Abstract
The vasa vasorum form a network of microvasculature that originate primarily in the adventitial layer of large arteries. These vessels supply oxygen and nutrients to the outer layers of the arterial wall. The expansion of the vasa vasorum to the second order is associated with neovascularization related to progression of atherosclerosis. Immunohistological analysis of human plaques from autopsied aortas have defined plaque progression and show a significant correlation with vasa vasorum neovascularization. Recent technological advances in microcomputed tomography have enabled investigation of vasa vasorum structure and function in nondiseased large arteries from pigs and dogs. Smaller mammals, particularly mice with genetic modifications that enable disease development, have been used extensively to study the vasa vasorum in diseased vessels. Despite the fact that most mouse models that are used to study atherosclerosis are unable to develop plaque to the extent found in humans, studies in both humans and mice underscore the importance of angiogenic vasa vasorum in progression of atherosclerosis. Those who have examined the vasa vasorum in occluded vessels of nondiseased pigs and dogs find that inhibition of the vasa vasorum makes the animals atheroprone. Atherosclerosis is a multifactorial disease. There is increasing evidence that factors, produced in response to changes in the arterial wall, collaborate with the vasa vasorum to enhance the disease process.
Keywords: vasa vasorum, angiogenesis, atherosclerosis, inflammation
Vasa Vasorum Structure and Function
The vasa vasorum form a network of microvasculature that originates primarily in the adventitial layer of large arteries [vasa vasorum externa (VVE)] with the function of supplying oxygen and nutrients to the outer layers of the arterial wall (35). In larger mammals, the vasa vasorum can also originate from the luminal surface [vasa vasorum interna (VVI)] (45) or from the media (34). Additionally, there are the venous vasa vasorum (VVV) that drain the arterial wall in companion veins (75).
The vasa vasorum can be found as two anatomically distinct types. One type, the first-order vasa vasorum, originates from large vessels and runs longitudinally between the adventitia and media of the main vessel. Small vessel branches from the first-order vasa vasorum form circumferential arches around the vessel wall; these have been classified as second-order vasa vasorum (45). The vasa vasorum originate from various arteries; the vasa vasorum in the ascending aorta come from the brachiocephalic and coronary arteries while those in the descending thoracic aorta come from the intercostal arteries, and vasa vasorum in the abdominal artery originate from the lumbar and mesenteric arteries (7).
There is evidence that the vasa vasorum develop in humans during the first weeks of gestation and continue to expand during fetal growth beginning at the 12th week (6, 87). A study that examined the growth of coronary arteries in postnatal pigs found that the lumen radius and vessel wall area of 1- and 6-mo-old pigs increased with age (28). Reconstructed microcomputed tomography (microCT) images of the vessels were skeletonized to create a vessel tree structure that was then analyzed for diameter, length, and branching angles for each interbranch segment of vasa vasorum volume. The measurements of the vasa vasorum tree structure were smaller in the younger pigs, thus providing evidence that the vessels continue to branch with age.
MicroCT images of vasa vasorum branching patterns in pig coronary arteries have been studied extensively. One group compared the first- and second-order vasa vasorum ratio, density, and diameter in nondiseased vs. diseased hearts (46). The pigs with normal hearts had significantly greater first-order vasa vasorum density compared with the second order (ratio 3:2). The mean diameter in the first-order vasa vasorum was more than twofold greater than the second order. However, the pattern was altered dramatically in hypercholesterolemic pigs. The first-order vessel diameter was 1.4-fold greater than the second order, and the vessel density ratio shifted to one first-order per two second-order vasa vasorum. The increase in vessel density correlated with increased arterial wall area but preceded development of plaque. The most striking difference was the formation of a dense vasa vasorum plexus in the adventitia of the hypercholesterolemic pigs that was not evident in the coronary arteries of healthy animals.
Others, who analyzed vasa vasorum branching patterns in nondiseased pigs with the use of microCT, show a dichotomous tree structure that is similar to the vasculature of systemic circulation; a parent vessel segment divides into two branches, which in turn divide into two additional branches that form a hierarchical structure in which each branch pair is at a higher generation than the parent branch (27). However, the branching lacks uniformity in that some continue to branch while others discontinue the process. This is thought to be because of variability in the needs of localized blood flow.
This same group used microCT to identify the origin of the vasa vasorum structural tree based on the parental vessel (27). Scanned vessels were cross sectioned to analyze the vessel diameter gradient. Measurements of the rate at which the vessel diameter decreases from the parental vessel to the branching tree were used to categorize the various vasa vasorum. Based on these measurements and using the earlier described classification for human hearts (88), the authors placed the VVE in the distributing vessel category, a muscular vessel with few side branches and the same vessel diameter throughout many generations. On the other hand, they considered the VVI to be more similar to delivering vessels, those with symmetrical branches that decrease at each bifurcation, ultimately forming capillaries. Their overall conclusion was that the branching of the VVE and VVI is not significantly different from all other vasculature.
Gossl et al. (27) hypothesized that if the vasa vasorum branching pattern is like a dichotomous tree structure then they must be endarteries rather than a plexus. Perfusion of the coronary artery wall would be quite different in each instance. If the artery was occluded, a plexus would still be able to perfuse the more distal vasa vasorum by shunting and retrograde perfusion while endarteries could not perfuse the distal branches. They took two approaches to examine this hypothesis (26a). The left anterior descending coronary artery (LAD) of nondiseased pigs was injected with either 300- or 100-μm-diameter microspheres to investigate the extent of perfusion when proximal VVE and VVI segments were occluded. Following the occlusion procedure, the hearts were removed from euthanized pigs, and the LAD was infused with Microfil at 100 mmHg. Control animals received either an antegrade infusion of Microfil under physiological pressure or retrograde infusion under atmospheric pressure. MicroCT and histological analysis of the perfused vessels showed that occlusion of the proximal vasa vasorum resulted in decreased perfusion of arterial vasa vasorum and increased the size of low vasa vasorum density areas in the coronary artery wall. The same distribution of low vasa vasorum density areas was also found in the controls that were perfused antegradely under physiological pressure. On the other hand, retrograde filling of the LAD at atmospheric pressure resulted in greater spatial density of perfused vasa vasorum in all quadrants of the vessel wall. Based on these data, it was concluded that the vasa vasorum are not connected by a plexus, but are endarteries. The argument to support this conclusion was that a plexus would have compensated for the occlusion by redirecting the blood flow through a network.
The combined studies clearly demonstrate that the adventitial vasa vasorum acquire a significantly altered branching pattern in response to early cues in the disease process. One theory is that thickening of the vessel wall stimulates a hypoxic environment, which activates the angiogenic process in the adventitial vasa vasorum (16, 64). For the hypercholesterolemic pig coronary artery vasa vasorum to expand to the extent shown by Kwon et al. (45, 46), the extracellular matrix (ECM) composition must undergo changes to provide an environment suitable for activation of angiogenic factors, provide an appropriate scaffold to support endothelial cell (EC) adhesion, vessel formation, stability, maturation, and increased density (73). The necessary factors are secreted by various cell types in the stroma of the vessel wall and/or by the vascular cells within the vessel wall. The specific events of the disease process that stimulate these events remain largely unknown.
The VVV in the porcine coronary artery has a much more complex tree structure. It is not a major arterial branch but develops inside the coronary artery and drains to branches of concomitant coronary veins. The VVV drain a larger area of the arterial vessel than the VVI and VVE perfuse. The authors interpreted this to mean that the area covered by VVV is about the size of a new plaque and that the VVV keep the wall oxygenated and fed such that it does not become hypoxic, a condition considered to be a major factor in development of atherosclerosis. Based on these findings in nondiseased vessels, the authors took a stance similar to others: increased vasa vasorum is atheroprotective (2, 26, 35, 65).
The notion that expansion of the vasa vasorum is atheroprotective is based on experiments in dogs and pigs where the parental vessel that supplies blood to the vasa vasorum was ligated so that the role of the vasa vasorum in the vessel wall could be determined. The vasa vasorum are in the outer and inner layers of the media in pigs and dogs, as well as humans, with little blood flow occurring within the middle layer. The outermost third of the media is supplied by the advential vasa vasorum while the innermost third is nourished by the luminal blood flow. One study examined the extent of vasa vasorum nourishment of the descending aorta wall in dogs (35). The intercostals arteries branching from the descending aorta were ligated to block the blood flow to the vasa vasorum. The media of the arterial wall was examined for tissue viability 10 days postligation. The middle layer of the media was found to be necrotic, whereas the inner third and outer third layers were not. These data demonstrated that the vasa vasorum supply nutrients to the outer and middle layers of the artery, and the luminal blood nourishes the inner third layer. It provided evidence that the nourishment of the media is dependent on the vasa vasorum (34).
Similar experiments were performed in pigs. The side branches of the femoral artery were ligated to occlude blood flow to the vasa vasorum. Later (3 wk), the ligated arteries of six of eight experimental pigs had significant intimal hyperplasia, which was absent in control animals (2). The authors' interpretation of these results was that inhibition of the vasa vasorum promotes intimal thickening. Although oxygenation measurements were not possible following the ligation, their rationale was that loss of oxygenation by the vasa vasorum would induce a hypoxic environment, a condition that makes the vessel wall atheroprone. The hematoxylin- and eosin-stained cells observed in the thickened intima were assumed to be smooth muscle cells (SMC). Histology was not performed to determine if the cells were in fact SMC. The authors predicted that SMC proliferation was a response to a hypoxic environment following the ligation. However, they did not consider that hypoxia would also induce expression of transcription factor hypoxia inducible factor-1α, which binds the vascular endothelial growth factor (VEGF) promoter to stimulate a proangiogenic and arteriogenic response in ECs (16, 64); VEGF expression was not examined. The conclusions from this study are not consistent with what others have found in human autopsy tissue and mouse models of atherosclerosis.
Vasa Vasorum in Human Diseased Vessels
In 1965, Wilens and Plair (86) showed that vasa vasorum are the source of neovascularization in the media of the arterial wall. Since then, studies have shown that neoangiogenesis associated with more advanced stages of human atherosclerosis is found in plaque (67) and the vasa vasorum (84).
Studies conducted by Barger (1) in 1984 found a strong correlation between the vasa vasorum in the media and intima and atherosclerosis progression in human coronary arteries. Human hearts were perfused with a silicone polymer, and its passage through the capillaries was imaged. The vasa vasorum were associated with plaque and the arterial wall of diseased vessels, but were not present in nondiseased vessels. The vasa vasorum in the diseased vessels extended from the adventitia through the media and into the thickened intima. The vascular pattern changed dramatically between diseased and nondiseased areas. Although it was apparent that these vessels contributed to the disease process, it was unclear if their proliferation occurs before or is a result of plaque formation. That question still remains unanswered.
Others corroborated Barger's findings through immunohistochemical analysis of human carotid atherosclerotic lesions. They found that microvessels were absent in normal intima and in lesions associated with nonspecific intimal thickening. As lesions developed into fatty streaks, microvessels became present but still remained at 30% compared with type III lesions at the preatheroma stage, which had 100% neovascularization (36).
Moreno et al. (57) emphasized the correlation between neovascularized plaque and plaque progression. Two hundred sixty-nine aortic lesions from 24 autopsied human males were examined to determine if there was a correlation between neovascularization from the adventitial vasa vasorum through the media into the plaque. Additionally, they investigated a potential correlation between microvessel density in plaque and plaque rupture based on the knowledge that there is an increase in microvessel density in coronary atherosclerotic lesions from patients with acute myocardial infarction. They established a criteria for isolating uniform plaque samples for sequential histological sectioning. Microvessel density in fibrocalcific and lipid-rich plaques were compared with ruptured plaques. They found that microvessel density was higher in lipid-rich and ruptured plaques as well as in lesions with intraplaque hemorrhage and a thin fibrous cap. Furthermore, the microvessel density in plaques increased proportionately with the level of inflammation. This study underscored the correlation between neovascularized plaque and the severity of plaque progression in the human aorta.
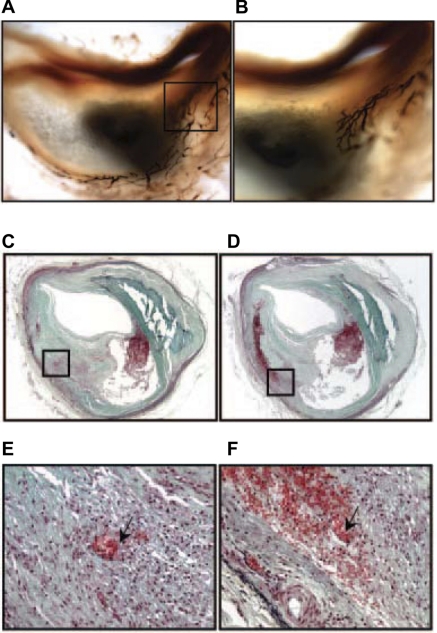
Virmani et al. (43, 83) showed a significant correlation between vasa vasorum density and plaque progression in humans. Serial sections from more than 100 patients' plaques were examined for vasa vasorum and morphological indicators of plaque progression (Fig. 1, A and B). The vessel density increased twofold in plaques categorized as vulnerable and fourfold in those that were ruptured when compared with stable plaques having significant luminal narrowing. Furthermore, they showed that increased microvesssel density is associated with intraplaque hemorrhage, a characteristic of unstable lesions (42) (Fig. 1, C–F).
Fig. 1.
Intraplaque vasa vasorum in human coronary artery. The vasa vasorum identified with endothelial marker Ulex europaeus I is shown in the adventitial and extending through the media and into the intima (magnification ×20) (A) and the border area of the necrotic core (×40) (B). Note the multiple branches of the vasa vasorum in B. Recent hemorrhage in human thin-cap vulnerable plaque is shown in C–E. C: ×20 magnification of intraplaque hemorrhage is noted by black box; E: ×200 amplification of C to illustrate spillage of erythrocytes from vasa vasorum; D: ×20 magnification of serial section of lesion shown in C; F: ×200 amplification of D to illustrate the pool of extravasated erythrocytes surrounding microvessels (arrow). Courtesy of R. Virmani et al., Arterioscler Thromb Vasc Biol 2005 (84).
The importance of microvessels in adventitial vasa vasorum was also shown in human coronary arteries obtained from 68 autopsied subjects who died from acute myocardial infarction, other cardiovascular diseases, infections, neoplasms, alcoholism, and suicide-accident (89). Serial sections from paraffin-embedded blocks were examined for factor VIII, fibrinogen, and albumin. Immunofluorescence and high-resolution confocal microscopy detected neovascularization in thickened intimas before atherosclerotic plaque had developed. The density of the neovascularized area increased as plaque size increased and lumen diameter decreased. The new intimal vessels could be traced back to the vasa vasorum. Albumin and fibrinogen immunoreactivity were closely associated with microvessels in the intima and plaque, indicating that the microvessels were hyperpermeable, a characteristic of angiogenic vessels.
Couffinhal et al. (10) examined expression of VEGF in normal human thoracic aorta, saphenous vein, and internal mammary artery and compared them with tissue from sites of coronary arterial luminal narrowing. Immunohistochemical staining showed that 97% of the specimens were positive for VEGF, but its expression was at higher levels in atherogenic arteries. Although it localized to the ECM and was found in close proximity to infiltrating macrophages, they did not colocalize. Instead, VEGF colocalized with cells expressing CD45RO, a CD45 isoform involved with lymphocyte activation (82). A correlation between VEGF expression and neovascularized vasa vasorum was not found. These data suggested that VEGF plays an inflammatory role rather than an angiogenic role in the wall of medium to large vessels in human tissue.
Altogether, the studies conducted in human tissue clearly indicate that angiogenic vasa vasorum contribute to plaque growth and progression. One could argue that much of the data were collected from autopsied vessels, which are not adequate for controlled experiments. Nevertheless, the trends are confirmatory.
The Vasa Vasorum in Mouse Models of Atherosclerosis
The findings from human studies are strongly supported by data from experiments performed in small mammals. The presence and extent of vasa vasorum correlate with atherosclerotic lesion size and lumen diameter in hypercholesterolemic mouse models (17, 40, 41, 48, 49, 58, 59). The similarities between the human and mouse might be surprising considering that the vessel wall thickness in large arteries of humans is >0.5 mm, thus requiring the presence of the vasa vasorum in the arterial wall for nutrient supply, whereas mice have a vessel wall <0.5 mm and normally have few or no small vessels in the media (34). These differences in vessel thickness do not appear to modify the angiogenic effect of vasa vasorum on plaque development. Therefore, it becomes apparent that disease-related factors stimulate the robust vasa vasorum neovascularization.
As atherosclerotic plaque growth continues, it becomes infiltrated with proinflammatory molecules that are thought to enter the plaque by way of permeable angiogenic vessels (19, 22). Hyperpermeable angiogenic vessels secrete profibrotic and prothrombotic molecules that contribute to plaque progression (80). Inflammatory cells, proteases, matrix proteins, and fibrinolytic and angiogenic factors are all linked to plaque progression toward a more advanced phenotype. Formation of angiogenic vessels is regulated by growth factors, integrins, membrane-bound proteinases, and the composition of the ECM (12, 77, 81). Each of these factors stimulates intracellular signaling pathways that contribute to EC proliferation, branching, sprouting, and lumen formation (12).
Proangiogenic factors and vasa vasorum density in small mammals.
Rat and mouse models have been used extensively to investigate factors that potentially influence the vasa vasorum during intimal thickening and development of plaque. Mouse models are generally preferred because many are genetically modified to enhance the disease process when fed an atherogenic diet. These mice can be crossed with other genetically modified mice to study the role of a specific gene product in the progression or inhibition of the disease process.
VEGF-A164/165 is the more studied angiogenic growth factor relative to the role of the vasa vasorum in intimal thickening and plaque growth. This is in part because of the angiogenesis therapy trials that have tested VEGF effects in cardiovascular disease and cancer. However, VEGF is a complicated growth factor; it stimulates a transient angiogenic response, stable arteriovenous malformations, arteriogenesis, lymphangiogenesis, vascular permeability, edema, fibrin deposition, EC proliferation, migration and, survival (64).
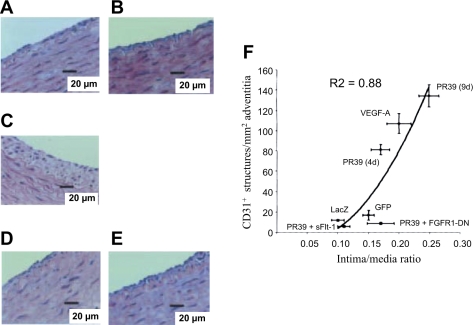
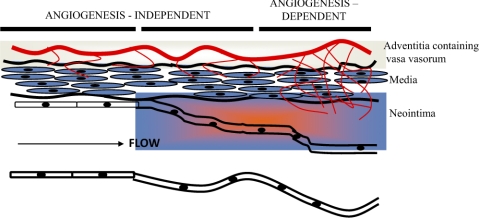
Studies in rats directly addressed VEGF stimulation of angiogenic vasa vasorum and intimal thickening (41). Khurana et al. (40, 41) used two models to induce neointimal formation, each by a different mechanical stimulation. One model used a silicone collar placed around the right carotid artery and exposed the contralateral artery control to stretch similar to that of the collared artery. The second model was a carotid artery balloon injury. In both models, proangiogenic molecules, antiangiogenic molecules, or a combination of both were applied to the adventitial space to examine SMC accumulation and arterial wall angiogenesis. Proangiogenic molecule PR39, a proline-arginine-rich angiogenic response peptide (51), and VEGF-A165, delivered via an adenoviral construct, induced a significant increase in intimal thickening. PR39 is thought to mediate its proangiogenic effects by stimulating VEGF and fibroblast growth factor (FGF) receptors (60). Therefore, VEGF activity was inhibited by a VEGF trap (soluble Flt-1), and FGF functions were inhibited with a dominant-negative FGF receptor (DN-FGFR1). In both cases, intimal thickening was reduced to control levels. However, there was no effect on intimal thickening when soluble Flt-1 and DN-FGFR1 were added to the periadventitial space in the absence of angiogenesis, thus demonstrating a significant correlation between adventitial angiogenesis and intimal thickening (41). Additionally, mechanical injury to the artery in the absence of proangiogenic stimulators promoted a mild adventitial angiogenic response that was not altered by angiogenesis inhibitors (Fig. 2). The authors concluded that angiogenesis is not sufficient to induce neointima thickening, but once the event is initiated proangiogenic molecules can promote the growth of the intimal lesion. This study demonstrated that there is an angiogenesis-independent and an angiogenesis-dependent component to mechanical injury of an artery (depicted in Fig. 3). These data confirm human studies, which suggested that adventitial vasa vasorum of human coronary arteries have a role in atherosclerotic plaque growth (1) and vessel stenosis following injury (45).
Fig. 2.
Vascular endothelial growth factor (VEGF) and fibroblast growth factor receptor (FGFR1) inhibitors block proangiogenic molecule (PR39)-induced intimal thickening and adventitial angiogenesis. Adventitia of collared carotid artery transduced with adenovirus (Ad)-expressing dominant negative (DN) FGFR (Ad-FGFR1-DN) was stained with hematoxylin and eosin 9 days posttransduction. A: VEGF inhibitor sFlt-1 (A) and FGFR1 inhibitor Ad-FGFR1-DN (B) transfer alone had no effect on neointima formation, whereas Ad-PR39 (C) increased intimal thickening after 9 days. Intimal thickening induced by Ad-PR39 was inhibited by cotransfection with sFlt-1 (D) or Ad-FGFR1-DN (E) to levels comparable to control gene transfection. F: adventitial angiogenesis is significantly correlated with degree of intimal thickening in collared arteries after treatment with angiogenesis stimulators and/or inhibitors. GFP, green fluorescent protein. Courtesy of R. Khurana et al., Circulation 2004 (41).
Fig. 3.
Adventitial angiogenesis in intimal thickening. Distinct phases of intimal thickening occur regardless of endothelial integrity. Adventitial angiogenesis contributes to intimal hyperplasia in a later phase that occurs after an injury-induced and angiogenesis-independent early phase. Courtesy of R. Khurana et al., Circulation 2005 (40).
Others examined the role of the angiogenic growth factor VEGF in relationship to intimal thickening and plaque progression in atherogenic mouse and rabbit models (5). They reported that VEGF enhances plaque growth. However, the mechanism is seemingly through an inflammatory-mediated pathway. The atherogenic ApoE−/−ApoB100+/+ mice administered a single low dose of VEGF or albumin (permeability control) had a 14-fold increase in plaque area, but not until after 2 wk of treatment. The plaque growth increased proportionately with macrophage levels in bone marrow and peripheral blood 3 wk after VEGF injection. The results in ApoE−/−ApoB100−/− mice were comparable to those found in atherogenic New Zealand White rabbits treated with VEGF or albumin (4, 5). These studies were the subject of debate because of the length of time it took for a proatherosclerotic effect to occur after a single injection of VEGF. This contradicted the results of others who showed that a single dose of VEGF does not remain in circulation for more than a few hours (20). Furthermore, the investigators did not examine VEGF expression levels after the single bolus injection.
Additional evidence that VEGF has a proinflammatory cytokine function in plaque development was provided by Zhao et al. (90). This study used the cuff-induced periarterial injury model in hypercholesterolemic mice. Soluble Flt-1 was introduced in the periadventitial space to test its inhibition of VEGF, VEGF receptor (VEGFR)-1, VEGFR-2, monocyte chemotactic protein-1, and placental growth factor (PlGF) expression. They found that inhibition of VEGF reduced an early inflammatory response that was accompanied by a later reduction in neointimal thickening.
Others have not been able to detect any proatherogenic correlation with VEGF in low-density lipoprotein receptor-deficient (LDLR−/−) ApoB48-deficient hypercholesterolemic mice (50). Mice received VEGF-A, -B, -C, or -D treatment at different time intervals after initiation of a Western diet. In all cases, the investigators did not detect any significant differences in atherosclerotic lesion size, neovascularization, nor lesion composition when compared with controls.
Mouse studies that investigated VEGF's angiogenic activity in the vasa vasorum suggest that it functions not only as a proangiogenic factor but also as an inflammatory factor in the setting of atherosclerosis. Determining the timing of VEGF proangiogenic and proinflammatory function could provide valuable insight into the sequence of events that initiate intimal thickening leading to plaque development.
PlGF, a member of the VEGF family of proteins (64), signals through VEGFR-1 on ECs to stimulate postembryonic pathophysiological angiogenesis and monocyte chemotaxis (8). This growth factor has raised interest in terms of its ability to stimulate angiogenesis and recruit monocytes (76). The silicone collar model was used to examine these PlGF functions in the carotid artery of rabbits fed normal chow or high-cholesterol diet. Adenovirus expressing PlGF (Ad-PlGF) was introduced to the periadventitial space. The same functions were examined in atherogenic ApoE−/−PlGF−/− mice. The investigators found that neovascularization was increased significantly in the adventitia but was not present in the media or the atherosclerotic lesions of the PlGF-transduced carotid arteries of rabbits fed either diet. However, Ad-PlGF induced adventitial and neointimal macrophage accumulation in collared atherogenic rabbits. A comparison of the ApoE−/−PlGF−/− with ApoE−/− mice showed that the absence of PlGF significantly reduced plaque size and macrophage number. This effect was only detected in early atherosclerotic plaques and was dramatically reduced at 25 wk of high-fat diet. Neovascularization was not a significant determinant in this model, presumably because of the small lesion size in early atherogenesis. These data show that PlGF, a VEGFR1 ligand, promotes adventitial angiogenesis and an inflammatory response, but only in early stages of atherosclerosis.
The work of Drinane et al. (17) indicates that FGF-2 is the primary angiogenic growth factor expressed in the vasa vasorum of LDLR−/−ApoB48-deficient mice fed an atherogenic diet for 20 wk. Vessels perfused with fluorescein isothiocyanate-labeled lectin showed that adventitial vasa vasorum have a lumen and follow a well-defined pattern of FGF-2-bound vascular structures. FGF-2 detected in plaque was also associated with vessels. Quantitative PCR showed an eightfold increase in FGF-2 mRNA copy number in mice fed an atherogenic diet compared with age- and gender-matched chow-fed mice. Quantitative PCR analysis of VEGF-A165 mRNA levels showed no significant differences among the test groups. These data suggest that FGF-2 has a significant role in stimulating angiogenesis in the vasa vasorum and contributes to plaque growth while VEGF appears to have no influence, at least within the examined time frame. Studies that examine earlier time points may show that VEGF plays a role in early plaque progression.
FGF-1 has also been implicated in promoting intimal hyperplasia and angiogenesis in a porcine model. In this study, Nabel et al. (63) introduced a eukaryotic gene expression vector encoding secreted FGF-1 in the arterial wall while β-galactosidase served as the control gene. After the gene transfer (21 days), arteries were examined for intimal thickening and angiogenesis. In the case of the FGF-1-transduced arteries, the ratio of the intima to media was sixfold greater than in the β-galactosidase-transduced arteries. Angiogenesis was also increased in the intima of some, but not all, of the FGF-1-expressing pigs.
Granulocyte macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) can mobilize endothelial progenitor cells in some animal models and thus have been tested in clinical trials for their ability to improve cardiac function in myocardial infarctions (38). A study designed to examine the therapeutic effects of GM-CSF and G-CSF on atherosclerosis in ApoE−/− mice showed very surprising results (29). Both factors increased plaque area by greater than twofold and neovascularization of the arterial wall was greater than sevenfold when compared with the normal chow-fed mice. Inflammatory markers were not increased nor was plaque morphology altered in response to the treatment. These results give pause to clinical applications of GM-CSF and G-CSF given the potential risks demonstrated in this study.
Inhibition of the vasa vasorum with antiangiogenic proteins.
Others have studied the effects of angiogenesis inhibitors on adventitial vasa vasorm and plaque growth in genetically modified atherogenic mouse models. Three of these inhibitors, which are all breakdown products of ECM proteins, have proven to be effective inhibitors of the vasa vasorum and plaque progression (17, 58, 59). Endostatin, a naturally occurring 20-kDa COOH-terminal fragment of type XVIII collagen (70), delivered daily to atherogenic ApoE−/− mice over a 16-wk period, inhibited plaque growth by 85% and vasa vasorum density by nearly sixfold, but did not have any detectable impact on cholesterol levels (58) . The investigators also found that endostatin treatment was less effective in smaller lesions where significant intimal neovascularization was absent, which is consistent with the conclusions reported by Khurana et al. (41) in rat models of carotid artery mechanical injury (Fig. 2).
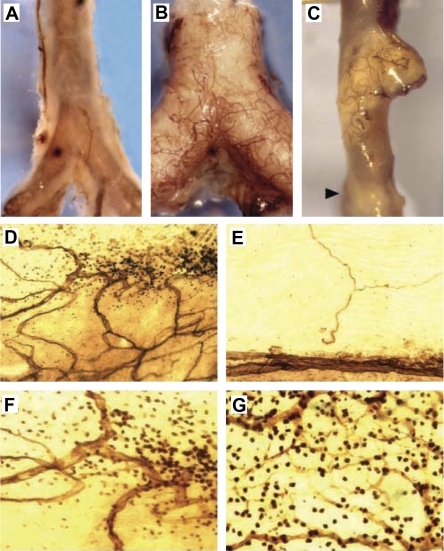
Studies that examined the antiangiogenic effects of angiostatin, a cleavage product of plasminogen (71), in atherogenic ApoE−/− mice found that the vasa vasorum was expanded in some but not all plaques. Interestingly, the neovascularized vasa vasorum was not uniformly distributed within the same plaque (Fig. 4). Further investigation determined a significant linear correlation between infiltrated mononuclear cells and vasa vasorum density (r2 = 0.815), but not with plaque size (59). Angiostatin treatment reduced plaque angiogenesis, plaque growth, and accumulation of macrophages at sites of neovascularized vasa vasorum, but did not reduce cholesterol levels. This study demonstrated that the neovascularization of advanced atherosclerotic plaques in this particular mouse model is more closely associated with the presence and extent of inflammatory cells rather than plaque size.
Fig. 4.
Vasa vasorum associated with inflammatory cells in atherosclerotic plaque. CD31-probed whole-mounted aortas from either C57BL6/J (A) or ApoE−/− (B and C) mice have varied levels of vasa vasorm network associated with plaque. The normal C57BL6/J mouse in A does not have many vasa vasorum, whereas the atherogenic ApoE−/− mouse in B has extensive vasa vasorum networks associated with plaque (opaque). The vasa vasorum are not uniformly associated with advanced atheroma, as shown in C and D. E: vasa vasorum are absent in areas without plaque. F: lesion in D at ×400 magnification shows CD31+ leukocytes accumulated around the vasa vasorum. G: intense mononuclear staining associated with vasa vasorum. Courtesy of K. S. Moulton et al., Proc Natl Acad Sci USA 2003 (59).
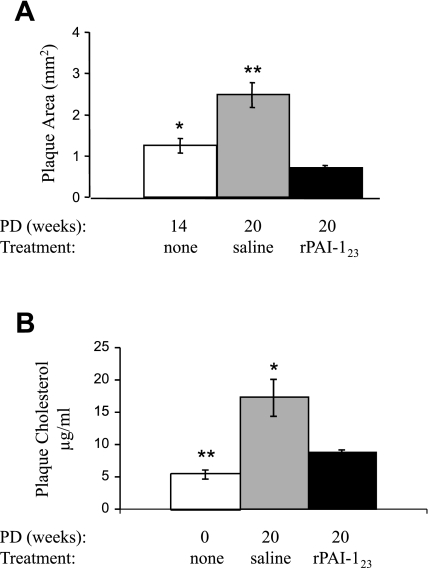
More recent studies using the angiogenesis inhibitor rPAI-123, a plasminogen activator inhibitor (PAI)-1 cleavage product (18, 61, 62), in atherogenic LDLR−/−ApoB48-deficient mice (21) resulted in a 37% reduction in total vasa vasorum area, a 43% reduction in vessel length, a 73% reduction in plaque area, and a remarkable 49% reduction in plaque cholesterol (Fig. 5) (17). This study demonstrates that rPAI-123 activity promotes regression of vasa vasorum and plaque (Fig. 6).
Fig. 5.
rPAI-123 promotes regression of plaque area and plaque cholesterol in the descending aorta of atherogenic mice. Twelve-week-old female low-density lipoprotein receptor-deficient (LDLR−/−) ApoB48-deficient mice were fed Paigen's diet without cholate (PD) for varied time periods and received variable treatment in the final 6 wk of diet. A: plaque area was measured in en face preparations of descending aortas stained with Sudan IV. Plaque area relative to total area of the descending aorta was calculated in mice receiving PD for 14 wk (n = 14), PD for 20 wk [saline weeks 14–20 (n = 16)], and PD for 20 wk [rPAI-123 weeks 14–20 (n = 21)]. B: plaque cholesterol measured in the descending aorta from fasted atherogenic mice. White bars: chow diet in age-matched female mice (n = 6); gray bars, PD for 20 wk [saline weeks 14–20 (n = 6)]; black bars, PD for 20 wk [rPAI-123 weeks 14–20 (n = 6)]. Data are shown as means ± SE, and P values were determined by ANOVA. *P < 0.05 vs. rPAI-123. **P < 0.001 vs. rPAI-123. Courtesy of M. Drinane et al., Circ Res 2009 (17).
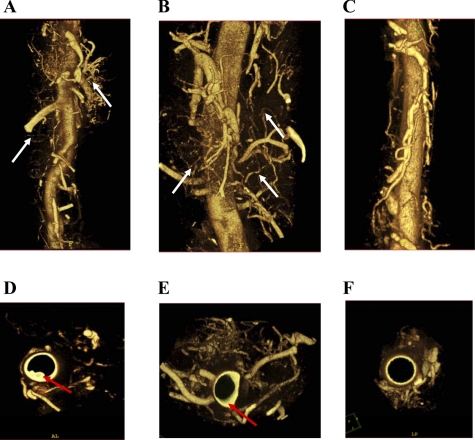
Fig. 6.
Microcomputed tomography images of second-order vasa vasorum. LDLR−/− ApoB48-deficient mice fed PD without cholate for either 14 or 20 wk were infused with Microfil. Descending aortas were scanned ex vivo at 6.5 μm, and three-dimensional volumetric images of vasa vasorum were reconstructed from mice receiving PD for 14 wk [no treatment (n = 3); A]; PD for 20 wk [saline weeks 14–20 (n = 3); B]; and PD for 20 wk [rPAI-123 weeks 14–20 (n = 3); C]. Reconstructed images were rotated to obtain luminal images from mice receiving PD for 14 wk (no treatment; D), PD for 20 wk (saline weeks 14–20; E), and PD for 20 wk (rPAI-123 weeks 14–20; F). White arrows, second-order vasa vasorum; red arrows, plaque. Courtesy of M. Drinane et al., Circ Res 2009 (17).
Endostatin, rPAI-123, and angiostatin all have a similar effect on neovascularized vasa vasorum and plaque progression. The other common denominator for all three inhibitors is that they are breakdown products of an ECM/basement membrane protein. The ECM provides a support scaffold that is essential for blood vessel stability. Adhesion of EC to the ECM enables them to undergo migration, proliferation, and morphogenesis, which are all necessary for neovascularization. Degradation of the ECM leads to vessel collapse/regression (13, 14). These three antiangiogenic proteins may in part inhibit angiogenic vasa vasorum through breakdown of the ECM followed by loss of vessel stability that leads to vessel collapse.
Vasa Vasorum and Inflammation
A dysfunctional endothelium is one of the initial signatures of early atherosclerosis that is stimulated by multiple factors to include elevated and modified low-density lipoproteins, sheer stress, free radicals, and hypertension (47). The altered endothelium induces an inflammatory response.
Atherosclerosis is a cardiovascular and fibroproliferative inflammatory disease (30, 31). The inflammatory process entails interactions between vascular cells and leukocytes along with production of reactive oxygen species (ROS) (11). Monocytes/macrophages, T lymphocytes, and neutrophils secrete cytokines that stimulate EC activation, proliferation, and migration, which are all part of the angiogenic process (66).
The original theory regarding the interaction between the endothelium and inflammatory cells in the initial stages of atherosclerosis was that various risk factors stimulate leukocyte infiltration into the subendothelial space. This event promotes endothelial production of ROS. Moreover, the accumulation of oxidized lipids on the intimal surface causes injury to the endothelium and initiates an inflammatory response (53). In this “inside-out” theory, the process would begin in the intima and work its way outward into the adventitia. However, there is now evidence that an “outside-in” theory may be more plausible. In this theory, vessel wall inflammation begins in the adventitia and works its way into the media and intima. The data that support this theory are increased vasa vasorum neovascularization, increased numbers of adventitial leukocytes (25), and proliferation and differentiation of adventitial fibroblasts into myofibroblasts (78, 79).
The adventitial vasa vasorum are thought to be a conduit for entry of inflammatory mediators and cells to the vessel wall and eventually to the developing plaque where they contribute significantly to disease progression (15, 37). This is made possible by vascular permeability factors such as VEGF. The permeability leads to edema, which attracts inflammatory cells to the permeable vessels. There is also evidence to support the idea that the inflammatory cells accumulate in the adventitia first and in turn induce the angiogenic process. The actual sequence of events remains to be determined.
Obesity, angiogenesis, and atherosclerosis.
Obesity in the United States has reached unacceptable levels; body mass index measurements show that 30.5% of adults are obese, 64.5% are overweight, and 15.5% of 15- to 19-yr-olds are overweight (23, 33, 68). Obesity is associated with cardiovascular disease (44, 55, 74). There is increasing evidence to link excess visceral fat content with atherosclerosis, particularly in young adults (55, 56). The mechanisms by which obesity accelerates atherosclerosis in adolescents and young adults are unknown, but are the subject of intense research. The hormonal milieu sets up adiposity by producing leptin, a circulating hormone that regulates adipose tissue mass (72). It induces vascular permeability (3), chronic inflammation in adipose tissue (69), and low-grade vascular inflammation (9) and increases oxidative stress (54). Growth of body fat requires angiogenesis and ECM remodeling to support the expansion of adipose tissue that becomes a depot for expression and secretion of factors required for these processes to occur (52). Adipose tissue contains angiogenic growth factors, inflammatory mediators, and endothelial and inflammatory cells that are necessary for angiogenesis. VEGF is responsible for most adipose tissue angiogenesis (24, 32, 85), whereas FGF-2 enhances adipocyte differentiation (39). Visceral fat content could have a paracrine effect on the development of atherosclerosis. However, local surrounding adiposity may be a more likely source of factors that exert a paracrine effect on the vessel wall.
Conclusions
The diseased vessel wall in atherosclerosis is a complex combination of many intertwined biological processes. It is clear that angiogenesis and inflammation are major contributors to the diseased vessel wall and that the adventitial vasa vasorum facilitate both processes. However, many questions remain unanswered in terms of the sequence of events and the factors that initiate the neovascularization to promote disease progression. Part of the reason for this lack of information is that the existing published data lack uniformity in mouse models and their diet as well as timing of examined events. Additionally, there was not a mouse model that exemplifies the vulnerable plaque that occurs in advanced human atherosclerosis until recently (48). The human plaque data have significantly impacted our understanding of plaque progression. However, the tissue comes from autopsied specimens, which prevent controlled experiments to follow the order of disease events.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-69948 (M. J. Mulligan-Kehoe).
DISCLOSURES
No conflicts of interest are declared by the author.
REFERENCES
- 1.Barger AC, Beeuwkes R, 3rd, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med 310: 175–177, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Barker SG, Talbert A, Cottam S, Baskerville PA, Martin JF. Arterial intimal hyperplasia after occlusion of the adventitial vasa vasorum in the pig. Arterioscler Thromb 13: 70–77, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci USA 98: 6390–6395, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celletti FL, Hilfiker PR, Ghafouri P, Dake MD. Effect of human recombinant vascular endothelial growth factor165 on progression of atherosclerotic plaque. J Am Coll Cardiol 37: 2126–2130, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med 7: 425–429, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Clarke JA. An x-ray microscopic study of the development of the vasa vasorum in the human foetal aorta and pulmonary trunk. Acta Anat (Basel) 63: 55–70, 1966 [DOI] [PubMed] [Google Scholar]
- 7.Clarke JA. An x-ray microscopic study of the postnatal development of the vasa vasorum in the human aorta. J Anat 99: 877–889, 1965 [PMC free article] [PubMed] [Google Scholar]
- 8.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 271: 17629–17634, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Cleland SJ, Sattar N, Petrie JR, Forouhi NG, Elliott HL, Connell JM. Endothelial dysfunction as a possible link between C-reactive protein levels and cardiovascular disease. Clin Sci (Lond) 98: 531–535, 2000 [PubMed] [Google Scholar]
- 10.Couffinhal T, Kearney M, Witzenbichler B, Chen D, Murohara T, Losordo DW, Symes J, Isner JM. Vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in normal and atherosclerotic human arteries. Am J Pathol 150: 1673–1685, 1997 [PMC free article] [PubMed] [Google Scholar]
- 11.Csanyi G, Taylor WR, Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med 47: 1254–1266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis GE, Bayless KJ, Mavila A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat Rec 268: 252–275, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Davis GE, Saunders WB. Molecular balance of capillary tube formation versus regression in wound repair: role of matrix metalloproteinases and their inhibitors. J Investig Dermatol Symp Proc 11: 44–56, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 97: 1093–1107, 2005 [DOI] [PubMed] [Google Scholar]
- 15.de Boer OJ, van der Wal AC, Teeling P, Becker AE. Leucocyte recruitment in rupture prone regions of lipid-rich plaques: a prominent role for neovascularization? Cardiovasc Res 41: 443–449, 1999 [DOI] [PubMed] [Google Scholar]
- 16.de Muinck ED, Simons M. Re-evaluating therapeutic neovascularization. J Mol Cell Cardiol 36: 25–32, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Drinane M, Mollmark J, Zagorchev L, Moodie K, Sun B, Hall A, Shipman S, Morganelli P, Simons M, Mulligan-Kehoe MJ. The antiangiogenic activity of rPAI-1(23) inhibits vasa vasorum and growth of atherosclerotic plaque. Circ Res 104: 337–345, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drinane M, Walsh J, Mollmark J, Simons M, Mulligan-Kehoe MJ. The anti-angiogenic activity of rPAI-1(23) inhibits fibroblast growth factor-2 functions. J Biol Chem 281: 33336–33344, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol 237: 97–132, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Eppler SM, Combs DL, Henry TD, Lopez JJ, Ellis SG, Yi JH, Annex BH, McCluskey ER, Zioncheck TF. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin Pharmacol Ther 72: 20–32, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Farese RV, Jr, Veniant MM, Cham CM, Flynn LM, Pierotti V, Loring JF, Traber M, Ruland S, Stokowski RS, Huszar D, Young SG. Phenotypic analysis of mice expressing exclusively apolipoprotein B48 or apolipoprotein B100. Proc Natl Acad Sci USA 93: 6393–6398, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng D, Nagy JA, Pyne K, Hammel I, Dvorak HF, Dvorak AM. Pathways of macromolecular extravasation across microvascular endothelium in response to VPF/VEGF and other vasoactive mediators. Microcirculation 6: 23–44, 1999 [PubMed] [Google Scholar]
- 23.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288: 1723–1727, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res 93: e88–e97, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 27: 165–197, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glagov S, Ts'ao CH. Restitution of aortic wall after sustained necrotizing transmural ligation injury. Role of blood cells and artery cells. Am J Pathol 79: 7–30, 1975 [PMC free article] [PubMed] [Google Scholar]
- 26a.Gossl M, Malyar NM, Rosol M, Beighley PE, Ritman EL. Impact of coronary vasa vasorum functional structure on coronary vessel wall perfusion distribution. Am J Physiol Heart Circ Physiol 285: H2019–H2026, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Gossl M, Rosol M, Malyar NM, Fitzpatrick LA, Beighley PE, Zamir M, Ritman EL. Functional anatomy and hemodynamic characteristics of vasa vasorum in the walls of porcine coronary arteries. Anat Rec A Discov Mol Cell Evol Biol 272: 526–537, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Gossl M, Zamir M, Ritman EL. Vasa vasorum growth in the coronary arteries of newborn pigs. Anat Embryol (Berl) 208: 351–357, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Haghighat A, Weiss D, Whalin MK, Cowan DP, Taylor WR. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein E-deficient mice. Circulation 115: 2049–2054, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6: 508–519, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol 1: 297–329, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci 82: 925–934, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291: 2847–2850, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Heistad DD, Marcus ML. Role of vasa vasorum in nourishment of the aorta. Blood Vessels 16: 225–238, 1979 [DOI] [PubMed] [Google Scholar]
- 35.Heistad DD, Marcus ML, Larsen GE, Armstrong ML. Role of vasa vasorum in nourishment of the aortic wall. Am J Physiol Heart Circ Physiol 240: H781–H787, 1981 [DOI] [PubMed] [Google Scholar]
- 36.Jeziorska M, Woolley DE. Neovascularization in early atherosclerotic lesions of human carotid arteries: its potential contribution to plaque development. Hum Pathol 30: 919–925, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Kaartinen M, Penttila A, Kovanen PT. Accumulation of activated mast cells in the shoulder region of human coronary atheroma, the predilection site of atheromatous rupture. Circulation 90: 1669–1678, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Soo Lee D, Sohn DW, Han KS, Oh BH, Lee MM, Park YB. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet 363: 751–756, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi N, Toriyama K, Nicodemou-Lena E, Inou K, Torii S, Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci USA 95: 1062–1066, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation 112: 1813–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Khurana R, Zhuang Z, Bhardwaj S, Murakami M, De Muinck E, Yla-Herttuala S, Ferrara N, Martin JF, Zachary I, Simons M. Angiogenesis-dependent and independent phases of intimal hyperplasia. Circulation 110: 2436–2443, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 349: 2316–2325, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Kolodgie FD, Virmani R, Burke AP, Farb A, Weber DK, Kutys R, Finn AV, Gold HK. Pathologic assessment of the vulnerable human coronary plaque. Heart 90: 1385–1391, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopelman PG. Obesity as a medical problem. Nature 404: 635–643, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Kwon HM, Sangiorgi G, Ritman EL, Lerman A, McKenna C, Virmani R, Edwards WD, Holmes DR, Schwartz RS. Adventitial vasa vasorum in balloon-injured coronary arteries: visualization and quantitation by a microscopic three-dimensional computed tomography technique. J Am Coll Cardiol 32: 2072–2079, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR, Jr, Schwartz RS, Lerman A. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest 101: 1551–1556, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation 109: II27–33, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Langheinrich AC, Michniewicz A, Bohle RM, Ritman EL. Vasa vasorum neovascularization and lesion distribution among different vascular beds in ApoE−/−/LDL−/− double knockout mice. Atherosclerosis 191: 73–81, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Langheinrich AC, Sedding DG, Kampschulte M, Moritz R, Wilhelm J, Haberbosch WG, Ritman EL, Bohle RM. 3-Deazaadenosine inhibits vasa vasorum neovascularization in aortas of ApoE(−/−)/LDL(−/−) double knockout mice. Atherosclerosis 202: 103–110, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Leppanen P, Koota S, Kholova I, Koponen J, Fieber C, Eriksson U, Alitalo K, Yla-Herttuala S. Gene transfers of vascular endothelial growth factor-A, vascular endothelial growth factor-B, vascular endothelial growth factor-C, and vascular endothelial growth factor-D have no effects on atherosclerosis in hypercholesterolemic low-density lipoprotein-receptor/apolipoprotein B48-deficient mice. Circulation 112: 1347–1352, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Li J, Post M, Volk R, Gao Y, Li M, Metais C, Sato K, Tsai J, Aird W, Rosenberg RD, Hampton TG, Sellke F, Carmeliet P, Simons M. PR39, a peptide regulator of angiogenesis. Nat Med 6: 49–55, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Lijnen HR. Angiogenesis and obesity. Cardiovasc Res 78: 286–293, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res 75: 640–648, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maingrette F, Renier G. Leptin increases lipoprotein lipase secretion by macrophages: involvement of oxidative stress and protein kinase C. Diabetes 52: 2121–2128, 2003 [DOI] [PubMed] [Google Scholar]
- 55.McGill HC, Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, Strong JP. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation 105: 2712–2718, 2002 [DOI] [PubMed] [Google Scholar]
- 56.McMahan CA, Gidding SS, Malcom GT, Tracy RE, Strong JP, McGill HC., Jr Pathobiological determinants of atherosclerosis in youth risk scores are associated with early and advanced atherosclerosis. Pediatrics 118: 1447–1455, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O'Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation 110: 2032–2038, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation 99: 1726–1732, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA 100: 4736–4741, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muinck ED, Nagy N, Tirziu D, Murakami M, Gurusamy N, Goswami SK, Ghatpande S, Engelman RM, Simons M, Das DK. Protection against myocardial ischemia-reperfusion injury by the angiogenic Masterswitch protein PR 39 gene therapy: the roles of HIF1alpha stabilization and FGFR1 signaling. Antioxid Redox Signal 9: 437–445, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Mulligan-Kehoe MJ, Kleinman HK, Drinane M, Wagner RJ, Wieland C, Powell RJ. A truncated plasminogen activator inhibitor-1 protein blocks the availability of heparin-binding vascular endothelial growth factor A isoforms. J Biol Chem 277: 49077–49089, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Mulligan-Kehoe MJ, Wagner R, Wieland C, Powell R. A truncated plasminogen activator inhibitor-1 protein induces and inhibits angiostatin (kringles 1–3), a plasminogen cleavage product. J Biol Chem 276: 8588–8596, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Nabel EG, Yang ZY, Plautz G, Forough R, Zhan X, Haudenschild CC, Maciag T, Nabel GJ. Recombinant fibroblast growth factor-1 promotes intimal hyperplasia and angiogenesis in arteries in vivo. Nature 362: 844–846, 1993 [DOI] [PubMed] [Google Scholar]
- 64.Nagy JA, Dvorak AM, Dvorak HF. VEGF-A(164/165) and PlGF: roles in angiogenesis and arteriogenesis. Trends Cardiovasc Med 13: 169–175, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Nakata Y, Shionoya S, Matsubara J, Shinjo K. An experimental study on the vascular lesions caused by disturbance of the vasa vasorum. 3. Influence of obstruction of the venous side of the vasa vasorum and the periaortic vein. Jpn Circ J 36: 945–951, 1972 [DOI] [PubMed] [Google Scholar]
- 66.Naldini A, Carraro F. Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy 4: 3–8, 2005 [DOI] [PubMed] [Google Scholar]
- 67.O'Brien ER, Garvin MR, Dev R, Stewart DK, Hinohara T, Simpson JB, Schwartz SM. Angiogenesis in human coronary atherosclerotic plaques. Am J Pathol 145: 883–894, 1994 [PMC free article] [PubMed] [Google Scholar]
- 68.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 288: 1728–1732, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 117: 798–805, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88: 277–285, 1997 [DOI] [PubMed] [Google Scholar]
- 71.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79: 315–328, 1994 [DOI] [PubMed] [Google Scholar]
- 72.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543, 1995 [DOI] [PubMed] [Google Scholar]
- 73.Rhodes JM, Simons M. The extracellular matrix and blood vessel formation: not just a scaffold. J Cell Mol Med 11: 176–205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roth J, Qiang X, Marban SL, Redelt H, Lowell BC. The obesity pandemic: where have we been and where are we going? Obes Res 12, Suppl 2: 88S–101S, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Schoenenberger F, Mueller A. On the vascularization of the bovine aortic wall. Helv Physiol Pharmacol Acta 18: 136–150, 1960 [PubMed] [Google Scholar]
- 76.Scholz D, Elsaesser H, Sauer A, Friedrich C, Luttun A, Carmeliet P, Schaper W. Bone marrow transplantation abolishes inhibition of arteriogenesis in placenta growth factor (PlGF) −/− mice. J Mol Cell Cardiol 35: 177–184, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Senger DR. Molecular framework for angiogenesis: a complex web of interactions between extravasated plasma proteins and endothelial cell proteins induced by angiogenic cytokines. Am J Pathol 149: 1–7, 1996 [PMC free article] [PubMed] [Google Scholar]
- 78.Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation 94: 1655–1664, 1996 [DOI] [PubMed] [Google Scholar]
- 79.Shi Y, Pieniek M, Fard A, O'Brien J, Mannion JD, Zalewski A. Adventitial remodeling after coronary arterial injury. Circulation 93: 340–348, 1996 [DOI] [PubMed] [Google Scholar]
- 80.Smith EB. Fibrinogen, fibrin and fibrin degradation products in relation to atherosclerosis. Clin Haematol 15: 355–370, 1986 [PubMed] [Google Scholar]
- 81.Stupack DG, Cheresh DA. Apoptotic cues from the extracellular matrix: regulators of angiogenesis. Oncogene 22: 9022–9029, 2003 [DOI] [PubMed] [Google Scholar]
- 82.Trowbridge IS, Thomas ML. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol 12: 85–116, 1994 [DOI] [PubMed] [Google Scholar]
- 83.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 20: 1262–1275, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 25: 2054–2061, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology 146: 4545–4554, 2005 [DOI] [PubMed] [Google Scholar]
- 86.Wilens SL, Plair CM. Blood cholesterol, nutrition, atherosclerosis. A necropsy study. Arch Intern Med 116: 373–380, 1965 [DOI] [PubMed] [Google Scholar]
- 87.Wolinsky H, Glagov S. Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res 20: 409–421, 1967 [DOI] [PubMed] [Google Scholar]
- 88.Zamir M. Distributing and delivering vessels of the human heart. J Gen Physiol 91: 725–735, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y, Cliff WJ, Schoefl GI, Higgins G. Immunohistochemical study of intimal microvessels in coronary atherosclerosis. Am J Pathol 143: 164–172, 1993 [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Q, Egashira K, Hiasa K, Ishibashi M, Inoue S, Ohtani K, Tan C, Shibuya M, Takeshita A, Sunagawa K. Essential role of vascular endothelial growth factor and Flt-1 signals in neointimal formation after periadventitial injury. Arterioscler Thromb Vasc Biol 24: 2284–2289, 2004 [DOI] [PubMed] [Google Scholar]