Abstract
Spatial variation in hemodynamic stresses acting on the arterial wall may explain the nonuniform distribution of atherosclerosis. In thoracic aortas of LDL receptor/apolipoprotein E double knockout mice, lesions develop preferentially around the entire circumference of intercostal branch ostia, regardless of age, with the highest prevalence occurring upstream. Additional chevron-shaped lesions occur further upstream of the ostia. This pattern differs from the age-related ones occurring in people and rabbits. In the present study, patterns of near-wall blood flow around intercostal ostia in wild-type mice were estimated from the morphology of endothelial nuclei, which were shown in vitro to elongate in response to elevated shear stress and to align with the flow, and wall structure was assessed from confocal and scanning electron microscopy. A triangular intimal cushion surrounded the upstream part of most ostia. Nuclear length-to-width ratios were lowest over this cushion and highest at the sides of branches, regardless of age. Nuclear orientations were consistent with flow diverging around the branch. The pattern of nuclear morphology differed from the age-related ones observed in rabbits. The intimal cushion and the distribution of shear stress inferred from these observations can partly account for the pattern of lesions observed in knockout mice. Nuclear elongation in nonbranch regions was approximately constant across animals of different size, demonstrating the existence of a mechanism by which endothelial cells compensate for the dependence of mean aortic wall shear stress on body mass.
Keywords: hemodynamics, shear stress, intimal cushion, age, atherosclerosis
the patchy distribution of atherosclerosis within the arterial system, particularly at sites of branching and curvature, is consistent with a controlling influence of local hemodynamic stresses. Characterization of the precise nature of the stresses that predispose to disease has been hampered by confusion over the distribution of lesions. This confusion stems in part from a failure to recognize that the distribution can change with age. In human fetuses, neonates, and infants, lipid deposition develops downstream of aortic branch points, but, at later ages, it is seen at the lateral margins and then upstream of the ostia, with the downstream region being spared (33, 34, 38). An age-related switch from the downstream to the lateral pattern also occurs in spontaneous (3) and diet-induced (4, 5) lipid deposition in the rabbit aorta.
Determining hemodynamic correlates of lesion patterns has also been hampered by difficulties in assessing blood flow. Existing techniques for directly measuring flow under physiological conditions lack the necessary spatial resolution, while the accuracy of computational modeling is restricted by the incomplete characterization of boundary conditions. Our laboratory recently assessed hemodynamic shear stresses exerted on the endothelium around branch ostia in rabbits by examining the shape of endothelial nuclei (1). It is well established from in vitro and in vivo studies that endothelial cells (ECs) align with the predominant flow direction and elongate with increasing shear stress (9, 11, 25, 27, 28, 37). The cells can therefore be considered a high-resolution array of in situ flow sensors, and their shape has been used to characterize shear stress patterns in several previous studies (26, 30, 32). Their nuclei appear to show the same behavior (13); indeed, nuclear shape and alignment correlate with cell shape and alignment in vivo (6). We found that endothelial nuclei are more elongated downstream than upstream of the origins of intercostal arteries in immature rabbit aortas, and that this pattern reverses with age (1).
Knockout mice are widely and increasingly used as models of human atherosclerosis and provide a powerful means of investigating disease-localizing factors at the molecular level. It is therefore important to determine the relation between hemodynamic stress and lesion prevalence in their arteries. The pattern of lipid deposition around intercostal branch ostia in the mouse aorta differs from that in rabbits and people. In low-density lipoprotein receptor/apolipoprotein E double knockout mice, lesions develop all around the ostium. There is a significantly higher frequency upstream of the branch than downstream and a trend for this difference to decrease with increasing age (31). There is additionally a chevron-shaped lesion some distance upstream of many ostia (31).
The difference between mice and rabbits in the pattern of lesions around intercostal branch ostia, and in the change of this pattern with age, may be explained by a different pattern of wall shear stress and a different change in the pattern of shear with age. Here we report a study that investigated this hypothesis by examining endothelial nuclear shape and orientation around intercostal branch ostia in aortas from wild-type mice of different ages. Because the techniques were not the same as those used in our laboratory's earlier investigation (1), we also report a small study to determine whether they give results consistent with our previous data for rabbits. We, additionally, report an in vitro examination of the effect of shear stress on nuclear elongation and alignment, using mouse aortic endothelial cells (MAECs), since hitherto only elongation and alignment of the whole cell have rigorously been shown to depend on shear in this way. Finally, we describe the geometry of a raised intimal cushion found at most branches that appears to explain features of the observed pattern of lesions. A brief preliminary report has been published (7).
METHODS
In vitro studies.
Endothelial cells (ECs) were isolated from the thoracic aortas of 4-wk-old male C57BL/6 mice (Jackson Laboratories) using a modification of the explant method described by Hwang et al. (21). The thoracic aorta was cut into pieces ∼1 mm × 1 mm in area, which were cultured intimal side down on a collagen gel droplet (20 μl of 0.175% Type I rat tail collagen) in EGM2-MV medium (Lonza) in a six-well plate. Typically, luminal ECs adhered to the collagen gel within 3 days of incubation. After the explants were removed, adherent cells were cultured for another day in MAEC growth medium [DMEM supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 50 μg/ml EC growth supplement (Sigma), and 1× nonessential Eagle's amino acid].
The methods for immortalization and characterization of endothelial markers will be reported in detail elsewhere. Briefly, adherent cells were infected with polyoma middle T antigen, which specifically immortalizes ECs, but not other cell types, using established methods (2, 16, 40). Polyoma middle T-infected cells were then selected by subculturing in MAEC growth medium supplemented with the neomycin analog G418 (1 mg/ml). The antibiotic selection was maintained for 2 mo. Immortalized MAEC (iMAEC) were further purified by cell sorting based on uptake of Dil-labeled acetylated low density lipoprotein, as previously described (21). iMAEC expressed EC markers (VE-cadherin, platelet EC adhesion molecule-1, endothelial nitric oxide synthase, and Kruppel-like factor-2), but not the smooth muscle cell marker, smooth muscle cell α-actin (data not shown).
iMAEC were maintained at 37°C and 5% CO2 in MAEC growth medium. Confluent iMAEC monolayers on 0.1% gelatin-coated 100-mm dishes (Falcon) were exposed to unidirectional laminar shear stress (10 or 20 dyn/cm2) by rotating a Teflon cone (0.5° cone angle) over them for 48 h, as previously described (23, 35), or were maintained under static conditions.
Cells were fixed with 4% paraformaldehyde, and nuclei were stained with Hoechst 33258. Eight images per dish were acquired by epifluorescence microscopy (Zeiss, Axiovert 200M).
Animal procedures.
All animal procedures complied with the Animal (Scientific Procedures) Act 1986 and were approved by the Local Ethical Review Panel of the University of Reading. Endothelial nuclei were examined in normal mice (University of Reading strain), aged 6–10 wk (n = 4) or 18–20 wk (n = 5), and in male New Zealand White rabbits (Harlan Interfauna strain), aged 6 wk (n = 3), 17 wk (n = 3), 38 wk (n = 3), or >100 wk (n = 2). One additional wild-type mouse (C57BL/6 strain) was used to make a corrosion cast of the aortic lumen. All animals were fed a standard laboratory diet without added fat.
Mice were killed by carbon dioxide inhalation. Following a ventral midline thoracotomy and laparotomy, a cannula was inserted into the left ventricle and secured with tissue adhesive (Vetbond, 3M). Thoracic aortas for nuclear staining were flushed with 5 ml of Ringer solution (composition in g/l: 9.0 NaCl, 0.2 KCl, 0.2 Ca2Cl, 0.1 NaHCO3) containing heparin (Sigma, 30 USP units/ml) before being perfused for 30 min with Karnovsky's fixative (4% glutaraldehyde plus 5% formaldehyde, n = 7) or 10% neutral buffered formalin (Sigma, n = 2) from a reservoir 100–120 cm above the animal. They were excised and postfixed for at least 16 h.
The aorta used for corrosion casting was heparinized but not fixed. Instead, the ventricular cannula was used to inject a methacrylate resin (Batson's no. 17, Anatomical Corrosion Kit, Polysciences, mixed according to the manufacturer's instructions) from a syringe. Perfusion pressure was monitored with a mercury manometer and kept at 95–100 mmHg until the resin set sufficiently so that no more could be injected. The resin was allowed to harden overnight, and the tissue was then dissolved by immersing the carcass for 7 days in 30% wt/vol KOH. The cast was cleansed in detergent (Decon), and vessels other than the aorta and its immediate branches were removed.
Rabbits were heparinized (Sigma, 2200 USP units iv) before being killed with an overdose of sodium pentobarbitone (Euthatal, Rhone Merieux, approx 160 mg/kg iv). A ventral midline thoracotomy and laparotomy were performed, and a retrograde cannula was tied into the aorta at the level of the diaphragm. The thoracic aorta was flushed with 50 ml of Ringer solution containing heparin and then perfused for 30 min with Karnovksy's fixative, as described above. The aortic arch was clamped after 1 min to reduce the volume of fixative required and to maintain in vivo pressure. Aortas were postfixed for at least 16 h.
Tissue preparation and histology.
Loose connective tissue on the adventitial surface of fixed aortas was removed, and side branches were cut as close to their origin as possible so that the remaining stubs did not distort the main vessel when it was mounted for en face viewing. Mouse aortas were examined whole, whereas the larger rabbit aortas were cut perpendicular to the longitudinal axis to produce rings, with each containing a pair of intercostal branches.
Aortas and aortic rings were permeabilized with Triton X-100 (Sigma, 0.2%, 30 s), incubated in ribonuclease A (Sigma, 0.01%, 10 min at 37°C) to remove RNA but not DNA, and stained with propidium iodide (Molecular Probes, 1 mg/ml, ∼0.5 s). They were then cut along the longitudinal axis opposite the branch sites before being mounted in saline, luminal surface down, in coverslip bottomed petri dishes.
The luminal surface around intercostal branch ostia was viewed en face with an inverted confocal microscope (TCS NT, Leica Microsystems) using a ×20 immersion objective. Propidium iodide fluorescence was imaged using 543-nm excitation and 600- to 640-nm emission wavelengths. For most samples, autofluorescence was also imaged, using 488-nm excitation and 500- to 580-nm emission wavelengths. A stack of optical slices was obtained, with each slice being 750 × 750 μm in area. This field of view allowed the whole mouse branch to be visualized. For rabbit branches, regions upstream and downstream of the ostium had to be imaged separately.
Control regions at least one branch diameter away from the ostia were also imaged. Because of the low Reynolds occurring in the mouse, influences of the intercostal branch on wall shear stress do not extend this far (24).
Image processing and analysis.
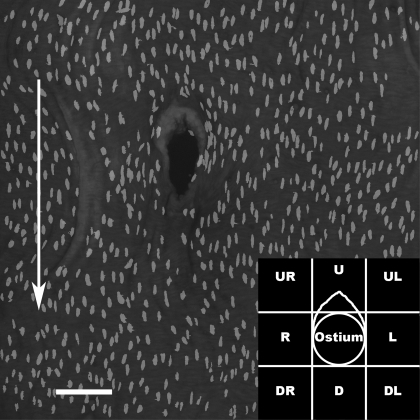
Images of aortic nuclei were processed using Photoshop (version 7.0, Adobe Systems). Individual slices from each stack were converted to gray-scale images, and noise was reduced with a median filter (one-pixel radius). They were then binarized, and EC nuclei were selected manually. EC nuclei from every slice in a stack were copied onto a blank canvas to produce a montage of the endothelial surface in a single image, despite the irregular surface height in the original three-dimensional data set (Fig. 1).
Fig. 1.
En face montage of propidium iodide-stained endothelial nuclei around the origin of a mouse aortic branch. Arrow indicates direction of mean aortic blood flow. Bar = 100 μm. Inset: regions for which mean nuclear shape and orientation were calculated (U, upstream; D, downstream; L, anatomic left; R, anatomic right). Region U excludes the intimal cushion, which was analyzed separately.
For quantitative analysis of nuclei around mouse ostia, montages were divided into eight regions of 200 × 200 μm (Fig. 1, inset). The region upstream of the branch frequently contained an intimal cushion; nuclei covering this cushion were analyzed separately. For the rabbits, analysis was restricted to single 600 × 600-μm areas upstream and downstream of the ostium, each offset ∼75 μm from the ostial lip to avoid regions of high curvature. The length of each region was approximately equal to the branch diameter for both mice (Table 1) and rabbits (1).
Table 1.
Dimensions of mouse intimal cushion and branch ostium
| Younger | Older | |
|---|---|---|
| Intimal cushion | ||
| Length | 88 ± 9 | 82 ± 6 |
| Width | 139 ± 6 | 143 ± 6 |
| Branch ostium | ||
| Length | 179 ± 5 | 196 ± 6 |
| Width | 118 ± 5 | 119 ± 7 |
Values are means ± SE in μm.
All images were analyzed in ImageTool (version 3.00, UTHSCSA) to obtain the length of the major axis (“length”) and minor axis (“width”), and thus the length-to-width ratio (L/W), of nuclei, and the angle between the major axis and the vertical axis of the image (“orientation”). The average of all the nuclear orientations around a branch was subtracted from all individual values for the same branch. This procedure removed biases that would otherwise have been caused by inaccuracies in cutting or mounting the tissue.
Images of nuclei sheared in vitro were processed in a similar manner. After their conversion to gray scale, noise reduction and high-pass filtering were applied. Analysis was performed with ImageTool, as described above.
Analysis of intimal cushions and overlying nuclei.
Outlines of the ostia and intimal cushions were traced from confocal images of autofluorescence onto transparencies and scanned. Manual measurements were made of ostial length and width and cushion length (the distance from the most proximal region of the cushion to the most proximal region of the branch ostium) and width (maximum distance across the cushion perpendicular to its length).
To further examine cushion geometry, segments of the corrosion cast of the aortic lumen were mounted on metal stubs, coated with a thin layer of gold using a sputter coater (Emitech K550), and then imaged by scanning electron microscopy (Joel JSM-5610 OLV).
EC nuclei overlying the intimal cushion had more variable orientations and, due to the high degree of surface curvature, were distributed over more slices of the confocal stack than nuclei in other regions. Consequently, it was not possible to carry out an automated analysis of their shape. Instead, they were traced onto transparencies, scanned, and analyzed in ImageTool to determine L/W.
Statistical analysis.
Data are presented as means ± SE. Effects of shear stress on L/W and orientations in vitro were assessed by linear regression using the number of fields of view as n. The significance of effects of location and age on L/W (including control regions and intimal cushion) and orientations of mouse nuclei were assessed by univariate analysis of variance (SPSS version 14.0) and Tukey post hoc tests, using the number of animals as n. Effects of age on the dimensions of the branch and intimal cushion were assessed using Student's unpaired t-test.
RESULTS
Nuclear L/W in vitro.
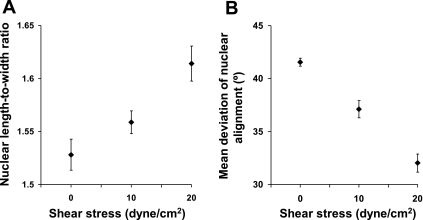
Seven or eight fields of view, each containing >70 cells, were analyzed at each shear level in each of three independent experiments. Figure 2A shows nuclear L/W as a function of the shear applied to the iMAEC. Higher shear stresses caused elongation of the nuclei (P < 0.005 by linear regression).
Fig. 2.
Effect of applied shear stress on elongation (A) and deviation (B) from the most frequently occurring alignment of immortalized mouse aortic endothelial cell nuclei in vitro. Both effects were highly significant (P < 0.005 and P < 0.0005 by linear regression, respectively, n = 71 fields of view).
Nuclear orientations in vitro.
To analyze variation in alignment, the absolute (unsigned) angular deviation between the long axis of each nucleus and the modal nuclear orientation was averaged for 70 randomly selected cells in each field of view. (This is equivalent to the standard error of alignment, but is based on the mode rather than the mean. The mode was used rather than the mean because angles are modular; for example, lines at 179° and 1° to a reference line themselves differ in alignment by only 2°.) The mean angular deviations decreased with increasing shear (Fig. 2B, P < 0.0005 by linear regression), indicating a decrease in the scatter of alignments.
Nuclear lengths, widths, and L/W in mice.
Nuclear lengths for 42 younger and 57 older mouse branches, averaged across the 8 regions in Fig. 1, inset, were 17 and 18 μm, respectively. Equivalent widths were 8 and 8 μm.
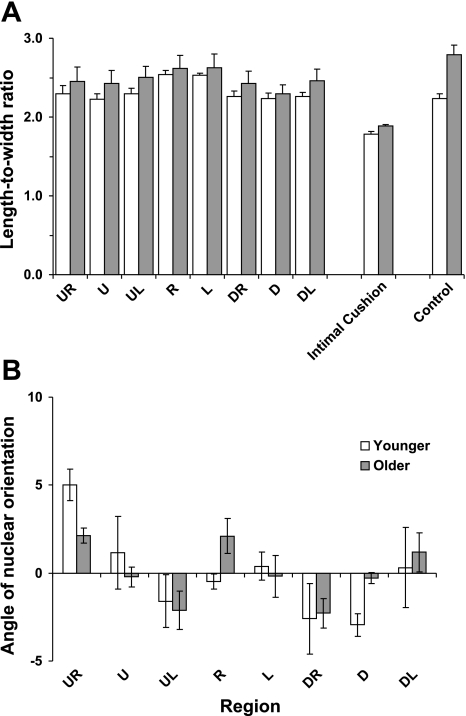
L/W are shown by region in Fig. 3A. Overall, there was a significant difference in mean L/W between the eight regions defined in Fig. 1, inset (P < 0.0005). Although younger L/W were lower than older ones in every region, the overall effect was not significant (P = 0.46), reflecting a large variation in mean values between older mice. The interaction between region and age approached significance (P = 0.054), suggesting that the pattern of L/W may change with age. L/W in the L (left) and R (right) regions were the highest in both age groups; they were significantly different from the remaining six regions in younger mice and from five of the remaining six regions in older mice (P < 0.0005), with the exception being region UL (upstream left) (P > 0.1). In younger mice, mean L/W in the remaining six regions were similar to one another. In older mice, L/W in the D (downstream) region were the lowest; the difference from the other regions reached significance (P < 0.05) for seven of the eight comparisons, with region U being the exception (P > 0.05).
Fig. 3.
A: length-to-width ratios of endothelial nuclei from the 8 regions defined in Fig. 1, inset, over the intimal cushion, and in control regions away from the branch, in younger and older mice. B: angle between the long axis of nuclei and the average nuclear orientation around the branch for the regions defined in Fig. 1, inset, in the same mice. Negative angles indicate that the proximal end of the nucleus was displaced toward the anatomic right; positive angles, that it was displaced toward the left. Bars indicate means ± SE (n = 4 younger and 5 older mice, 99 branches in total).
In both age groups, L/W overlying the intimal cushion were significantly lower than ratios in all other regions (P < 0.0005). In older but not younger mice, L/W in control regions were significantly higher than in all regions other than L and R (P < 0.05); means for the control regions were 2.24 ± 0.06 in younger mice and 2.79 ± 0.12 in older mice.
Nuclear orientations in mice.
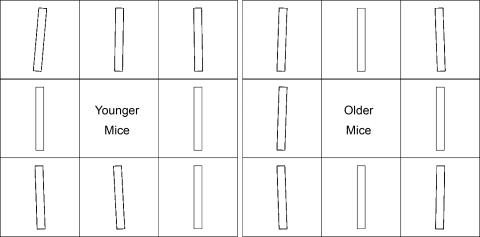
Figure 3B shows the mean orientation of nuclei in each of the eight regions defined in Fig. 1, inset. Although there was a significant effect of region (P < 0.0005), divergences from the average orientation for the same branch were small, ranging only between −3.0 ± 0.6 and 5.0 ± 0.9° in younger mice, and between −2.3 ± 0.8 and 2.1 ± 0.4° in older mice. There was no significant effect of age on nuclear orientation (P = 0.345). Furthermore, there was no interaction between age and location (P = 0.096), suggesting that the pattern of orientations remains constant with age. Orientations were positive on the anatomic right side and negative on the left side upstream of the branch, while the reverse pattern was seen downstream of the branch; regions L, R, U, and D, on the whole, had intermediate values. The pattern is shown in diagrammatic form in Fig. 4.
Fig. 4.
Bars representing endothelial nuclear orientation, relative to the mean for the branch, in different regions around branch ostia in younger and older mice. Time-averaged blood flow is from top to bottom. The ostium is located in the central square in each image. Each square represents a region measuring 200 μm × 200 μm, defined in Fig. 1, inset.
The statistical conclusions were not altered when the region overlying the intimal cushion was included (effect of region, P < 0.0005; effect of age, P = 0.552; interaction between region and age, P = 0.145). Nuclei in nonbranch regions were not included in the analysis, since the normalization procedure gave them an average orientation of 0°.
Dimensions of ostia and intimal cushions in mice.
Average lengths and widths of ostia in younger and older mice are shown in Table 1. There were no significant effects of age (length, P = 0.220; width, P = 0.678). The intimal cushion resembled the bow of a boat, extending upstream from the ostium (Fig. 5A). Its shape could be analyzed in 29 out of 42 branches (69%) from immature mice, and in 45 out of 57 branches (79%) from mature mice. The dimensions of the cushion (Table 1) were unaffected by age (length, P = 0.882; width, P = 0.456).
Fig. 5.
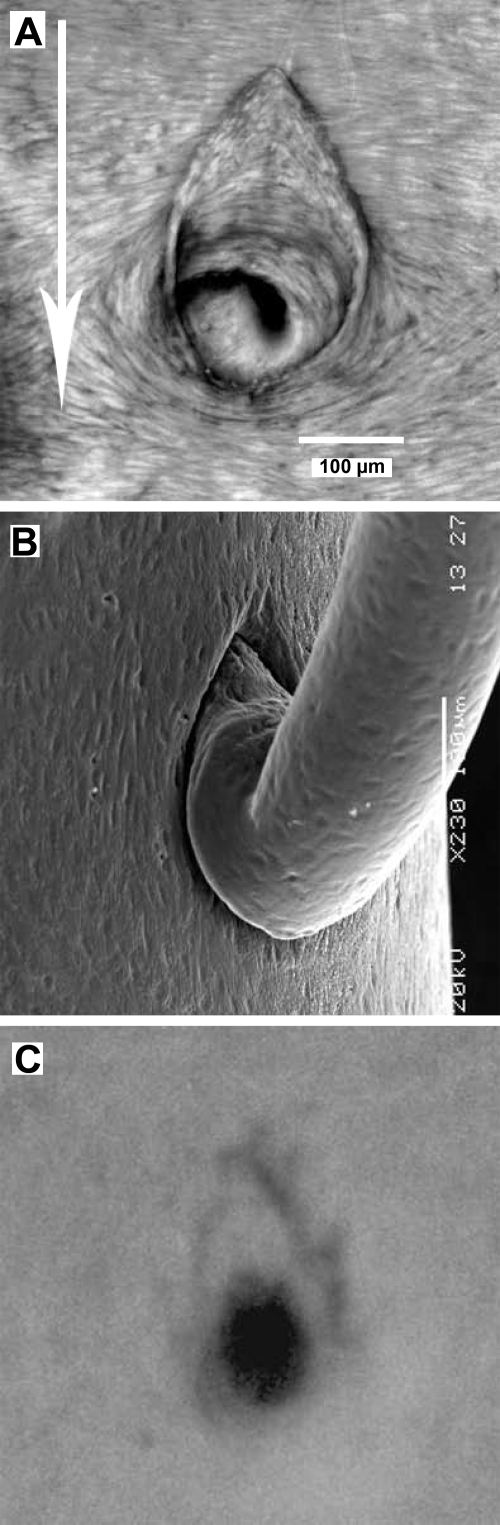
A: projection of a confocal image stack showing en face view of tissue autofluorescence around the origin of a mouse aortic branch. A raised intimal cushion resembling the prow of a boat is present upstream of the ostium. Arrow indicates direction of mean aortic blood flow. B: scanning electron microscope image of a corrosion cast of the mouse aortic lumen at an intercostal branch. The intimal cushion, which protrudes into the lumen, is seen as a deep trench in the cast around the upstream (uppermost) side of the branch mouth. Raised endothelial nuclei leave pits in the cast. The downstream side of the branch is undercut where the flow divider projects into the lumen. C: chevron-shaped lesion, revealed by staining with oil red O, upstream of the mouth of an intercostal artery from an apolipoprotein E/low-density lipoprotein receptor double knockout mouse (detail from Fig. 2 of Ref. 31).
Scanning electron microscopy images (Fig. 5B) confirmed the shape of the cushion and additionally demonstrated the abrupt nature of the structure, which appeared to have walls at its outer margins that, in places, are essentially perpendicular to the aortic surface and that overhang the aortic wall at the upstream margin of the cushion. Because the spacing of ECs is apparent from the impressions left by their nuclei in the cast, the images also demonstrated that the dimensions of the cushion were of the same order as the length of a single cell.
Nuclear lengths, widths, and L/W in rabbits.
Nuclear lengths for rabbits aged 6, 17, 38, and ≥100 wk, averaged across the upstream and downstream regions, were 18, 19, 20, and 21 μm, respectively. Equivalent widths were 8, 8, 7, and 7 μm.
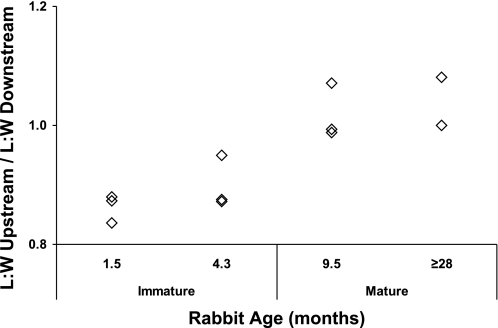
L/W in rabbits (Fig. 6) were greater downstream of the branch than upstream in the youngest age group: the ratio of the mean upstream value to the mean downstream value was 0.86. The difference decreased in the second age group (ratio = 0.90), essentially disappeared in the third group (ratio = 0.99), and reversed in the oldest group (ratio = 1.04). (Statistical analyses were not carried out because only 24 branches in total were studied.) L/W in both regions tended to increase with age, as did L/W in control regions; the latter averaged 2.60 ± 0.06, 2.96 ± 0.11, 3.14 ± 0.09, and 3.25 ± 0.17 with increasing age in the four groups of rabbits (which had mean weights of 1.43 ± 0.03, 2.90 ± 0.15, 3.30 ± 0.06, and 4.95 ± 0.45 kg).
Fig. 6.
Changes with age in the pattern of endothelial nuclear length-to-width ratios (L/W) upstream and downstream of rabbit aortic branches. The upstream L/W has been divided by the downstream L/W, so values <1 indicate greater nuclear elongation downstream, and values >1 indicate greater elongation upstream. Each point indicates the data for a single rabbit (24 branches in total, 5–8 in each age group). Ages are the mean for each group.
DISCUSSION
This study used endothelial nuclear morphology to assess the magnitude and direction of aortic wall shear stress. Because effects of shear have been rigorously demonstrated in vitro only for the morphology of whole ECs, not for the morphology of their nuclei, we first examined whether nuclei of cultured MAECs align and elongate in response to shear. Significant correlations were obtained for both alignment and elongation. These experiments were conducted at constant strain. Several in vitro studies have shown that ECs elongate when exposed to cyclic strain. However, when the cells are exposed simultaneously to strain and shear, as in vivo, strain has little effect at mean shears >5 dyn/cm2 and, even where there is an effect, it is observed only within a narrow band of strains (2–4%) (41). Shear therefore appears to have the dominant effect. Our in vitro experiments were also restricted to steady flow. Pulsatile nonreversing flow has a more elongatory effect on cells than the equivalent steady flow, and pulsatile reversing flow has less effect than the equivalent steady flow. However, the influences of unsteadiness are much smaller than the influence of the mean shear (one-third to one-fifth of the effect) (20). Mean shear appears to be dominant.
In our previous study of rabbits (1), we found greater nuclear elongation downstream than upstream of the origins of intercostal arteries in immature aortas, but observed the opposite trend in animals aged 10–11 mo. In the small study of rabbits reported here, conducted to determine whether the new techniques for measuring nuclear L/W gave comparable results, we observed the same trend (Fig. 6). Furthermore, both studies showed an increase with age in the elongation of nuclei away from branches. There were minor quantitative differences between the two sets of data: the present study gave a bigger difference between downstream and upstream regions in immature animals, a smaller difference in mature animals, and a later age at which the pattern switched. These discrepancies may reflect the limited number of branches examined or the difficulty in delineating the same sampling areas with the two different microscope systems, as well as genuine differences between the methods. The discrepancies are sufficiently small that, if the new method revealed a markedly different pattern of nuclear elongation in mice, this could be attributed to a real effect of species rather than to a methodological problem.
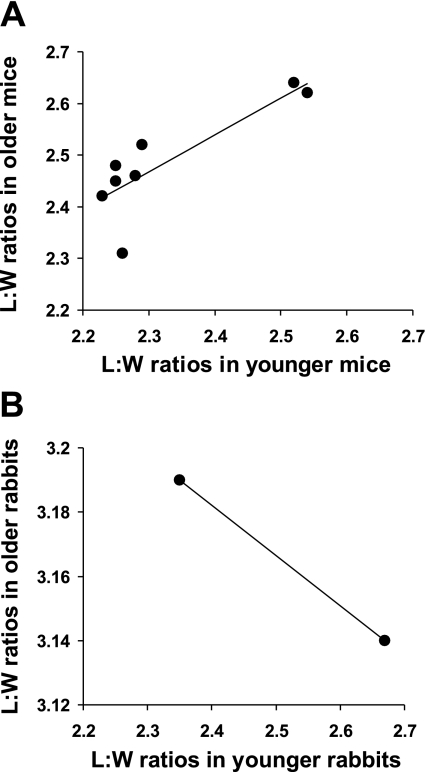
The pattern of elongation observed in mice was indeed markedly different from those previously observed in rabbits. Most strikingly, L/W in both mouse age groups were greatest in regions at the sides of branches, whereas ratios in the rabbit were highest either upstream or downstream, depending on age (1). In general, the pattern of ratios around branches in the mouse (Fig. 3A) did not alter substantially with age. (The changes had only borderline statistical significance.) This is clearly shown by plotting the mean L/W for each region in younger mice against the equivalent ratio in older mice (Fig. 7A). The data are strongly and positively related (best-fit regression line y = 0.71x + 0.83, R2 = 0.71), indicating that regions with, say, high L/W in young mice also had high L/W in older mice. The equivalent line for rabbits in our previous study had a gradient close to −1, with all data points lying close to it (1), and the line obtained with the more limited data from the rabbits in the present study also had a negative gradient (Fig. 7B). The offset of the line in Fig. 7A shows the general (although not statistically significant) increase in L/W with age in mice. The outlier, corresponding to the downstream region (D), reveals the only obvious change in pattern: L/W in this region did not increase with age to the extent expected from the regression line. Consequently, although L/W were essentially identical upstream and downstream of branches in younger mice (where the L/Wupstream-to-L/Wdownstream ratio averaged 0.99), they became somewhat greater upstream than downstream in older mice (average L/Wupstream-to-L/Wdownstream ratio = 1.05), as in rabbits.
Fig. 7.
Effect of age on the pattern of endothelial nuclear L/W around the branch. A: the mean L/W obtained in younger mice for each of the regions defined in Fig. 1, inset, plotted against the equivalent ratio in older mice. The positive correlation shows the broadly similar pattern with age, with the outlier (corresponding to the downstream region, D) revealing the only obvious change. The offset of the best-fit regression line shows the general increase in L/W with age. B: equivalent plot for the data obtained in regions upstream and downstream of rabbit ostia, showing a best-fit regression line of the opposite sign.
Angles between nuclei and the average nuclear orientation around each branch in the mouse vessel also differed from the angles previously observed in rabbits (1). First, they were substantially smaller in mice. Rabbits had mean orientations for lateral regions in the range 10–20°, whereas in mice the largest mean for a region was ∼5°, and most regions had values considerably smaller than this. Second, orientations in the upstream and downstream left and right regions (UL, UR, DL, DR) differed between the species: in rabbits, the long axis of nuclei deviated from the aortic axis toward the ostium (1), and in mice it deviated away from the ostium (albeit with smaller angles). This discrepancy is broadly consistent with the observation of Langille and Adamson (26) that ECs orient toward large branches in the rabbit abdominal aorta, but do not do so at small branches in the mouse femoral circulation. Reynolds numbers were ∼500 and <1 in these two cases, respectively (20), and were ∼100 in the mouse aorta studied here (12).
Our laboratory's recent computational study of wall shear stress around idealized branches (24) may explain some of these differences. It shows that, at Reynolds numbers appropriate for the rabbit, fluid enters the branch from regions adjacent to the aortic wall; fluid nearer the center of the aorta has too much inertia to do so. The branch, consequently, has a profound effect on the direction of near-wall flow and on wall shear stress, and is expected to affect endothelial orientation and elongation. For example, movement of fluid into the branch from regions lateral to the ostium can account for the endothelial nuclei in these regions being oriented toward the branch. At the lower Reynolds numbers occurring in the mouse, on the other hand, fluid in regions further away from the wall can enter the branch. The branch consequently has a smaller and less extensive influence on near-wall flow, which is more uniform; such flow, and endothelial morphology, may be dominated by other factors, including local anatomy.
A raised cushion with a shape resembling the prow of a boat was observed upstream of most mouse ostia. Intimal cushions have been reported to occur at a range of arterial branches in a number of species; they are particularly prevalent in mice. We have also observed them around intercostal branch ostia in apolipoprotein E knockout mice (data not shown), but did not observe them in the rabbit aorta. The cushion contains smooth muscle cells that are aligned with the aortic longitudinal axis, and elastin fibers that branch from the internal elastic laminae of the aorta and the intercostal artery (15). This specialized structure, which is quite distinct from that of the parent and daughter vessels, demonstrates that the cushion is not a fixation artifact or a projection of the side branch into the aorta. EC nuclei under the “prow,” which were not visualized in the present study, are particularly tightly packed (15). Cushions may reduce plasma skimming: in their absence, but not in their presence, the hematocrit in side branches is reduced by a few percent (14).
Endothelial nuclei overlying this cushion had significantly lower L/W, implying lower wall shear stresses, than in other regions around the branch. The cushion may channel flow around the branch, accounting for the diverging pattern of endothelial orientation. The resulting compression of streamlines could elevate wall shear stress in lateral regions, which in turn may account for the higher L/W observed there.
The cushion is not itself an early lesion, since it occurs in disease-free, wild-type mice, but it may explain two features of the lesion distribution seen in knockout mice. First, although lesions tend to surround ostia, they are significantly more prevalent or more extensive upstream of ostia than downstream of them (31). Second, a chevron-shaped lesion occurs further upstream of many ostia (31); its spatial correspondence with the intimal cushion can be seen by comparing Fig. 5, A and C. Particular patterns of hemodynamic stress associated with the cushion (indicated by the reduced nuclear L/W) and with its upstream lip may account for these phenomena.
Further evidence for an influence of hemodynamic stresses on disease in mice is that the relative decrease with age in L/W in region D correlates with the trend toward an increased lesion prevalence with age in this region (31). [A high prevalence of lesions is observed on the inner curve of the ascending aorta (22), a site that is also characterized by less elongated ECs (18)]. On the other hand, there is neither a particularly high nor a particularly low prevalence of lesions at the sides of branches (31), where the most elongated nuclei were observed. Furthermore, the lack of a consistent difference in L/W between regions near the branch and regions away from the branch does not correlate with the strong tendency for lesions to occur around branch ostia at both ages. Overall, the data do not therefore support a simple relation of disease prevalence with nuclear L/W (and, by implication, hemodynamic shear stress at the luminal surface) around intercostal branches.
A limitation of our study is that spatial patterns of endothelial shape were determined in wild-type mice and normal rabbits rather than knockout mice and cholesterol-fed rabbits of the type used to map lesion prevalence. This was necessary because EC shape is disrupted by hypercholesterolemia (29). Some flow characteristics may differ between normal and diseased animals. For example, although wild-type and apolipoprotein E−/− mice are indistinguishable in their blood pressure, heart rate, and descending aortic velocity waveform, the knockout mice have a 60–70% elevation of mean and peak aortic blood velocity (19). Our computational fluid dynamic studies show that substantial elevations of Reynolds number (which depends on velocity) increase shear stress upstream and downstream of the branch and decrease it at the sides of the branch. However, fundamental features of the shear stress pattern are unlikely to be changed by the relatively small difference in Reynolds number occurring between wild-type and hypercholesterolemic mice (24). Additional limitations are that changes in nuclear pattern may occur in mice outside the restricted range of ages we studied, and that apparent dimensions of nuclei viewed en face in regions of high curvature may be distorted by foreshortening.
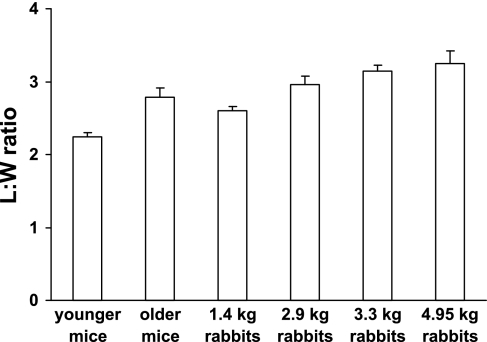
Our data are relevant to another question concerning the relation between shear stress and endothelial pathophysiology. Recent theoretical (39) and experimental (17) work and a literature survey (8) have demonstrated that arterial wall shear stress is not the same in all animals but increases with decreasing size. For the aorta of different species, it is inversely proportional to the three-eighths power of body mass (17, 39). Assuming typical weights for mice, rabbits, and people of 25 g, 3.5 kg, and 75 kg, respectively, this relation gives aortic wall shear stresses that are 20-fold higher in mice, and 3-fold higher in rabbits, than those in people. This raises an important question concerning the many shear-dependent features of endothelial biology: do they vary with body mass, or are they independent of body mass as the result of some adjustment for the mean shear experienced in each animal? We can investigate this question using the results from the present study: if nuclear L/W varies with shear stress, as inferred from our in vitro observations, and there is no adjustment, lower ratios should occur in larger animals. L/W in control regions, away from branch ostia, are ranked by body mass for the different experimental groups in Fig. 8. They do not decrease with increasing body size, in fact, they increase slightly, suggesting, at least for this endothelial property, that there is compensation for the effect of body mass on mean shear stress, both within and across species.
Fig. 8.
Mean endothelial nuclear L/W for control regions, away from branches, in mice and rabbits from different age groups, ranked according to body size. A decrease in mean aortic wall shear stress (∼10×) is expected with increasing size, but there is no corresponding decrease in L/W. Bars show means ± SE; n = 2–5 animals per group.
The existence of a compensatory mechanism allowing ECs to adapt to mechanical stresses may be relevant to the development and prevention of cardiovascular disease. However, the nature of the mechanism is obscure. The existence of spatial variation in nuclear elongation around branches demonstrates that the adaptive process does not accommodate differences in shear at a local level. Furthermore, since there appears to be continuing compensation for the changes in shear that occur with growth, within each species, compensation cannot be determined by genotype or fixed in early development. [Wall shear stress falls by 50% in the rabbit carotid artery between 3 and 15 wk of age (10), and a 36% fall is expected between the aortas of our smallest and largest rabbit groups, if the intraspecies body mass exponent is also −0.375.] Indeed, the effects on nuclear elongation and orientation of surgical rotation of aortic segments (13) demonstrate morphological plasticity in the adult. Further research is required to determine the molecular mechanisms underlying these phenomena.
GRANTS
This study was funded by the British Heart Foundation.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of T. Jenkinson and staff, University of Reading, and A. Hunt and M. Ardakani, Imperial College London.
Present address of A. R. Bond: Bristol Heart Institute, Level 7, Bristol Royal Infirmary, Bristol BS2 8HW, UK.
REFERENCES
- 1.Al-Musawi S, Bishton J, Dean J, Williams S, Cremers SG, Weinberg PD. Evidence for a reversal with age in the pattern of near-wall blood flow around aortic branches. Atherosclerosis 172: 79–84, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Balconi G, Spagnuolo R, Dejana E. Development of endothelial cell lines from embryonic stem cells: a tool for studying genetically manipulated endothelial cells in vitro. Arterioscler Thromb Vasc Biol 20: 1443–1451, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Barnes SE, Weinberg PD. Contrasting patterns of spontaneous aortic disease in young and old rabbits. Arterioscler Thromb Vasc Biol 18: 300–308, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Barnes SE, Weinberg PD. Two patterns of lipid deposition in the cholesterol-fed rabbit. Arterioscler Thromb Vasc Biol 19: 2376–2386, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Barnes SE, Weinberg PD. Strain-dependent differences in the pattern of aortic lipid deposition in cholesterol-fed rabbits. Exp Mol Pathol 71: 161–170, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Bond AR. Effect of Age and Species on Blood Flow Patterns at Arterial Branches in Relation to Atherosclerosis (PhD Thesis). London: University of London, 2007, p. 290–297 [Google Scholar]
- 7.Bond AR, Weinberg PD. Haemodynamic stresses and wall structure can account for the pattern of lipid deposition around aortic branches in mice (Abstract). Atherosclerosis Suppl 7: 200, 2007 [Google Scholar]
- 8.Cheng C, Helderman F, Tempel D, Segers D, Hierck B, Poelmann R, van Tol A, Duncker DJ, Robbers-Visser D, Ursem NT, van Haperen R, Wentzel JJ, Gijsen F, van der Steen AF, de Crom R, Krams R. Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis 195: 225–235, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103: 177–185, 1981 [DOI] [PubMed] [Google Scholar]
- 10.Di Stefano I, Koopmans DR, Langille BL. Modulation of arterial growth of the rabbit carotid artery associated with experimental elevation of blood flow. J Vasc Res 35: 1–7, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Eskin SG, Ives CL, McIntire LV, Navarro LT. Response of cultured endothelial cells to steady flow. Microvasc Res 28: 87–94, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Feintuch A, Ruengsakulrach P, Lin A, Zhang J, Zhou YQ, Bishop J, Davidson L, Courtman D, Foster FS, Steinman DA, Henkelman RM, Ethier CR. Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am J Physiol Heart Circ Physiol 292: H884–H892, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Flaherty JT, Pierce JE, Ferrans VJ, Patel DJ, Tucker WK, Fry DL. Endothelial nuclear patterns in the canine arterial tree with particular reference to haemodynamic events. Circ Res 30: 23–33, 1972 [DOI] [PubMed] [Google Scholar]
- 14.Fourman J, Moffat DB. The effect of intra-arterial cushions on plasma skimming in small arteries. J Physiol 158: 374–380, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorgas K, Bock P. Studies on intra-arterial cushions. I. Morphology of the cushions at the origins of intercostal arteries in mice. Anat Embryol (Berl) 148: 59–72, 1975 [DOI] [PubMed] [Google Scholar]
- 16.Garlanda C, Parravicini C, Sironi M, De Rossi M, Wainstok de Calmanovici R, Carozzi F, Bussolino F, Colotta F, Mantovani A, Vecchi A. Progressive growth in immunodeficient mice and host cell recruitment by mouse endothelial cells transformed by polyoma middle-sized T antigen: implications for the pathogenesis of opportunistic vascular tumors. Proc Natl Acad Sci USA 91: 7291–7295, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greve JM, Les AS, Tang BT, Draney Blomme MT, Wilson NM, Dalman RL, Pelc NJ, Taylor CA. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am J Physiol Heart Circ Physiol 291: H1700–H1708, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA 97: 9052–9057, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartley CJ, Reddy AK, Madala S, Martin-McNulty B, Vergona R, Sullivan ME, Halks-Miller M, Taffet GE, Michael LH, Entman ML, Wang YX. Hemodynamic changes in apolipoprotein E-knockout mice. Am J Physiol Heart Circ Physiol 279: H2326–H2334, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Helmlinger G, Geiger RV, Schreck S, Nerem RM. Effects of pulsatile flow on cultured vascular endothelial cell morphology. J Biomech Eng 113: 123–131, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem 278: 47291–47298, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res 85: 199–207, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Jo H, Song H, Mowbray A. Role of NADPH oxidases in disturbed flow- and BMP4-induced inflammation and atherosclerosis. Antioxid Redox Signal 8: 1609–1619, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Kazakidi A, Sherwin SJ, Weinberg PD. Effect of Reynolds number and flow division on patterns of haemodynamic wall shear stress near branch points in the descending thoracic aorta. J R Soc Interface 6: 539–548, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim DW, Gotlieb AI, Langille BL. In vivo modulation of endothelial F-actin microfilaments by experimental alterations in shear stress. Arteriosclerosis 9: 439–445, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Langille BL, Adamson SL. Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ Res 48: 481–488, 1981 [DOI] [PubMed] [Google Scholar]
- 27.Levesque M, Liepsch D, Moravec S, Nerem RM. Correlation of endothelial cell shape and wall shear stress in a stenosed dog aorta. Arteriosclerosis 6: 220–229, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Levesque MJ, Nerem RM. The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng 107: 341–347, 1985 [DOI] [PubMed] [Google Scholar]
- 29.Lewis JC, Taylor RG, Jones ND, St. Clair RW, Cornhill JF. Endothelial surface characteristics in pigeon coronary artery atherosclerosis. I. Cellular alterations during the initial stages of dietary cholesterol challenge. Lab Invest 46: 123–138, 1982 [PubMed] [Google Scholar]
- 30.Malinauskas RA, Sarraf P, Barber KM, Truskey GA. Association between secondary flow in models of the aorto-celiac junction and subendothelial macrophages in the normal rabbit. Atherosclerosis 140: 121–134, 1998 [DOI] [PubMed] [Google Scholar]
- 31.McGillicuddy CJ, Carrier MJ, Weinberg PD. Distribution of lipid deposits around aortic branches of mice lacking low density lipoprotein receptors and apolipoprotein E. Arterioscler Thromb Vasc Biol 21: 1220–1225, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Okano M, Yoshida Y. Influences of shear stress on endothelial cell shapes and junction complexes at flow dividers of aortic bifurcations in cholesterol-fed rabbits. Front Med Biol Eng 5: 95–120, 1993 [PubMed] [Google Scholar]
- 33.Sinzinger H, Silberbauer K, Auerswald W. Quantitative investigation of sudanophilic lesions around the aortic ostia of human fetuses, newborn and children. Blood Vessels 17: 44–52, 1980 [DOI] [PubMed] [Google Scholar]
- 34.Sloop GD, Perret RS, Brahney JS, Oalmann M. A description of two morphologic patterns of aortic fatty streaks, and a hypothesis of their pathogenesis. Atherosclerosis 141: 153–160, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem 278: 31128–31135, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Sucosky P, Padala M, Elhammali A, Balachandran K, Jo H, Yoganathan AP. Design of an ex vivo culture system to investigate the effects of shear stress on cardiovascular tissue. J Biomech Eng 130: 035001, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walpola PL, Gotlieb AI, Langille BL. Monocyte adhesion and changes in endothelial cell number, morphology and F-actin distribution elicited by low shear stress in vivo. Am J Pathol 142: 1392–1400, 1993 [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg PD. Disease patterns at arterial branches and their relation to flow. Biorheology 39: 533–537, 2002 [PubMed] [Google Scholar]
- 39.Weinberg PD, Ethier CR. Twenty-fold difference in hemodynamic wall shear stress between murine and human aortas. J Biomech 40: 1594–1598, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Williams RL, Risau W, Zerwes HG, Drexler H, Aguzzi A, Wagner EF. Endothelioma cells expressing the polyoma middle T oncogene induce hemangiomas by host cell recruitment. Cell 57: 1053–1063, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Zhao S, Suciu A, Ziegler T, Moore JE, Jr, Bürki E, Meister JJ, Brunner HR. Synergistic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Arterioscler Thromb Vasc Biol 15: 1781–1786, 1995 [DOI] [PubMed] [Google Scholar]