Abstract
Human studies of coronary circulation are limited because of methodological issues. Recently, a noninvasive transthoracic duplex ultrasound (TTD) technique has emerged as an important tool to measure coronary blood flow velocity (CBV) in conscious humans. We employed two protocols to determine whether noninvasive “native” coronary artery velocity responses to constrictor or dilator stimuli assessed by TTD provide reliable data. In the first protocol, coronary vascular resistance (CVR = diastolic blood pressure/CBV) responses to static handgrip were examined in the left internal mammary artery (LIMA) and native left anterior descending artery (LAD) into which the graft was inserted (patient age 63 ± 3 years). Our prior report documented increased CVR in the LIMA graft during static handgrip (Momen et al., J Appl Physiol 102: 735–739, 2007). We hypothesized that the magnitude of increases in CVR during handgrip would be similar in the LIMA graft and LAD in the same individual. Percent increases in CVR were similar in the LIMA and distal native LAD (27 ± 4% vs. 28 ± 6%). In the second protocol, we studied six patients (age 61 ± 3 years) who underwent cardiac catheterization of the LAD. We compared coronary vasodilator responses to intravenous adenosine infusion (0.14 mg·kg−1·min−1) obtained by intracoronary Doppler guidewire technique and TTD on separate studies. The relative increases in CBV with adenosine obtained by intracoronary Doppler guidewire and TTD were similar (62 ± 10% vs. 65 ± 12%). Noninvasive TTD provides reliable human coronary circulatory constrictor and dilator data.
Keywords: coronary flow velocity, duplex ultrasound, handgrip
from a physiological standpoint there are relatively few human studies of coronary circulatory function. This has largely been due to methodological issues. Specifically, until recently, invasive measurements of coronary blood flow velocity (CBV) were needed to determine an index of coronary flow velocity. Although these invasive approaches are certainly possible, studies in large groups of patients and in those not already undergoing cardiac catheterization are not feasible. Thus important issues pertaining to the cardiac circulation have been impossible to study. Recently, transthoracic duplex ultrasound (TTD) has been introduced as a promising tool for the assessment of coronary circulatory parameters (5–12). This technique allows investigators to measure real-time changes in CBV in a segment of the left anterior descending artery (LAD) that is in close proximity to the chest wall. TTD has the potential to measure rapid and sequential changes in CBV during a variety of stressful maneuvers in a safe and noninvasive way.
We have recently employed the TTD method to examine left internal mammary artery (LIMA) blood velocity during static handgrip exercise (15). In the subjects that we studied, the LIMA was grafted to the coronary artery to bypass the stenosed coronary artery segments. We found that static handgrip evoked coronary vasoconstriction (15). In the present report, we speculated that vascular responses to static handgrip would be qualitatively similar in the LIMA graft and in the distal native LAD into which the LIMA graft was inserted. Thus, in our experiment, we recorded coronary flow velocity simultaneously from both the LIMA graft and the native coronary artery during handgrip exercise.
Another important objective was to compare the coronary measures of TTD with measures obtained from the intracoronary Doppler guidewire (ICDGW) technique. To accomplish this goal, we performed studies in patients undergoing cardiac catheterization followed by a percutaneous coronary intervention (PCI) involving the LAD. Immediately after the angioplasty procedure was successfully performed, we examined the vasodilator responses of the LAD coronary artery to an intravenous infusion of adenosine measured by the ICDGW technique. Within 24 h of the catheterization procedure, we employed the noninvasive TTD method to again measure coronary flow velocity responses to intravenous adenosine.
Our data reveal that 1) flow responses during static handgrip in the LIMA and the mid-distal segment of the LAD were similar and 2) measures of vasoresponsiveness to adenosine measured invasively and noninvasively are qualitatively similar.
METHODS
Study Population
Two different groups of patients were studied in the two different protocols. In the first study, patients who had previously undergone coronary artery bypass surgery (LIMA placed to LAD) were recruited to perform the handgrip protocol. Patients who had undergone a cardiac catheterization procedure and who had a high likelihood of occlusive coronary artery disease involving the LAD and who would be amenable to a PCI were examined in the second study. The Institutional Review Board at the Hershey Medical Center approved the study protocols, and all patients provided informed consent before participation. A physical examination was performed on all patients before they were studied.
LIMA graft recipients.
We studied five nonsmoking male LIMA graft recipients who were recruited from the Penn State Heart and Vascular Institute at the Hershey Medical Center. For another LIMA graft recipient that we recruited for this study, we could not obtain a LAD signal. All patients (age 63 ± 3 years; body mass index 29 ± 2 kg/m2) were clinically stable and were within 5 years of having had bypass surgery that included LIMA bypass graft. All LIMA recipients had total cholesterol values of <200 mg/dl and had normal left ventricular ejection fractions. Recent testing by stress echocardiography and/or nuclear perfusion/function imaging showed no infarction or wall motion abnormalities within the LAD territory. Patients with diabetes, renal failure, and chronic lung disease were excluded from the study. Medications used by the patients included the following: β1-blockers (n = 5), α-blockers (n = 2), angiotensin-converting enzyme inhibitors (n = 1), calcium channel blockers (n = 2), and statins (n = 5). Medications were withheld on the morning of the study.
PCI patients.
Six patients (4 men, 2 women, age = 61 ± 3 years, body-mass index = 26 ± 3 kg/m2) were studied. Patients were studied as part of their clinical care at the Penn State Heart and Vascular Institute. All patients had normal left ventricular function. Patients with dilated cardiomyopathy, multivessel disease, or diabetes mellitus were excluded. At the time of study, all patients were stable and without pain, and no patients had evidence of a myocardial infarction. Four of six PCI patients had a history of hypertension; however, at the time of the study, blood pressure (BP) was under control. None of the patients had diabetes or chronic kidney disease.
All patients had angiographically documented proximal-mid-LAD stenosis (arterial lumen diameter stenosis range = 70–90%). Medications used by the patients included the following: β-blockers (n = 6), α-blockers (n = 3), angiotensin-converting enzyme inhibitors (n = 2), calcium channel blockers (n = 2), and statins (n = 6).
Coronary Blood Velocity by TTD Technique
CBV was measured simultaneously in the LIMA graft and the LAD branch of the left coronary artery with two separate TTD machines (HDI 5000, ATL ultrasound, Bothell, WA). A linear-array, high-frequency transducer (7–10 MHz) with a 6-MHz-pulsed Doppler frequency was used for these studies. The LIMA was scanned along the left sternal border in the second or third intercostal space (15), whereas the blood velocity in the LAD was measured along the left midclavicular line in the 4th or 5th intercostal space with the patients lying in the left lateral decubitus position.
The distal part of LAD as well the LIMA graft was first identified with color flow mapping, and the focal zone was then set at the depth of the respective arteries. The insonation angle to the artery was ≤60°.
The velocity range was set at 0–30 cm/s. The sample volume of the pulsed wave was adjusted according to the size of the corresponding vessel. Care was taken to ensure that patients did not perform Valsalva maneuvers during the static handgrip protocols.
Handgrip Exercise Protocol
For this protocol, two investigators simultaneously recorded coronary blood velocity by using two separate echo/Doppler machines. One investigator measured CBV from the LIMA graft and the other investigator measured CBV from the LAD.
Maximal voluntary contraction (MVC) of the nondominant arm was determined in each patient with a handgrip dynamometer (Stoelting, Wood Dove, IL). Baseline data for heart rate (HR), BP, and CBV were collected for 5 min. Each patient then performed static handgrip exercise at 50% of their respective MVC and continued for 1 min. A visual feedback of the amount of tension generated was provided to each patient while they performed handgrip exercise.
Each cardiac cycle (diastolic component) Doppler tracing was analyzed using HDI 5000 ATL software to measure coronary diastolic blood velocity. Beat-to-beat recordings of BP (Finapres, Ohmeda, Madison, WI) were also obtained with a Power Lab data-acquisition system (AD Instruments) and analyzed using the Power Lab Chart software suite. Therefore, for each diastolic period, a diastolic velocity value and a corresponding diastolic BP were obtained. Subsequently, an index of coronary vascular resistance (CVR) was calculated by dividing corresponding diastolic BP by CBV (cm/s). CVR is expressed in arbitrary units.
Continuous recordings of HR (electrocardiogram) were also obtained throughout the protocols. An automated sphygmomanometer (Dinamap, Critikon, Tampa, FL) was used to determine resting BP as well as to verify the BP measurements obtained by Finapres.
Pharmacological (Adenosine) Stress Protocol
Patients undergoing PCI procedure performed the following protocol. Fifteen minutes after the procedure was completed, Flow Map 5500 and the Doppler FloWire (Volcano, Rancho Cordova, CA) were used to measure coronary blood velocity in the LAD. The wire was 0.014 in. in diameter. Care was taken to ensure that the distal end of the wire where the Doppler transducer was located was distal to the site of the intervention and stent placement. The wire was maneuvered so that the Doppler sensor was not adjacent to the vessel wall and was within the stream of flow in the vessel.
Midazolam and/or fentanyl were given to the patients for sedation before and throughout the intervention. Heparin and/or other medications were given as per standard of care. After baseline coronary flow velocity data were obtained, an intravenous infusion of adenosine (0.14 mg·kg−1·min−1) was started so that we could examine the hyperemic response. The infusion was continued for 6 min during which time spectral tracings of CBV were obtained, characterized by a biphasic pattern with prominent diastolic component.
The adenosine infusion protocol was repeated with a noninvasive flow velocity assessment in all patients using TTD within 24 h of the intracoronary assessment. CBV was measured during baseline condition followed by a 6-min adenosine infusion. In one subject, the infusion was stopped after 3 min because of shortness of breath.
Peak diastolic blood velocity was determined during baseline conditions and during the adenosine infusion. HR and BP were monitored continuously during both the invasive and noninvasive studies.
Data Analysis and Statistics
Beat-by-beat sequential analysis of HR, BP, CBV, and CVR were performed for all LIMA graft recipients. Averages of these variables during baseline and during handgrip exercise were considered for statistical analysis.
During the adenosine infusion protocol, the averages of peak diastolic blood velocity (from the two separate methods) and HR and BP measurements were considered for statistical analysis.
Data are presented as means ± SE. Paired t-test was used to compare diastolic peak velocity responses obtained by invasive vs. noninvasive methodologies. Paired t-test was also used to compare the responses during adenosine infusion and static handgrip protocols. A probability value of <5% was considered significant to reject null hypothesis.
RESULTS
Responses to Handgrip Exercise Protocol (LIMA Graft vs. LAD)
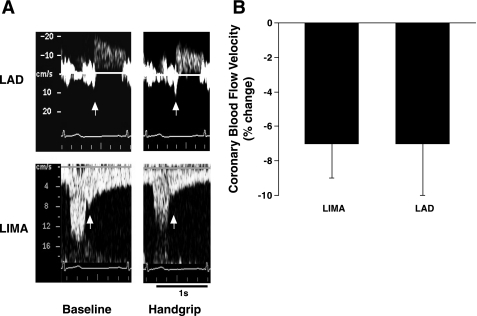
As compared with baseline, CBV decreased and CVR was increased during static handgrip exercise (Table 1). HR and BP also increased during handgrip (Table 1). The relative magnitude of the decrease in CBV (percent change from the baseline values) was similar in the LIMA graft and the LAD (Fig. 1, Table 1). The magnitude of the increases in CVR during handgrip (percent change from the baseline values) was also similar (27 ± 4% vs. 28 ± 6%; LIMA grafts and LAD, respectively).
Table 1.
Values at baseline and during static handgrip
| Baseline | Handgrip | P Value | |
|---|---|---|---|
| SBP, mmHg | 128 ± 11 | 142 ± 13* | <0.05 |
| DBP, mmHg | 69 ± 9 | 81 ± 10* | <0.05 |
| HR, beats/min | 58 ± 4 | 65 ± 5 | 0.077 |
| MVC, kg | 29 ± 3 | ||
| CBV, cm/s (LIMA) | 10.1 ± 2.1 | 9.3 ± 2.2* | <0.05 |
| CVR unit (LIMA) | 7.35 ± 0.73 | 9.40 ± 1.09* | <0.05 |
| CBV cm/s (LAD) | 15.1 ± 2.2 | 14.0 ± 2.2 | 0.053 |
| CVR, unit (LAD) | 4.82 ± 0.63 | 6.31 ± 1.1* | <0.05 |
Values (means ± SE) were obtained from patients who had left internal mammary artery (LIMA) grafted to the left anterior descending coronary artery (LAD). All hemodynamic data (SBP, systolic blood pressure; DBP, diastolic blood pressure, HR, heart rate; CBV, coronary blood flow velocity; CVR, coronary vascular resistance index) were obtained during baseline and during static handgrip at 50% of patient's respective maximum voluntary contraction (MVC). CBV was measured simultaneously by 2 separate transthoracic duplex ultrasound machine from the LIMA graft and the distal LAD.
Significantly different (P < 0.05) from the corresponding baseline values.
Fig. 1.
A: representative Doppler coronary blood flow velocity signal tracings from a patient during baseline (left) and static handgrip exercise (right) obtained by noninvasive transthoracic duplex ultrasound technique (TTD) from the left internal mammary artery (LIMA; bottom) grafted to the coronary artery and the left anterior descending artery (LAD; top). White arrows on LAD and LIMA tracings indicate the beginning of the diastolic component used to obtain mean coronary blood flow velocity. B: results are means ± SE, shown as percent change from baseline in coronary blood flow velocity during static handgrip at 50% of patient's respective maximum voluntary contraction. The measurements were obtained by noninvasive TTD simultaneously from the LIMA graft and the LAD. Note: the magnitude of decreases in coronary blood flow velocity was similar in 2 locations of the coronary artery.
Responses to Pharmacologic Stress Protocol (Invasive vs. Noninvasive Technique)
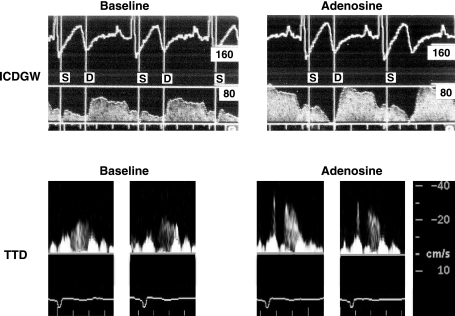
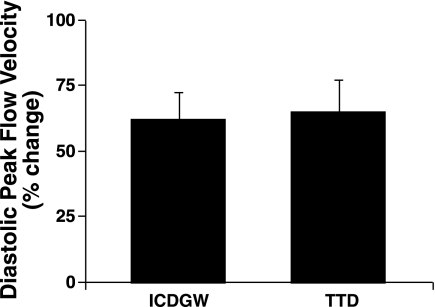
Adenosine had similar effects on HR and BP during the invasive and noninvasive studies (Table 2). Peak diastolic CBV was increased with adenosine. Doppler tracings obtained with ICDGW and TTD are shown in Fig. 2. The relative increases in peak diastolic coronary flow velocity were similar during the two study periods (62 ± 10% vs. 65 ± 12% by ICDGW and TTD, respectively, P = NS; Fig. 3 and Table 2).
Table 2.
Values at baseline and during adenosine infusion
| ICDGW (Invasive) |
TTD (Noninvasive) |
|||||
|---|---|---|---|---|---|---|
| Baseline | Adenosine | P Value | Baseline | Adenosine | P Value | |
| SBP, mmHg | 115 ± 8 | 111 ± 9 | NS | 119 ± 6 | 121 ± 8 | NS |
| DBP, mmHg | 66 ± 6 | 64 ± 7 | NS | 60 ± 5 | 60 ± 6 | NS |
| HR, beats/min | 69 ± 5 | 77 ± 6* | <0.05 | 65 ± 7 | 74 ± 6* | <0.05 |
| PDV, cm/s | 54 ± 7 | 85 ± 9* | <0.05 | 19 ± 3 | 31 ± 6* | <0.05 |
Values (means ± SE) were obtained from patients who underwent LAD percutaneous coronary intervention. The hemodynamic data were obtained during baseline and during intravenous infusion of adenosine (0.14 mg·kg−1·min−1 for 6 min) during the 2 study periods. The invasive studies were performed using an intracoronary Doppler guidewire (ICDGW) placed in the mid-LAD (distal to stent placement). The noninvasive data were obtained with transthoracic duplex ultrasound (TTD) of the distal LAD. Note, HR and peak diastolic velocity (PDV) both increased during adenosine infusion.
Significantly different (P < 0.05) from the corresponding baseline values. NS, not significant.
Fig. 2.
Representative Doppler coronary blood velocity signal tracings from a patient during baseline (left) and adenosine infusion (right) obtained by invasive intracoronary Doppler guidewire (ICDGW; top) and noninvasive TTD (bottom) technique. S and D represent the systolic and diastolic components of each velocity tracing.
Fig. 3.
Results are means ± SE, shown as percent change from baseline in diastolic peak blood flow velocity measured by invasive ICDGW and noninvasive TTD technique during 2 study periods. Note: magnitude of increases in velocity is similar with 2 techniques.
DISCUSSION
In the present report, we examined coronary vasoconstriction during static handgrip exercise in the LIMA as well as in the distal segment of the LAD. We have previously shown that the LIMA is relatively easy to interrogate with TTD (15). Moreover, previous reports have indicated that hemodynamics in the LIMA graft are reflective of that of the native coronary artery (9, 10). In the present report, we noted similar relative (and simultaneous) increases in CVR during handgrip exercise in both the LIMA graft and the LAD within the same individual. These vasoconstrictor responses are consistent with our recent work performed in a similar patient population (15).
The concept that handgrip can evoke vasoconstriction in humans is not new. Brown et al. (2) measured coronary vascular responses during static handgrip with quantitative arteriography in subjects with coronary artery disease. The authors noted coronary vasoconstriction during handgrip that was independent of the rate pressure product (HR times systolic BP, an index of myocardial oxygen demand) and suggested that reflex sympathetic activation might have contributed to these responses. After Marcus et al. (14) introduced the Doppler guidewire technique to measure CBV, Bortone et al. (1) performed studies in patients with ischemia-like symptoms and measured coronary flow velocity employing Doppler guidewire technique during exercise. Interestingly, the investigators noted coronary vasoconstriction in the distal segment of normal coronary arteries. The mechanism responsible for this vasoconstrictor response was not clear. Of note, animal studies have also suggested that coronary vasoconstriction occurs during exercise. It has been further suggested that sympathetic activation plays an important role in evoking this response (3, 8, 13).
In the second protocol, we found similar noninvasive and invasive coronary diastolic flow velocity responses to adenosine. In line with our data, prior studies performed in the clinical settings have indicated that noninvasive TTD-derived measures of coronary hyperemic responses to adenosine were similar to the measures obtained from the invasive ICDGW technique (6, 11, 12, 17). However, unlike some of the studies cited above (6), CBV values obtained by the TTD in the present report yielded lower velocity values than the values obtained by the ICDGW (Table 2). Kim et al. (11) observed lowered CBV measures by TTD than the ICDGW techniques. These investigators suggested that some important methodological issues explain the observed differences in the absolute coronary velocity measured with invasive and noninvasive methods. First, the tip of the Doppler guidewire is positioned directly within the vessel. On the other hand, with the TTD technique, there was always an angle (≤60°) between the projected Doppler beam and the sampled segment of the LAD. This might lead to an underestimation of the TTD-derived measures. Second, during the ICDGW procedure, most of the Doppler flow tracings were obtained from the midportion of the LAD, immediately distal to the treated area, whereas the Doppler tracings from the TTD methods were obtained from the distal portion of the LAD where flow velocity is thought to be less than in the proximal or middle part of the LAD. Of note, the velocity values we report for the distal LAD are comparable to the data reported by Hozumi et al. (7). These investigators studied patients who underwent coronary angiography for evaluation of coronary artery disease and measured CBV in the distal LAD utilizing similar noninvasive TTD techniques to the ones that we employed.
A final explanation for the lower CBV values obtained with the noninvasive method is that Doppler tracings recorded during the ICDGW procedure represented the blood velocity from the center of the vessel lumen where the tip of the Doppler guidewire was positioned. On the other hand, CBV derived from the TTD technique represented the velocity signal from the sample volume, which was positioned over the LAD. Thus both central and peripheral velocity streams were measured. The blood velocity at the center of stream of flow is faster than the velocity adjacent to the vessel wall (18). Despite these issues, the present report demonstrates that the TTD-derived changes in the CBV measures from rest to stress were comparable to the changes determined by the invasive ICDGW technique. Thus our findings demonstrate that the hyperemic responses measured invasively and noninvasively yield qualitatively similar findings. This suggests that transthoracic coronary flow determinations are useful to measure coronary dilatory responses in human subjects.
Methodological Limitations
In these studies, changes in CBV have been measured as an index of changes in coronary blood flow. We did not measure absolute blood flow (BF). To measure BF, accurate and precise measurements of target vessel diameter are required since BF is a function of mean blood velocity and vessel cross-sectional area (BF = mean blood velocity × πr2, where r is the vessel radius). Due to the limited spatial resolution of presently available noninvasive methods, we were unable to obtain precise measurements of target coronary artery diameter. We would like to point out that prior reports have documented that changes in CBV represent a useful surrogate of changes in absolute coronary flow (4, 16). Therefore, in our report, we suggest that changes in CBV data as well as the indexes of CVR (calculated from CBV data) seen during adenosine infusion and handgrip exercise are reflective of changes in coronary flow.
In the present report, all subjects were taking both β-blockers and statin medications. Therefore, it is certainly possible that coronary responses that we observed could have been influenced by these medications.
Of note, however, are the observations in coronary velocity studies that we conducted in healthy subjects as they performed static handgrip exercise. The magnitude of CVR responses in these subjects is very similar to the magnitude of CVR responses that we noted during static handgrip exercise in this report (∼26% vs. 28% LAD in healthy vs. LAD in LIMA patients, respectively). Thus we suspect that patient medications did not have any major effects on the observed patterns of responses seen in our patients. However, this issue does not have an effect on the major reason that we performed these studies, namely, to determine whether noninvasive methods could be employed to examine coronary physiological responses to stress. The results of our studies suggest that this is the case.
Another concern is whether the observed results are reproducible. To address this issue, we examined LAD velocity during handgrip in five healthy patients on three consecutive weeks. These subjects performed 20-s static handgrip at 70% MVC. The average increases in CVR were quite similar (∼26%, ∼27%, and ∼25% in weeks 1, 2, and 3, respectively; unpublished observations). Furthermore, the magnitude of the increases in CVR in these normal subjects was similar to the increases seen with handgrip in the present report. These results suggest that the measurements obtained in our studies are reproducible.
In conclusion, our data demonstrate that the noninvasive TTD technique can be utilized to determine coronary circulatory responses in conscious humans during physiological as well as pharmacological studies where constrictor and dilator stimuli are employed.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL-070222 (L. I. Sinoway) and National Center for Research Resources Grants M01 RR-010732 and C06 RR-016499. This project is funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
ACKNOWLEDGMENTS
The authors are grateful to Jennie Stoner for expert manuscript preparation; Kris Gray, Steve Gugoff, Jonathon Yoder for technical assistance; and the staff of the catheterization laboratories of Penn State Heart and Vascular Institute and the General Clinical Research Center.
REFERENCES
- 1.Bortone AS, Hess OM, Eberli FR, Nonogi H, Marolf AP, Grimm J, Krayenbuehl HP. Abnormal coronary vasomotion during exercise in patients with normal coronary arteries and reduced coronary flow reserve. Circulation 79: 516–527, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Brown BG, Lee AB, Bolson EL, Dodge HT. Reflex constriction of significant coronary stenosis as a mechanism contributing to ischemic left ventricular dysfunction during isometric exercise. Circulation 70: 18–24, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Chilian WM, Ackell PH. Transmural differences in sympathetic coronary constriction during exercise in the presence of coronary stenosis. Circ Res 62: 216–225, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Di Mario C, Moses JW, Anderson TJ, Bonan R, Muramatsu T, Jain AC, Suarez de Lezo J, Cho SY, Kern M, Meredith IT, Cohen D, Moussa I, Colombo A. Randomized comparison of elective stent implantation and coronary balloon angioplasty guided by online quantitative angiography and intracoronary Doppler. DESTINI Study Group (Doppler Endpoint STenting INternational Investigation). Circulation 102: 2938–2944, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Hirata K, Amudha K, Elina R, Hozumi T, Yoshikawa J, Homma S, Lang CC. Measurement of coronary vasomotor function: getting to the heart of the matter in cardiovascular research. Clin Sci (Lond) 107: 449–460, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Hozumi T, Yoshida K, Akasaka T, Asami Y, Ogata Y, Takagi T, Kaji S, Kawamoto T, Ueda Y, Morioka S. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol 32: 1251–1259, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Hozumi T, Yoshida K, Ogata Y, Akasaka T, Asami Y, Takagi T, Morioka S. Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation 97: 1557–1562, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Huang AH, Feigl EO. Adrenergic coronary vasoconstriction helps maintain uniform transmural blood flow distribution during exercise. Circ Res 62: 286–298, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Katz WE, Zenati M, Mandarino WA, Cohen HA, Gorcsan J., 3rd Assessment of left internal mammary artery graft patency and flow reserve after minimally invasive direct coronary artery bypass. Am J Cardiol 84: 795–801, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Kern MJ, Deligonul U, Tatineni S, Serota H, Aguirre F, Hilton TC. Intravenous adenosine: continuous infusion and low dose bolus administration for determination of coronary vasodilator reserve in patients with and without coronary artery disease. J Am Coll Cardiol 18: 718–729, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Kim SM, Shim WJ, Lim HE, Hwang GS, Song WH, Lim DS, Kim YH, Seo HS, Oh DJ, Ro YM. Assessment of coronary flow reserve with transthoracic Doppler echocardiography: comparison with intracoronary Doppler method. J Korean Med Sci 15: 139–145, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lethen H, Tries HP, Brechtken J, Kersting S, Lambertz H. Comparison of transthoracic Doppler echocardiography to intracoronary Doppler guidewire measurements for assessment of coronary flow reserve in the left anterior descending artery for detection of restenosis after coronary angioplasty. Am J Cardiol 91: 412–417, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Longhurst JC, Aung-Din R, Mitchell JH. Static exercise in anesthetized dogs, a cause of reflex alpha-adrenergic coronary vasoconstriction. Basic Res Cardiol 76: 530–535, 1981 [DOI] [PubMed] [Google Scholar]
- 14.Marcus ML, Doty DB, Hiratzka LF, Wright CB, Eastham CL. Decreased coronary reserve: a mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N Engl J Med 307: 1362–1366, 1982 [DOI] [PubMed] [Google Scholar]
- 15.Momen A, Gahremanpour A, Mansoor A, Kunselman A, Blaha C, Pae W, Leuenberger UA, Sinoway LI. Vasoconstriction seen in coronary bypass grafts during handgrip in humans. J Appl Physiol 102: 735–739, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serruys PW, de Bruyne B, Carlier S, Sousa JE, Piek J, Muramatsu T, Vrints C, Probst P, Seabra-Gomes R, Simpson I, Voudris V, Gurne O, Pijls N, Belardi J, van Es GA, Boersma E, Morel MA, van Hout B. Randomized comparison of primary stenting and provisional balloon angioplasty guided by flow velocity measurement. Doppler Endpoints Balloon Angioplasty Trial Europe (DEBATE) II Study Group. Circulation 102: 2930–2937, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Ueno Y, Nakamura Y, Takashima H, Kinoshita M, Soma A. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the right coronary artery by transthoracic Doppler echocardiography: comparison with intracoronary Doppler guidewire. J Am Soc Echocardiogr 15: 1074–1079, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Zwiebel WJ, Pellerito J. Basic concepts of Doppler frequency spectrum analysis and ultrasound blood flow imaging. In: Introduction to Vascular Ultrasonography (5th ed.), edited by Zwiebel WJ, Pellerito J. Philadelphia, PA: Elsevier Science, 2005, chapt. 3, p. 61–89 [Google Scholar]