Abstract
Aging, vascular function, and exercise are thought to have a common link in oxidative stress. Of the 28 subjects studied (young, 26 ± 2 yr; old, 71 ± 6 yr), 12 took part in a study to validate an antioxidant cocktail (AOC: vitamins C, E, and α-lipoic acid), while the remaining 8 young and 8 old subjects performed submaximal forearm handgrip exercise with placebo or AOC. Old subjects repeated forearm exercise with placebo or AOC following knee-extensor (KE) exercise training. Brachial arterial diameter and blood velocity (Doppler ultrasound) were measured at rest and during exercise. During handgrip exercise, brachial artery vasodilation in the old subjects was attenuated compared with that in young subjects following placebo (maximum = ∼3.0 and ∼6.0%, respectively). In contrast to the previously documented attenuation in exercise-induced brachial artery vasodilation in the young group with AOC, in the old subjects the AOC restored vasodilation (maximum = ∼7.0%) to match the young. KE training also improved exercise-induced brachial artery vasodilation. However, in the trained state, AOC administration no longer augmented brachial artery vasodilation in the elderly, but rather attenuated it. These data reveal an age-related pro-/antioxidant imbalance that impacts vascular function and show that exercise training is capable of restoring equilibrium such that vascular function is improved and the AOC-mediated reduction in free radicals now negatively impacts brachial artery vasodilation, as seen in the young.
Keywords: aging, free radicals, oxidative stress, arterial function
a reduction in nitric oxide (NO) bioavailability, perhaps secondary to free radical generation, has been implicated in the predominantly endothelium-mediated attenuation in vasodilatory capacity with age (34, 35). The role of free radicals in this process is supported by the observation that plasma ascorbic acid (vitamin C), a powerful antioxidant, administered by intra-arterial or venous infusion transiently restores resting blood flow and endothelial function in aged subjects (13, 18, 36). Presumably by a similar action, exercise training has been associated with a reduction in oxidative stress (33) and improved vascular function (11, 34). However, relatively little is currently known about the direct interaction among free radicals, antioxidants, exercise, and vascular function in the elderly population.
Despite the successful amelioration of the age-related endothelial dysfunction with high plasma levels of ascorbic acid, to date orally administered vitamin C, despite elevating plasma levels, appears to have little impact (13). This observation may be a consequence of the method employed to assess vascular function [flow-mediated vasodilation (FMD) (13)] and the potentially limited perturbation of the balance between pro- and antioxidant forces in the conduit vessel of interest utilizing this model. In contrast, exercise significantly increases circulating free radical outflow from the active muscle bed and subsequently elevates oxidative stress systemically (1, 4). Because activities of daily living require exercise and an elevation in circulating free radicals appears to be an unavoidable consequence of physical activity (20), most likely more pronounced with age (21), antioxidant supplementation in this population preceding physical activity may facilitate the maintenance of normal vascular function. In contrast to acute exercise, habitual physical activity is associated with improved vascular function (11, 34) and reduced oxidative stress in the vasculature (33) and skeletal muscle (21), likely due to upregulated endogenous antioxidants in both the vasculature and muscle (21, 33). However, the importance of these acute and adaptive processes in the vasculature and the efficacy of antioxidant use in the trained and untrained elderly has yet to be elucidated.
Therefore, with already published handgrip exercise data with and without an antioxidant cocktail (AOC) in young subjects as a reference (31), this study sought to examine exercise-induced brachial artery vasodilation in old subjects and the effect of the same AOC on blood-borne free radicals both before and after exercise training in this population. Specifically, we tested the following five hypotheses: 1) aging will attenuate exercise-induced brachial artery vasodilation, 2) an AOC will significantly attenuate plasma oxidative stress in old subjects, 3) this AOC will restore exercise-induced brachial vasodilation in old subjects, 4) chronic single-leg knee-extensor training will augment exercise-induced brachial vasodilation in old subjects, and 5) exercise training and an oral AOC in old subjects will synergistically improve exercise-induced brachial vasodilation.
MATERIALS AND METHODS
Subjects and general procedures:.
A total of 28 [14 young (18–30 yr) and 14 old (65–80 yr)] healthy men participated in the current study. All subjects were normally active, nonsmokers, free of overt cardiovascular disease, and not hypertensive (<140/90 mmHg). The protocol was approved by, and informed consent was obtained according to, the University of California San Diego Human Subjects Protection Program requirements and was performed in San Diego. Six of the young and six of the old subjects partook solely in the validation of the AOC's ability to reduce oxidative stress and therefore attenuate radical-mediated lipid peroxidation, whereas the remaining eight young and eight old subjects reported to the laboratory to perform a maximal work rate (WRmax) test on the knee-extensor ergometer and a single maximal voluntary contraction (MVC) using a hydraulic handgrip dynamometer (Rolyan Ability One, Germantown, WI) and later took part in the interventional trials. It should be noted that the results for the eight young subjects during handgrip exercise with and without the AOC have already been published (31) and are included in this study simply for reference purposes, whereas the data for the six young subjects who performed the AOC validation studies have not been previously published. Subjects always reported to the laboratory in the fasted state, and data collection was performed with subjects in the supine position with the arm at heart level in a thermoneutral environment.
Preliminary validation of antioxidant efficacy.
The measurements of plasma ascorbate and ex vivo spin trapping in combination with electron paramagnetic resonance (EPR) spectroscopy were performed on venous blood samples with and without antioxidant supplementation. Samples were evaluated at rest and within 15 s of the subject performing maximal cycle exercise to establish the efficacy of the AOC in reducing baseline and exercise-induced free radicals and subsequent radical-mediated lipid peroxidation.
Forearm handgrip exercise:.
Forearm handgrip exercise was performed at low absolute workloads attainable by both groups (rest, 3, 6, and 9 kg at 0.5 Hz), workloads that have previously been documented to elicit similar blood flow responses in both young and old subjects (12). Subjects exercised for 3 min to ensure the attainment of steady-state hemodynamics, with ultrasound measurements performed continuously, but only the last 60 s were analyzed.
Antioxidant supplementation.
On separate study days at least 4 days apart, all subjects received either the AOC or placebo in a balanced, double-blind design. The formulation and timing of this AOC was the result of our pilot work employing vascular sampling and EPR spectral analysis to document its efficacy but was restrained by the intent to not vastly exceed the common “over the counter” dosage for each individual antioxidant. Consequently, supplements were taken in two doses separated by 30 min, with the first dose ingested 2 h before the graded handgrip exercise protocol. The first dose consisted of 300 mg of α-lipoic acid, 500 mg of vitamin C, and 200 IU of vitamin E, whereas the second included 300 mg of α-lipoic acid, 500 mg of vitamin C, and 400 IU of vitamin E. Placebo microcrystalline cellulose capsules were of similar taste, color, and appearance and were likewise consumed in two doses.
Plasma ascorbate.
For ascorbic acid measurements, plasma was stabilized and deproteinated by adding 900 μl of 5% metaphosphoric acid (Sigma Chemical, Dorset, UK) to 100 μl of EDTA plasma. Ascorbic acid was subsequently assayed by fluorimetry based on the condensation of dehydroascorbic acid with 1,2-phenylenediamine (38). The intra-/interassay coefficient of variance (CV) was <5%.
EPR and spin trapping.
To directly assess vascular free radical levels, we performed EPR on venous blood samples, as described previously (2). Briefly, 4.5 ml of venous blood were collected into a vacutainer that contained 1.5 ml of the spin trap α-phenyl-tert-butylnitrone (PBN; 0.140 M). After centrifugation, the PBN adduct was extracted from the serum supernatant with toluene, and the adduct (200 μl) was pipetted into a precision-bore quartz EPR sample tube (Wilmad, Old Glossop, UK) that had been flushed with compressed N2. EPR was performed at 21°C using an EMX X-band spectrometer (Bruker, Billerica, MA) using commercially available software (Bruker Win EPR System, version 2.11) with the analyst blinded to experimental condition.
Lipid hydroperoxide.
As an indirect marker or “footprint” of oxidative stress, serum lipid hydroperoxide (LOOH) was determined using the ferrous iron/xylenol orange (FOX) assay with modification. The intra-/interassay CV was <2 and <4%, respectively.
Exercise training regimen.
After the initial testing days, the older group reported to the laboratory three times each week for 6 wk to complete single-leg knee-extensor exercise training of varied 1-h protocols. The exercise regime combined short, high-intensity intervals (5–10 min at 70–95% of WRmax) with longer, low-intensity work bouts (15–45 min at 40–65% of WRmax). Graded maximal knee-extensor work rate tests were performed to reevaluate WRmax after weeks 2, 4, and 6 of exercise training, with relative work rates adjusted as improvements in WRmax were achieved. This training regime has previously resulted in a significant improvement in maximum O2 consumption in young and old healthy subjects (27, 42).
Ultrasound Doppler measurements and calculations.
The ultrasound system (Logiq 7; GE Medical Systems, Milwaukee, WI) was equipped with a linear array transducer operating at an imaging frequency of 10 MHz. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area, where the best spatial resolution was achieved. Anatomic landmarks, skin notations, and printed ultrasound images were all utilized to ensure a similar site of measurement between scans and across experimental days. The brachial artery was continuously insonated (rest and exercise) approximately midway between the antecubital and axillary regions, medial to the biceps brachii muscle. For each 20-s ultrasound Doppler segment, mean flow velocity (Vmean) was averaged across the first and last 10 s of the recorded clip with diameter measurements (adventitia to adventitia) evaluated during diastole, as described previously (12, 40).
Because blood viscosity was not measured but shear stress has been identified as an important mechanism that stimulates the vascular endothelium, resulting in vasodilation (30), shear rate was calculated using the following equation (7): shear rate (s−1) = 4Vmean (cm/s)/diameter (cm).
Statistics.
Statistics were performed with the use of commercially available software (SPSS). The data related to the main hypotheses of this study were analyzed using a two-factor (drug and exercise intensity) mixed analysis of variance model and did not incorporate the data for young subjects, which are included for reference purposes only and have been published previously (31). After a significant main effect and interaction, Bonferroni-corrected paired sample t-tests were employed to make post hoc comparisons at each level of the within-subjects factor. Between-group comparisons (subject characteristics) were assessed using independent sample t-tests. All group data are means ± SE. Significance was established at P < 0.05.
RESULTS
Subject characteristics.
The young and old subjects were of similar height and weight, but body mass index (BMI) was significantly greater in the old subjects (Table 1). The young subjects had significantly greater maximum handgrip and knee-extensor WRmax than the old subjects (Table 1). In addition, resting heart rate and systolic and diastolic blood pressures were significantly higher in the old subjects (Table 1). Although there was a tendency for resting brachial artery diameter to increase with age, this trend did not achieve statistical significance (young: 0.49 ± 0.02 cm; old: 0.54 ± 0.02 cm; P = 0.08).
Table 1.
Young and old (pretraining) subject characteristics
| Young | Old | |
|---|---|---|
| Age, yr | 24 ± 2 | 71 ± 6* |
| Height, cm | 178 ± 3 | 173 ± 2 |
| Weight, kg | 78 ± 3 | 79 ± 4 |
| Body mass index, kg/m2 | 24 ± 1 | 27 ± 1* |
| Forearm muscle mass, kg | 0.98 ± 0.04 | 0.84 ± 0.08 |
| Quadriceps muscle mass, kg | 2.1 ± 0.08 | 1.6 ± 0.04* |
| Systolic blood pressure (supine), mmHg | 120 ± 4 | 129 ± 3* |
| Diastolic blood pressure (supine), mmHg | 72 ± 3 | 82 ± 4* |
| Heart rate, beats/min | 62 ± 8 | 70 ± 3* |
| Maximum handgrip, kg | 53 ± 3 | 28 ± 2* |
| Maximum knee extensor, W | 62 ± 3 | 31 ± 7* |
| Maximum O2 consumption (cycle), ml·kg−1·min−1 | 38 ± 2 | 24 ± 3* |
Values are means ± SE.
P < 0.05, young vs. old subjects.
Antioxidant cocktail validation and efficacy.
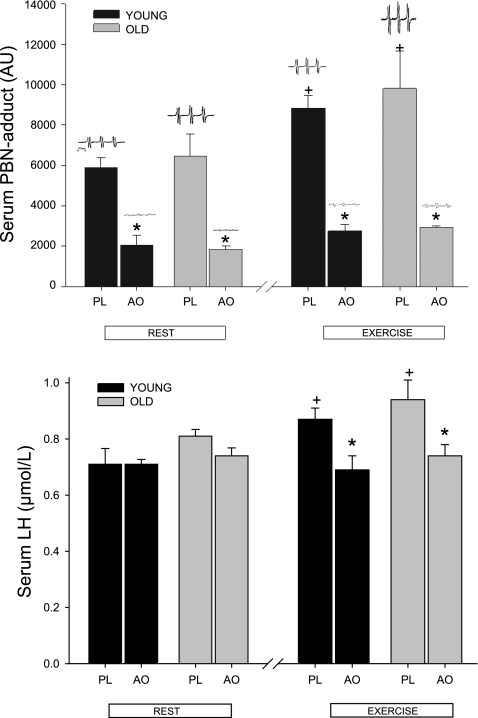
A clear and characteristic “triplet of doublets” EPR signal proportional to free radical concentration was detected at rest in the PBN spin-trapped venous blood of all 12 subjects who took part in the AOC validation study, but there was no difference between young and old subjects (young: 5,872 ± 492 AU; old: 6,453 ± 1,098 AU, where AU is arbitrary units). However, this signal was significantly reduced (young: 2,060 ± 265 AU; old: 1,852 ± 169 AU) in resting venous samples after ingestion of the antioxidant cocktail (Fig. 1, top). Maximal cycling exercise increased the concentration of PBN adducts, resulting in a significantly greater signal magnitude (young: 8,834 ± 646 AU; old: 9,804 ± 1,893 AU) (Fig. 1, top). Again, the ingestion of the AOC significantly attenuated this signal (young: 2,755 ± 323 AU; old: 2,928 ± 83 AU) (Fig. 1, top); however, there also were no age-related differences. The dominant signal exhibited hyperfine coupling constants of aN =13.7 G and aβH = 1.9 G, consistent with published values for an O2-centered alkoxyl species (PBN-LO·) using similar extraction solvents (1).
Fig. 1.
α-Phenyl-tert-butylnitrone (PBN) spin adducts assessed by electron paramagnetic resonance (EPR) spectroscopy (top) and concentration of serum lipid hydroperoxides (LH; bottom) under the conditions of rest and after exercise with placebo (PL) and the oral antioxidant (AO) cocktail in young and old subjects (n = 12). Values are means ± SE. Inset at top are representative individual examples of PBN EPR spectra in each scenario. +P < 0.05, significantly different from resting levels. *P < 0.05, significantly different levels during AO trial from levels in matched PL condition.
The concentration of serum lipid hydroperoxides (LH) were not significantly affected by ingestion of the AOC at rest (young: 0.71 ± 0.06 to 0.71 ± 0.02 μM; old: 0.81 ± 0.02 to 0.74 ± 0.03 μM) (Fig. 1, bottom). However, exercise significantly increased serum LH concentration, and the AOC reduced the effect of exercise (young: 0.87 ± 0.04 to 0.69 ± 0.05 μM; old: 0.94 ± 0.08 to 0.74 ± 0.04 μM) (Fig. 1, bottom). There were no age-specific differences in terms of LH concentration (Fig. 1, bottom).
The AOC significantly elevated plasma ascorbate concentrations in the young and old 2 h after the ingestion of the first dose (Table 2). Exercise also significantly elevated plasma ascorbate concentrations in both groups from rest to postexercise with and without the AOC (Table 2). However, again, there were no age-related differences (Table 2).
Table 2.
Effect of antioxidant cocktail on plasma ascorbate levels in young and old subjects at rest and postexercise
| Young |
Old |
|||
|---|---|---|---|---|
| Placebo | Antioxidant | Placebo | Antioxidant | |
| Resting ascorbic acid, μM | 63 ± 8 | 123 ± 10* | 74 ± 11 | 136 ± 7* |
| Postexercise ascorbic acid, μM | 68 ± 8† | 130 ± 7*† | 78 ± 12† | 141 ± 7*† |
Values are means ± SE.
P < 0.05, placebo vs. antioxidant.
P < 0.05, rest vs. exercise.
Exercise training effects.
The 6 wk of knee-extensor exercise training in the old subjects significantly improved knee-extensor WRmax (pretraining: 29 ± 7 W; posttraining: 40 ± 8 W; P < 0.05) but did not alter maximal handgrip strength. In addition, the exercise training did not change resting brachial artery diameter in the placebo (pretraining: 0.55 ± 0.02 cm; posttraining: 0.54 ± 0.02 cm) or AOC trials (pretraining: 0.53 ± 0.02 cm; posttraining: 0.55 ± 0.02 cm).
Exercise-induced brachial artery vasodilation and shear rate.
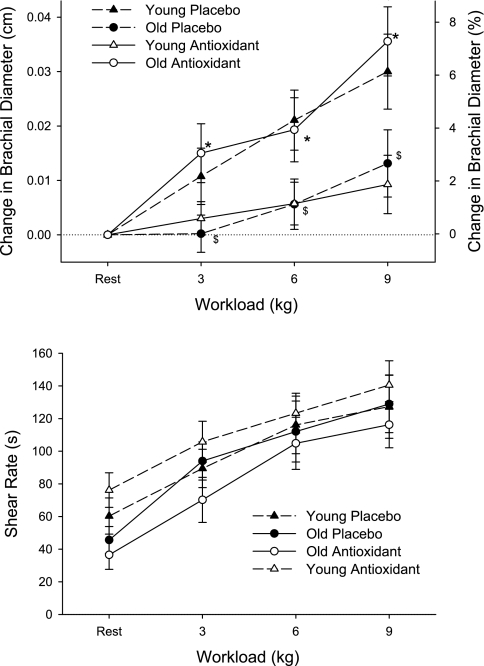
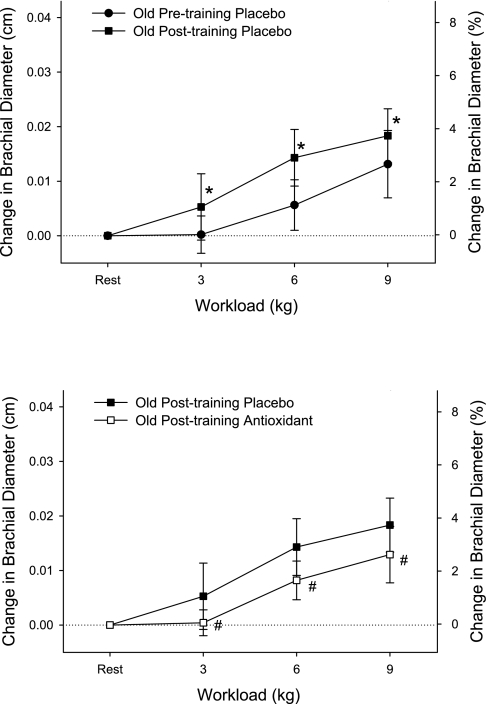
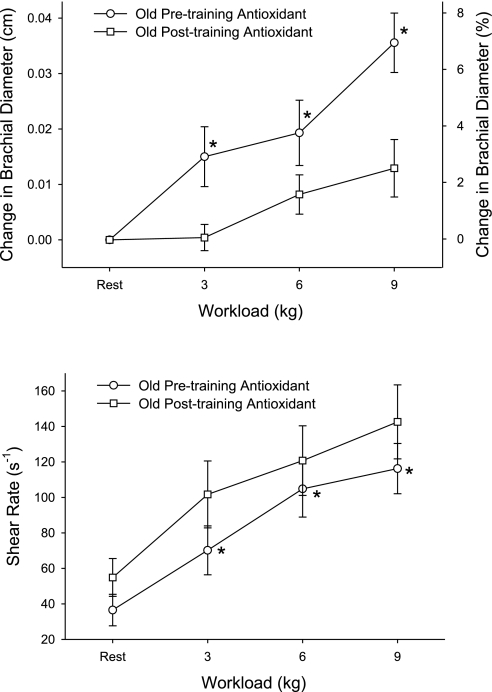
There was an age-related attenuation in exercise-induced brachial vasodilation, and the AOC restored this vasodilatory response in the old subjects back to the level of the young subjects (Fig. 2). Thus antioxidants abolished the age-related difference in exercise-induced brachial artery vasodilation. Unlike in the untrained state, antioxidant administration in the exercise-trained state diminished exercise-induced brachial artery vasodilation compared with the placebo trial (Fig. 3, bottom). The differing effect of the antioxidant treatment was even more clearly revealed when brachial artery vasodilation was compared with the antioxidant treatment in the exercise-trained and untrained state (Fig. 4, top). Across all trials, shear rate increased with increasing handgrip workload (P = 0.04, Figs. 2 and 4, bottom). However, shear rates were not significantly different between young and old and thus could not explain age-related differences in exercise-induced brachial artery vasodilation (Fig. 2, bottom). In the majority of comparisons of vasodilation with and without the AOC and age, the shear rate did not change significantly but tended to inversely reflect alterations in vessel diameter (Figs. 2–4). The exception to this was the old subjects with the AOC before and after the exercise training period (Fig. 4, bottom), where shear rates were significantly lowered when the diameters were significantly increased. Again, however, the altered shear rate could not explain the observed antioxidant-induced change in vasodilation, since they were in opposing directions (Fig. 3, top).
Fig. 2.
Change in brachial artery diameter (top) and shear rates (bottom) in young and old subjects at rest and at 3 levels of handgrip exercise either after the ingestion of the AO cocktail or placebo. Values for %change in brachial diameter (top) are not exact and are displayed solely for reference purposes. Shear rate in the brachial artery was calculated for young and old under each of these conditions (bottom). Values are means ± SE. $P < 0.05, diameter changes in old PL trial significantly decreased from those in young PL trial. *P < 0.05, diameter changes during AO trial significantly increased from PL in old subjects. Note young subject data (dashed lines) are presented for reference purposes only (31).
Fig. 3.
Effect of 6 wk of single-leg knee-extensor exercise training on the change in brachial artery diameter (top) and the impact of the AO cocktail on this response after training in old subjects at rest and at 3 levels of handgrip exercise (bottom). Values for %change in brachial diameter are not exact and are displayed solely for reference purposes. It should be noted that baseline resting diameters did not differ as a consequence of exercise training. *P < 0.05, diameter changes during posttraining trial significantly increased from pretraining trial. #P < 0.05, diameter changes during posttraining AO trial significantly decreased from pretraining PL trial.
Fig. 4.
Effect of the oral AO cocktail on the change in brachial artery diameter in old subjects at rest and at 3 levels of handgrip exercise in the exercise-trained and untrained states. Values for %change in brachial diameter are not exact and are displayed solely for reference purposes (top). Shear rate in the brachial artery was calculated under these conditions (bottom). *P < 0.05, significantly different from posttraining AO condition.
DISCUSSION
There are several major findings from this study. First, we have directly documented the efficacy of an oral AOC (vitamin C, vitamin E, and α-lipoic acid) in terms of reducing blood-borne free radicals at rest and when oxidative stress is elevated in response to acute exercise in both young and old subjects. Second, these data support the documented age-related decline in vascular function with aging (11, 35), extending this finding to vascular function during acute exercise, but in addition, this study reveals the novel observation that acute oral antioxidant supplementation preceding acute exercise improved exercise-induced vascular reactivity in older subjects. Third, after exercise training, acute antioxidant supplementation no longer restores vascular function during acute exercise, but instead diminishes it. This suggests that exercise training in older subjects alters the balance between pro- and antioxidant forces, leading to a greater reliance on free radical-mediated vasodilation, resulting in a response that is now similar to that in the untrained young.
Age-related decline in arterial vasodilation:.
The age-related decline in vascular function has been well documented in human and animal models (24). One of the predominant vascular phenotypes with advancing sedentary aging is impaired endothelial function in conduit and small resistance arteries (8, 11, 29, 35, 39). The majority of data on aging and endothelial dysfunction support the theory that an elevated production of reactive oxygen species or other free radicals inactivate NO, thus attenuating arterial vasodilation (13, 36). The present study implemented an acute exercise model initially documented by Shoemaker et al. (33a), which provides the opportunity to study conduit arterial function that is likely in a state of elevated oxidative stress suggested as a consequence of elevated skeletal muscle metabolic activity and free radical outflow to interstitial fluid and vascular plasma (1, 9, 19).
The results of the current study demonstrate that there is a severe age-related impairment in brachial artery vasodilation during rhythmic handgrip exercise (Fig. 2). Specifically, the highest workload produced a ∼7% brachial artery vasodilation in young subjects and ∼3% brachial artery vasodilation in older subjects. To our knowledge, these data are the first to demonstrate that brachial artery vasodilation is markedly compromised with advanced age during submaximal exercise. These data are comparable to data produced by our laboratory and by others who have documented similar age-related reductions in brachial artery FMD (8, 13, 14, 42). Although endothelial dysfunction cannot be isolated in this more complex exercise model, with similar age-related vasodilator deficiencies it is tempting to speculate that these findings utilizing the exercise model may represent endothelial dysfunction similar to that observed in other FMD studies of aging.
Antioxidants and arterial vasodilation in the old.
Chronic oral antioxidant therapy has been documented to be largely ineffective in reducing mortality associated with cardiovascular disease (23). Conversely, the acute supraphysiological infusion of vitamin C in old subjects appears to transiently restore endothelium-dependent vasodilation and blood flow, suggesting that elevations in free radicals have a deleterious effect on endothelial function, most likely through a reduction in NO bioavailability (13, 18, 36). The current data demonstrate that in a likely pro-oxidant state [i.e., elevated circulating free radicals, such as occurs during acute exercise (Fig. 1)], oral antioxidants are effective at ameliorating the age-related reduction in brachial artery vasodilation (Fig. 2). The current data are in contrast to the findings of Eskurza et al. (13), who failed to see any improvement in brachial artery FMD of resting older subjects with chronic oral vitamin C supplementation. These disparate results may be explained by several important differences between these studies. First, our study used an AOC composed of several potent antioxidants (vitamin C, vitamin E, and α-lipoic acid), which was directly validated by using EPR spectroscopy to reduce blood free radical concentrations (Fig. 1, top), rather than a vitamin C supplement alone. Second, our experimental design employed acute small muscle mass exercise (handgrip), which in addition to locally generating free radicals that may or may not recirculate in a great enough concentration to play a role, produces greater blood flows and therefore more shear stress-mediated oxidants in the vessel being studied than a FMD protocol (3, 4, 19). Thus the potential role for antioxidants could be greater in this type of experimental paradigm than studies using FMD. Indeed, in support of this contention, we have recently published that this AOC had no effect on FMD in older subjects in an untrained state (41). Together, these data suggest that oral antioxidant supplementation has a greater beneficial effect on arterial vasodilation in older subjects in a scenario that elevates oxidative stress, rather than at rest.
Small muscle mass exercise training and arterial vasodilation.
Habitual exercise that recruits a large muscle mass has been shown to improve endothelial function in multiple cardiovascular disease states (17, 22) and healthy aging (11, 16), and often the beneficial effects of this habitual exercise are even seen in the arteries of non-exercise-trained limbs (11, 15, 26, 28). The mechanisms responsible for the beneficial effects of habitual exercise on arterial vasodilation are multifactorial and likely include regular increases in systemic shear stress and pulse pressure, leading to greater mRNA and endothelial NO synthase protein expression (15, 34), augmented endogenous muscle (21), and vascular antioxidants (32), leading to an increase in NO bioavailability. However, the extent to which a relatively small muscle mass can invoke such systemic adaptations is not well known. In this study, 6 wk of exercise training that only recruited the knee extensors of a single leg and yielded a 38% increase in work rate maximum also significantly increased exercise-induced brachial artery vasodilation (Fig. 3). Although this exercise modality (small muscle mass) stimulates a somewhat modest cardiovascular response, it is interesting to note that, as published by others (15) during cycle exercise, we have recognized a more pronounced anterograde/retrograde oscillation in the arm during knee-extensor exercise that results an augmented cumulative shear profile experienced by the resting limb (unpublished observations). Of note, it is the commonly accepted that an exercise-induced increase in anterograde shear within an exercising limb has beneficial vascular effects, whereas there is significant evidence that oscillatory flow patterns may, in fact, be proatherogenic (10, 25). The finding of improved exercise-induced vascular function with a relatively small muscle mass is in agreement with our previous observation that brachial artery FMD in older subjects is augmented in response to 6 wk of single-leg knee-extensor exercise training (42), suggesting potentially similar contributing mechanisms responsible for these two improvements in vasodilatory response. However, the mechanism responsible for these changes remains unknown.
Interaction among exercise training, antioxidants, and arterial vasodilation.
Exercise training has been documented to increase endogenous antioxidants (21); therefore, it is possible that the administration of supplemental antioxidants in the trained state would have little or no effect, and the current data not only support this contention but, in fact, reveal a negative effect of the AOC in the trained state (Fig. 3, bottom). This very different response to the AOC in the trained and untrained state is highlighted in Fig. 4 (top), where the positive consequences of the antioxidants when untrained contrast with the effects after exercise training. This is in agreement with our recent observation that in subjects in an untrained state, the AOC had little effect on endothelial function as measured by FMD and blood pressure; however, following exercise training, both were negatively impacted by an AOC-induced reduction in free radicals (41).
The current study does not address the mechanisms responsible for the deleterious combined effect of exercise training and acute antioxidant administration on exercise-induced brachial vasodilation. However, we speculate that this is likely the result of a disturbance in the balance between pro- and antioxidant forces. Oxidative stress is typically regarded as an unwanted by-product of cellular oxidation and is seen as a negative risk factor for cellular and vascular health (5). However, the downstream consequences of free radicals, such as H2O2 and ONOO−, may act as potent vasodilators (6) and as such possess the capacity to alter vascular responsiveness. Thus it is tempting to speculate that whereas exercise training alone evokes an appropriate adaptation to the increase in oxidative stress, the even greater reduction in free radical concentration following antioxidant administration may have removed oxidative species, which possess some beneficial vasoactive properties. This possible vasodilatory role of free radicals (31) may explain the loss of exercise-induced brachial artery vasodilation in the posttraining antioxidant condition.
Direct and indirect markers of oxidative stress.
It is important to note that in terms of assessing oxidative stress, direct (EPR) and indirect (FOX assay) measurements may not always lead to the same conclusions (Fig. 1), especially with the use of an acute AOC intervention as performed in this study. As documented in Fig. 1, the top panel (EPR) reflects the acute measurable response to the AOC in terms of directly measured free radicals, whereas the bottom panel reflects little acute change in the lipid peroxidation footprint of these (and other) free radicals. Now this relationship is undoubtedly also influenced by the magnitude of the oxidative challenge, which is increased by the acute exercise (Fig. 1, top) and now yields a greater footprint and greater impact of the AOC on LOOH levels (Fig. 1, bottom right).
Experimental considerations.
Currently, the exercise model employed in these studies of arterial vasodilation has not been proven to isolate the effects of endothelial function from metabolic vasodilation. However, even in cuff occlusion FMD studies, a well-accepted technique to evaluate endothelium-dependent vasodilation, there is still a potential for retrograde conducted vasodilation, myogenic responses, and changes in sympathetic activity, all of which may modify conduit artery vasodilation (37). Importantly, the exercise-induced experimental model of vasodilation emulates the physiological conditions present during activities of daily living. Also, unlike the “single-shot” FMD assessment of vascular function, this graded exercise approach facilitates multiple assessments, increasing confidence in the data.
We acknowledge that this investigation utilized several different exercise modalities, each initially adopted with clear experimental goals in mind. This ultimately raised additional questions regarding the potential interaction between the exercise type and, for example, the AOC efficacy. However, as per the original experimental design, the whole body cycle exercise was employed in the AOC validation study because this was deemed to be the most metabolically challenging exercise, the premise being that if the AOC could reduce oxidative stress under these conditions, it was highly likely to function similarly in the much less systemically perturbing handgrip model. As already noted, the third exercise modality, single-leg knee-extension training, was adopted because it stimulates a somewhat modest cardiovascular response and because the extent to which such a relatively small muscle mass can invoke systemic adaptations is not well known. Such knowledge may be useful in the maintenance of cardiovascular health in the elderly, especially those who are unable to perform whole body exercise. Finally, we also acknowledge that the single sex and small number of subjects involved in this study limits the generalization of the results to the population at large.
Conclusions.
This study demonstrates that the brachial artery of older individuals vasodilated less than that in their young counterparts during submaximal exercise, despite similar shear rates, and that acute oral antioxidant administration ameliorated the underlying age-related reduction in conduit artery vasodilation. This improvement in arterial vascular function is likely due to an antioxidant-induced reduction in circulating free radicals, as supported by vascular EPR measurements. Exercise training of a relatively small muscle mass in the leg improved vascular function in the arm, and then antioxidant administration no longer restored the exercise-induced brachial artery vasodilation, with evidence of a negative impact on vasodilatory capacity in the exercise-trained state. This suggests that exercise training in older subjects alters the balance between pro- and antioxidant forces, resulting in a greater reliance on free radical-mediated vasodilation in the exercise-trained state.
GRANTS
This study was funded in part by National Institutes of Health Grants PO1-HL-091830 and K01-AG-029337, Tobacco-Related Disease Research Program 15RT-0100, the Francis Family Foundation, American Heart Association Grant 0835209N, and the Salt Lake City Veterans Affairs Medical Center Geriatric Research, Education, and Clinical Center.
ACKNOWLEDGMENTS
We thank the subjects for their time and effort in volunteering for this study.
REFERENCES
- 1.Bailey DM, Ainslie PN, Jackson SK, Richardson RS, Ghatei M. Evidence against redox regulation of energy homoeostasis in humans at high altitude. Clin Sci (Lond) 107: 589–600, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bailey DM, Davies B, Young IS, Jackson MJ, Davison GW, Isaacson R, Richardson RS. EPR spectroscopic detection of free radical outflow from an isolated muscle bed in exercising humans. J Appl Physiol 94: 1714–1718, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bailey DM, Davies B, Young IS, Jackson MJ, Davison GW, Isaacson R, Richardson RS. EPR spectroscopic evidence of free radical outflow from an isolated muscle bed in exercising humans: functional significance of decreasing intracellular Po2 vs. increasing O2 flux. Adv Exp Med Biol 540: 297–303, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bailey DM, Young IS, McEneny J, Lawrenson L, Kim J, Barden J, Richardson RS. Regulation of free radical outflow from an isolated muscle bed in exercising humans. Am J Physiol Heart Circ Physiol 287: H1689–H1699, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Beckman JS, Koppenol WH. Nitric oxide, superoxide, peroxynitrite: the good, the bad, ugly. Am J Physiol Cell Physiol 271: C1424–C1437, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Benkusky NA, Lewis SJ, Kooy NW. Attenuation of vascular relaxation after development of tachyphylaxis to peroxynitrite in vivo. Am J Physiol Heart Circ Physiol 275: H501–H508, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Betik AC, Luckham VB, Hughson RL. Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. Am J Physiol Heart Circ Physiol 286: H442–H448, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun 107: 1198–1205, 1982 [DOI] [PubMed] [Google Scholar]
- 10.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res 82: 1094–1101, 1998 [DOI] [PubMed] [Google Scholar]
- 11.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'Driscoll G, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol 562: 617–628, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation 93: 210–214, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol 103: 1715–1721, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Jackson MJ, Edwards RH, Symons MC. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim Biophys Acta 847: 185–190, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Ji LL. Exercise at old age: does it increase or alleviate oxidative stress? Ann NY Acad Sci 928: 236–247, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Ji LL, Leeuwenburgh C, Leichtweis S, Gore M, Fiebig R, Hollander J, Bejma J. Oxidative stress and aging. Role of exercise and its influences on antioxidant systems. Ann NY Acad Sci 854: 102–117, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi N, Tsuruya Y, Iwasawa T, Ikeda N, Hashimoto S, Yasu T, Ueba H, Kubo N, Fujii M, Kawakami M, Saito M. Exercise training in patients with chronic heart failure improves endothelial function predominantly in the trained extremities. Circ J 67: 505–510, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation 110: 637–641, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107: 346–354, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol 104: 588–600, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavrencic A, Salobir BG, Keber I. Physical training improves flow-mediated dilation in patients with the polymetabolic syndrome. Arterioscler Thromb Vasc Biol 20: 551–555, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Lawrenson L, Hoff J, Richardson RS. Aging attenuates vascular and metabolic plasticity but does not limit improvement in muscle V̇o2 max. Am J Physiol Heart Circ Physiol 286: H1565–H1572, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Linke A, Schoene N, Gielen S, Hofer J, Erbs S, Schuler G, Hambrecht R. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol 37: 392–397, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986 [DOI] [PubMed] [Google Scholar]
- 31.Richardson RS, Donato AJ, Uberoi A, Wray DW, Nishiyama SK, Bailey DM. Exercise induced brachial artery vasodilation: the role of free radicals. Am J Physiol Heart Circ Physiol 292: H1516–H1522, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol 279: H2068–H2076, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol 284: H1378–H1387, 2003 [DOI] [PubMed] [Google Scholar]
- 33a.Shoemaker JK, MacDonald MJ, Hughson RL. Time course of brachial artery diameter responses to rhythmic handgrip exercise in humans. Cardiovasc Res 35: 125–131, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Tschakovsky ME, Pyke KE. Counterpoint: flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol 99: 1235–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Vuilleumier JP, Keck E. Fluorimetric assay of vitamin C in biological materials using a centrifugal analyser with fluorescence attachment. J Micronutr Anal 5: 25–34, 1993 [Google Scholar]
- 39.Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 93: 1685–1690, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol 565: 1053–1060, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 116: 433–441, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol 290: H1271–H1277, 2006 [DOI] [PubMed] [Google Scholar]