Abstract
Previously, we showed that sulfaphenazole (SUL), an antimicrobial agent that is a potent inhibitor of cytochrome P4502C9, is protective against ischemia-reperfusion (I/R) injury (Ref. 15). The mechanism, however, underlying this cardioprotection, is largely unknown. With evidence that activation of autophagy is protective against simulated I/R in HL-1 cells, and evidence that autophagy is upregulated in preconditioned hearts, we hypothesized that SUL-mediated cardioprotection might resemble ischemic preconditioning with respect to activation of protein kinase C and autophagy. We used the Langendorff model of global ischemia to assess the role of autophagy and protein kinase C in myocardial protection by SUL during I/R. We show that SUL enhanced recovery of function, reduced creatine kinase release, decreased infarct size, and induced autophagy. SUL also triggered PKC translocation, whereas inhibition of PKC with chelerythrine blocked the activation of autophagy in adult rat cardiomyocytes. In the Langendorff model, chelerythrine suppressed autophagy and abolished the protection mediated by SUL. SUL increased autophagy in adult rat cardiomyocytes infected with GFP-LC3 adenovirus, in isolated perfused rat hearts, and in mCherry-LC3 transgenic mice. To establish the role of autophagy in cardioprotection, we used the cell-permeable dominant-negative inhibitor of autophagy, Tat-Atg5K130R. Autophagy and cardioprotection were abolished in rat hearts perfused with recombinant Tat-Atg5K130R. Taken together, these studies indicate that cardioprotection mediated by SUL involves a PKC-dependent induction of autophagy. The findings suggest that autophagy may be a fundamental process that enhances the heart's tolerance to ischemia.
Keywords: protein kinase C, ischemia-reperfusion, cytochrome P-450
effective therapies to reduce or prevent ischemia-reperfusion (I/R) injury in humans remain elusive, despite a better understanding of the triggers, signaling pathways, and effectors that may be involved in preconditioning and postconditioning (14). These phenomena and the many pharmacological interventions that have been shown to condition the heart and confer protection appear to involve survival kinases, redox-sensitive mechanisms, PKC and mitochondrial ATP-sensitive K+ activation, and inhibition of mitochondrial permeability transition pore opening. Our laboratory and others (15, 27) have reported another approach to cardioprotection using sulfaphenazole (SUL), chloramphenicol, and cimetidine. Although these agents are known inhibitors of cytochrome P-450 (CYP) enzymes, their mechanism of cardioprotection is unknown.
Our laboratory recently reported that autophagy appears to be a necessary process involved in the cardioprotection conferred by 2-chloro-N6-cyclopentyladenosine, an adenosine receptor A1 agonist that has been shown to mimic ischemic preconditioning (45). Because of the possibility that SUL might share a common mechanism with 2-chloro-N6-cyclopentyladenosine and with ischemic preconditioning, we elected to investigate the role of autophagy in the myocardial protection afforded by SUL. Many studies of cardioprotection have demonstrated a role for protein kinase C. While there is controversy over the roles of various isozymes, most studies agree that chelerythrine blocks preconditioning mediated by a variety of inducing stimuli (3, 10, 11). I/R injury is associated with the formation of protein aggregates and damaged mitochondria, which can only be removed by autophagy. Autophagy may also benefit the cell by generating metabolic substrates (amino acids, free fatty acids, and glycogen) from intracellular stores through breakdown of proteins, organelles, and glycogen granules. For these reasons, we considered it likely that protection mediated by SUL would involve autophagy.
MATERIALS AND METHODS
Langendorff perfusion.
The isolated, perfused rat heart model was utilized as previously described (8, 16). In brief, after anesthesia and heparinization (pentobarbital sodium 60 mg/kg ip and heparin 500 U ip), rat hearts were excised into ice cold Krebs-Henseleit solution (in mM: 118.5 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.18 MgSO4, 25 NaHCO3, 11.1 glucose, 2.5 CaCl2) and perfused with oxygenated buffer within 30 s. Hearts were perfused at constant pressure (60 mmHg) for 5 min before administration of any drugs. Where indicated, SUL dissolved in dimethyl sulfoxide (SUL, 10 μM) was administered throughout the perfusion. For hemodynamic analysis, a balloon made by plastic wrap was inserted into the ventricle through the left atrium. Hemodynamic parameters were recorded with the EMKA system. All procedures were approved by the Animal Care and Use Committee at The Scripps Research Institute and at San Diego State University, and conform to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication no. 85-23, revised 1996).
Tat-Atg5K130R (∼200 nM), or Tat-β-galactosidase (∼200 nM) was infused for 15 min before ischemia. Inhibition of autophagy was accomplished using a cell-permeable agent, Tat-Atg5K130R, to selectively inhibit autophagy. This was necessary because the two widely used inhibitors of autophagy, 3-methyladenine and wortmannin, have broad, nonspecific effects that can confound the interpretation of the results. 3-Methyladenine alters intermediary metabolism and could have beneficial effects unrelated to its effects on autophagy (6), and wortmannin will inhibit not only the phosphatidylinositol 3-kinase involved in regulating autophagy, but also the phosphatidylinositol 3-kinase that is responsible for activating Akt (1, 33).
Where indicated, chelerythrine was added for 15 min before the onset of ischemia. Control hearts were perfused with a similar amount of DMSO (final concentration 0.01%). Global no-flow ischemia was maintained for 30 min, and reperfusion was accomplished by restoring flow. Creatine kinase (CK) release was measured in the coronary effluent of the first 15 min of reperfusion using the CK EC 2.7.3.2 UV test kit (Stanbio Laboratories). Infarct size determination by triphenyl tetrazolium chloride staining was performed on hearts reperfused for 120 min (8). Other biochemical analyses of ischemic and reperfused heart tissue were performed on hearts flash-frozen in liquid nitrogen at the times indicated.
Induction of autophagy in vivo and ex vivo.
mCherry-LC3 transgenic mice were given SUL (10 mg/kg) or vehicle by intraperitoneal injection; after 30 min, hearts were removed and processed for cryosection images and/or cadaverine assay. To quantitate the autophagosomes, cryosections were washed with PBS for 5 min. Red dots (mCherry-LC3-labeled autophagosomes) were then counted under the microscope. Hearts were subjected to global no-flow ischemia for 30 min, followed by 120-min reperfusion, then harvested and prepared for different assays, as described below, or tetrazolium chloride staining, as described above.
Preparation of recombinant Tat-Atg5K130R.
Recombinant protein expression and purification was performed as described by Becker-Hapak et al. (4). Briefly, a 100-ml LB-ampicillin overnight culture of Tat-Atg5K130R was grown at 37°C and 225 rpm to an 600-nm optical density of 0.9–1.2. The overnight culture was diluted into 1 liter of fresh LB-ampicillin and incubated to a 600-nm optical density of 0.6–0.9. Isopropylthiogalactoside (0.5 mM; Roche) was added to the culture and incubated for an additional 3 h. The bacterial pellet was harvested by centrifugation at 6,000 rpm for 15 min and resuspended in 20 ml 1× PBS. This was repeated twice, with the final pellet dissolved in 15 ml buffer Z (8 M urea, 100 mM NaCl, and 20 mM HEPES, pH 8.0) and left overnight at 4°C. The lysate was sonicated on ice three times for 15-s pulses, followed by centrifugation at 16,000 rpm for 30 min. The supernatant was saved and equilibrated in 10 mM imidazole. One-half was applied to a 25-ml column packed with 6 ml of Ni-NTA resin (Qiagen), equilibrated in buffer Z with 10 mM imidazole. The mixture was allowed to incubate at room temperature on a rocker for 1 h. The suspension was collected by gravity flow, and the flow through was reapplied onto the column twice. The column was washed with 50 ml of buffer Z containing 10 mM imidazole, and proteins were eluted in buffer Z containing 250 mM imidazole followed by another elution with buffer Z containing 1 M imidazole. Both elution fractions were pooled together and concentrated to one-half the volume using an Amicon Ultra centrifugation device (Millipore). The proteins were then desalted into 1× PBS plus 10% glycerol in 2.5-ml aliquots, eluted with 3.5 ml on a PD-10 column (GE Healthcare), and filtered through a 0.22-μm filter. Aliquots (200 μl) of purified fusion proteins were stored at −80°C until use.
Isolation and treatment of adult rat cardiomyocytes.
Isolation of adult rat cardiomyocytes was performed as previously described (21). Briefly, rat hearts were perfused with perfusion buffer (modified Krebs-Heinseleit buffer: 10 mM HEPES, 30 mM taurine, 2 mM carnitine, and 2 mM creatine in 500 ml Joklik's MEM, pH 7.3) for 4 min at 3 ml/min and then digested with digestion buffer (1 mg/ml of collagenase II, 6.25 μM CaCl2 in 50 ml perfusion buffer) for 18 min at 3 ml/min. The heart was then removed and minced in digestion buffer, to which stop buffer (perfusion buffer containing 12.5 μM CaCl2 and 5% newborn calf serum) was added. Cells were allowed to sediment by gravity for 8–10 min in a 50-ml Falcon tube. The supernatant was removed, and the pellet was resuspended in 30 ml of room temperature stop buffer. Calcium was then reintroduced to myocytes gradually to achieve a concentration of 1 mM, while being monitored by microscopy. Rod-shaped myocytes (100,000 per 2 ml) were plated in laminin-coated 35-mm dishes and allowed to recover for 6 h. Cells were infected with GFP-LC3 adenovirus for 2 h, washed, and cultured for 16 h in full medium containing 10% fetal calf serum and 10% newborn calf serum before exposure to SUL and chelerythrine. Chelerythrine was added to medium at a final concentration of 5 μM 10 min before the addition of SUL. Cells were treated with 10 μM SUL for 30 min, and autophagosomes (green dots) were quantified by fluorescence microscopy.
For assessment of subcellular distribution of PKC-δ, rod-shaped cardiomyocytes were plated in laminin-coated 35-mm MatTek glass bottom dishes (14-mm glass microwell). Following 15-min treatment with SUL or vehicle (control), cells were fixed with 4% paraformaldehyde for 15 min. Fixed cells were permeabilized with 0.3% Triton X-100/PBS for 10 min, blocked for 45 min in 3% BSA/0.3% Triton X-100/PBS, and stained with mouse anti-α-actinin (Sigma) and rabbit anti-PKC-δ (Sigma) and the respective secondary antibodies (mouse Alexa Fluor 488 and rabbit Alexa Fluor 546; Invitrogen). Imaging was performed at ×60 magnification using a Nikon TE300 fluorescence microscope.
Histological analysis and immunostaining.
Hearts were embedded in optimum cutting temperature, and 7-μm frozen sections were prepared. For immunostaining, tissue sections were immersed in acetone for 1–2 min at room temperature and then allowed to air dry. Samples were incubated in Tris-buffered saline buffer with 5% horse serum, 5% goat serum, and 0.3% Triton X-100 for 20 min, and then incubated for 2 h with primary antibody following the manufacturer's instruction (1:200 of LC3 antibody from Novus Bio and 1:500 anti-HA from Santa Cruz). Stained sections were observed through a Nikon TE300 fluorescence microscope (Nikon) equipped with a cooled charge-coupled device camera (Orca-ER, Hamamatsu).
Subcellular fractionation.
Frozen heart samples were thawed on ice in homogenization buffer containing (in mmol/l: 20 Tris·HCl, 2 EDTA, 10 EGTA, 1 PMSF, 0.1 leupeptin, 0.01 E-64, and 250 sucrose). The tissue was then minced and Polytron homogenized (Kinematica, Basel, Switzerland) on ice for 15 s for three passes. The homogenates were centrifuged at 600 g for 5 min at 4°C, and the crude supernatants were further centrifuged at 10,000 g for 10 min at 4°C. The supernatant, designated as crude cytosol, was divided, and one fraction was further centrifuged at 100,000 g for 1 h at 4°C. The resulting supernatant was designated as cytosolic fraction. The pellet was resuspended in homogenization buffer with 1% Triton X-100, incubated on ice for 1 h, and then centrifuged at 100,000 g for 1 h at 4°C. The resulting supernatant was designated as the particulate fraction. Samples were stored at −80°C until use.
Western blot analysis.
Proteins prepared from rat hearts were quantified by Bio-Rad protein assay. For immunodetection, 50 μg of crude cytosol, prepared as above, were resolved on SDS-PAGE 10% denaturing gels and transferred to PVDF nylon membranes. The membranes were blocked with 5% nonfat dry milk in TNT buffer [in mM: 100 NaCl, 10 Tris·HCl (pH 7.4), and 0.1% Tween 20] for 1 h. The blots were then incubated with 200-fold diluted primary antibodies against LC3 (Novus Biologicals, Littleton, CO) at 4°C overnight, or with 1,000-fold diluted primary antibodies against PKC-δ (Sigma) and PKC-ε (BD) at room temperature for 2 h. Membranes were washed with TNT buffer at room temperature and incubated with appropriate peroxidase-conjugated secondary antibody (1:2,000 dilution). Immunoreactive bands were visualized by chemiluminescence (ECL kit, Amersham) on X-ray film. Each immunoblotting experiment was repeated three to five times, and the results were averaged. To quantify the protein, intensity of bands was assessed with Scion Image Software.
Measurement of autophagy by cadaverine uptake.
Heart tissue from Langendorff-perfused rat hearts was minced in homogenization buffer (250 mM sucrose, 1 mM Na2EDTA, 10 mM HEPES, pH 7.0, plus fresh protease inhibitors), and homogenized by Polytron for 5 s at half speed. Nuclei and heavy membranes were removed by centrifugation at 1,000 g for 5 min at 4°C. The postnuclear supernatant was moved to new 1.5-ml centrifuge tubes and incubated with Alexa Fluor 488 cadaverine (Molecular Probes) at 25 μM final concentration for 10 min. The samples were spun at 20,000 g for 20 min at 4°C, and the pellet was washed twice with resuspension buffer (140 mM KCl, 10 mM MgCl2, 5 mM KH2PO4, 1 mM EGTA, 10 mM MOPS, pH 7.4, plus fresh protease inhibitors). The pellet was resuspended in 350-μl resuspension buffer, and the fluorescence intensity read on a 96-well plate reader at excitation/emission of 495/519 nm in triplicate. The relative fluorescence units were standardized to the protein concentration of each sample, which was determined by Bradford assay (Pierce). Our laboratory previously described the highly specific colocalization of monodansylcadaverine with mCherry-LC3 puncta (24 46) and subsequently found that the labeling could be performed on frozen heart tissue or homogenates (38). We also found that AlexaFluor488-cadaverine and BODIPY-TR-cadaverine (Invitrogen) were preferable to monodansylcadaverine because of greater selectivity, lower background signal, improved fluorescence properties, and slight improvement in the ability to preserve the signal after tissue fixation (38). These various approaches were consistent in their ability to reflect autophagy, and the advantage of the cadaverine incorporation method is that it can be used on frozen tissue samples and provides a quantitative result without the need for laborious point counting of microscopy fields. To further validate this method, we probed the pellet obtained after the 20,000 g spin for the presence of the autophagy marker protein, LC3. We detected LC3-II in the pellet (consistent with autophagosome membranes) and confirmed that the amount of LC3-II was proportional to the amount of cadaverine dye binding (data not shown).
Statistical analysis.
Statistical analysis was performed between groups by ANOVA by using INSTAT 4.10 software (GraphPad). A P value < 0.05 was considered significant.
RESULTS
SUL protects isolated, perfused rat hearts from I/R injury.
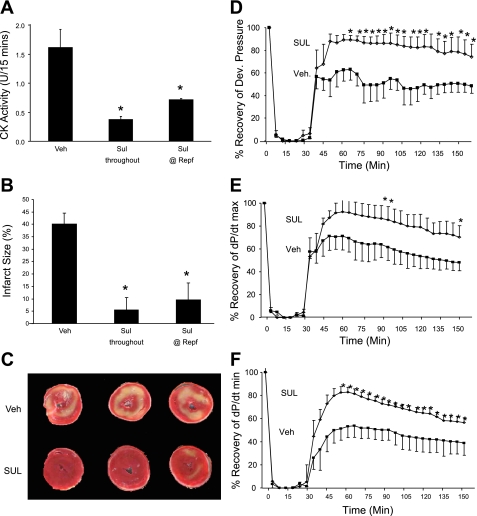
Here, we confirmed our laboratory's previous study that showed that SUL attenuated CK release and reduced infarct size (15). We extended the findings to measure hemodynamics and infarct size using 10 μM SUL introduced into the perfusion buffer 10 min before ischemia and maintained throughout reperfusion, or added only at the onset of reperfusion. As shown in Fig. 1, A–C, SUL administration attenuated CK release and reduced infarct size; the reduction of infarct size was sustained even when SUL was introduced at the onset of reperfusion. SUL had no effect on contractility before ischemia. SUL enhanced recovery of contractile function after I/R to ∼90% of preischemic values, whereas vehicle control hearts recovered only to ∼50% of preischemic values (Fig. 1, D–F).
Fig. 1.
Effects of sulfaphenazole (SUL) on ischemia-reperfusion (I/R) injury in isolated, perfused rat hearts. A: SUL or vehicle (Veh) was infused before 30 min of global no-flow ischemia, and coronary effluent was collected for the first 15 min of reperfusion (Repf) for determination of creatine kinase (CK) release. Mean and SD from at least five hearts per condition are shown. *P < 0.05. B: hearts treated as above were reperfused for 120 min, and infarct size was measured by triphenyl tetrazolium chloride (TTC) staining. C: representative slices of TTC-stained hearts are shown. D–F: preischemic SUL administration enhances recovery of function, as measured by recovery of developed pressure (D), maximum change in pressure over time (dP/dtmax; E), and minimum change in pressure over time (dP/dtmin; F). Means and SD from at least five hearts per condition are shown. *P < 0.05.
SUL induces autophagy.
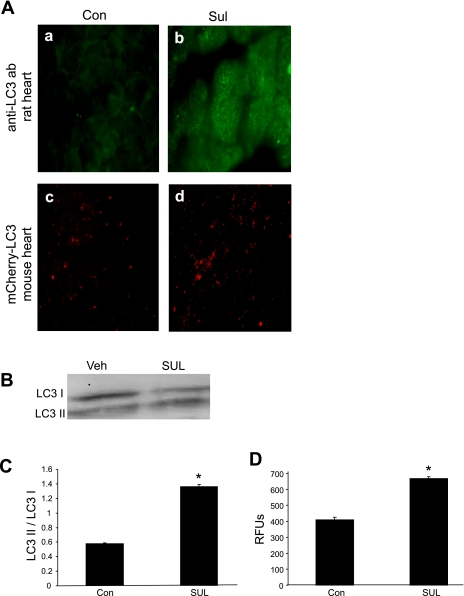
To determine whether SUL induced autophagy in the heart, isolated, perfused rat hearts were exposed to SUL for 30 min, and the distribution of autophagosomes (LC3 dots) was assessed by immunostaining (Fig. 2A, a and b). During the induction of autophagy, LC3 is proteolytically processed by Atg4 to expose a terminal glycine (LC3-I) and then is conjugated to phosphatidylethanolamine by Atg7, a specialized ubiquitin ligase. The lipidated LC3 is membrane-associated and has an altered mobility on SDS-PAGE (LC3-II). The conversion of LC3-I to LC3-II reflects autophagic flux. SUL administration resulted in a doubling of the ratio of LC3-II/I (Fig. 2, B and C). To confirm that the autophagy was upregulated specifically in cardiomyocytes, we used mCherry-LC3 transgenic mice, in which the transgene is under the control of the α-myosin heavy chain promoter, thereby restricting expression of the red fluorescent LC3 fusion protein to cardiomyocytes. There was a significant increase in the number of autophagosomes in the hearts of SUL-treated mice (Fig. 2A, c and d) and quantified by cadaverine assay in Fig. 2D. These results demonstrate that SUL induces autophagy in adult rat cardiomyocytes, in the isolated, perfused rat heart, and in the mouse heart in vivo.
Fig. 2.
SUL induces autophagy in rat and mouse hearts. A: rat hearts were perfused with Veh or SUL for 30 min and then fixed and immunostained for LC3 antibody (a and b). Veh or SUL was administered by intraperitoneal injection to mCherry-LC3 transgenic mice, and hearts were removed for tissue processing 60 min later (c and d). B: representative Western blot to detect LC3-I and LC3-II in rat hearts perfused with Veh or SUL. C: quantification of LC3-II/LC3-I. Experiments were repeated 4 times. *P < 0.05. D: quantification of autophagosomes (mCherry-LC3 puncta) in hearts of mice that received Veh or SUL. Values are means and SD; n = 6. RFU, relative fluorescence units. *P < 0.01.
SUL triggers redistribution of PKC-δ in the perfused heart and in adult rat myocytes.
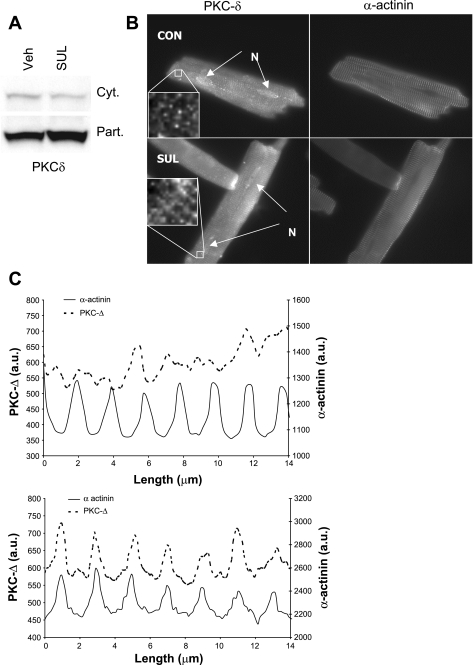
Cardioprotection is associated with signaling through PKC (2, 10, 11, 17, 22). PKC activation is typically accompanied by translocation from the cytosol to a membrane compartment. To determine whether SUL could activate PKC, we sought evidence for redistribution of PKC-δ and -ε after SUL administration. SUL infusion into the Langendorff-perfused heart resulted in translocation of PKC-δ to the particulate fraction (Fig. 3A). PKC-ε did not show a consistent pattern of translocation (data not shown). Additionally, we studied the effect of SUL on PKC distribution in isolated adult rat cardiomyocytes. Immunostaining for PKC-δ revealed a somewhat random punctate pattern under resting conditions, but, after SUL administration, the distribution of PKC-δ was much more closely aligned with α-actinin (Fig. 3B), which was further verified using pseudoline scanning (Fig. 3C). Curiously, a similar analysis for PKC-ε did not yield a clear pattern of distribution or a detectable change in response to SUL administration (data not shown).
Fig. 3.
Effect of SUL on PKC-δ translocation. A: immunoblots of cytosol and particulate fractions of rat hearts 30 min after SUL infusion (Langendorff). PKC-δ increased in the particulate (Part) fraction and decreased in the cytosol (Cyt). This blot is representative of 3 similar results. B: fluorescence micrograph of adult rat cardiomyocytes treated with SUL or Veh (control) for 15 min, then fixed and immunostained with antibody to PKC-δ and α-actinin. Inset shows a higher resolution field. N, nuclei. C: pseudoline scan derived from the myocytes shown in B, in which the fluorescence intensity (y-axis; au, arbitrary units) is measured along a defined segment of the myocyte on the longitudinal axis (x-axis). Solid line denotes the fluorescence intensity obtained with antibody to α-actinin, whereas the dotted line denotes the signal from antibody to PKC-δ on the same segment. The increased regularity of PKC-δ distribution (colocalization with α-actinin) after SUL administration was a consistent finding (N = 3). PKC-δ distribution coincided with Z-lines, which may be consistent with association with T-tubules.
PKC mediates the induction of autophagy triggered by SUL in adult rat myocytes.
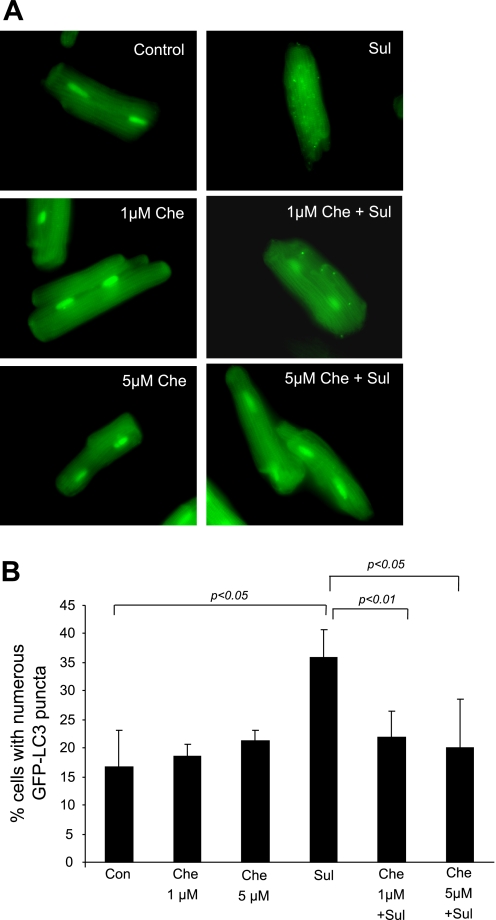
To determine whether PKC signaling is required for the induction of autophagy by SUL, we examined adult cardiomyocytes infected with GFP-LC3 adenovirus and treated with 10 μM SUL for 30 min. SUL significantly increased the percentage of cells with numerous autophagosomes, which was suppressed by the PKC inhibitor, chelerythrine (Fig. 4).
Fig. 4.
Role of PKC in autophagy induction by SUL in rat cardiomyocytes. A: isolated adult cardiomyocytes were infected with GFP-LC3 adenovirus. The next day, cells were treated with SUL, with or without the PKC inhibitor, chelerythrine (Che). Autophagy is induced by SUL in adult rat cardiomyocytes but is suppressed by Che. B: quantification of autophagy by percentage of cells displaying numerous puncta. Values are means and SD. Experiments were repeated 3 times.
Cardioprotection and autophagy induction by SUL depends on PKC.
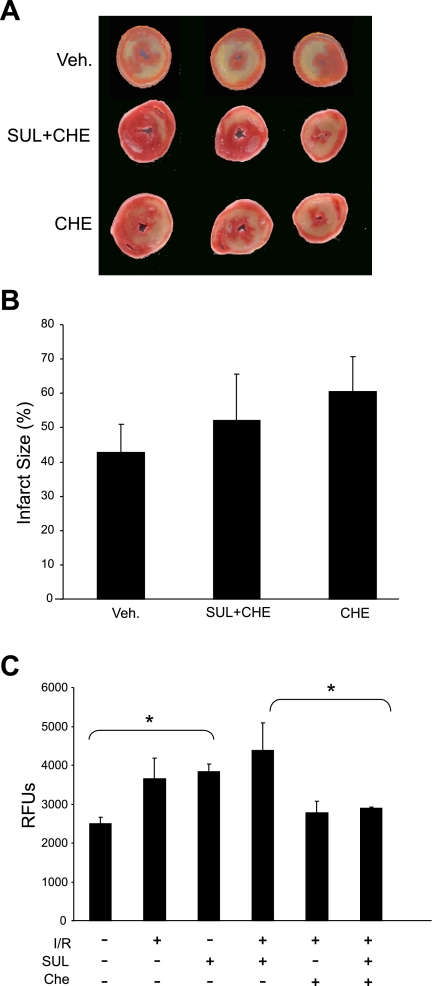
To determine whether PKC signaling is required for cardioprotection mediated by SUL in the ex vivo heart, we evaluated the effect of chelerythrine on infarct size in hearts treated with SUL. As shown in Fig. 5, A and B, in the presence of chelerythrine, there is no difference in infarct size whether SUL is present or absent, indicating that cardioprotection by SUL has been abolished. To measure autophagy in these same tissues, we used a fluorescent conjugate of cadaverine, which incorporates into autophagosomes (33) and serves as an accurate reporter of autophagy in heart tissue (24, 38). Chelerythrine suppressed autophagy induced by SUL (Fig. 5C). These results suggest that PKC is required for the induction of autophagy and cardioprotection by SUL.
Fig. 5.
Role of PKC in autophagy and cardioprotection in isolated, perfused rat hearts. A: hearts were treated with Che, with or without SUL, then subjected to I/R and stained with TTC for infarct size determination. B: quantification of infarct size after administration of Che is shown (P = nonsignificant, n = 4). C: quantification of autophagy in perfused hearts treated as indicated and measured by cadaverine dye binding assay. Values are means and SD; n = 3. *P < 0.03.
Tat-Atg5K130R blocks autophagy induced by SUL in isolated, perfused hearts.
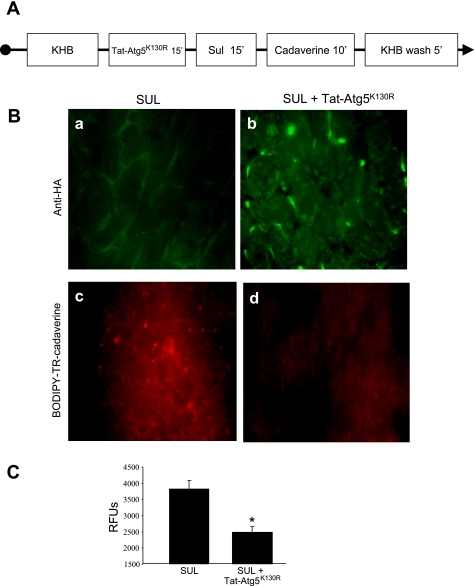
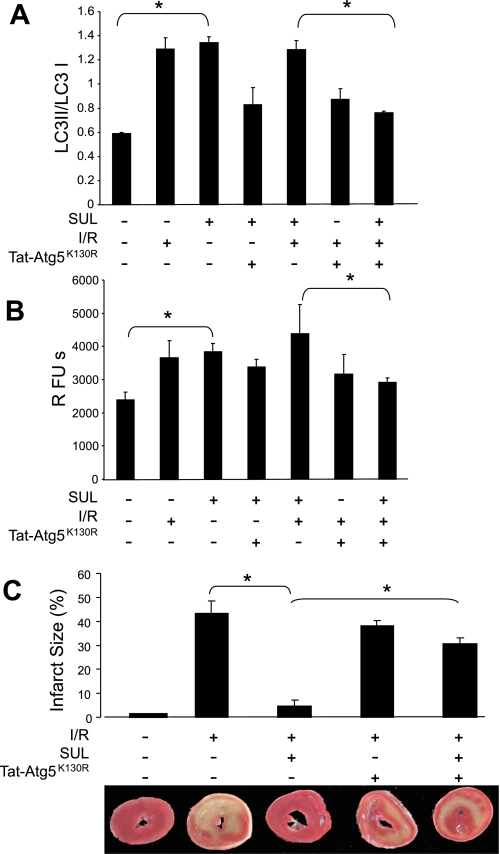
Atg5K130R is a point mutant of Atg5, which functions as a dominant negative to inhibit autophagosome formation (20, 39). We expressed Atg5K130R as a fusion protein with the protein transduction domain derived from HIV Tat (Tat-Atg5K130R) and used this reagent to inhibit autophagy. We perfused rat hearts with Tat-Atg5K130R and assessed its ability to block autophagy induced by SUL. For these studies, rat hearts were perfused with Tat-Atg5K130R followed by SUL (Fig. 6A). We confirmed uptake of Tat-Atg5K130R into cardiomyocytes by immunostaining for the hemagglutinin epitope incorporated into the Tat fusion protein (Fig. 6B, a and b). To measure autophagy, we used the cadaverine binding assay (Fig. 6B, c and d, and quantified in 6C). We further characterized autophagy in the setting of SUL administration and I/R and assessed the effects of Tat-Atg5K130R using immunoblotting of LC3 and cadaverine dye binding assays (Fig. 7, A and B). Results were similar using LC3-II/I ratios or cadaverine dye binding, thus further validating this method to measure autophagy. These results also show that Tat-Atg5K130R potently suppressed autophagy induced by SUL in the isolated, perfused heart.
Fig. 6.
Effects of Tat-Atg5K130R and SUL on autophagy in isolated, perfused rat hearts. A: protocol for Langendorff perfusion. Rat hearts were stabilized for 15 min, followed by treatments as indicated. KHB, Krebs-Henseleit buffer. B: Tat-Atg5K130R in cardiomyocytes is detected by anti-HA antibody (green immunofluorescence; a and b). BODIPY-TR-cadaverine incorporation into autophagosomes (red fluorescence; c and d) was increased by SUL administration (reflecting increased autophagy) and diminished by pretreatment with Tat-Atg5K130R. C: quantification of autophagy by cadaverine dye binding in heart tissue. Values are means and SD. *P < 0.005.
Fig. 7.
Induction of autophagy by SUL is abolished by administration of Tat-Atg5K130R. Rat hearts were perfused with Tat-Atg5K130R, as indicated in Fig. 6, followed by addition of SUL or Veh to perfusion buffer and treatment, as indicated. A: quantification of the LC3-II-to-LC3-I ratio from Western blots. Values are means and SD; N = 3. *P < 0.01. B: quantification of autophagy by cadaverine binding assay. Values are means and SD; N = 6. *P < 0.02. C: hearts treated as above were reperfused for 120 min, and infarct size was determined by TTC staining. Shown are quantification of infarct size and representative TTC-stained heart sections. Values are means and SD; N = 5. *P < 0.01.
Tat-Atg5K130R blocks cardioprotection induced by SUL in isolated, perfused hearts.
To determine whether autophagy was required for cardioprotection, we perfused rat hearts with Tat-Atg5K130R and assessed its effect on cardioprotection by SUL (Fig. 7C). Whereas administration of SUL reduced infarct size to 5% of the area at risk, pretreatment with Tat-Atg5K130R reduced the protection afforded by SUL infusion, resulting in an infarct size of 30% of the area at risk. The fact that cardioprotection is only partially eliminated may be due to incomplete suppression of autophagy by Tat-Atg5K130R or to additional cardioprotective mechanisms that are independent of autophagy. In the absence of SUL, Tat-Atg5K130R did not alter infarct size relative to the vehicle control (42.0 vs. 38.5%, P = nonsignificant). These results demonstrate that autophagy is required for SUL-mediated cardioprotection against I/R injury.
DISCUSSION
The results of this study extend our previous finding that SUL is cardioprotective, which has subsequently been confirmed by other groups (23, 26, 27). Here, we show that SUL induces autophagy and is dependent on signaling through PKC. The connection between SUL, PKC, and autophagy is novel. PKC has been demonstrated to be essential for preconditioning, although controversy exists over which isozyme is responsible for the protective signal. For instance, preconditioning exacerbated I/R injury in PKC-δ null mice (32). On the other hand, most studies have implicated PKC-ε in cardioprotection (5, 7, 40). Our studies suggest a link between SUL and PKC-δ.
Several groups have linked autophagy to cardioprotection mediated by preconditioning (18, 37, 44, 45). Effective autophagy depends on efficient fusion of autophagosomes with functional lysosomes, which, in turn, requires lysosomal acidification accomplished by the vacuolar proton ATPase (VPATPase). Our laboratory previously reported that inhibition of the VPATPase with bafilomycin A1 abolishes ischemic preconditioning (13, 25). Other investigators have confirmed that bafilomycin A1 blocks preconditioning (28, 41). Both PKC and PKA have been reported to trigger phosphorylation of a regulatory subunit of the VPATPase (34, 36, 42). The VPATPase is required for lysosomal acidification, a prerequisite for autophagosome-lysosome fusion and is, therefore, a critical factor in regulating autophagic flux.
A number of observations link SUL and CYP inhibition to cardioprotective signaling. Shimamoto's group showed that SUL inhibited a CYP activity in rat heart microsomes (23). The SUL-sensitive CYP enzyme might participate in arachidonic acid (AA) metabolism. Since AA can activate some PKC isozymes (29), inhibition of CYP-dependent conversion of AA to other products could increase AA levels and support PKC activation. AA can also be metabolized by a lipoxygenase to a cardioprotective product, so inhibiting CYPs that consume AA might increase the availability of AA to a cardioprotective lipoxygenase (9). Furthermore, the AA metabolite 20-hydroxyeicosatetraenoic acid (20-HETE) increases after I/R, and inhibition of CYPs that metabolize AA to 20-HETE is cardioprotective (35). Interestingly, 20-HETE is an inhibitor of AMP-activated protein kinase (AMPK) (43). AMPK is known to induces autophagy, but would be inhibited by 20-HETE. Preventing the CYP-dependent formation of 20-HETE would, therefore, allow AMPK to activate autophagy and achieve cardioprotection. Thus there are a number of possible links between SUL, CYP inhibition, and myocardial protection.
We have shown that SUL induces autophagy, and that autophagy is required for its cardioprotective effect. We also observed an increase in autophagy after I/R; however, based on our laboratory's previously published studies of autophagic flux in HL-1 cells (19), we suspect that this is due to impaired clearance of autophagosomes rather than increased autophagosome formation. We used the cell-permeable Tat-Atg5K130R to block autophagy and observed an increase in infarct size in hearts concurrently treated with SUL. Interestingly, we did not see an increase in infarct size in hearts subjected to I/R and Tat-Atg5K130R. It is possible that the heart does not mount an effective autophagic response in the absence of preconditioning, or that infarct size measurements above 50% of the area at risk are not linearly related to the extent of injury. Decreased infarct size was observed in Beclin1(+/−) mice subjected to 20-min ischemia and 24-h reperfusion (30). It is possible that defective autophagy during reperfusion contributes to cell injury and inflammation, in which case less autophagy might be preferable to frustrated autophagy in vivo. Our studies in the Langendorff system do not shed light on this possibility. More work is needed to assess the role of autophagy in the context of long-term functional recovery and remodeling.
Our results with SUL clearly demonstrate a protective role for autophagy in the acute setting. It has been suggested that autophagy may be beneficial during ischemia by providing metabolic substrates (31). However, SUL is also effective when administered at reperfusion (Fig. 1), which suggests that induction of autophagy during reperfusion is sufficient. It will be important to verify these findings in an in vivo model. In addition to the generation of metabolic substrates, activation of autophagy and the VPATPase can serve as a sink for protons, thereby limiting Na+/H+ exchange and preventing Ca+2 overload (25). Autophagy may also be important for removing damaged mitochondria, which might otherwise trigger cell death. Alternatively, the amino acids generated in the autophagolysosome may provide the driving force for glutathione resynthesis, thereby supporting repair of oxidized protein sulfhydryls. Regardless of the mechanism by which autophagy protects the heart subjected to I/R, the findings indicate that PKC signaling and autophagy are linked to SUL-mediated cardioprotection.
These findings reveal that SUL induces autophagy in adult rat cardiomyocytes, isolated perfused rat hearts, and intact mouse hearts. Stimulation of autophagy by SUL is mediated by a PKC-dependent pathway. The results obtained with the selective autophagy inhibitor, Tat-Atg5K130R, indicate that autophagy is an important element of cardioprotection in the setting of I/R injury. Given that other cardioprotective interventions, such as ischemic preconditioning, and an adenosine A1 agonist also induce autophagy, it is reasonable to infer that autophagy represents a common process utilized by cardiomyocytes to withstand I/R injury (12, 44, 45). Induction of autophagy may represent a new therapeutic approach to myocardial protection in humans.
GRANTS
This work was supported by National Institutes of Health Grants P01 HL85577, R01 HL071091, R01 AG033283 (to R. A. Gottlieb), R01 HL034579 (to R. M. Mentzer), and NIH F31 HL091723 (to C. N. Perry).
DISCLOSURES
One or more authors is employed by and/or has a total financial interest worth more than US $10,000 (from consultancy honoraria/expert testimony/corporate grants/patents pending/royalties/other) and/or has a 5% equity in an entity related to the subject matter discussed in the paper. R. A. Gottlieb is CEO and majority owner of Radical Therapeutix, Inc., a drug discovery company developing cardioprotective agents based on autophagy. R. M. Mentzer, Jr. has a significant financial interest in Radical Therapeutix, Inc.
REFERENCES
- 1.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551, 1996 [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong S, Downey JM, Ganote CE. Preconditioning of isolated rabbit cardiomyocytes: induction by metabolic stress and blockade by the adenosine antagonist SPT and calphostin C, a protein kinase C inhibitor. Cardiovasc Res 28: 72–77, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Arnaud C, Joyeux-Faure M, Bottari S, Godin-Ribuot D, Ribuot C. New insight into the signalling pathways of heat stress-induced myocardial preconditioning: protein kinase C epsilon translocation and heat shock protein 27 phosphorylation. Clin Exp Pharmacol Physiol 31: 129–133, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods 24: 247–256, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Budas GR, Churchill EN, Mochly-Rosen D. Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res 55: 523–536, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Caro LHP, Plomp PJAM, Wolvetang EJ, Kerkhof C, Meijer AJ. 3-Methyladenine, an inhibitor of autophagy, has multiple effects on metabolism. Eur J Biochem 175: 325–329, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, 2nd, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA 98: 11114–11119, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem 277: 29181–29186, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Glasgow W, Murphy E, Steenbergen C. Lipoxygenase metabolism of arachidonic acid in ischemic preconditioning and PKC-induced protection in heart. Am J Physiol Heart Circ Physiol 276: H2094–H2101, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Das A, Ockaili R, Salloum F, Kukreja RC. Protein kinase C plays an essential role in sildenafil-induced cardioprotection in rabbits. Am J Physiol Heart Circ Physiol 286: H1455–H1460, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Fryer RM, Wang Y, Hsu AK, Gross GJ. Essential activation of PKC-delta in opioid-initiated cardioprotection. Am J Physiol Heart Circ Physiol 280: H1346–H1353, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb RA, Finley KD, Mentzer RM., Jr Cardioprotection requires taking out the trash. Basic Res Cardiol 104: 169–180, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottlieb RA, Gruol DL, Zhu JY, Engler RL. Preconditioning in rabbit cardiomyocytes: role of pH, vacuolar proton ATPase, and apoptosis. J Clin Invest 97: 2391–2398, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granfeldt A, Lefer DJ, Vinten-Johansen J. Protective ischaemia in patients: preconditioning and postconditioning. Cardiovasc Res 83: 234–246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granville DJ, Tashakkor B, Takeuchi C, Gustafsson AB, Huang C, Sayen MR, Wentworth P, Jr, Yeager M, Gottlieb RA. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc Natl Acad Sci USA 101: 1321–1326, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granville DJ, Tashakkor B, Takeuchi C, Gustafsson AB, Huang C, Sayen MR, Wentworth P, Jr, Yeager M, Gottlieb RA. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc Natl Acad Sci USA 101: 1321–1326, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray MO, Zhou HZ, Schafhalter-Zoppoth I, Zhu P, Mochly-Rosen D, Messing RO. Preservation of base-line hemodynamic function and loss of inducible cardioprotection in adult mice lacking protein kinase C epsilon. J Biol Chem 279: 3596–3604, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Gurusamy N, Lekli I, Gherghiceanu M, Popescu LM, Das DK. BAG-1 induces autophagy for cardiac cell survival. Autophagy 5: 120–121, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 281: 29776–29787, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ 14: 146–157, 2007 [DOI] [PubMed] [Google Scholar]
- 21.He H, Li HL, Lin A, Gottlieb RA. Activation of the JNK pathway is important for cardiomyocyte death in response to simulated ischemia. Cell Death Differ 6: 987–991, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Hirotani S, Sadoshima J. Preconditioning effects of PKCδ. J Mol Cell Cardiol 39: 719–721, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Ishihara Y, Sekine M, Nakazawa M, Shimamoto N. Suppression of myocardial ischemia-reperfusion injury by inhibitors of cytochrome P450 in rats. Eur J Pharmacol 611: 64–71, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Iwai-Kanai E, Yuan H, Huang C, Sayen MR, Perry-Garza CN, Kim L, Gottlieb RA. A method to measure cardiac autophagic flux in vivo. Autophagy 4: 322–329, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karwatowska-Prokopczuk E, Nordberg J, Li HL, Engler RL, Gottlieb RA. Effect of the vacuolar proton ATPase on intracellular pH, calcium, and on apoptosis in neonatal cardiomyocytes during metabolic inhibition and recovery. Circ Res 82: 1139–1144, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Khan M, Iyyapu KM, Kutala V, Kotha S, Parinandi NL, Hamlin RL, Kuppusamy P. Sulfaphenazole protects heart against ischemia-reperfusion injury and cardiac dysfunction by overexpression of iNOS leading to enhancement of nitric-oxide bioavailability and tissue oxygenation. Antioxid Redox Signal 11: 725–738, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan M, Mohan IK, Kutala VK, Kumbala D, Kuppusamy P. Cardioprotection by sulfaphenazole, a cytochrome p450 inhibitor: mitigation of ischemia-reperfusion injury by scavenging of reactive oxygen species. J Pharmacol Exp Ther 323: 813–821, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Long X, Crow MT, Sollott SJ, O'Neill KL, Menees DS, Hipolito L, Boluyt MO, Asai T, Lakatta EG. Enhanced expression of p53 and apoptosis induced by blockade of the vacuolar proton ATPase in cardiomyocytes. J Clin Invest 101: 1453–1461, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackay K, Mochly-Rosen D. Arachidonic acid protects neonatal rat cardiac myocytes from ischaemic injury through epsilon protein kinase C. Cardiovasc Res 50: 65–74, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100: 914–922, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100: 914–922, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Mayr M, Metzler B, Chung YL, McGregor E, Mayr U, Troy H, Hu Y, Leitges M, Pachinger O, Griffiths JR, Dunn MJ, Xu Q. Ischemic preconditioning exaggerates cardiac damage in PKC-delta null mice. Am J Physiol Heart Circ Physiol 287: H946–H956, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Munafo DB, Colombo MI. A novel assay to study autophagy: regulation of autophagosome vacuole size by amino acid deprivation. J Cell Sci 114: 3619–3629, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Nanda A, Gukovskaya A, Tseng J, Grinstein S. Activation of vacuolar-type proton pumps by protein kinase C. Role in neutrophil pH regulation. J Biol Chem 267: 22740–22746, 1992 [PubMed] [Google Scholar]
- 35.Nithipatikom K, Gross ER, Endsley MP, Moore JM, Isbell MA, Falck JR, Campbell WB, Gross GJ. Inhibition of cytochrome P450 omega-hydroxylase: a novel endogenous cardioprotective pathway. Circ Res 95: e65–e71, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Nordstrom T, Grinstein S, Brisseau GF, Manolson MF, Rotstein OD. Protein kinase C activation accelerates proton extrusion by vacuolar-type H(+)-ATPases in murine peritoneal macrophages. FEBS Lett 350: 82–86, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Park HK, Chu K, Jung KH, Lee ST, Bahn JJ, Kim M, Lee SK, Roh JK. Autophagy is involved in the ischemic preconditioning. Neurosci Lett 451: 16–19, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Perry CN, Kyoi S, Hariharan N, Takagi H, Sadoshima J, Gottlieb RA. Novel methods for measuring cardiac autophagy in vivo. Methods Enzymol 453: 325–342, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JIL, Woo HN, Cho DH, Choi B, Lee H, Kim JH, Mizushima N, Oshumi Y, Jung YK. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem 280: 20722–20729, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Sivaraman V, Hausenloy DJ, Kolvekar S, Hayward M, Yap J, Lawrence D, Di Salvo C, Yellon DM. The divergent roles of protein kinase C epsilon and delta in simulated ischaemia-reperfusion injury in human myocardium. J Mol Cell Cardiol 46: 758–764, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Tong H, Rockman HA, Koch WJ, Steenbergen C, Murphy E. G protein-coupled receptor internalization signaling is required for cardioprotection in ischemic preconditioning. Circ Res 94: 1133–1141, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Voss M, Vitavska O, Walz B, Wieczorek H, Baumann O. Stimulus-induced phosphorylation of vacuolar H(+)-ATPase by protein kinase A. J Biol Chem 282: 33735–33742, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Ward N, Chen K, Croft K, Keaney J., Jr 20-Hydroxyeicosatetraenoic acid mediated AMP activated protein kinase suppression induces endothelial nitric oxide synthase uncoupling (Abstract). Circulation 118: S274b, 2008 [Google Scholar]
- 44.Yan L, Sadoshima J, Vatner DE, Vatner SF. Autophagy in ischemic preconditioning and hibernating myocardium. Autophagy 5: 709–712, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Yitzhaki S, Huang C, Liu W, Lee Y, Gustafsson AB, Mentzer RM, Jr, Gottlieb RA. Autophagy is required for preconditioning by the adenosine A1 receptor-selective agonist CCPA. Basic Res Cardiol 104: 157–167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan H, Perry CN, Huang C, Iwai-Kanai E, Carreira RS, Glembotski CC, Gottlieb RA. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol 296: H470–H479, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]