Abstract
Advanced age, independent of concurrent cardiovascular disease, can be associated with increased extracellular matrix (ECM) fibrillar collagen content and abnormal diastolic function. However, the mechanisms causing this left ventricular (LV) remodeling remain incompletely defined. We hypothesized that one determinant of age-dependent remodeling is a change in the extent to which newly synthesized procollagen is processed into mature collagen fibrils. We further hypothesized that secreted protein acidic and rich in cysteine (SPARC) plays a key role in the changes in post-synthetic procollagen processing that occur in the aged myocardium. Young (3 mo old) and old (18–24 mo old) wild-type (WT) and SPARC-null mice were studied. LV collagen content was measured histologically by collagen volume fraction, collagen composition was measured by hydroxyproline assay as soluble collagen (1 M NaCl extractable) versus insoluble collagen (mature cross-linked), and collagen morphological structure was examined by scanning electron microscopy. SPARC expression was measured by immunoblot analysis. LV and myocardial structure and function were assessed using echocardiographic and papillary muscle experiments. In WT mice, advanced age increased SPARC expression, myocardial diastolic stiffness, fibrillar collagen content, and insoluble collagen. In SPARC-null mice, advanced age also increased myocardial diastolic stiffness, fibrillar collagen content, and insoluble collagen but significantly less than those seen in WT old mice. As a result, insoluble collagen and myocardial diastolic stiffness were lower in old SPARC-null mice (1.36 ± 0.08 mg hydroxyproline/g dry wt and 0.04 ± 0.005) than in old WT mice (1.70 ± 0.10 mg hydroxyproline/g dry wt and 0.07 ± 0.005, P < 0.05). In conclusion, the absence of SPARC reduced age-dependent alterations in ECM fibrillar collagen and diastolic function. These data support the hypothesis that SPARC plays a key role in post-synthetic procollagen processing and contributes to the increase in collagen content found in the aged myocardium.
Keywords: secreted protein acidic and rich in cysteine, aging
left ventricular (LV) structural remodeling, such as changes in LV mass, volume, and geometry, are important predictors of functional and clinical outcomes (21, 22, 27, 45). Advancing age, independent of any concurrent cardiovascular disease, can itself be associated with significant LV structural remodeling (16, 24). These age-dependent changes in LV structure may play an important role in the functional limitations that occur in advancing age (16, 24). Previous studies have shown that with increasing age, the LV develops concentric remodeling (characterized by an increased LV mass-to-volume ratio), increased extracellular matrix (ECM) fibrillar collagen content, and significant abnormalities in diastolic function (16, 24, 29). However, the pathophysiological mechanisms by which advancing age leads to cardiac remodeling, particularly a net increase in myocardial collagen content and the development of diastolic dysfunction, have not been completely defined.
Fibrillar collagen biosynthesis begins within a fibroblast with the synthesis of a procollagen molecule (6). After synthesis, the procollagen molecule is secreted into the extracellular space, where it must undergo a series of ordered, time-sensitive, and location-sensitive processing steps to become a mature cross-linked insoluble structural collagen fibril (for reviews, see Refs. 19 and 36). Secreted protein acidic and rich in cysteine (SPARC; also called osteonectin or BM-40), a collagen-binding matricellular protein, has been implicated in post-synthetic procollagen processing (9, 33, 38). Changes in SPARC-dependent collagen deposition have been investigated in animal models of pressure-overload hypertrophy and myocardial infarction (10, 40); however, this aspect of myocardial fibrillar collagen biosynthesis has not been examined in aging. Therefore, we hypothesized that one fundamental mechanism by which advanced age increases myocardial fibrillar collagen content and causes the development of abnormal diastolic function is an alteration in post-synthetic procollagen processing.
Post-synthetic procollagen processing and subsequent collagen assembly is dependent on and influenced by soluble factors including matricellular proteins (8, 9, 11). Previous studies examining SPARC have suggested that SPARC participates in the coordination of procollagen processing and facilitates the formation and assembly of mature cross-linked insoluble structural collagen fibrils (11, 38). However, it is not known whether a change in SPARC expression is a fundamental mechanism by which advanced age increases myocardial fibrillar collagen content and causes diastolic dysfunction. Therefore, we further hypothesized that one determinant of an age-dependent change in post-synthetic procollagen processing is an increase in the extracellular protein SPARC.
Thus, the purpose of this study was to test the following hypotheses: 1) SPARC is significantly increased in the aged myocardium and 2) the absence of SPARC significantly alters the effects of advanced age on myocardial fibrillar collagen content and diastolic function.
METHODS
Animals
Four groups of mice were studied: young wild-type (WT) mice, old WT mice, young SPARC-null mice, and old SPARC-null mice. “Young” was defined as 3 mo old; “old” was defined as 18–24 mo old. Transgenic mice did not express SPARC (SPARC-null mice, produced by targeted gene deletion) were compared with WT mice on the same background (C57Bl6/SV129). All procedures performed were approved by the Institution Animal Care and Use Committee of the Medical University of South Carolina in accordance with National Institutes of Health guidelines.
Echocardiography
Mice from each genotype and age group (n = 7 mice each) underwent echocardiography to examine in vivo LV structure and function using previously described methods and measurements (10). Echocardiographic measurements were made using a 15-MHz transducer and a Sonos 5500 echocardiograph (Agilent Technologies, Andover, MA). Three to six beats were averaged for each measurement. LV dimension and wall thickness measurements were made at end diastole and end systole using American Society of Echocardiography criteria (25). Mean wall thickness was calculated as the average of the interventricular septal wall thickness (IVS) and LV posterior wall thickness (LVPW). Relative wall thickness (RWTH) was calculated as the mean LV wall thickness divided by the LV internal dimension (LVID) at end diastole. LV mass was calculated using the following formula: LV mass = 1.005 × [(IVS at end diastole + LVPW at end diastole + LVID at end diasole)3 − (LVID)3]. LV mass was normalized to body weight (LV-to-body weight ratio) and tibial length (LV-to-tibial length ratio). LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were determined using the Simpson's method of discs. The ejection fraction (EF; in %) was calculated as follows: EF = 100 × [(LVEDV − LVESV)/LVEDV]. Blood pressure measurements were made as described in Ref. 10.
Extraction and Biochemical Quantification of Collagen
Young SPARC-null (n = 10), young WT (n = 8), old SPARC-null (n = 11), and old WT (n = 11) mice were anesthetized using inhalation isoflurane and given 200 units of heparin intraperitoneally. The LV was isolated, weighed, and frozen in liquid nitrogen. Frozen LV tissue was lyophilized, weighed (dry weight), pulverized, resuspended in 1 M NaCl with protease inhibitors, tumbled overnight at 4°C, and centrifuged. The supernatant then contained “NaCl-soluble” collagen (i.e., largely noncross-linked collagen); the pellet contained “NaCl-insoluble” collagen (mature cross-linked fibrillar collagen). Each collagen fraction was processed separately. Collagen fractions underwent complete acid hydrolysis with 6 N HCl for 18 h at 120°C, and each was then neutralized to pH 7 with 4 N NaOH. One milliliter of chloramine T was added to 2-ml volumes of the collagen sample and incubated at room temperature for 20 min. One milliliter of Ehrlich's reagent (60% perchloric acid, 15 ml 1-propanol, and 3.75 g p-dimethyl-amino-benzaldehyde in 25 ml) was added, and samples were incubated at 60°C for 20 min. Absorbance at a wavelength of 558 was read on a spectrophotometer. Collagen was quantified as milligrams of hydroxyproline per gram dry weight of the LV myocardium. Thus, two collagen measurements were made by this hydroxyproline quantification of differentially isolated collagen fractions: NaCl-insoluble collagen (mature, fully processed, cross-linked collagen) and NaCl-soluble collagen (not processed or incompletely processed but noncross-linked collagen). Total collagen was the sum of soluble collagen with insoluble collagen.
Western Blot Analysis
Immunoblots were performed on LV tissue samples from four separate young and old WT and SPARC-null mice extracted with triton extraction buffer. SDS-PAGE gels were transferred to nitrocellulose membranes and probed with rabbit anti-murine SPARC polyclonal (1:20,000 dilution, R&D Systems, Minneapolis, MN) and anti-actin (Sigma, St. Louis, MO) antibodies. Chemiluminescence was used to detect secondary antibodies conjugated to horseradish peroxidase and exposed to film. The density of bands was determined using the ImageJ software program.
Papillary Muscle Preparation and Myocardial Function Measurements
Mice (6 mice of each genotype and age) were anesthetized and given 200 units of heparin intraperitoneally. The LV was isolated, the aorta was cannulated, the LV was perfused with 2,3-butanedione monoxime (BDM), and the papillary muscle was isolated to determine in vitro myocardial diastolic function. One half of the LV was fixed in 4% paraformaldehyde at 4°C to undergo quantitative collagen content analysis using light microscopy experiments (described below). The other half of the LV was fixed in 2% glutaraldehyde to undergo morphometric analysis of fibrillar collagen using scanning electron microscopy experiments. The methods used to isolate and study murine papillary muscle were as previously described (10). Passive diastolic stiffness was examined two ways: 1) define rest stress at maximum length (Lmax) and 2) perform a muscle stretch at a very slow stretch rate (1 mm/min) beginning from near slack length (very lightly preloaded muscle at 0.1 g) to a muscle length of 15% greater than that at slack length (equivalent to Lmax preload). The myocardial stress-vs.-strain relationship during this muscle stretch was used to calculate the passive stiffness constant (β) as follows: stress = Ae(β × strain) + C, where A and C are curve-fitting constants. Myocardial stress was calculated from muscle force divided by muscle cross-sectional area, and strain was calculated as L − L0/L0, where L is the muscle length during stretch and L0 is the muscle length at 0.1-g preload.
Collagen content by light microscopy.
LV sections were stained with picrosirius red (PSR) to detect collagen fibers and viewed with polarized light under dark-field optics to detect the birefringence of collagen fibers. Quantitative analysis of PSR-stained images captured with polarized light were performed. Five fields chosen at random from each of the 6 mice/group were scanned using Sigma Scan software. Fields with large blood vessels were excluded from the analysis. The areas examined were distributed throughout the myocardium from the subendocardium to subepicardium and excluded the epicardial surface. The collagen volume fraction was calculated as the area stained by PSR divided by the total area of interest using previously published techniques (7). PSR birefringent staining was used to identify and quantify mature fully processed cross-linked insoluble fibrillar collagen within the myocardium (23, 48). A previous study (10) has confirmed that PSR birefringent staining does not detect procollagen and is specific for mature collagen fibrils.
Collagen Morphological Structure by Scanning Electron Microscopy
LV samples from three separate mice of each genotype and age were processed using previously published techniques that included immersion fixation in 2% cacodylate glutaraldehyde, postfixation in 2% osmium tetroxide, dehydration with 100% alcohol, and drying with hexamethyldisilazine using a critical point dryer (43). Samples were mounted on scanning electron microscopy stubs, sputter coated with gold palladium, and imaged using a JEOL JSM-5410 scanning microscope at 15 KV, with a lens current of 46 and a working distance of 14.
Statistical Analysis
Data are presented as means ± SE. Differences between young WT, old WT, young SPARC-null, and old SPARC-null groups were determined using one-way ANOVA followed by Tukey testing of pairwise analysis. P values of <0.05 were considered significant. The primary outcome variable for this study was the concentration of insoluble collagen measured by biochemically determined hydroxyproline levels. The other measurements presented were secondary endpoints that were included as an exploratory analysis to examine pathophysiological mechanisms. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
Effects of Aging
SPARC immunoblot analysis.
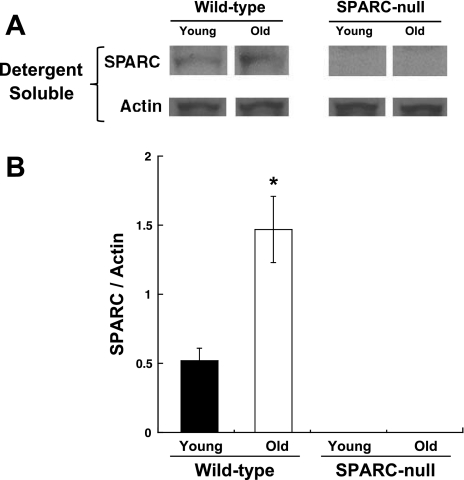
There was a significant increase in the levels of SPARC protein in old WT hearts compared with the young WT hearts as measured in LV myocardial tissue samples taken from four separate mice at each age (Fig. 1).
Fig. 1.
Increased levels of secreted protein acidic and rich in cysteine (SPARC) in the aged myocardium. A: immunoblots showing SPARC abundance in the left ventricular (LV) myocardium. B: optical density analysis was used to semiquantitate SPARC abundance. Young mice were 3 mo of age; old mice were 18–24 mo of age. Wild-type (WT) mice were from the C57Bl6/SV129 background, and SPARC-null mice were created using targeted gene insertion to prevent the expression of SPARC. *P < 0.05 vs. young WT mice.
Fibrillar collagen content by light microscopy.
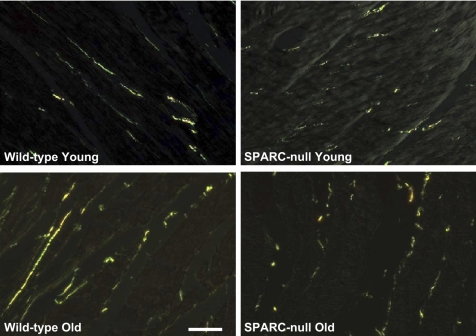
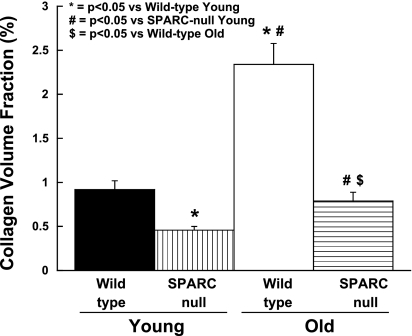
There was a significant increase in the collagen volume fraction in hearts of old WT mice compared with young WT mice (n = 6 mice/group; Figs. 2 and 3). In addition, collagen fibers in old mice were morphometrically dissimilar from those in young mice. Collagen fibers from old mice detected by PSR staining appeared red/orange when viewed under polarized light, characteristic of mature, cross-linked fibers. Young collagen fibers stained with PSR appeared green/yellow when viewed under polarized light, suggesting that the young collagen fibers were less cross-linked than the collagen fibers from old mice.
Fig. 2.
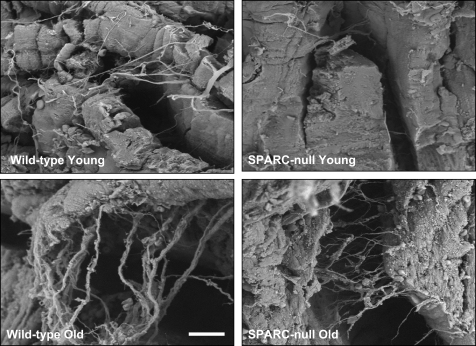
Altered collagen fiber morphology in hearts from old SPARC-null mice. Collagen fibers were visualized using picrosirius red staining and polarized light microscopy. Scale bar = 20 μm.
Fig. 3.
Reduced collagen content in hearts from old SPARC-null mice. The absence of SPARC decreased age-dependent increases in collagen content. Collagen content was examined using picrosirius red staining and light microscopy to quantify the collagen volume fraction.
Sections containing the epicardial surface, endocardial surface, or large blood vessels were excluded from analysis. However, there were not demonstrable differences in the regional expression of collagen by PSR across the myocardium, and, qualitatively, the distribution appeared uniform. Particularly, no differences in sites of collagen accumulation between young versus old hearts were apparent.
Collagen composition by biochemistry.
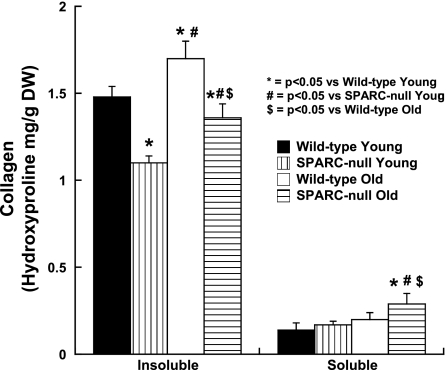
In WT mice, advanced age was associated with an increase in the total collagen and salt-insoluble collagen as measured by hydroxyproline analysis compared with young mice (n > 8 mice/group; Fig. 4 and Table 1).
Fig. 4.
Changes in collagen composition in hearts from old SPARC-null mice. The effects of the absence of SPARC on age-dependent changes in collagen composition are shown. Collagen composition was examined by measuring NaCl-insoluble collagen versus NaCl-soluble collagen by hydroxyproline quantification.
Table 1.
Effects of advanced age on collagen composition in WT versus SPARC-null mice
| Young |
Old |
|||
|---|---|---|---|---|
| WT mice | SPARC-null mice | WT mice | SPARC-null mice | |
| Total collagen content | 1.63 ± 0.08 | 1.27 ± 0.04* | 1.94 ± 0.10*† | 1.66 ± 0.07† |
| NaCl-insoluble collagen | 1.48 ± 0.06 | 1.10 ± 0.04* | 1.70 ± 0.10*† | 1.36 ± 0.08†‡ |
| NaCl-soluble collagen | 0.14 ± 0.04 | 0.17 ± 0.02 | 0.20 ± 0.04 | 0.29 ± 0.06*†‡ |
Values are means ± SE (in mg hydroxyproline/g dry wt of the starting material). Hydroxyproline analysis was performed on NaCl-extracted left ventricles (LVs) from young and old wild-type (WT) and secreted protein acidic and rich in cysteine (SPARC)-null mice. The total collagen content is the sum of the amount of hydroxyproline in NaCl-soluble and -insoluble fractions.
P < 0.05 vs. young WT mice;
P < 0.05 vs. young SPARC-null mice;
P < 0.05 vs. old WT mice.
Collagen morphology by scanning electron microscopy.
The morphological structure and distribution of fibrillar collagen were qualitatively examined in LV samples from young and old WT mice (Fig. 5). Fibrillar collagen struts and weaves appeared more frequently in old WT mice compared with young WT mice.
Fig. 5.
Distinct collagen fibril morphology in hearts from old SPARC-null compared with WT mice. The morphological structure of collagen was qualitatively examined using scanning electron microscopy. Scale bar = 10 μm.
LV structure and function.
There were no significant differences in LV volume or EF in old WT mice compared with young WT mice (n = 7; Table 2). Likewise, no significanct differences in blood pressure were found between young and old WT animals (n = 6; Table 2). However, old WT mice had an increase in wall thickness, LV mass, and the LV mass-to-LV volume ratio compared with young WT mice.
Table 2.
Effects of aging on LV structure and function in WT versus SPARC-null mice
| Young |
Old |
|||
|---|---|---|---|---|
| WT mice | SPARC-null mice | WT mice | SPARC-null mice | |
| Heart rate, beats/min | 440 ± 18 | 426 ± 11 | 415 ± 15 | 400 ± 15 |
| Body weight, g | 25 ± 1 | 24 ± 1 | 32 ± 2* | 27 ± 1† |
| Tibia length, mm | 20 ± 1 | 20 ± 1 | 21 ± 1 | 21 ± 1 |
| LV end-diastolic volume, μl | 36 ± 1 | 38 ± 2 | 40 ± 2 | 37 ± 1 |
| LV ejection fraction, % | 70 ± 3 | 72 ± 1 | 70 ± 2 | 72 ± 1 |
| Wall thickness, mm | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.1* | 0.9 ± 0.1† |
| LV mass, mg | 85 ± 5 | 88 ± 4 | 115 ± 4* | 104 ± 5† |
| LV mass/volume, mg/μl | 2.4 ± 0.05 | 2.3 ± 0.06 | 2.9 ± 0.07* | 2.8 ± 0.06† |
| Relative wall thickness at end diastole | 0.43 ± 0.02 | 0.45 ± 0.02 | 0.60 ± 0.02* | 0.57 ± 0.02† |
| Blood pressure, mmHg | 102 ± 7 | 104 ± 6 | 101 ± 12 | 100 ± 8 |
Values are means ± SE. Gravimetric measurements were collected at the time of euthanization. LV end-diastolic volume, LV ejection fraction, wall thickness, and LV volume were determined from echocardiographic measurements as described in methods. Relative wall thickness at end diastole was determined as follows: (LVPWd + IVSd)/LVIDd, where LVPWd is the LV posterior wall thickness at end diastole, IVSd is the intravetricular septal wall thickness at end diastole, and LVIDd is the LV internal dimension at end diastole.
P < 0.05, young WT vs. old WT mice;
P < 0.05, young SPARC-null vs. old SPARC-null mice.
Myocardial function.
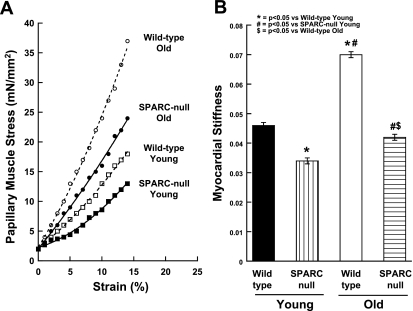
Myocardial stiffness constant β was significantly increased in papillary muscles isolated from the old WT mice compared with young WT mice (n = 6 mice/group; Fig. 6).
Fig. 6.
Reductions in diastolic Stiffness in aged papillary muscle of SPARC-null mice. A: examples of passive diastolic myocardial stress-vs.-strain curves for the four groups of animals studied. B: mean ± SE values of the passive stiffness constant for the four groups of animals studied.
Effects of Aging in the Absence of SPARC
SPARC immunoblot analysis.
As expected, there was no SPARC expression in SPARC-null mice (Fig. 1).
Fibrillar collagen content by light microscopy.
In old SPARC-null mice, there was a significant increase in the collagen volume fraction (n = 6 mice/group; Figs. 2 and 3). However, the increase in the collagen volume fraction in old SPARC-null mice was substantially less than that which occurred in old WT mice. There were no appreciable differences in sites of collagen accumulation in old versus young SPARC-null mice or with respect to differences in the regional distribution of collagen in old WT hearts. These data indicate that the age-dependent increase in mature cross-linked NaCl-insoluble collagen fibrils was blunted by the absence of SPARC.
Collagen composition by biochemistry.
In SPARC-null mice, advanced age was associated with an increase in total, NaCl-insoluble, and NaCl-soluble collagen concentrations compared with young SPARC-null mice (n > 10 mice/group; Fig. 4 and Table 1). The absence of SPARC during the aging process did not appear to alter the age-dependent increase in total collagen as measured by hydroxyproline assays. There were no statistical differences between total collagen in the old WT mice versus old SPARC-null mice. In addition, there was a comparable increase in total collagen in old hearts from both genotypes compared with the respective values of young hearts. However, with advanced age, the relative proportion of collagen that was insoluble was less and the relative proportion of collagen that was soluble in 1 M NaCl was greater in old SPARC-null mice compared with old WT mice. The fraction of total collagen soluble in 1 M NaCl has been predicted to reflect collagen that is not incorporated into cross-linked collagen (20, 34). Thus, these data suggest that, with advanced age, in the absence of SPARC, less procollagen was processed into mature cross-linked collagen, resulting in less NaCl-insoluble collagen and more NaCl-soluble collagen.
Collagen morphology by scanning electron microscopy.
Collagen fibrils from old SPARC-null hearts appeared to be present in higher density than in young SPARC-null mice (Fig. 5). However, collagen fibrils in old SPARC-null mice did not appear to undergo as substantial an increase in collagen fibril thickness as those observed in old WT mice.
LV structure and function.
There were no significant differences in LV volume, LV mass, the LV mass-to-volume ratio, EF, or blood pressure in young SPARC-null mice compared with young WT mice (n = 7 mice for echocardiography and 6 mice for blood pressure measurements; Table 2). Advanced age resulted in an equivalent increase in the LV mass in old WT and old SPARC-null mice (Table 2). LV mass, wall thickness, and the LV mass-to-volume ratio increased in both old WT and old SPARC-null mice. No significant changes in blood pressure in old WT or SPARC-null mice were detected.
Myocardial function.
Myocardial diastolic stiffness increased in old SPARC-null mice compared with young SPARC-null mice; however, this increase was significantly less than that which occurred in old WT mice (n = 6 mice/group; Fig. 6). Therefore, these experiments suggested that age-dependent increases in stiffness were blunted by the absence of SPARC.
DISCUSSION
The present study demonstrated the following: 1) in WT mice, advanced age was associated with increased SPARC expression, increased mature cross-linked NaCl-insoluble collagen fibrils, increased fibrillar collagen content, and increased myocardial diastolic stiffness; and 2) in SPARC-null mice, advanced age resulted in a significantly reduced increase in NaCl-insoluble collagen, collagen content, and myocardial stiffness than it did in WT mice. These data support the hypothesis that a SPARC-mediated increase in post-synthetic procollagen processing may be one factor that contributes to the LV ECM remodeling and diastolic dysfunction that occurs with advanced age.
Age-Dependent LV Remodeling: Effects on Structure, Function, and Clinical Status
Cross-sectional studies (18, 44) of human subjects with wide ranges in age, but without any detectable cardiovascular disease, have indicated that advanced age is associated with increased LV mass, increased wall thickness, unchanged or decreased LV volume, and a preserved EF. In the absence of cardiovascular disease, these increases in LV mass do not reach levels defined as LV hypertrophy; however, it is sufficient to result in the development of concentric remodeling as evidenced by an increase in the LV mass-to-volume ratio (16, 17, 24). Similarly, studies in aging animals have shown that with increasing age, the LV develops concentric remodeling. In addition, these studies have shown that advanced age was associated with increased concentrations of ECM fibrillar collagen and significant abnormalities in diastolic function (16, 17, 24). Likewise, the present study found increases in collagen consistent with values reported previously for WT mice and significant abnormalities in diastolic function in mice of advanced age (1, 15, 49).
Although the age-dependent changes in LV structure and function do not result in clinical cardiovascular disease per se, they do compromise cardiovascular reserve capacity, decrease exercise tolerance, and increase vulnerability to the effects of comorbid diseases (35). The age-dependent mechanisms that limit exercise include changes in myocardial structure, diastolic function, responses to catecholamine stimulation, and chronotropic reserve (41). During maximum exercise in younger subjects, end-systolic volume is decreased and end-diastolic volume, EF, heart rate, and cardiac output are all increased. In older subjects, however, the exercise-induced increases in end-diastolic volume, EF, heart rate, and cardiac output are markedly blunted. One important mechanism limiting cardiovascular reserve in older subjects is abnormalities in diastolic function, which result, at least in part, from changes in fibrillar collagen content and composition (14, 17). Age-dependent increases in diastolic stiffness associated with increased collagen content and increased NaCl-insoluble collagen result in a limited ability to recruit Frank-Starling reserve mechanisms to increase LV diastolic volume and augment EF and cardiac output.
However, the pathophysiological mechanisms by which advancing age leads to cardiac remodeling, particularly a net increase in myocardial collagen content and the development of diastolic dysfunction, remain incompletely defined. Examining one potential factor, age-dependent alterations in SPARC-mediated post-synthetic procollagen processing into mature NaCl-insoluble fibrillar collagen, was the central focus of the present study.
Determinants of ECM Fibrillar Collagen Content
Fibrillar collagen homeostasis is influenced by at least three regulatory control mechanisms: procollagen biosynthesis, post synthetic procollagen processing, and collagen degradation (31, 36, 42). The balance between synthesis, processing, and degradation determines the total fibrillar collagen content. Changes in each of these three determinants may play a significant role in age-dependent alterations in ECM fibrillar collagen and diastolic function. However, previous studies that have suggested that procollagen synthesis and collagen degradation may be altered in advanced age have found that some of these changes favored age-dependent collagen accumulation, whereas others did not (4, 28, 42).
In aged hearts, levels of mRNA encoding collagen I and III were generally found to be decreased compared with young hearts (2, 5, 46). Hence, increased transcription of fibrillar collagens in aged hearts is not likely to be the primary contributor to elevated collagen content. SPARC has been hypothesized to enhance tissue collagen concentration by facilitating collagen deposition into the ECM. The absence of SPARC during the aging process did not appear to alter the age-dependent increase in total collagen as measured by hydroxyproline assays. There were no statistical differences between total collagen in the old WT versus old SPARC-null mice. In addition, there was a comparable increase in total collagen from young to old age in both WT and SPARC-null mice.
However, significant increases in collagen soluble in 1 M NaCl, a relatively small proportion of total cardiac collagen that is predicted to include newly synthesized, noncross-linked collagen, was observed in the absence of SPARC expression (20, 34). Although extraction of insoluble collagen with more stringent agents such as pepsin/acetic acid or cyanogen bromide releases a greater amount of cross-linked collagen from cardiac tissue, 1 M NaCl was used in these studies to monitor the amounts of noncross-linked collagen (34). Increases in NaCl-soluble collagen in SPARC-null mice were similar to the response to transverse aortic constriction (TAC)-induced pressure overload (10). Increases in insoluble collagen associated with pressure overload resulted in similar increases in diastolic stiffness in TAC mice as those observed here in aged animals (10). Likewise, the absence of SPARC decreased both collagen concentrations and diastolic stiffness associated with TAC comparable to the decreases shown here in the aged myocardium.
The proteolytic determinants of collagen degradation appear to change in an age-dependent fashion in ways that favor less collagen degradation and more collagen accumulation (42). Collagen-degrading enzymes, matrix metalloproteinases (MMPs), are generally decreased and their endogenous tissue inhibitors [tissue inhibitors of metalloproteinases (TIMPs)] are generally increased as a function of advancing age. Therefore, the balance between MMPs and TIMPs promotes a decrease in the degradative capacity of the aged myocardium (42). As SPARC is also a substrate for a number of different MMPs, we predict that the extracellular half-life of SPARC is likely increased in the aged myocardium due, at least in part, to decreases in specific MMP activity (39). As SPARC is a collagen-binding protein, the increase in collagen content in old hearts might also increase levels of extracellular SPARC through interactions with collagen in the ECM. Levels of mRNA encoding SPARC were not found to be significantly altered in old versus young hearts, and, therefore, increased transcription of SPARC is not considered to contribute appreciably to more SPARC protein in the aged myocardium (30). Whether SPARC, in turn, affects MMP activity in cardiac cells is of significant interest, and future experiments to address this are planned. Studies (3, 32) carried out primarily in transformed cells in culture have suggested that SPARC acts to increase the activity of certain MMPs, notably MMP-2, MMP-9, and MT1-MMP.
Although SPARC is a primary secreted product of endothelial cells in culture, significant differences in the vascularity of SPARC-null tissues, including the heart, have not been demonstrated. Angiogenesis, as monitored in an implanted sponge model, was enhanced in young SPARC-null mice (12). However, similar experiments in old WT and SPARC-null mice revealed that the differences in angiogenesis disappeared with age (37). Although subtle differences in endothelial biology and blood vessel ultrastructure cannot be ruled out in hearts of old SPARC-null mice, to date evidence that SPARC has overt effects on cardiac vascularity has not been found.
Proposed Mechanisms by Which SPARC Affects Procollagen Processing
Once secreted into the extracellular space, post-synthetic procollagen processing is predicted to have two major outcomes: 1) ordered procollagen processing within the extracellular space that favors the formation of or incorporation into a mature cross-linked insoluble structural collagen fibril; or 2) procollagen association with cell surface receptors, which leads to the degradation of procollagen before its incorporation into mature cross-linked insoluble collagen fibrils, and/or premature disordered procollagen processing associated with the cell surface, which does not promote the formation of or incorporation into mature cross-linked insoluble collagen fibrils (9, 38). The process of extracellular procollagen processing and collagen fiber maturation can be influenced by multiple factors including SPARC, a matricellular protein. Matricellular proteins are defined as nonstructural proteins that associate with the ECM and modulate the interaction of classical ECM components, such as collagen, with cell surfaces (8).
SPARC is a collagen-binding protein with counteradhesive activity that has been hypothesized to coordinate procollagen processing and facilitate collagen fibril assembly and formation (9). Although a number of activities have been ascribed to SPARC in vitro, including the modulation of growth factor activity, regulation of cell cycle progression, and gene expression, the function of SPARC in tissues appears complex as well as contextual (13). The primary phenotypes characterized to date in SPARC-null mice involve aberrant ECM assembly and maintenance (for a review, see Ref. 9). Consistently, reductions in fibrillar collagen content of connective tissues have been observed, particularly in response to fibrotic injury.
The mechanism by which SPARC influences collagen deposition has been studied in vitro using primary dermal fibroblast cultures from WT and SPARC-null mice (38). In the absence of SPARC, procollagen secreted from fibroblasts had a greater tendency to associate with and bind to the fibroblast cell surface, to undergo either degradation or premature, disordered processing, and did not efficiently or effectively develop into mature cross-linked insoluble collagen. If recombinant SPARC was added to SPARC-null primary fibroblasts cultures, then procollagen processing was restored toward normal and collagen association with the fibroblast cell surface was decreased (38). These in vitro results suggest that SPARC limits procollagen binding to cell surface receptors and promotes the processing of procollagen to mature collagen fibrils. In the absence of SPARC, the regulation of procollagen processing is disrupted and the interaction of collagen with receptors is enhanced, leading to an increased degradation of procollagen at the expense of incorporation of processed collagen into insoluble collagen fibrils.
The balance between procollagen processing and procollagen degradation is a fundamental mechanism by which fibrillar collagen content is regulated. A study (6) by Bishop and Laurent has suggested that a substantial portion of newly synthesized procollagen is degraded before complete processing and formation of or incorporation into a mature collagen fibril. Even in normal tissues, as much as 5–60% of the newly synthesized procollagen is degraded (14). Degradation of nascent procollagen was particularly high in the cardiac interstitium compared with levels in skin. Therefore, even small changes in the balance between procollagen processing and procollagen degradation may act as a critical regulatory control mechanism effecting myocardial fibrillar collagen content. Age-dependent increases in SPARC could alter this balance, increase procollagen processing, and be one mechanism responsible for the age-dependent increases in ECM fibrillar collagen.
Relationship Among Advanced Age, SPARC, and ECM Fibrillar Collagen
Data from the present study support the proposed SPARC-mediated mechanism affecting procollagen processing as described above. The increase in SPARC that occurred with advanced age promoted procollagen processing, as evidenced by an increase in insoluble collagen in the old versus young WT mice. In the absence of SPARC, advanced age was associated with significantly less procollagen incorporation, as evidenced by a decrease in NaCl-insoluble collagen and an increase in NaCl-soluble collagen in the old SPARC-null versus old WT mice. Other studies examining noncardiovascular tissues have also implicated the influence of SPARC in aging. For example, SPARC expression was increased in dermal fibroblasts aged in vitro and in those from patients with Werner's syndrome (a condition characterized by premature aging) (26, 47). However, in primary murine dermal fibroblasts, SPARC expression was found to decrease with age (37). In contrast to the myocardium, dermal collagen concentrations have been shown to dimimish with age (37). Hence, age and tissue-specific mechanisms that control collagen deposition and accumulation likely differ and perhaps reflect differences in organ function adversely affected by age.
Myocardial Collagen: Effects of the Absence of SPARC
LV myocardial collagen was examined in the present study using three independent methods: 1) collagen content was examined using light microscopy and PSR birefringent staining and quantified by measuring the collagen volume fraction, 2) collagen composition was examined biochemically using hydroxyproline assays to quantify NaCl-insoluble versus NaCl-soluble collagen, and 3) collagen morphology was examined by scanning electron microscopy. These data showed that the absence of SPARC reduced the age-dependent increase in myocardial collagen content, changed the composition of myocardial collagen in favor of more NaCl-soluble collagen and less NaCl-insoluble collagen, and altered collagen morphology. Although the differences in the collagen volume fraction were directionally similar to the hydroxyproline analysis, the magnitude of the change in fibrillar collagen differed somewhat from the biochemically derived collagen measurements. When collagen content was assessed by collagen volume fraction, there was a more robust response to advanced age in mice than was measured by hydroxyproline analysis. We believe that both the measurements of collagen volume fraction and hydroxyproline are complimentary: that each contributes to the overall understanding of collagen homeostasis in advanced age and confers an ability to examine the effects of SPARC on procollagen processing in advanced age. Importantly, both collagen measurements support the hypothesis that the absence of SPARC influences postsynthetic procollagen processing and alters age-dependent changes in collagen concentrations in the LV.
A similar difference between the measurements of collagen volume fraction and hydroxyproline was seen in TAC induced by pressure-overload hypertrophy in WT versus SPARC-null mice (10). The potential factors that contributed to these differences in collagen measurements were outlined in this previous TAC study (10). We attribute the differences in collagen measurements in the present study of advanced age to similar mechanisms.
Conclusions
Data from the present study support the conclusion that SPARC is one factor contributing to the increases in myocardial fibrillar collagen content in advanced age and, as such, augments the development of diastolic dysfunction.
GRANTS
This work was supported by the Research Service of the Department of Veterans Affairs (to M. R. Zile and A. D. Bradshaw) and by National Heart, Lung, and Blood Institute Grant PO1-HL-48788 (to M. R. Zile).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Alwardt CM, Yu Q, Brooks HL, McReynolds MR, Vazquez R, Watson RR, Larson DF. Comparative effects of dehydroepiandrosterone sulfate on ventricular diastolic function with young and aged female mice. Am J Physiol Regul Integr Comp Physiol 290: R251–R256, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Annoni G, Luvara G, Arosio B, Gagliano N, Fiordaliso F, Santambrogio D, Jeremic G, Mircoli L, Latini R, Vergani C, Masson S. Age-dependent expression of fibrosis-related genes and collagen deposition in the rat myocardium. Mech Ageing Dev 101: 57–72, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Arnold S, Mira E, Muneer S, Korpanty G, Beck AW, Holloway SE, Mañes S, Brekken RA. Forces expression of MMP9 rescues the loss of angiogenesis and abrogates metastasis of pancreatic tumors triggered by the absence of host SPARC. Exp Biol Med 233: 860–873, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bátkai S, Rajesh M, Mukhopadhyay P, Haskó G, Liaudet L, Cravatt BF, Csizár A, Ungvari Z, Pacher P. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol 293: H909–H918, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besse S, Robert V, Assayag P, Declayre C, Swynghedauw B. Non-synchronous changes in myocardial collagen mRNA and protein during aging:effect of DOCA salt hypertension. Am J Physiol Heart Circ Physiol 267: H2237–H2244, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Bishop JE, Laurent GJ. Collagen turnover and its regulation in the normal and hypertrophying heart. Eur Heart J 16: 38–44, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Blom AS, Mukherjee R, Pilla JJ, Lowry AS, Yarbrough WM, Mingoia JT, Hendrick JW, Stroud RE, McLean JE, Affuso J, Gorman RC, Gorman JH, 3rd, Acker MA, Spinale FG. Cardiac support device modifies left ventricular geometry and myocardial structure after myocardial infarction. Circulation 112: 1274–1283, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bornstein P, Sage EH. Matricellular proteins: extracellular modulatoris of cell function. Curr Opin Cell Biol 14: 608–616, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, Zile MR. Pressure-overload induced alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in post-synthetic procollagen processing. Circulation 119: 269–280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Sage EH. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol 120: 949–955, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw AD, Reed MJ, Carbon JG, Pinney E, Brekken RA, Sage EH. Increased fibrovascular invasion of subcutaneous polyvinyl alcohol sponges in SPARC-null mice. Wound Repair Regen 9: 522–530, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 107: 1049–1054, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Souza RR. Aging of myocardial collagen. Biogerentology 3: 325–335, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Du XJ, Samuel CS, Gao XM, Zhao L, Parry LJ, Tregear GW. Increased myocardial collagen and ventricular diastolic dysfunction in relaxin deficient mice: a gender-specific phenotype. Cardiovasc Res 57: 395–404, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Ferrari AU, Radaelli A, Centola M. Aging and the cardiovascular system. J Appl Physiol 95: 2591–2597, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Gaasch WH, Zile MR. Left ventricular diastolic dysfunction and diastolic heart failure. Annu Rev Med 55: 373–394, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Ganau A, Saba PS, Roman MJ, de Simone G, Realdi G, Devereux RB. Ageing induces left ventricular concentric remodelling in normotensive subjects. J Hypertens 13: 1818–1822, 1995 [PubMed] [Google Scholar]
- 19.Greenspan DS. Biosynthetic processing of collagen molecules. Top Curr Chem 247: 149–183, 2005 [Google Scholar]
- 20.Gross J, Highberger JH, Schmitt FO. Extraction of collagen from connective tissue by neutral salt solutions. Proc Natl Acad Sci USA 41: 1–7, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heart Failure Society of America Executive summary: HFSA 2006 comprehensive heart failure practice guidelines. J Card Fail 12: 10–38, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B, American College of Cardiology. American Heart Association Task Force on Practice Guidelines. American College of Chest Physicians. International Society for Heart and Lung Transplantation. Heart Rhythm Society ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to update the 2001 guidelines for the evaluation and management of heart failure). Circulation 112: e154–e235, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11: 447–455, 1979 [DOI] [PubMed] [Google Scholar]
- 24.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation 107: 346–354, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, St John Sutton M, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Lecka-Czernik B, Moerman EJ, Jones RA, Goldstein S. Identification of gene sequences overexpressed in senescent and Werner syndrome human fibroblasts. Exp Gerontol 31: 159–174, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Kalon KL, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 347: 1397–1402, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingonia JT, Sweterlitsch SE, Spinale FG. Age-dependent changes in myocardial matrix metalloproteinase profiles and fibroblast function. Cardiovasc Res 66: 410–419, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Martos R, Baugh J, Ledwidge M, O'Loughlin C, Conlon C, Patle A, Donnelly SC, McDonald K. Diastolic heart failure. Evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation 115: 888–895, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Masson S, Arosio B, Fiordaliso F, Gagliano N, Calvillo L, Santambrogio D, D'Aquila S, Vergani C, Latini R, Annoni G. Left ventricular response to β-adrenergic stimulation in aging rats. J Gerontol 55A: B35–B41, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Mays PK, McAnulty RJ, Campa JS, Laurent GJ. Age-related changes in collagen synthesis and degradation in rat tissues. Biochem J 276: 307–313, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClung HM, Thomas SL, Osenkowski P, Toth M, Menon P, Rãz A, Fridman R, Rempel SA. SPARC upregulates MT1-MMP expression, MMP-2 activation, and the secretion and cleavage of galectin-3 in U87MG glioma cells. Neurosci Lett 29: 172–177, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCurdy S, Baicu CF, Heyman S, Bradshaw AD. Cardiac extracellular matrix remodeling: fibrillar collagens and secreted protein acidic and rich in cysteine (SPARC). J Mol Cell Cardiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukerjee D, Sen S. Alteration of collagen phenotypes in ischemic cardiomyopathy. J Clin Invest 88: 1141–1146, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Najjar SS, Schulman SP, Gerstenblith G, Fleg JL, Kass DA, O'Connor F, Becker LC, Lakatta EG. Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol 44: 611–617, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Prockop DJ, Kivirkko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 64: 403–434, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Reed MJ, Bradshaw AD, Shaw M, Sadoun E, Han N, Ferara N, Funk S, Puolakkainen P, Sage EH. Enhanced angiogenesis characteristic of SPARC-null mice disappears with age. J Cell Physiol 204: 800–807, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Rentz TJ, Poobalarahi F, Bornstien P, Sage EH, Bradshaw AD. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem 282: 22062–22071, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Sasaki T, Göhring W, Mann K, Maurer P, Hohenester E, Knäuper V, Murphy G, Timpl R. Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens. J Biol Chem 272: 9237–9243, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Schellings MWM, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen REW, d'Hooge J, Van de Werf F, Carmeliet P, Pinto YM, Sage EH, Heymans S. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med 206: 113–123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulman SP, Fleg JL, Goldberg AP, Busby-Whitehead J, Hagberg JM, O'Connor FC, Gerstenblith G, Becker LC, Katzel LI, Lakatta LE, et al. Continuum of cardiovascular performance across a broad range of fitness levels in healthy older men. Circulation 94: 359–367, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev 87: 1285–1342, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Spinale FG, Tomita M, Zellner JL, Cook JC, Crawford FA, Zile MR. Collagen remodeling and changes in ventricular function during the development and regression of supraventricular tachycardia induced cardiomyopathy in swine. Am J Physiol Heart Circ Physiol 261: H308–H318, 1991 [DOI] [PubMed] [Google Scholar]
- 44.Sumimoto T, Mukai M, Murakami E, Koubu T, Lin M, Shigematsu Y, Hamada M, Hiwada K. Effect of age on left ventricular geometric patterns in hypertensive patients. J Hypertens 13: 1813–1817, 1995 [PubMed] [Google Scholar]
- 45.Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Levy S, Linde C, Lopez-Sendon JL, Nieminen MS, Pierard L, Remme WJ, Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 26: 1115–1140, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Thomas DP, Zimmerman SD, Hansen TR, Martin DT, McCormick RJ. Collagen gene expression in rat left ventricle: interactive effect of age and exercise training. J Appl Physiol 89: 1462–1468, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Moerman EJ, Jones RA, Thweatt R, Goldstein S. Characterization of IGFBP-3, PAI-1 and SPARC mRNA expression in senescent fibroblasts. Mech Ageing Dev 92: 121–132, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol 89: 397–410, 1994 [DOI] [PubMed] [Google Scholar]
- 49.Yu Q, Horak K, Larson DF. Role of T lymphocyte in hypertension-induced cardiac extracellular matrix remodeling. Hypertension 48: 98–104, 2006 [DOI] [PubMed] [Google Scholar]