Abstract
Chronic mucocutaneous candidiasis (CMC) is frequently associated with T cell immunodeficiencies. Specifically, the proinflammatory IL-17A–producing Th17 subset is implicated in protection against fungi at epithelial surfaces. In autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED, or autoimmune polyendocrine syndrome 1), CMC is often the first sign, but the underlying immunodeficiency is a long-standing puzzle. In contrast, the subsequent endocrine features are clearly autoimmune, resulting from defects in thymic self-tolerance induction caused by mutations in the autoimmune regulator (AIRE). We report severely reduced IL-17F and IL-22 responses to both Candida albicans antigens and polyclonal stimulation in APECED patients with CMC. Surprisingly, these reductions are strongly associated with neutralizing autoantibodies to IL-17F and IL-22, whereas responses were normal and autoantibodies infrequent in APECED patients without CMC. Our multicenter survey revealed neutralizing autoantibodies against IL-17A (41%), IL-17F (75%), and/ or IL-22 (91%) in >150 APECED patients, especially those with CMC. We independently found autoantibodies against these Th17-produced cytokines in rare thymoma patients with CMC. The autoantibodies preceded the CMC in all informative cases. We conclude that IL-22 and IL-17F are key natural defenders against CMC and that the immunodeficiency underlying CMC in both patient groups has an autoimmune basis.
Candida albicans can be both a commensal microorganism and a pathogen in humans, infecting epithelial sites or internally. Chronic mucocutaneous candidiasis (CMC), i.e., persistent or recurrent infections of nail beds, skin, and mucosal surfaces, occurs in primary or secondary immunodeficiencies, including HIV. Protection is thought to be T cell mediated, particularly by IL-17–producing Th17 cells at epithelial surfaces (LeibundGut-Landmann et al., 2007; Curtis and Way, 2009). Indeed, the natural Th17 memory repertoire includes many C. albicans–specific Th17 cells (Acosta-Rodriguez et al., 2007). CMC and impaired Th17 production are also prominent in the autosomal dominant hyper-IgE syndrome caused by STAT3 mutations (de Beaucoudrey et al., 2008; Ma et al., 2008; Milner et al., 2008) and in patients with defective CARD9 (Glocker et al., 2009) or dectin-1 (Ferwerda et al., 2009). In addition, heterogeneous patients with isolated CMC show reduced production of Th17-associated cytokines (Eyerich et al., 2008).
In addition to protecting against infections, Th17 cells have been implicated in several autoimmune diseases (Korn et al., 2009). Th17 cells characteristically express the transcription factor RORC (retinoic acid orphan receptor γt; Ivanov et al., 2006), IL-23R (IL-23 receptor), CCR6 (CC chemokine receptor 6), and CCR4 (Acosta-Rodriguez et al., 2007), which facilitate their migration to epithelial areas. By producing IL-17A and IL-17F (as homo- or heterodimers), as well as IL-22, IL-21, and IL-26, they trigger the subsequent production of neutrophil-recruiting and -activating cytokines and chemokines, proinflammatory cytokines, and antimicrobial peptides in many cell types (Liang et al., 2006; Wolk et al., 2006; Fouser et al., 2008; Korn et al., 2009). Recently, a skin-homing IL-22+/IL-17A− subset of Th cells has been described (Duhen et al., 2009; Trifari et al., 2009). IL-22 has important protective functions at epithelial surfaces, critically regulating antimicrobial genes and maintaining barrier integrity (Wolk et al., 2006; Aujla and Kolls, 2009).
Usually, CMC is the earliest and most common sign of autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED; Husebye et al., 2009). This autosomal recessive syndrome results from defects in the autoimmune regulator (AIRE) gene (Nagamine et al., 1997), in which ∼60 different mutations are now known. The commonest are p.R257X in exon 6 and a 13-bp deletion in exon 8. Many point substitutions, small insertions, deletions, and splice-site mutations have also been reported. The AIRE protein is principally expressed in medullary thymic epithelial cells where it normally prevents autoimmunity by regulating expression of peripheral tissue-specific antigens that induce self-tolerance in developing T cells (Anderson et al., 2002; Liston et al., 2003; Peterson et al., 2008; Mathis and Benoist, 2009). The known defects in central tolerance induction in this highly variable syndrome contrast strikingly with the consistent and selective C. albicans susceptibility. Several groups have noted modest and sometimes contradictory abnormalities in peripheral dendritic cell function in APECED patients. These did not correlate with their CMC and so still cannot explain the susceptibility to it (Lindh et al., 2008; Pöntynen et al., 2008; Ryan et al., 2008).
We have recently reported high-titer neutralizing autoantibodies against all 12 IFN-α subtypes, and especially IFN-ω, in almost all APECED patients, whatever their exact AIRE mutation or clinical picture (Meager et al., 2006; Meloni et al., 2008). These are rarely seen in other disorders, although patients with thymomas uniquely show intriguing parallels; ∼65% have anti-IFN autoantibodies at diagnosis (Meager et al., 1997) and rare cases develop CMC.
In this paper, we describe decreased IL-22 and IL-17F responses in patients with APECED or thymomas, which correlate with both CMC and neutralizing autoantibodies against these cytokines. We therefore conclude that even the CMC can have an autoimmune basis.
RESULTS AND DISCUSSION
Th17 responsiveness of APECED patient PBMCs
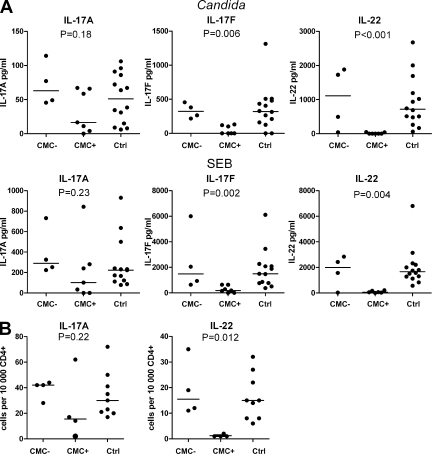
Th17 cells have been implicated in protection against C. albicans infection (Ma et al., 2008; Conti et al., 2009), so we first investigated their role in APECED. We compared responses of PBMCs from patients with and without CMC (Table I) and age-matched healthy controls to C. albicans antigens or staphylococcal enterotoxin B (SEB) as polyclonal stimulus. Production of IL-17A was not significantly altered in the patients overall, whether measured by ELISA in culture supernatants or by quantitative RT-PCR on cell mRNA (Fig. 1 A and Fig. S1). In contrast, IL-17F and especially IL-22 responses were significantly and consistently reduced in the patients with CMC but unaltered in the rare cases without it (Fig. 1 A). These reductions were seen at both time points tested and in levels of both protein and mRNA (Fig. 1 A and Fig. S1). Among other Th17-associated cytokines, IL-21 showed marginal increases in CMC patients, whereas IL-26 was unchanged (Fig. S1).
Table I.
APECED patients whose PBMCs were tested for cytokine responses
| Patient | AIRE mutation | Age | CMC | FACS sample | Sample at 72 h | Neutralizing antibody titer against: | Binding antibodies against: a | |||||
| IFN-ω | IL-17A | IL-17F | IL-22 | IL-17A | IL-17F | IL-22 | ||||||
| yr | ||||||||||||
| A | c.[769C>T] + [769C>T]b | 29 | Yes | No | No | 20,000 | <32 | 38 | 9,000 | 0.12 | 3.11 | 0.17 |
| Bc | c.[21_43dup23] + [21_43dup23] | 10 | Yes | Yes | Yes | >51,200 | 250,000 | 75 | 9,000 | 3.85 | 2.43 | 0.55 |
| C | c.[769C>T] + [769C>T] | 17 | Yes | Yes | Yes | >51,200 | <32 | 56 | 5,500 | 0.07 | 1.30 | 0.28 |
| D | c.[1064-1068dupCCCGG] + [1064-1068dupCCCGG] | 18 | Yes | Yes | Yes | >51,200 | <32 | 1,250 | >525,000 | 0.83 | 3.64 | 1.81 |
| E | c.[769C>T] + [769C>T] | 18 | Yes | No | No | >51,200 | <32 | 350 | 160,000 | 0.70 | 3.52 | 1.01 |
| F | c.[769C>T] + [769C>T] | 23 | Yes | No | Yes | 20,000 | 15,000 | 1,300 | 95,000 | 3.90 | 3.90 | 0.79 |
| G | c.[769C>T] + [769C>T] | 60 | Yes | Yes | Yes | 12,800 | <32 | 300 | 13,000 | 0.39 | 3.89 | 0.64 |
| H | c.[769C>T] + [967_979del13] | 20 | Yes | No | Yes | 100,000 | <32 | 5,500 | 4,500 | 2.98 | 3.90 | 0.25 |
| I | c.[967_979del13] + [1163_1164insA] | 24 | Yes | No | Yes | 256,000 | nd | 9,200 | 3,300 | 0.09 | 3.90 | 0.56 |
| J | c.[879 + 1G>A] + [879 + 1G>A] | 48 | No | Yes | No | 30,000 | <32 | <32 | <32 | 0.30 | 0.29 | 0.18 |
| Kc | c.[967_979del13] + [967_979del13] | 21 | No | Yes | Yes | >51,200 | <32 | <32 | <32 | 0.17 | 0.40 | 0.24 |
| L | c.[769C>T] + [c.274C>T] | 55 | No | No | Yes | 12,000 | <32 | <32 | <32 | 0.04 | 0.03 | 0.16 |
| M | c.[967_979del13] + [c.274C>T] | 29 | No | Yes | Yes | 200,000 | <32 | <32 | 930 | 0 | 0.03 | 0.21 |
| Nd | c. [682T>G] + [=] | 40 | No | Yes | Yes | 9,000 | <32 | <32 | <32 | 0.01 | 0.16 | ND |
Positive is a value >0.4 for anti–IL-17A and anti–IL-17F and >0.2 for anti–IL-22.
The mutation c.769C>T causes truncated AIRE protein p.R257X.
PBMCs from patients B and K were tested repeatedly 6 mo apart. The results (unpublished data) were similar to the first stimulation.
Dominant mutation p.G228W.
Figure 1.
Decreased IL-17F and IL-22 responses associate with CMC in APECED patients. (A) Secretion of IL-17A, IL-17F, and IL-22 by PBMCs from APECED patients without (CMC−) or with (CMC+) CMC and age-matched controls (Ctrl, n = 14 at 72-h time points) after stimulation with heat-killed C. albicans hyphae or SEB for 72 h was measured by ELISA. The patients are detailed in Table I. (B) SEB-stimulated PBMCs were stained intracellularly for IL-17A and IL-22 and FACS analyzed. Horizontal bars represent median values. A Kruskal-Wallis test was used to compare the group medians. This figure represents three independent experiments. Each symbol corresponds to a value obtained from an individual.
Notably, the reductions in IL-17F and IL-22 responses were not C. albicans antigen specific but were also seen after SEB stimulation. This could reflect loss of cytokine-producing cells, which we checked by intracellular cytokine staining. Indeed, frequencies of IL-22–secreting Th cells were very low in APECED patients with CMC, whereas those without CMC showed no differences from healthy controls (Fig. 1 B). Percentages of IL-17A–secreting cells were variable though not significantly changed overall (Fig. 1 B). Collectively, these results show significant decreases in IL-17F and IL-22 production and an almost complete lack of IL-22–producing cells in APECED patients with CMC. Thus, they imply that protection against CMC in humans depends more on IL-22 and IL-17F than on the more extensively studied IL-17A.
Autoantibodies against Th17-associated cytokines
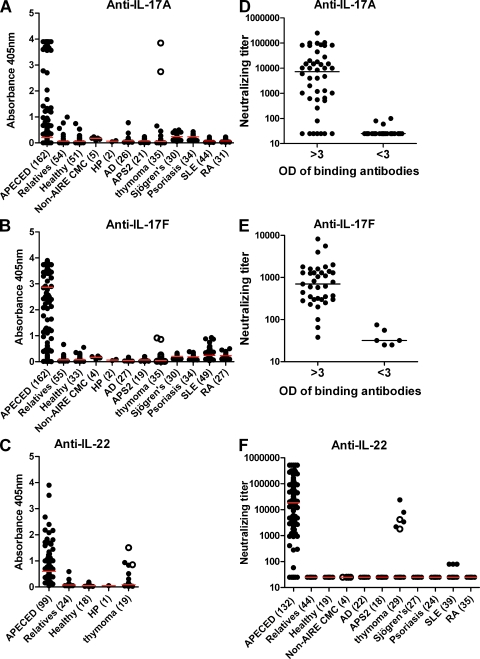
We next sought to understand the basis for these cytokine secretion defects. Recalling the high prevalences and titers of neutralizing autoantibodies against type I IFNs in APECED (Meager et al., 2006; Meloni et al., 2008), we hypothesized that analogous autoimmunity against Th-17–related cells and cytokines is one likely mechanism underlying these defects and predisposing to CMC. Remarkably, we detected serum autoantibodies against IL-17A, IL-17F, and/or IL-22 in the great majority of the 162 patients, with diverse AIRE mutations. In ELISAs, we found binding IgG autoantibodies to IL-17A in 67 of 162 (41%), to IL-17F in 121 of 162 (75%), and to IL-22 in 86 of 99 (87%) APECED patients (Fig. 2, A–C). In sharp contrast, their levels were low or undetectable in healthy subjects and unaffected relatives heterozygous for AIRE mutations. The few patients negative for IL-22 autoantibodies also tested negative against IL-17F and IL-17A (with a single exception). To test whether these responses were primarily mucosal, we checked their Ig isotypes. In fact, they proved to be predominantly IgG. Binding signals for IgA appeared weaker for every sample tested (Fig. S2 A).
Figure 2.
Disease-specificity of autoantibodies to IL-17A, IL-17F, and/ or IL-22. (A–C) Autoantibodies binding human IL-17A, IL-17F, and/ or IL-22 in sera from APECED or thymoma patients and disease or healthy controls tested by ELISA. (D and E) Comparisons of neutralizing and binding activity of anti–IL-17A and anti–IL-17F. (F) Because ELISA underestimates antibodies against IL-22, we used neutralizing assays for systematic comparisons. 12 sera were exhausted before exact titration of anti-IL-22 could be done. Red and black bars indicate group medians. SLE, systemic lupus erythematosus; RA, rheumatoid arthritis. Open circles represent patients T1 and T2 (Fig. 3 C and Table S2).
Importantly, both IL-17A and IL-17F were clearly neutralized in functional assays by most of the sera with binding values over three (Fig. 2, D and E). The corresponding binding and neutralizing titers correlated very well (r = 0.90 and r = 0.84, P < 0.001; Fig. S2 B). Interestingly, against IL-22, binding signals rarely reached saturation (Fig. 2 C), suggesting a relatively low gain in this particular ELISA (see Fig. S2). In sharp contrast, 136 of 150 (91%) patients tested positive in IL-22 neutralization assays, nearly all with titers >1,000 (Fig. 2 F). These included many with only weak or undetectable autoantibodies against IL-17s. We therefore use neutralizing titers against IL-22 for optimal quantification and sensitivity in most subsequent analyses. Sera from unaffected heterozygous relatives of APECED patients and healthy controls were negative in all three neutralizing assays (Fig. 2 F, shown against IL-22). Further screening revealed no additional positives against IL-17A/F heterodimer and also very few low positives (by ELISA) against IL-17B, IL-17C, or IL-17D (unpublished data), which are produced in other tissues and not by Th17 cells. We also noted only occasional or weakly binding autoantibodies against IL-6, IL-9, IL-12, IL-21, IL-23, IL-26, IL-29, or RANTES and none against IL-1α/β, IL-2, IL-4, IL-8, IL-10, IL-18, TGF-β1, or TNF (unpublished data).
We next assessed the disease specificity of the anti–IL-17 and –IL-22 autoantibodies. Their levels proved to be minimal or negative in other patients with just one of the APECED triad, i.e., CMC without AIRE mutations, isolated hypoparathyroidism (HP), or Addison’s disease (AD), autoimmune polyendocrine syndrome II, and also in diseases where Th17 cells or type I IFNs are implicated, such as Sjögren’s syndrome, psoriasis, systemic lupus erythematosus, and rheumatoid arthritis (Fig. 2, A–C and F). The only clear exceptions were in thymoma patients (see next section).
73 patients were studied serially. In nearly all the positives, autoantibodies against IL-17 and/or IL-22 were already present (or maximal) in the earliest samples, and, in two cases, even before the anti–type I IFN autoantibodies. However, titers against these Th17 cytokines were frequently lower two to three decades later, whereas those against the IFNs appear more stable (Meager et al., 2006; Meloni et al., 2008). Indeed, the anti–IL-22 and –IL-17F antibodies correlated negatively with age-at sampling (r = −0.44, P < 0.001; r = −0.25, P = 0.002).
Clinical correlates of the autoantibodies
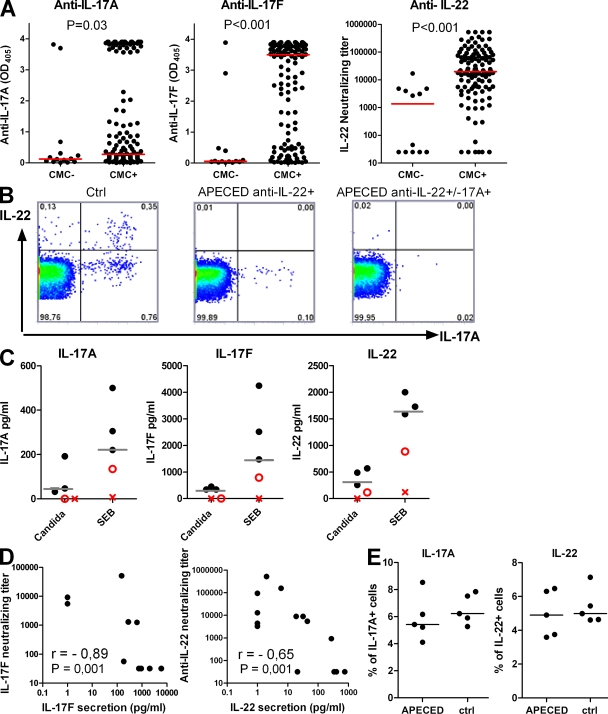
We next checked for associations of these autoantibodies with the patients’ CMC. These proved to be highly significant for anti–IL-17F and –IL-22; the titers were higher (Fig. 3 A) and they occurred more frequently (Table S1) in APECED patients with CMC than without it. This association was not absolute; 8 of the 15 cases without anti–IL-22 autoantibodies nonetheless had CMC. However, in at least 3 of these 8, the candidiasis had never recurred after early childhood, and autoantibody titers may then have declined before sampling. Furthermore, autoantibody levels appeared to be lower against IL-17F in 11 patients with unusually late-onset CMC (at 18–30 yr of age) than in 68 patients with childhood-onset CMC sampled as adults (P = 0.02).
Figure 3.
Autoimmunity to Th17-related cytokines is associated with CMC and cytokine productivity in vitro. (A) Associations between CMC and anti-cytokine autoantibodies in APECED patients. Red bars indicate group medians. (B) Representative FACS plots for intracellular IL-22 and IL-17A in a healthy subject (left) or in APECED patients with CMC and antibodies against IL-22 alone (middle) or against both IL-17A and IL-22 (right) after 6 h of SEB stimulation. (C) Production of IL-17A, IL-17F, and IL-22 by C. albicans– and SEB-stimulated (72 h) PBMCs from five random myasthenia gravis/thymoma patients was measured with ELISA. Patients T1 (X) and T2 (O) proved to be the only two with CMC. Median values for healthy controls are indicated with gray bars. (D) The concentration of secreted IL-17F (left) and IL-22 (right) after stimulating APECED patients’ PBMCs with SEB (for 72 and 18 h, respectively) correlates negatively with the respective neutralizing antibody titer. (E) Normal naive T cells were differentiated to Th17 cells with IL-1β, IL-6, IL-23, and IL-21 in the presence of sera (1:10) from either controls or APECED patients with antibodies to IL-22, IL-17F, and/or IL-17A. The IL-22– and IL-17A–producing cells were enumerated by flow cytometry. Data are representative of three independent experiments. Horizontal bars show median values.
Very rarely, thymoma patients develop CMC, whose pathogenesis remains unknown (Asherson and Webster, 1980). Strikingly, 2 of the 35 patients screened showed high binding and neutralizing titers against both IL-17A and IL-22 (Fig. 2, A and F; and Table S2). They were the only patients with documented CMC (cases T1 and T2). We also found similar autoantibodies in several other thymoma patients selected because of known C. albicans infections (Table S2).
Notably, the autoantibody titers were already high before CMC onset in the informative sera from four patients with APECED (Fig. S2 C, left) and one with thymoma (Fig. S2 C, right). Their titers showed no clear increases after CMC onset, suggesting that they are not solely an effect of candidiasis. Possibly, some of the rare autoantibody-positive patients without CMC in our analysis may develop it later. If so, the autoantibodies against IL-17 and IL-22 might prove to be useful predictors of CMC susceptibility that could be assessed in prospective serial studies in AIRE mutant siblings of APECED probands (or thymoma patients). These autoantibodies were also evident in nearly every informative sample taken before onset of HP (n = 2) or AD (n = 16) and, thus, seem not to be effects either of these components or of their treatments. In addition, the autoantibodies did not correlate clearly with any other APECED manifestation.
Intriguingly, we noted that certain mutations associated more strongly with both the autoantibodies and CMC. At one extreme, every p.R257X homozygote (61 from Finland, 5 from Slovenia, and 2 from Norway) had CMC and was positive against IL-22, and 64 were also positive against IL-17F. In contrast, among 20 c.967_979del13 homozygous patients (13 from Norway, 6 from USA, and 1 from Pakistan), 7 were negative against IL-17F and 1 against IL-22, and these also included the 7 without CMC. Moreover, autoantibodies and CMC were both rare in a unique family with a dominant-negative p.G228W substitution (Cetani et al., 2001) that cosegregates mainly with autoimmune thyroiditis and autoantibodies to type I IFNs (Meloni et al., 2008). Only two of these six relatives have had CMC (and only one with the full APECED triad). All six were negative against both IL-22 and IL-17A in single bleeds, and just one of the two with CMC was weakly positive against IL-17F. These apparent associations between AIRE genotype and autoantibodies or CMC require confirmation with larger patient numbers to exclude possible confounding factors, whether genetic, environmental, or clinical (e.g., CMC onset age or duration).
Possible implications of anti-cytokine autoantibodies
We immediately noticed that neutralizing titers were high against IL-17F and IL-22 in all the CMC+ APECED patients whose PBMC had shown reduced productivity of either cytokine in Fig. 1 (Table I). In contrast, they were weak or negative against IL-22, IL-17F, and IL-17A in the five CMC− patients with IL-17F and IL-22 responses in the normal range (Table I). Notably, although anti–IL-17A autoantibodies were less prevalent overall, the one patient (Table I, B) with very high titers against it had undetectable IL-17A responses, lacking both IL-22– and IL-17A–producing cells (Fig. 3 B, right). Indeed, we noted strong negative correlations between the amounts of secreted IL-17F and IL-22 and the corresponding neutralizing antibody titers in APECED (Fig. 3 D). Moreover, PBMC from the two thymoma patients with CMC and autoantibodies to all three cytokines likewise showed the lowest cytokine productivity (Fig. 3 C).
Brucklacher-Waldert et al., (2009) have recently detected IL-17A and IL-17F on the surface of activated Th17 cells. We therefore tested for effects of the autoantibodies themselves on Th17 cell development. We cultured naive CD4+ T cells from healthy donors in Th17-driving conditions in the presence of control sera or APECED sera containing high titers of anti-cytokine autoantibodies. Interestingly, we saw no effect on Th17 polarization (Fig. 3 E), arguing against direct inhibition by these antibodies in vitro. However, we cannot rule out opsonization or antibody-dependent cellular cytotoxicity reactions in vivo. Additional cell-mediated attack on Th17 subsets might also contribute to the deficient IL-17F and IL-22 production (Fig. 1 and Fig. 3 C), possibly even in patients without the antibodies.
Conclusions
Clinically, APECED is highly variable, including in the CMC onset age (even in p.R257X homozygotes), so there must be multiple predisposing factors, whether genetic, immunological, or environmental. Nevertheless, Th17 cells that produce IL-17 and IL-22 are crucial in protection against oral or mucocutaneous candidiasis (de Beaucoudrey et al., 2008; Ma et al., 2008; Milner et al., 2008; Conti et al., 2009; Curtis and Way, 2009).
We now show high-titer anti-Th17 cytokine autoantibodies and decreased Th17-related responses in APECED and thymoma patients with CMC. We therefore propose that their CMC susceptibility is primarily a result of antecedent immune attack on IL-22–expressing cells and on the IL-17A, IL-17F, and IL-22 that they produce. Because IL-22 especially protects epithelial surfaces (Liang et al., 2006; Aujla and Kolls, 2009) and IL-22+/IL17− cells show skin-homing properties (Duhen et al., 2009; Trifari et al., 2009), our findings may help to explain the mucocutaneous rather than systemic focus of the candidiasis in both patient groups. The apparent specificity for C. albicans remains unexplained but probably highlights the reserve capacity in human immune defenses. Indeed, similarly restricted susceptibilities to certain organisms, such as atypical mycobacteria, are seen in patients genetically deficient in multifaceted pathways (Bustamante et al., 2008) such as those involving IL-12 and IFN-γ. Even more importantly, this susceptibility is likewise phenocopied in patients with autoantibodies against IFN-γ (Notarangelo and Casanova, 2009). In contrast, the neutralizing autoantibodies against type I IFNs in APECED seem neither to predispose to viral infections (Meager et al., 2006) nor to affect type I IFN production by APECED DCs (Kisand et al., 2008). However, they might conceivably bias toward conditions favorable for C. albicans and so might the anti–IL-12/23 autoantibodies in thymoma patients (Table S2).
The striking serological and cellular parallels between APECED and thymoma patients seem to implicate shared thymic aberrations in autoimmunization against cytokines. Tissue-specific autoimmunity in APECED is thought to develop to antigens that are not expressed in the AIRE-deficient thymus (Anderson et al., 2002; Liston et al., 2003; Mathis and Benoist, 2009). In contrast, type I IFNs and Th17-related cytokines are made in the thymus by several cells of hematopoietic origin (Meager et al., 2006; Cooper et al., 2009; Marks et al., 2009), and their expression levels are most likely not directly regulated by AIRE. This suggests a different mechanism of autoimmunization. We recently proposed that thymic microenvironments, which are rendered dangerous by the tumor or by the absence of AIRE, can be responsible for breakdown of tolerance to cytokines (Meager et al., 2008).
Finally, our findings have several implications for therapies. Immunosuppressive treatments should at least be considered for the CMC in APECED, as it now appears to have an autoimmune basis analogous to the bone marrow aplasias in thymoma patients, for which these treatments are routine. They have not been tested systematically in APECED, although cyclosporin A has been shown to reverse pancreatic insufficiency, keratitis, and alopecia (Ward et al., 1999). Moreover, regression of disease manifestations, including candidiasis and autoantibody levels, was observed in a patient receiving tacrolimus, mycophenolate mofetil, and prednisone (Ulinski et al., 2006). Next, our data argue that IL-22 is more protective than IL-17A against CMC, which persisted in many APECED patients despite normal IL-17A responses. This raises the interesting possibility of IL-22 replacement therapy for CMC. Having no receptors on immune cells (Aujla and Kolls, 2009), IL-22 should have fewer side effects than IL-17A or IL-17F. However, these patients’ neutralizing autoantibodies might vitiate treatment with IL-22 itself, demanding development of alternative IL-22 receptor agonistic drugs/antibodies.
In conclusion, we show that the CMC in APECED associates with high-titer autoantibodies to Th17-related cytokines in APECED patients and dramatically decreased IL-22 and IL-17F productivity. We propose that the autoimmunity in APECED targets not only endocrine tissues but also components of the acquired immune system involved in mucosal protection against C. albicans.
MATERIALS AND METHODS
Subjects.
The patients with APECED (n = 162), thymomas (41), AD (26), HP (2), autoimmune polyendocrine syndrome II (21), isolated CMC (5), and the unaffected AIRE heterozygous relatives (54) and healthy controls (51) were summarized by Meloni et al. (2008; but the UK/Irish APECED patients are now being studied elsewhere). 54 Finnish and 19 Norwegian patients were studied serially (two to six serum samples each; mean interval between first and last = 13 yr; range, 0.7–32 yr). Sera from Sjögren’s syndrome (30 patients) or psoriasis (34 patients) were gifts of S. Bowman (University of Birmingham, Birmingham, England, UK) or J. Barker (University of London, London, England, UK). Sera from systemic lupus erythematosus (49 patients) and rheumatoid arthritis (31 patients) were gifts from D. Isenberg (University College Hospital, London, England, UK) and P. Subrahmanyam and B. Dasgupta (Southend University Hospital, Essex, England, UK). All were studied in accordance with the Helsinki Declaration with informed consent and local Ethics Committee approval.
Cell stimulation and differentiation.
PBMCs from 14 APECED patients and matched controls were incubated in RPMI 1640 + 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% FBS (all from PAA Laboratories) at 106 cells/ml. They were stimulated with heat-killed C. albicans hyphae (1:1, strain 28366; American Type Culture Collection), 10 µg/ml C. albicans yeast lysate, 0.1 µg/ml SEB from Staphylococcus aureus (Sigma-Aldrich), or in medium alone for 18 and/or 72 h. Parallel samples were stimulated for 6 h with 0.1 µg/ml SEB in the presence of 10 µg/ml Brefeldin A (Sigma-Aldrich), fixed in lysing solution (BD), and permeabilized with 1% Saponin (Sigma-Aldrich). Cytokine expression was determined using anti–IL-17-A–Alexa Fluor 647 (eBioscience), anti–IFN-γ–PE (BD), and IL-22–PE (R&D Systems) using a FACSCalibur (BD) and FlowJo software (Tree Star, Inc.). We assayed the following cytokines in culture supernatants with commercial ELISA sets: IL-22 and IL-17-F (R&D Systems) and IL-17-A (eBioscience).
Naive T cells (CD4+CD45RA+) were isolated by immunomagnetic depletion of CD4−/CD45RO+ T cells according to the manufacturer’s instructions (Miltenyi Biotec). 105 naive T cells were cultured in 10% APECED or control serum in 96-well U-bottom plates in X-VIVO 15 serum-free medium (Lonza) in the presence of magnetic beads coated with antibodies to CD2, CD3, and CD28 (one bead per cell; Miltenyi Biotec) and 20 ng/ml IL-1β, 20 ng/ml IL-6, 20 ng/ml IL-23, and 20 ng/ml IL-21 (eBioscience) for 6 d. The cells were then extensively washed and restimulated for 6 h with 10 ng/ml PMA and 1 µg/ml ionomycin plus 10 µg/ml Brefeldin A (all from Sigma-Aldrich) and stained as described in the previous paragraph.
Quantitative RT-PCR.
RNA was extracted using Trizol (Invitrogen) and the RNeasy Micro kit (QIAGEN) with concomitant DNase treatment. Complementary DNA was synthesized using SuperScript III (Invitrogen). RT-PCR was performed using ABI Prism 7900 Sequence Detection System (Applied Biosystems) and qPCR SYBR Green Core kit (Eurogentec). All samples were normalized to expression of β-actin.
ELISAs.
Wells were coated with IL-17A, IL-17F, IL-17A/F, and IL-22 (R&D Systems) at 2 µg of protein/ml in PBS, pH 7.0, and blocked with 3% HSA. Sera were added for 2 h at 22°C before washing and development with anti–human IgG or IgA–alkaline phosphatase conjugate (Sigma-Aldrich). P-nitrophenyl phosphate substrate, and then 3 M NaOH, were added, and the absorbances were read at 405 nm. Any values >2 SD above the mean of 120 healthy controls were considered positive.
Cell-based IL-17 and IL-22 neutralization assays.
HFB4 human foreskin fibroblast cells (Schering-Plough Corporation) were maintained in DME plus 10% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. HFB4 were seeded at 104 cells/well, in which 2 ng/ml IL-17A or 20 ng/ml IL-17A/F had been preexposed to serially diluted patient sera for 2 h. After incubation at 37°C for 16–20 h, supernatants were collected and analyzed for growth-related oncogene α production by ELISA (R&D Systems). To assay any remaining IL-17F (10 ng/ml final; preexposed to serially diluted patient sera for 2 h), we additionally preincubated NCTC 2544 keratinocytes (Interlab Cell Line Collection) with 0.1 ng/ml TNF before the same read-out as with IL-17A neutralization assay (extensive checks revealed no antibodies against TNF alone). For IL-22, we instead used the cell line Colo205 and measured the IL-10 response. Antibody titers were estimated from graphs of ELISA absorbances as the reciprocal serum dilution yielding a value halfway between the positive and negative controls.
Statistical analysis.
Mann-Whitney or Kruskal-Wallis tests were used to compare the median values between groups. Spearman correlation and Fisher’s exact test was used to study associations between the variables.
Online supplemental material.
Fig. S1 shows additional time points/cytokine responses of stimulated T cells not shown in Fig. 1. Fig. S2 shows IgA autoantibodies against IL-17A, IL-17F, and IL-22 in a set of patients and controls, correlations between binding and neutralizing antibodies, anti-Th17 cytokine autoantibody levels in four APECED cases sampled before onset of CMC, and antibody levels, clinical evolution, and treatments in a thymoma patient. Table S1 shows associations between CMC and autoantibodies against Th17 cytokines. Table S2 summarizes clinical evolution and autoantibodies against Th17-related cytokines in thymoma patients with CMC or episodes of C. albicans infection. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091669/DC1.
Acknowledgments
We thank all the patients for their samples and many colleagues for ascertaining them, including Drs. S. Bowman and J Barker, Drs. Kari Lima, Kristian Fougner, Jens Bollerslev, and also Maire Pihlap for technical assistance, Katrin Eimra for the C. albicans strain and helpful technical hints, and an unknown referee of Meager et al. (2008) for very helpful suggestions.
The study was supported by EU Framework program 6 (Euraps), the European Regional Fund and Archimedes Foundation, Estonian Science Foundation (grants 6663 and 7197), the Estonian Ministry of Education and Research targeted funding SF0180035s08, The Wellcome Trust, Helse-Vest and The Norwegian Research Council, Slovene National Research Agency grant P3-0343, and Tampere University Hospital Research Fund.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- AD
- Addison's disease
- AIRE
- autoimmune regulator
- APECED
- autoimmune polyendocrinopathy candidiasis ectodermal dystrophy
- CMC
- chronic mucocutaneous candidiasis
- HP
- hypoparathyroidism
- SEB
- staphylococcal enterotoxin B
References
- Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8:639–646 10.1038/ni1467 [DOI] [PubMed] [Google Scholar]
- Anderson M.S., Venanzi E.S., Klein L., Chen Z., Berzins S.P., Turley S.J., von Boehmer H., Bronson R., Dierich A., Benoist C., Mathis D. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science. 298:1395–1401 10.1126/science.1075958 [DOI] [PubMed] [Google Scholar]
- Asherson G.L., Webster A.D.B. 1980. Thymoma and immunodeficiency. Diagnosis and Treatment of Immunodeficiency. Asherson G.L., Webster A.D.B., Blackwell Publishing, Oxford: 78–98 [Google Scholar]
- Aujla S.J., Kolls J.K. 2009. IL-22: a critical mediator in mucosal host defense. J. Mol. Med. 87:451–454 10.1007/s00109-009-0448-1 [DOI] [PubMed] [Google Scholar]
- Brucklacher-Waldert V., Steinbach K., Lioznov M., Kolster M., Hölscher C., Tolosa E. 2009. Phenotypical characterization of human Th17 cells unambiguously identified by surface IL-17A expression. J. Immunol. 183:5494–5501 10.4049/jimmunol.0901000 [DOI] [PubMed] [Google Scholar]
- Bustamante J., Boisson-Dupuis S., Jouanguy E., Picard C., Puel A., Abel L., Casanova J.L. 2008. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr. Opin. Immunol. 20:39–48 10.1016/j.coi.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Cetani F., Barbesino G., Borsari S., Pardi E., Cianferotti L., Pinchera A., Marcocci C. 2001. A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. J. Clin. Endocrinol. Metab. 86:4747–4752 10.1210/jc.86.10.4747 [DOI] [PubMed] [Google Scholar]
- Conti H.R., Shen F., Nayyar N., Stocum E., Sun J.N., Lindemann M.J., Ho A.W., Hai J.H., Yu J.J., Jung J.W., et al. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 206:299–311 10.1084/jem.20081463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M.A., Colonna M., Yokoyama W.M. 2009. Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep. 10:1103–1110 10.1038/embor.2009.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M.M., Way S.S. 2009. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 126:177–185 10.1111/j.1365-2567.2008.03017.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaucoudrey L., Puel A., Filipe-Santos O., Cobat A., Ghandil P., Chrabieh M., Feinberg J., von Bernuth H., Samarina A., Jannière L., et al. 2008. Mutations in STAT3 and IL12RB1 impair the development of human IL-17–producing T cells. J. Exp. Med. 205:1543–1550 10.1084/jem.20080321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhen T., Geiger R., Jarrossay D., Lanzavecchia A., Sallusto F. 2009. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10:857–863 10.1038/ni.1767 [DOI] [PubMed] [Google Scholar]
- Eyerich K., Foerster S., Rombold S., Seidl H.P., Behrendt H., Hofmann H., Ring J., Traidl-Hoffmann C. 2008. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J. Invest. Dermatol. 128:2640–2645 10.1038/jid.2008.139 [DOI] [PubMed] [Google Scholar]
- Ferwerda B., Ferwerda G., Plantinga T.S., Willment J.A., van Spriel A.B., Venselaar H., Elbers C.C., Johnson M.D., Cambi A., Huysamen C., et al. 2009. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 361:1760–1767 10.1056/NEJMoa0901053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouser L.A., Wright J.F., Dunussi-Joannopoulos K., Collins M. 2008. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol. Rev. 226:87–102 10.1111/j.1600-065X.2008.00712.x [DOI] [PubMed] [Google Scholar]
- Glocker E.O., Hennigs A., Nabavi M., Schäffer A.A., Woellner C., Salzer U., Pfeifer D., Veelken H., Warnatz K., Tahami F., et al. 2009. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 361:1727–1735 10.1056/NEJMoa0810719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husebye E.S., Perheentupa J., Rautemaa R., Kämpe O. 2009. Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J. Intern. Med. 265:514–529 10.1111/j.1365-2796.2009.02090.x [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Kisand K., Link M., Wolff A.S., Meager A., Tserel L., Org T., Murumägi A., Uibo R., Willcox N., Trebusak Podkrajsek K., et al. 2008. Interferon autoantibodies associated with AIRE deficiency decrease the expression of IFN-stimulated genes. Blood. 112:2657–2666 10.1182/blood-2008-03-144634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Oukka M., Kuchroo V.K. 2009. IL-17 and Th17 Cells. Annu. Rev. Immunol. 27:485–517 10.1146/annurev.immunol.021908.132710 [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S., Gross O., Robinson M.J., Osorio F., Slack E.C., Tsoni S.V., Schweighoffer E., Tybulewicz V., Brown G.D., Ruland J., Reis e Sousa C. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630–638 10.1038/ni1460 [DOI] [PubMed] [Google Scholar]
- Liang S.C., Tan X.Y., Luxenberg D.P., Karim R., Dunussi-Joannopoulos K., Collins M., Fouser L.A. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271–2279 10.1084/jem.20061308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh E., Lind S.M., Lindmark E., Hässler S., Perheentupa J., Peltonen L., Winqvist O., Karlsson M.C. 2008. AIRE regulates T-cell-independent B-cell responses through BAFF. Proc. Natl. Acad. Sci. USA. 105:18466–18471 10.1073/pnas.0808205105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A., Lesage S., Wilson J., Peltonen L., Goodnow C.C. 2003. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 4:350–354 10.1038/ni906 [DOI] [PubMed] [Google Scholar]
- Ma C.S., Chew G.Y., Simpson N., Priyadarshi A., Wong M., Grimbacher B., Fulcher D.A., Tangye S.G., Cook M.C. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 205:1551–1557 10.1084/jem.20080218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks B.R., Nowyhed H.N., Choi J.Y., Poholek A.C., Odegard J.M., Flavell R.A., Craft J. 2009. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat. Immunol. 10:1125–1132 10.1038/ni.1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D., Benoist C. 2009. Aire. Annu. Rev. Immunol. 27:287–312 10.1146/annurev.immunol.25.022106.141532 [DOI] [PubMed] [Google Scholar]
- Meager A., Vincent A., Newsom-Davis J., Willcox N. 1997. Spontaneous neutralising antibodies to interferon—alpha and interleukin-12 in thymoma-associated autoimmune disease. Lancet. 350:1596–1597 10.1016/S0140-6736(05)64012-3 [DOI] [PubMed] [Google Scholar]
- Meager A., Visvalingam K., Peterson P., Möll K., Murumägi A., Krohn K., Eskelin P., Perheentupa J., Husebye E., Kadota Y., Willcox N. 2006. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 3:e289 10.1371/journal.pmed.0030289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meager A., Peterson P., Willcox N. 2008. Hypothetical review: thymic aberrations and type-I interferons; attempts to deduce autoimmunizing mechanisms from unexpected clues in monogenic and paraneoplastic syndromes. Clin. Exp. Immunol. 154:141–151 10.1111/j.1365-2249.2008.03739.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni A., Furcas M., Cetani F., Marcocci C., Falorni A., Perniola R., Pura M., Bøe Wolff A.S., Husebye E.S., Lilic D., et al. 2008. Autoantibodies against type I interferons as an additional diagnostic criterion for autoimmune polyendocrine syndrome type I. J. Clin. Endocrinol. Metab. 93:4389–4397 10.1210/jc.2008-0935 [DOI] [PubMed] [Google Scholar]
- Milner J.D., Brenchley J.M., Laurence A., Freeman A.F., Hill B.J., Elias K.M., Kanno Y., Spalding C., Elloumi H.Z., Paulson M.L., et al. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 452:773–776 10.1038/nature06764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine K., Peterson P., Scott H.S., Kudoh J., Minoshima S., Heino M., Krohn K.J., Lalioti M.D., Mullis P.E., Antonarakis S.E., et al. 1997. Positional cloning of the APECED gene. Nat. Genet. 17:393–398 10.1038/ng1297-393 [DOI] [PubMed] [Google Scholar]
- Notarangelo L.D., Casanova J.L. 2009. Primary immunodeficiencies: increasing market share. Curr. Opin. Immunol. 21:461–465 10.1016/j.coi.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Peterson P., Org T., Rebane A. 2008. Transcriptional regulation by AIRE: molecular mechanisms of central tolerance. Nat. Rev. Immunol. 8:948–957 10.1038/nri2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöntynen N., Strengell M., Sillanpää N., Saharinen J., Ulmanen I., Julkunen I., Peltonen L. 2008. Critical immunological pathways are downregulated in APECED patient dendritic cells. J. Mol. Med. 86:1139–1152 10.1007/s00109-008-0374-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K.R., Hong M., Arkwright P.D., Gennery A.R., Costigan C., Dominguez M., Denning D., McConnell V., Cant A.J., Abinun M., et al. 2008. Impaired dendritic cell maturation and cytokine production in patients with chronic mucocutanous candidiasis with or without APECED. Clin. Exp. Immunol. 154:406–414 10.1111/j.1365-2249.2008.03778.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifari S., Kaplan C.D., Tran E.H., Crellin N.K., Spits H. 2009. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 10:864–871 10.1038/ni.1770 [DOI] [PubMed] [Google Scholar]
- Ulinski T., Perrin L., Morris M., Houang M., Cabrol S., Grapin C., Chabbert-Buffet N., Bensman A., Deschênes G., Giurgea I. 2006. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome with renal failure: impact of posttransplant immunosuppression on disease activity. J. Clin. Endocrinol. Metab. 91:192–195 10.1210/jc.2005-1538 [DOI] [PubMed] [Google Scholar]
- Ward L., Paquette J., Seidman E., Huot C., Alvarez F., Crock P., Delvin E., Kämpe O., Deal C. 1999. Severe autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy in an adolescent girl with a novel AIRE mutation: response to immunosuppressive therapy. J. Clin. Endocrinol. Metab. 84:844–852 10.1210/jc.84.3.844 [DOI] [PubMed] [Google Scholar]
- Wolk K., Witte E., Wallace E., Döcke W.D., Kunz S., Asadullah K., Volk H.D., Sterry W., Sabat R. 2006. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur. J. Immunol. 36:1309–1323 10.1002/eji.200535503 [DOI] [PubMed] [Google Scholar]