 2) and backward pressure comprised 71% of the AIx (Fig. 3, left). However, when the pressure constituents of the AIx were calculated accounting for the arterial reservoir, the calculated backward (reflected) pressure was no longer the principal constituent of augmented pressure. Backward (reflected) pressure only accounted for 5.8 ± 4.3 mmHg of augmentation pressure and only 25% of the AIx (a reduction of 64% compared with findings ignoring the reservoir pressure). The reservoir pressure was the largest component of the augmentation pressure (19.8 ± 9.2 mmHg, P < 0.001, Table 2) and AIx (87%, Fig. 3, right), and there was little or no contribution from the forward-pressure component.
Reservoir phenomenon and wave speed.
We examined the relationship between the magnitude of the reservoir pressure and aortic pulse wave velocity measured using the foot-to-foot technique over a 50-cm length of aorta (distal from the aortic root). Reservoir pressure was closely correlated to pulse-wave velocity squared (r = 0.67, P < 0.001), which is not surprising since the square of the wave speed is inversely related to the distensibility of the aorta. With the use of regression analysis, pulse-wave velocity was found to increase by 3.42 m/s for every 10 mmHg increase in reservoir pressure.
Changes in magnitude and timing of pressure constituents with age.
We applied the same analysis over the entire pressure waveform to assess the changes in forward, backward, and reservoir pressures that occur with age. The whole group included subjects with characteristically different-shaped pressure waveforms (i.e., type A, type B, and type D beats) (2). The augmentation pressure and AIx both increased with aging. When the aortic reservoir was ignored, both the forward and backward pressures increased with aging (Table 3). However, when the aortic reservoir was accounted for, the forward pressure continued to increase with aging (Table 3, and Fig. 4A) but the backward pressure was no longer found to increase (Table 3, and Fig. 4B). The reservoir pressure was found to markedly increase with aging (Table 3, and Fig. 4C). These findings are essentially identical when applied using either pulse pressure or systolic pressure waveforms.
Table 3.
The relationship between age and forward, backward, and reservoir components of the pulse pressure waveform
| Component | Age Dependence Pulse (Systolic) Pressure, mmHg/decade | r2 | P |
|---|---|---|---|
| Ignoring reservoir | |||
| Forward | 11.8 (11.1) | 0.4 | 0.009 |
| Backward | 4.8 (4.5) | 0.41 | 0.008 |
| Accounting for reservoir | |||
| Forward | 3.6 (4.1) | 0.36 | <0.02 |
| Backward | 0.13 (0.15) | 0.004 | 0.82 |
| Reservoir | 9.9 (11.3) | 0.69 | <0.001 |
The pulse pressure and systolic pressure waveforms were separated into wave and reservoir components before and after accounting for the reservoir. Before the accounting for the reservoir pressure, both forward and backward pressure increased with increasing age. After the accounting for the arterial reservoir, reservoir pressure increased rapidly with age (note that aging explained 69% of the variance in reservoir pressure), exceeding the age-related increases in forward pressure. However, backward pressure was found to no longer increase with age. Observations were very similar when using either pulse pressure or systolic pressure waveforms.
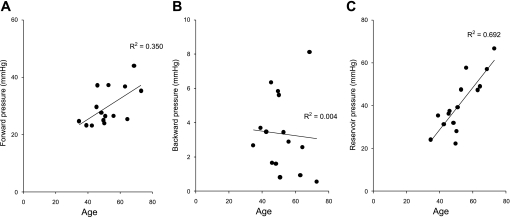
Fig. 4.
Relationship between separated pressure components and aging after accounting for the aortic reservoir pressure. The pressure waveform was separated into forward (A) and backward (B) and reservoir (C) pressure components. With increasing age, both forward and arterial reservoir pressure increased. However, the backward pressure component was not significantly correlated with age.
DISCUSSION
In this study, we have used a novel “wave reservoir” model to analyze the components making up the aortic pressure waveform in humans. In contrast to widely held assumptions, after accounting for the reservoir, the contribution from reflected waves is small, although not absent, and reservoir pressure is the dominant contributor to the AIx and augmentation pressure. Increases in both the arterial reservoir pressure and forward pressure and not in distal reflection explained the change in the aortic pressure waveform with aging.
Our study confirms previous observations regarding an increase in the AIx and augmentation pressure with aging but does not address the potential utility of the AIx as a clinical predictor of disease. Importantly, however, it provides a novel mechanistic insight into the factors responsible for the shape of the aortic pressure waveform in humans by demonstrating that while the onset of pressure augmentation (the shoulder) is determined by the arrival of the backward-traveling (reflected) wave, the degree of augmentation is principally determined by the arterial reservoir.
The concept of an arterial reservoir used in this study bears some similarities to the two-element windkessel concept introduced by Frank (12) and later developed into a three-element model (32). The two-element windkessel is now rarely used in hemodynamic modeling because of its zero-dimensional nature (i.e., it assumes an infinite wave speed) and its inability to accurately predict the pressure waveform in systole. Unlike the two-element windkessel, we do not propose that local loading involves instantaneous integration across the total compliance of the entire system but rather that the proximal aorta can be considered as one of a number of distributed elements, i.e., a transmission line model. Transmission line models give a more comprehensive description of the origins of the arterial pressure and flow waveforms but at the expense of computational and interpretative complexity. The wave-reservoir concept is a reduced model that draws a distinction between local (transverse) and distal (longitudinal) influences by employing a lumped model for the former while accommodating longitudinal wave travel in the latter. Wave speed is not assumed to be infinite in the wave-reservoir model, except in the local segment, which is assumed to be hydrodynamically compact (i.e., short in relation to the wave speed), permitting the application of a windkessel-type analysis.
As a result of the limitations of the windkessel-only model applied to the whole circulation, the traveling-wave paradigm and its associated analytical techniques have been widely adopted. These approaches successfully describe the shape of the pressure and flow waveforms, but pressure waveform separation results in a biologically implausible phenomenon: simultaneous “self canceling” forward- and backward-traveling waves in diastole. This is an inevitable consequence of the linear assumptions employed in wave separation and the neglect of a reservoir. During diastole, inflow into the proximal aorta is nearly zero, while pressure falls in a quasiexponential fashion. Consequently, any linear separation technique will result in forward and backward pressure with nearly equal magnitudes in diastole.
We propose that this phenomenon of self-canceling flow waves and additive pressure waves arises as a result of neglecting the increase in volume resulting from radial distension, which is substantial in the aorta and elastic arteries (24). Indeed, around 40% of stroke volume ejected in systole is stored in the distended elastic arteries (30). The aortic reservoir pressure, by definition, is proportional to the volume of blood stored in the aorta, which in turn depends on the compliance of the aorta and the impedance to outflow. Downstream impedance mismatching and wave reflection in will therefore contribute to the magnitude of the reservoir pressure. In effect, the arterial reservoir acts like a water tower (or multiple interconnecting water towers) storing volume in ejection and damping the pressure pulse and discharging the stored volume once ejection ceases. A subtraction of the reservoir pressure accounts for the potential energy stored in the reservoir and permits linear wave separation techniques to be applied.
Wave reflection, previously thought to be the major constituent of augmentation pressure and diastolic pressure, arises at sites of impedance mismatch (e.g., at branches) (13, 22). While such discrete reflections do occur and may have substantial magnitude locally, the arrangement of the arterial tree markedly attenuates the backward travel of waves such that a limited amount of reflection is evident in the proximal aorta once the arterial reservoir is accounted for (30, 33, 35). In this regard, our observations are consistent with a previous invasive study of healthy children (27) and other studies in anesthetized dogs (16).
While wave reflection is not completely abolished after reservoir subtraction, our observations indicate that the AIx is not predominantly a measure of wave reflection but rather is largely due to the compliant properties of the aorta and other elastic arteries (15, 20). As the aorta becomes stiffer (i.e., its compliance falls) with increasing age or disease, pressure in the arterial reservoir rises more rapidly for a similar increase in volume and reaches higher levels (Fig. 4, and Table 3) (29). This results in an increase in the AIx or augmentation pressure. This phenomenon, namely a change in local properties causing a change in the aortic waveform, has been observed in experiments that demonstrated acute changes in the pressure augmentation (akin to the pattern seen in aging) by applying a relatively noncompliant Teflon graft to the elastic portion of the aorta of healthy pigs (14) and dogs (15), without changes being made to the distal reflection sites (14).
Study limitations.
In this study the mean age of our patients was 54 yr, ranging between 35–73 yr. While it was possible to demonstrate a clear difference in the timing of wave reflection between young, middle-aged, and elderly adult subjects, it is possible that the timing and magnitude of the reflected waves may differ in subjects even younger than those studied here. It is also possible that, in much younger subjects, with a fall in pressure after the shoulder (so-called C-type waveforms) or in subjects with markedly elevated heart rates, there may be greater difficulty in fitting the monoexponential segment of the reservoir pressure, although the concept of the reservoir phenomenon would still hold true. Ethically, it was only possible for us to make hemodynamic measurements in subjects already undergoing coronary angiography on clinical grounds, and subjects younger than 35 yr rarely require this invasive procedure.
Conclusions.
The arterial reservoir makes a large contribution to the aortic blood pressure waveform in humans and is the principal component of the AIx. In contrast, wave reflection only made a minor contribution to the AIx. This reservoir pressure is the aggregate pressure resulting from the net difference between the total arterial system inflow and outflow divided by an effective arterial compliance. Reservoir pressure increases markedly with aging, probably as a result of decreased compliance, and this is the major factor accounting for the associated change in morphology of the aortic pressure waveform. Modifying the behavior of the arterial reservoir rather than changing wave reflection may be a useful target for future therapy.
GRANTS
This work was funded by a grant from the Coronary Flow Trust. J. E. Davies (FS/05/006), D. P. Francis (FS/04/079), N. Hadjiloizou (FS/05/034), and Z. I. Whinnett (FS/05/068) are British Heart Foundation fellows. C. H. Manisty (077049/Z/05/Z) is funded by the Welcome Trust. J. E. Davies, D. P. Francis, A. D. Hughes, I. S. Malik, and J. Mayet acknowledge the support of the National Institute of Health Research Biomedical Research Centre funding scheme.
DISCLOSURES
None of the authors has a conflict of interest to disclose.
REFERENCES
- 1.Baksi AJ, Treibel TA, Davies JE, Hadjiloizou NF, Parker KH, Francis DP, Mayet J, Hughes AD. A meta-analysis of mechanism of blood pressure change with aging. J Am Coll Cardiol 54: 2087–2092, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Broemser PR. Ueber die Messung des Schlagvolumens des Herzens auf unblutigem Weg. Z Biol 90: 467–507, 1930 [Google Scholar]
- 3.Cameron JD, McGrath BP, Dart AM. Use of radial artery applanation tonometry and a generalized transfer function to determine aortic pressure augmentation in subjects with treated hypertension. J Am Coll Cardiol 32: 1214–1220, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 95: 1827–1836, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Chen CH, Ting CT, Nussbacher A, Nevo E, Kass DA, Pak P, Wang SP, Chang MS, Yin FC. Validation of carotid artery tonometry as a means of estimating augmentation index of ascending aortic pressure. Hypertension 27: 168–175, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, Perez G, Mendez AJ. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension 45: 980–985, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Covic A, Mardare N, Gusbeth-Tatomir P, Prisada O, Sascau R, Goldsmith DJ. Arterial wave reflections and mortality in haemodialysis patients—only relevant in elderly, cardiovascularly compromised? Nephrol Dial Transplant 21: 2859–2866, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 366: 895–906, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Dart AM, Cameron JD, Gatzka CD, Willson K, Liang YL, Berry KL, Wing LM, Reid CM, Ryan P, Beilin LJ, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Morgan TO, West MJ, Kingwell BA. Similar effects of treatment on central and brachial blood pressures in older hypertensive subjects in the Second Australian National Blood Pressure Trial. Hypertension 49: 1242–1247, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Dart AM, Gatzka CD, Kingwell BA, Willson K, Cameron JD, Liang YL, Berry KL, Wing LM, Reid CM, Ryan P, Beilin LJ, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Morgan TO, West MJ. Brachial blood pressure but not carotid arterial waveforms predict cardiovascular events in elderly female hypertensives. Hypertension 47: 785–790, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Davies JE, Whinnett ZI, Francis DP, Willson K, Foale RA, Malik IS, Hughes AD, Parker KH, Mayet J. Use of simultaneous pressure and velocity measurements to estimate arterial wave speed at a single site in humans. Am J Physiol Heart Circ Physiol 290: H878–H885, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Frank O. Die Grundform des Arteriellen Pulses. Erste AbhandlungMathematische Analyse Z Biol 37: 483–526, 1899 [Google Scholar]
- 13.Hope SA, Tay DB, Meredith IT, Cameron JD. Waveform dispersion, not reflection, may be the major determinant of aortic pressure wave morphology. Am J Physiol Heart Circ Physiol 289: H2497–H2502, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Ioannou CV, Stergiopulos N, Katsamouris AN, Startchik I, Kalangos A, Licker MJ, Westerhof N, Morel DR. Hemodynamics induced after acute reduction of proximal thoracic aorta compliance. Eur J Vasc Endovasc Surg 26: 195–204, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Kelly RP, Tunin R, Kass DA. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res 71: 490–502, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Khir AW, Zambanini A, Parker KH. Local and regional wave speed in the aorta: effects of arterial occlusion. Med Eng Phys 26: 23–29, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Lighthill M. Waves in Fluids Cambridge: Cambridge University Press, 1978 [Google Scholar]
- 18.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension 38: 434–438, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Milnor WR. Haemodynamics Baltimore-London: William & Wilson, 1982 [Google Scholar]
- 20.Mitchell GF, Conlin PR, Dunlap ME, Lacourciere Y, Arnold JM, Ogilvie RI, Neutel J, Izzo JL, Jr, Pfeffer MA. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension 51: 105–111, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol 17: 543–551, 2002 [DOI] [PubMed] [Google Scholar]
- 22.O'Rourke MF. Vascular impedance in studies of arterial and cardiac function. Physiol Rev 62: 570–623, 1982 [DOI] [PubMed] [Google Scholar]
- 23.O'Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension 45: 652–658, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Rushmer RF, Crystal DK. Changes in configuration of the ventricular chambers during the cardiac cycle. Circulation 4: 211–218, 1951 [DOI] [PubMed] [Google Scholar]
- 25.Savitzky A, Golay MJ. Smoothing and differentiation of data by simplified least squares procedures. Analytical Chemistry 36: 1627–1639, 1964 [Google Scholar]
- 26.Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De BD, Van Bortel LM, De BG, Gillebert TC, Verdonck PR. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension 49: 1248–1255, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Senzaki H, Chen CH, Ishido H, Masutani S, Matsunaga T, Taketazu M, Kobayashi T, Sasaki N, Kyo S, Yokote Y. Arterial hemodynamics in patients after Kawasaki disease. Circulation 111: 2119–2125, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Swillens A, Segers P. Assessment of arterial pressure wave reflection: Methodological considerations. Artery Res In press [Google Scholar]
- 29.Vermeersch SJ, Rietzschel ER, De Buyzere ML, Van Bortel LM, Gillebert TC, Verdonck PR, Segers P. The reservoir pressure concept: the 3-element windkessel model revisited? Application to the Asklepios population study. J Eng Math 64: 417–428, 2009 [Google Scholar]
- 30.Wang JJ, O'Brien AB, Shrive NG, Parker KH, Tyberg JV. Time-domain representation of ventricular-arterial coupling as a windkessel and wave system. Am J Physiol Heart Circ Physiol 284: H1358–H1368, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 109: 184–189, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Westerhof N, Bosman F, De Vries CJ, Noordergraaf A. Analog studies of the human systemic arterial tree. J Biomech 2: 121–143, 1969 [DOI] [PubMed] [Google Scholar]
- 33.Westerhof N, Sipkema P, Van Den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res 6: 648–656, 1972 [DOI] [PubMed] [Google Scholar]
- 34.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 113: 1213–1225, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Womersley JR. Mathematical theory of oscillating flow in an elastic tube. J Physiol 127: 37–8P, 1955 [PubMed] [Google Scholar]
- 36.Womersley JR. Oscillatory flow in arteries. II. The reflection of the pulse wave at junctions and rigid inserts in the arterial system. Phys Med Biol 2: 313–323, 1958 [DOI] [PubMed] [Google Scholar]