Abstract
The natural cytotoxicity receptor NKp46 (encoded by Ncr1) was recently shown to identify a subset of noncytotoxic, Rag-independent gut lymphocytes that express the transcription factor Rorc, produce interleukin (IL)-22, and provide innate immune protection at the intestinal mucosa. Intestinal CD3−NKp46+ cells are phenotypically heterogeneous, comprising a minority subset that resembles classical mature splenic natural killer (NK) cells (NK1.1+, Ly49+) but also a large CD127+NK1.1− subset of lymphoid tissue inducer (LTi)–like Rorc+ cells that has been proposed to include NK cell precursors. We investigated the developmental relationships between these intestinal CD3−NKp46+ subsets. Gut CD3−NKp46+ cells were related to LTi and NK cells in requiring the transcriptional inhibitor Id2 for normal development. Overexpression of IL-15 in intestinal epithelial cells expanded NK1.1+ cells within the gut but had no effect on absolute numbers of the CD127+NK1.1−Rorc+ subset of CD3−NKp46+ cells. In contrast, IL-7 deficiency strongly reduced the overall numbers of CD3−NKp46+NK1.1− cells that express Rorc and produce IL-22 but failed to restrict homeostasis of classical intestinal NK1.1+ cells. Finally, in vivo fate-mapping experiments demonstrated that intestinal NK1.1+CD127− cells are not the progeny of Rorc-expressing progenitors, indicating that CD127+NK1.1−Rorc+ cells are not canonical NK cell precursors. These studies highlight the independent cytokine regulation of functionally diverse intestinal NKp46+ cell subsets.
Hematopoiesis is regulated through dynamic cell–cell interactions between developing blood cells and stromal cells in the bone marrow. Soluble factors that are elaborated in this microenvironment are also critically involved in sustaining developing hematopoietic precursors and guiding the differentiation of their progeny. Concerning NK cell development, both stromal cells and cytokine/growth factors play essential roles. Among the latter, IL-15 has been identified as a dominant cytokine in NK cell development, driving the generation of NK cells from committed NK cell precursors, promoting the differentiation of immature NK cells, and maintaining the survival of mature NK cells in the peripheral lymphoid organs (Kennedy et al., 2000; Cooper et al., 2001; Ranson et al., 2003; Vosshenrich et al., 2005). IL-15 signals are delivered through transpresentation, where cell surface–bound IL-15–IL-15Rα complexes trigger responsive cells (Dubois et al., 2002; Mortier et al., 2008). Both hematopoietic cells (including macrophages and dendritic cells) and nonhematopoietic cells (stromal cells, epithelial cells) transpresent IL-15 to assure homeostasis of NK cells and other IL-15–dependent cells (Huntington et al., 2009). The demonstration that several sources of IL-15–IL-15Rα exist in peripheral tissues provides a possibility to promote NK cell differentiation from circulating NK cell precursors outside the bone marrow. This “NK-poeisis” has been suggested to occur in the thymus in mice (Di Santo and Vosshenrich, 2006) and the lymph nodes in mice and humans (Freud et al., 2005; Freud and Caligiuri, 2006; Veinotte et al., 2008), and could also operate at epithelial surfaces.
Although IL-15 is required for development of all NK1.1+ cells (Kennedy et al., 2000; Vosshenrich et al., 2005), additional cytokines have been identified that are critical for the homeostasis of distinct, tissue-resident NK cell subsets. Thymic NK1.1+ cells are characterized by CD127 (IL-7Rα chain) expression and develop independently of Rag-dependent T cell progenitors (Vosshenrich et al., 2006). Unlike bone marrow or splenic NK cells, the homeostasis of thymic CD127+ NK cells requires IL-7 (Vosshenrich et al., 2006). Whether other NK cell subsets have particular cytokine requirements for their homeostasis or for their effector functions is unknown.
The natural cytotoxicity receptor (NCR) NKp46 (encoded by the Ncr1 locus) has been shown to be highly and specifically expressed in NK cells from several species (Moretta and Moretta, 2004), and has been proposed in a universal definition for NK cells (CD3−NKp46+; Walzer et al., 2007). Although this may be applicable to CD3−NKp46+ cells from the bone marrow, blood, or spleen (which appear as relatively homogeneous populations of cytotoxic NK1.1+ NK cells), recent studies have identified substantial diversity in the intestinal CD3−NKp46+ compartment (Satoh-Takayama et al., 2008; Luci et al., 2009; Sanos et al., 2009). Using CD127 and NK1.1, we were able to discriminate several subsets of intestinal CD3−NKp46+ cells (CD127+NK1.1−, CD127+NK1.1+, and CD127−NK1.1+) that showed distinct cell-surface phenotypes and functional attributes (Satoh-Takayama et al., 2008). CD127−NK1.1+ cells were similar to CD3−NK1.1+ NK cells found in the spleen, bearing mature NK markers (NKG2D, CD11b, Ly49 family members) and effector functions (perforin, IFN-γ). A predominant CD127+NK1.1− subset was clearly different, bearing few or no NK cell markers, lacking perforin and IFN-γ expression, and, unlike mature splenic NK cells, expressing the transcription factor Rorc (Satoh-Takayama et al., 2008; Luci et al., 2009; Sanos et al., 2009).
Rorc has been previously shown to be essential for thymocyte survival (Sun et al., 2000), the development of lymphoid tissue inducer (LTi) cells (Eberl and Littman, 2003), and the differentiation of polarized T cells that express IL-17 family cytokines (including IL-22; for review see Ivanov et al., 2007). Curiously, intestinal CD3−NKp46+ cells failed to express constitutive or inducible IL-17A, whereas a CD127+NK1.1−/lo subset clearly expressed IL-22 transcripts (Satoh-Takayama et al., 2008; Luci et al., 2009; Sanos et al., 2009). Moreover, development of intestinal NKp46+IL-22+ cells required the presence of microflora and an intact Rorc gene (Satoh-Takayama et al., 2008; Luci et al., 2009; Sanos et al., 2009). These results identified a novel CD3−NKp46+ cell subset in the gut that appeared to be hard-wired for rapid IL-22 production in response to microbial signatures. This pathway of innate IL-22 production appears evolutionarily conserved, as a CD3−NKp44+ cell subset with similar properties has been identified in human lymph nodes and tonsils (Cella et al., 2009; Cupedo et al., 2009).
LTi cells are classically defined as CD3−CD4+ hematopoietic cells that promote the formation of lymphoid tissues through a cross talk with lymphoid tissue stromal cells, resulting in the recruitment of B and T lymphocytes that segregate into functionally distinct zones (for review see Mebius, 2003). Rorc is critical to this process, as LTi cells from Rorc-deficient mice are not generated and stromal cells in lymphoid tissue anlagen fail to induce stromal cell expression of vascular cell adhesion molecule and intracellular adhesion molecule, resulting in abortive lymphoid tissue genesis. Cytokines also play an important role in this process. LTi cells express CD127 and IL-7 is required for the activation of LTi cells to express membrane-bound lymphotoxin LTα1β2 heterotrimer (Luther et al., 2003). This form of lymphotoxin activates stromal cells through the LTβR in a process required for the development of lymph nodes, Peyer’s patches, and intestinal isolated lymphoid follicles (Eberl and Lochner, 2009). Similar to CD127+NK1.1− cells, LTi cells have been shown to express IL-22 and IL-17 (Cupedo et al., 2009; Takatori et al., 2009). However, a role for IL-17 and/or IL-22 in the development of lymphoid tissues remains to be demonstrated.
The developmental relationship of LTi cells to other hemato-lymphoid subsets is not fully understood. The transcriptional inhibitor Id2 plays a critical role in LTi generation and is also essential at an early stage of NK cell commitment (Yokota et al., 1999), suggesting a link between LTi and NK cell developmental pathways. Consistent with this notion, a population of fetal CD3−CD4+ cells with LTi activity could generate lytic NK1.1+ cells after culture in IL-2 (Mebius et al., 1997; Yoshida et al., 2001). Analysis of human lymph nodes and tonsils identified a population of immature NK cells that express CD127 and Rorc that can further develop into mature CD56+ NK cells that express inhibitory CD94–NKG2A complexes (Hughes et al., 2009). Lastly, recent results showed that human fetal tissues harbor a lineage-negative CD127+ LTi cell population enriched in Rorc expression that can generate CD56+ NK cells in vitro (Cupedo et al., 2009). Thus, data in both humans and mice suggest that LTi-like Rorc+ cell subsets potentially harbor precursors to mature NK cells.
Considering the immature NK cell phenotype of most intestinal CD3−NKp46+ cells and their phenotypic similarities to LTi cells, a hypothesis has emerged that intestinal CD3−NKp46+ cells expressing Rorc may include NK cell precursors that could develop locally into specialized NK cell subsets under the influence of microbial stimulation (Colonna, 2009; Vivier et al., 2009). In this report, we define the developmental relationships between the different identified intestinal NKp46+ cell subsets using cytokine-deficient mice and in vivo fate-mapping approaches.
RESULTS AND DISCUSSION
Essential role for the transcriptional inhibitor Id2 in the homeostasis of intestinal CD3−NKp46+ cells that express Rorc and Il22
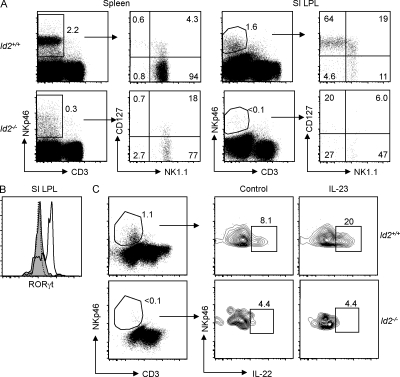
To understand the developmental relationship between distinct intestinal CD3−NKp46+ subsets, we first assessed the role for the transcriptional inhibitor Id2 in the homeostasis of these cells. Previous studies have shown that Id2 plays a critical role in the development of NK1.1+ NK cells (Yokota et al., 1999), and is essential for the development of LTi cells that express the transcription factor Rorc and coordinate programmed lymphoid tissue genesis during fetal life and inducible lymphoid structures in adult mice (Eberl and Littman, 2004). By comparing lamina propria lymphocytes (LPLs) from WT and Id2-deficient (Id2−/−) mice, we found that normal development of all intestinal CD3−NKp46+ cell subsets required Id2. Percentages of intestinal CD3−NKp46+ cell subsets that differentially express CD127 and/or NK1.1 were severely reduced in the absence of Id2 (Fig. 1 A), resulting in dramatically reduced absolute numbers of these cells (WT mice, 1.5 ± 0.2 × 105 cells; Id2−/− mice, ∼103 cells). In contrast, intestinal T cell homeostasis in the lamina propria was minimally perturbed (Fig. S1), whereas Id2 deficiency markedly reduced the frequency of splenic CD3−NKp46+ cells (essentially NK1.1+ NK cells; Fig. 1 A), consistent with previous reports (Yokota et al., 1999; Kim et al., 2004; Boos et al., 2007).
Figure 1.
Normal development of splenic and intestinal CD3−NKp46+ cell subsets requires Id2. (A) NK1.1 versus CD127 expression on gated CD3−NKp46+ splenocytes and small intestinal (SI) LPLs of WT and Id2−/− mice. Subset frequencies are indicated in one representative experiment out of six performed. (B) RORγt expression in intestinal CD3−NKp46+ subsets in WT (bold line) and Id2−/− mice (shaded histogram). The dotted line depicts staining of CD3−NKp46+ cells from Rorc−/− mice. (C) IL-23–induced IL-22 expression in intestinal CD3−NKp46+CD127+ cells from WT and Id2−/− mice. Results indicate frequencies from one out of two independent experiments.
Intestinal CD3−NKp46+ cells that express Rorc can produce IL-22 after stimulation with IL-23 in vitro (Cella et al., 2009). Id2 deficiency resulted in a strong reduction of RORγt expression (Fig. 1 B) within residual intestinal CD3−NKp46+ cells and a corresponding abrogation of IL-23–induced IL-22 production (Fig. 1 C), consistent with earlier studies showing an essential role for Rorc in constitutive and inducible IL-22 expression by gut CD3−NKp46+ cells (Satoh-Takayama et al., 2008; Luci et al., 2009; Sanos et al., 2009). Thus, differentiation of gut CD3−NKp46+ cells requires careful titration of E-box protein activity via Id2. These results demonstrate that the development and normal homeostasis of all intestinal CD3−NKp46+ cell subsets requires Id2, and reinforce the notion that intestinal CD3−NKp46+ cells are developmentally related to classical NK1.1+ NK cells and LTi cells.
Local overexpression of IL-15 expands intestinal CD3−NKp46+ cells expressing NK1.1 cells but fails to perturb homeostasis of the CD127+NK1.1−Rorc+ subset
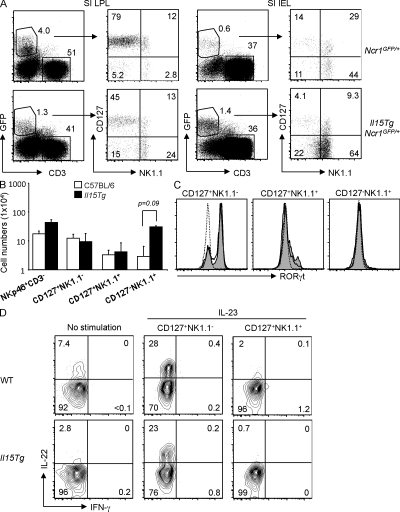
We previously demonstrated that intestinal CD3−NK1.1+ cells were strongly reduced in mice lacking IL-15 or the IL-2Rβ chain (Satoh-Takayama et al., 2008), reflecting their similar cytokine dependency with classical NK cells found in other tissues. In contrast, homeostasis of intestinal CD3−NKp46+ cells with the CD127+NK1.1− phenotype was not affected by an absence of IL-15 signaling. Although these observations rule out a role for IL-15 in the generation of the intestinal CD3−NKp46+NK1.1− cell subset, it nevertheless remained possible that these cells might represent IL-15–responsive precursors that could be recruited to develop into NK1.1+ NK cells under conditions where IL-15 availability increases. To assess the local inductive effects of IL-15 on the composition of intestinal CD3−NKp46+ cell subsets, we analyzed transgenic (Tg) mice in which IL-15 is overexpressed in the intestinal epithelium (Ohta et al., 2002). In these IL-15 Tg mice, local production of IL-15 has been previously shown to augment numbers of CD8+ T cells that express NK1.1 (Ohta et al., 2002). Total absolute numbers of LPLs were increased in IL-15 Tg mice (WT mice, 6.5 ± 3.3 × 106 cells; IL-15 Tg mice, 17.9 ± 9.4 × 106 cells), which increased the absolute numbers of intestinal lamina propria CD3−NKp46+ cells two- to threefold compared with non-Tg littermates (WT mice, 1.5 ± 0.2 × 105 CD3−NKp46+ cells; IL-15 Tg mice, 4.3 ± 0.4 × 105 CD3−NKp46+ cells). When comparing CD127 versus NK1.1 expression profiles, increased IL-15 availability strongly perturbed the relative frequencies of CD127/NK1.1 subsets with amplified percentages of NK1.1+CD127− cells in both the lamina propria and intraepithelial compartments (Fig. 2 A). This resulted in significantly increased absolute numbers of lamina propria NK1.1+CD127− cells compared with WT mice (P = 0.09; Fig. 2 B). Remarkably, IL-15 overexpression had no effect on the absolute numbers of CD127+NK1.1− or CD127+NK1.1+ cells (Fig. 2 B). Moreover, Rorc expression and IL-23–induced IL-22 production from intestinal CD3−NKp46+ cells were similar between WT and IL-15 Tg mice (Fig. 2, C and D). These results reinforce the concept that NK1.1− and NK1.1+ subsets of intestinal CD3−NKp46+ cells represent two distinct and independent lineages with respect to IL-15 responsiveness. Nevertheless, these results do not formally rule out the possibility that the CD127+NK1.1−Rorc+ subset of intestinal CD3−NKp46+ cells harbors IL-15–independent NK cell precursors.
Figure 2.
Perturbed development of intestinal CD3−GFP+ cell subsets in Ncr1GFP/+ mice that overexpress IL-15 in the intestinal epithelium. (A) NK1.1 versus CD127 expression on gated CD3−GFP+ cells from small intestinal (SI) LPL and intraepithelial (IEL) compartments. Subset frequencies are indicated. Representative results from one out of six independent experiments are shown. (B) Absolute numbers of total CD3−NKp46+ cells and CD127/NK1.1 subsets from small intestinal LPLs in WT and IL-15 Tg mice (n = 5–8 mice of each genotype analyzed). Only CD127−NK1.1+ cells were significantly increased in IL-15 Tg mice. Values are presented as means ± SD. (C) RORγt expression in intestinal CD3−NKp46+ subsets in WT (bold line) and IL-15 Tg mice (shaded histogram). The dotted line depicts staining of CD3−NKp46+ cells from Rorc−/− mice. (D) IL-22 production from intestinal CD3−NKp46+ subsets in WT and IL-15 Tg mice. Results indicate frequencies from one out of three independent experiments.
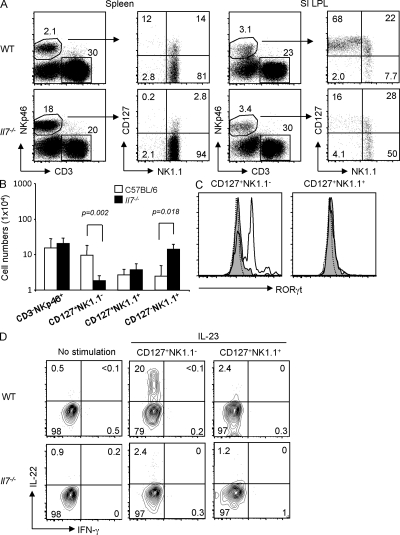
IL-7 is critical for the homeostasis of the CD127+NK1.1− subset of intestinal CD3−NKp46+ cells that produces IL-22
Our previous studies demonstrated that development of the CD127+NK1.1− subset of intestinal CD3−NKp46+ cells was dependent on γc cytokine signaling (Satoh-Takayama et al., 2008). As these cells express CD127, we assessed whether IL-7 was required for their homeostasis. Although the frequency of intestinal CD3−NKp46+ cells was unchanged in Il7−/− mice (Fig. 3, A and B), absolute numbers were slightly but not significantly increased (WT mice, 1.5 ± 0.2 × 105 cells; Il7−/− mice, 2.1 ± 0.9 × 105 cells; P = 0.24). We found that the CD127+NK1.1− subset of intestinal CD3−NKp46+ cells was strongly reduced in the absence of IL-7 compared with WT mice (Fig. 3, A and B). In contrast, the generation of intestinal CD3−NK1.1+ cells was IL-7 independent (in fact, intestinal CD127−NK1.1+ cells were significantly increased in Il7−/− mice), thereby distinguishing these cells from thymic NK cells (Vosshenrich et al., 2006). Intracellular staining revealed an absence of RORγt expression in residual CD3−NKp46+CD127+NK1.1− cells from IL-7–deficient mice (Fig. 3 C), demonstrating that IL-7 is required for normal development of this subset of Rorc+ intestinal cells. As such, NKp46+CD127+NK1.1− cells appear similar to fetal LTi cells in their strong IL-7 dependency (Meier et al., 2007; Schmutz et al., 2009). Lastly, we demonstrate that IL-23–induced IL-22 production by intestinal CD3−NKp46+ cells is abrogated in Il7−/− mice (Fig. 3 D). These observations identify IL-7 as an essential cytokine for IL-22–producing CD3−NKp46+Rorc+ cells in the gut. Moreover, they demonstrate that IL-7 and IL-15 have divergent roles in regulating the homeostasis of intestinal CD3−NKp46+ cell subsets via distinct precursors.
Figure 3.
Altered development of intestinal CD3−NKp46+ cells in Il7−/− mice. (A) NK1.1 versus CD127 expression on gated CD3−NKp46+ splenocytes and small intestinal (SI) LPLs of WT versus Il7−/− mice. Subset frequencies are indicated in one representative experiment out of six performed. (B) Absolute numbers of total CD3−NKp46+ cells and CD127/NK1.1 subsets from small intestinal LPLs in WT and Il7−/− mice (n = 6 mice of each genotype analyzed). Significant differences are indicated. Values are presented as means ± SD. (C) RORγt expression in the indicated intestinal CD3−NKp46+ subsets in WT (bold line) and Il7−/− mice (shaded histogram). The dotted line depicts staining of CD3−NKp46+ cells from Rorc−/− mice. (D) IL-22 production from intestinal CD3−NKp46+ subsets in WT and Il7−/− mice. Results indicate frequencies from one out of three independent experiments.
Relationships between intestinal CD3−NKp46+ cell subsets: insights from fate mapping of Rorc-expressing hematopoietic progenitors
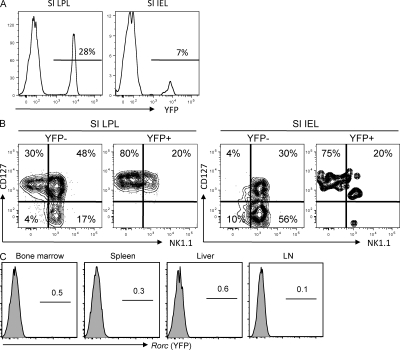
Although analysis of Rorc−/− mice showed that this transcription factor was not essential for normal NK cell development (Satoh-Takayama et al., 2008; Luci et al., 2009; Sanos et al., 2009), previous studies in both humans and mice demonstrated that lineage-negative populations of immature NK cells from diverse sources (lymph nodes, bone marrow, spleen, tonsils) did express Rorc and had NK cell precursor activity (Mebius et al., 1997; Chiossone et al., 2009; Cupedo et al., 2009; Hughes et al., 2009). As such, it remained possible that Rorc expression marked immature NK cells in the gut and elsewhere. To directly determine if Rorc+ cells in mice include precursors that can give rise to classical NK1.1+ cells, we performed a genetic cell fate–mapping experiment. Bacterial artificial chromosome Tg mice expressing the Cre recombinase under control of the Rorc(t) gene (Rorc(t)-CreTG mice; Lochner et al., 2008) were bred to mice bearing a loxP-flanked YFP reporter gene (Srinivas et al., 2001). In this context, YFP+ cells either express RORγt or have expressed RORγt at some previous stage in their developmental history. We confirmed previous results (Eberl and Littman, 2004) showing that all splenic T cells in these Tg mice express GFP (unpublished data), consistent with their development from Rorc+ double-positive thymocytes. We next examined intestinal CD3−NKp46+ cells in these mice. We found clear populations of YFP+NKp46+ cells in the intestinal lamina propria (51 ± 18%) and intraepithelial compartments (18 ± 12%) that were characterized by uniform CD127 expression and little or low NK1.1 expression (Fig. 4, A and B). This result is in accordance with our previous studies using Rorc-GFP mice that identified intestinal NKp46+ cell subsets (CD127+NK1.1−/lo) that constitutively express Rorc (Lochner et al., 2008; Satoh-Takayama et al., 2008). In contrast, intestinal cells with the mature NK cell phenotype (identified as CD3−NK1.1+CD127−) were not represented in the YFP+NKp46+ subset (Fig. 4 B). Concerning tissues outside of the intestine, we further characterized YFP expression in classical NK cells (defined as CD3−NKp46+NK1.1+CD127−) from the bone marrow, spleen, liver and lymph nodes (Fig. 4 C). Similar to that observed in the gut, frequencies of YFP+ cells among NK cells from these tissues were extremely low (<1%). These results are inconsistent with the hypothesis that Rorc+ cells are obligatory precursors to the NK1.1+ cells within the various tissues analyzed, and argue against a major pathway of classical NK cell development from Rorc+ precursors. Although we do not rule out the possibility that a small subset of NK1.1+ cells may be generated from Rorc+ precursors in vivo under steady-state conditions, any such cells are likely to be short-lived because they do not accumulate to appreciable levels. Collectively, the available data strongly suggest that intestinal CD3−NKp46+NK1.1− cells are a distinct lineage and not precursors to classical NK1.1+ NK cells.
Figure 4.
Fate-mapping analysis of Rorc-expressing precursors. (A) Frequency of YFP+ cells in gated CD3−NKp46+ cells from small intestinal (SI) LPLs and intraepithelial (IEL) cells. One representative experiment out of five is shown. Means of 51 ± 18% and 18 ± 12% YFP+ cells were detected in the LPL and intraepithelial compartments, respectively. (B) NK1.1 versus CD127 expression on YFP+ versus YFP− subsets of intestinal CD3−NKp46+ small intestinal LPL and intraepithelial cells. (C) Frequency of YFP+ cells in gated CD3−NK1.1+ cells from the indicated organs/tissues. Results indicate frequencies from one representative experiment out of five performed.
The recent discovery of CD3−NKp46+ cells in the intestinal mucosa that lack typical NK cell features (i.e., “natural” cytotoxicity) but express Rorc and IL-22 has raised questions about the biological roles and developmental origins of these unusual innate cells and their relationship (if any) to classical NK cells (Colonna, 2009; Vivier et al., 2009). Our results provide evidence for a lineage relationship between NCR-expressing, Rorc+, IL-22–producing (NCR22) cells, NK cells, and LTi cells, in that all of these cell subsets require the transcription factor Id2 for normal development. Still, our results clearly show that intestinal NK cells and NCR22 cells arise from independent developmental pathways that are driven by distinct cytokines (IL-7, IL-15). How IL-7 stimulates NCR22 cells (during development and/or as a peripheral survival factor) remains to be determined. Previous studies have considered Rorc expression as a potential marker for cells that harbor NK cell precursor activity (Chiossone et al., 2009; Hughes et al., 2009). In contrast, using an in vivo fate-mapping approach, we found that the vast majority of classical NK cells do not pass through a Rorc+ stage. It is likely that the NK cell precursor populations characterized in previous studies (Chiossone et al., 2009; Hughes et al., 2009) were heterogeneous mixtures of Rorc+ and Rorc− cells, with only the latter having the potential to differentiate into mature NK cells. This conclusion is consistent with the results presented in the paper by Crellin et al. in this issue. These authors found that lineage-negative RORC+ LTi-like cells in the human tonsil are precursors to CD56+NKp46+RORC+ cells that can produce IL-22 but lack conventional NK cell precursor activity. Their observations in the human system mirror our results demonstrating that mouse NCR22 cells expressing Rorc are not canonical NK cell precursors. NCR22 cells are also clearly different from the earliest immature NK cells that express NK1.1 (but not NKp46 or DX5) and are committed to the NK cell lineage (Rosmaraki et al., 2001; Vosshenrich et al., 2005; Walzer et al., 2007). These more precise phenotypic definitions may provide new access points to further dissect the signals that direct the specification of NK versus NCR22 innate cell lineages at the precursor stage.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from Janvier. Ncr1GFP/+ mice with the GFP reporter under the Ncr1 promoter elements (provided by O. Mandelboim, Hebrew University, Jerusalem, Israel; Gazit et al., 2006), as well as mice deficient in Id2 (provided by Y. Yokota, University of Fukui, Fukui, Japan; Yokota et al., 1999), IL-7 (von Freeden-Jeffry et al., 1995), or IL-15 (Kennedy et al., 2000), have been previously described. Mice with a human IL-15 transgene driven by the intestinal epithelial cell–specific T3b promoter (Ohta et al., 2002) were the gift of H. Kiyono (Institute of Medical Science, Tokyo, Japan) and were generously provided by N. Cerf-Bensussan (Institut National de la Santé et de la Recherche Médicale U793, Paris, France). For fate-mapping studies, mice harboring a recombinant bacterial artificial chromosome encoding RORγt-Cre (Lochner et al., 2008) were crossed with mice containing a loxP-flanked EYFP reporter in the ROSA locus (B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J; Srinivas et al., 2001). All mice were housed under specific pathogen-free conditions at the Laboratory Animal Facilities of the Institut Pasteur. Mice were analyzed at 6–12 wk of age. All animal experiments were approved by the Animal Care and Use Committee of the Institut Pasteur and were performed in accordance with French law.
Cell isolation and flow cytometric analysis.
Total splenocytes and small intestinal LPLs were prepared for flow cytometry as previously described (Satoh-Takayama et al., 2008). The cell-surface phenotype was analyzed using commercially available fluorochrome-conjugated mAbs (BD and eBioscience). Acquisitions were made using a flow cytometer (FACSCanto II; BD), interfaced to FACSDiva software (BD), and analyzed using FlowJo software (Tree Star, Inc.).
In vitro stimulation and intracellular cytokine staining.
Total LPL preparations diluted in RPMI 1640 with 10% FCS (106 cells/well) were left unstimulated or stimulated with 40 ng/ml IL-23 for 4 h in the presence of GolgiStop (BD). After surface staining and incubation with a fixable reagent (LIVE/DEAD; Invitrogen), cells were fixed in 4% paraformaldehyde and processed for intracellular cytokine detection. Cells were stained with allophycocyanin-conjugated anti–IL-22 mAb (MH22B2.2; provided by J.-C. Renauld, Ludwig Institut, Brussels, Belgium) in staining buffer (0.1% saponin in HBSS) before extensive washing in PBS. For RORγt detection, an anti–mouse/human RORγt-PE (clone AFKJS-9; eBioscience) was used. Nonspecific staining of the anti-RORγt was assessed using Rorc-deficient mice (Eberl et al., 2004).
Statistical analysis.
Values are presented as means ± SD. The statistical significance of differences between groups was determined by the unpaired Student’s t test. P < 0.05 was considered significant.
Online supplemental material.
Fig. S1 shows the minor effect of Id2 deficiency on T cell development in the lamina propria. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20092029/DC1.
Acknowledgments
We would like to thank O. Mandelboim, N. Cerf-Bensussan, H. Kiyono, and Y. Yokota for providing access to Tg mice, and J.-C. Renauld for IL-22 antibodies. N. Satoh-Takayama and J.P. Di Santo designed the research; N. Satoh-Takayama, S. Lesjean-Pottier, and C.A.J. Vosshenrich performed research and analyzed data; P. Vieira, S. Sawa, and G. Eberl provided new reagents and analytical tools; and N. Satoh-Takayama and J.P. Di Santo wrote the paper.
This work was supported by grants to J.P. Di Santo from the Institut Pasteur and the Institut National de la Santé et de la Recherche Médicale, and as an Equipe Labelisé by the Ligue Nationale Contre le Cancer. N. Satoh-Takayama was the recipient of fellowships from the Association Pasteur Japon and the Uehara Memorial Foundation, Japan.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- LPL
- lamina propria lymphocyte
- LTi
- lymphoid tissue inducer
- NCR
- natural cytotoxicity receptor
- Tg
- transgenic
References
- Boos M.D., Yokota Y., Eberl G., Kee B.L. 2007. Mature natural killer cell and lymphoid tissue–inducing cell development requires Id2-mediated suppression of E protein activity. J. Exp. Med. 204:1119–1130 10.1084/jem.20061959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Fuchs A., Vermi W., Facchetti F., Otero K., Lennerz J.K., Doherty J.M., Mills J.C., Colonna M. 2009. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 457:722–725 10.1038/nature07537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiossone L., Chaix J., Fuseri N., Roth C., Vivier E., Walzer T. 2009. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 113:5488–5496 10.1182/blood-2008-10-187179 [DOI] [PubMed] [Google Scholar]
- Colonna M. 2009. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 31:15–23 10.1016/j.immuni.2009.06.008 [DOI] [PubMed] [Google Scholar]
- Cooper M.A., Fehniger T.A., Turner S.C., Chen K.S., Ghaheri B.A., Ghayur T., Carson W.E., Caligiuri M.A. 2001. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 97:3146–3151 10.1182/blood.V97.10.3146 [DOI] [PubMed] [Google Scholar]
- Crellin N.K., Trifari S., Kaplan C.D., Cupedo T., Spits H. 2010. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional NK cells. J. Exp. Med. 207:■■■–■■■ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupedo T., Crellin N.K., Papazian N., Rombouts E.J., Weijer K., Grogan J.L., Fibbe W.E., Cornelissen J.J., Spits H. 2009. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat. Immunol. 10:66–74 10.1038/ni.1668 [DOI] [PubMed] [Google Scholar]
- Di Santo J.P., Vosshenrich C.A. 2006. Bone marrow versus thymic pathways of natural killer cell development. Immunol. Rev. 214:35–46 10.1111/j.1600-065X.2006.00461.x [DOI] [PubMed] [Google Scholar]
- Dubois S., Mariner J., Waldmann T.A., Tagaya Y. 2002. IL-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity. 17:537–547 10.1016/S1074-7613(02)00429-6 [DOI] [PubMed] [Google Scholar]
- Eberl G., Littman D.R. 2003. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer’s patches. Immunol. Rev. 195:81–90 10.1034/j.1600-065X.2003.00074.x [DOI] [PubMed] [Google Scholar]
- Eberl G., Littman D.R. 2004. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 305:248–251 10.1126/science.1096472 [DOI] [PubMed] [Google Scholar]
- Eberl G., Lochner M. 2009. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2:478–485 10.1038/mi.2009.114 [DOI] [PubMed] [Google Scholar]
- Eberl G., Marmon S., Sunshine M.J., Rennert P.D., Choi Y., Littman D.R. 2004. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5:64–73 10.1038/ni1022 [DOI] [PubMed] [Google Scholar]
- Freud A.G., Caligiuri M.A. 2006. Human natural killer cell development. Immunol. Rev. 214:56–72 10.1111/j.1600-065X.2006.00451.x [DOI] [PubMed] [Google Scholar]
- Freud A.G., Becknell B., Roychowdhury S., Mao H.C., Ferketich A.K., Nuovo G.J., Hughes T.L., Marburger T.B., Sung J., Baiocchi R.A., et al. 2005. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 22:295–304 10.1016/j.immuni.2005.01.013 [DOI] [PubMed] [Google Scholar]
- Gazit R., Gruda R., Elboim M., Arnon T.I., Katz G., Achdout H., Hanna J., Qimron U., Landau G., Greenbaum E., et al. 2006. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 7:517–523 10.1038/ni1322 [DOI] [PubMed] [Google Scholar]
- Hughes T., Becknell B., McClory S., Briercheck E., Freud A.G., Zhang X., Mao H., Nuovo G., Yu J., Caligiuri M.A. 2009. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood. 113:4008–4010 10.1182/blood-2008-12-192443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington N.D., Legrand N., Alves N.L., Jaron B., Weijer K., Plet A., Corcuff E., Mortier E., Jacques Y., Spits H., Di Santo J.P. 2009. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J. Exp. Med. 206:25–34 10.1084/jem.20082013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., Zhou L., Littman D.R. 2007. Transcriptional regulation of Th17 cell differentiation. Semin. Immunol. 19:409–417 10.1016/j.smim.2007.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M.K., Glaccum M., Brown S.N., Butz E.A., Viney J.L., Embers M., Matsuki N., Charrier K., Sedger L., Willis C.R., et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15–deficient mice. J. Exp. Med. 191:771–780 10.1084/jem.191.5.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.K., Takeuchi M., Yokota Y. 2004. Impairment of intestinal intraepithelial lymphocytes in Id2 deficient mice. Gut. 53:480–486 10.1136/gut.2003.022293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner M., Peduto L., Cherrier M., Sawa S., Langa F., Varona R., Riethmacher D., Si-Tahar M., Di Santo J.P., Eberl G. 2008. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells. J. Exp. Med. 205:1381–1393 10.1084/jem.20080034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luci C., Reynders A., Ivanov I.I., Cognet C., Chiche L., Chasson L., Hardwigsen J., Anguiano E., Banchereau J., Chaussabel D., et al. 2009. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 10:75–82 10.1038/ni.1681 [DOI] [PubMed] [Google Scholar]
- Luther S.A., Ansel K.M., Cyster J.G. 2003. Overlapping roles of CXCL13, interleukin 7 receptor α, and CCR7 ligands in lymph node development. J. Exp. Med. 197:1191–1198 10.1084/jem.20021294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius R.E. 2003. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3:292–303 10.1038/nri1054 [DOI] [PubMed] [Google Scholar]
- Mebius R.E., Rennert P., Weissman I.L. 1997. Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 7:493–504 10.1016/S1074-7613(00)80371-4 [DOI] [PubMed] [Google Scholar]
- Meier D., Bornmann C., Chappaz S., Schmutz S., Otten L.A., Ceredig R., Acha-Orbea H., Finke D. 2007. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 26:643–654 10.1016/j.immuni.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Moretta L., Moretta A. 2004. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 23:255–259 10.1038/sj.emboj.7600019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier E., Woo T., Advincula R., Gozalo S., Ma A. 2008. IL-15Rα chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med. 205:1213–1225 10.1084/jem.20071913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta N., Hiroi T., Kweon M.N., Kinoshita N., Jang M.H., Mashimo T., Miyazaki J., Kiyono H. 2002. IL-15-dependent activation-induced cell death-resistant Th1 type CD8 alpha beta+NK1.1+ T cells for the development of small intestinal inflammation. J. Immunol. 169:460–468 [DOI] [PubMed] [Google Scholar]
- Ranson T., Vosshenrich C.A., Corcuff E., Richard O., Müller W., Di Santo J.P. 2003. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 101:4887–4893 10.1182/blood-2002-11-3392 [DOI] [PubMed] [Google Scholar]
- Rosmaraki E.E., Douagi I., Roth C., Colucci F., Cumano A., Di Santo J.P. 2001. Identification of committed NK cell progenitors in adult murine bone marrow. Eur. J. Immunol. 31:1900–1909 [DOI] [PubMed] [Google Scholar]
- Sanos S.L., Bui V.L., Mortha A., Oberle K., Heners C., Johner C., Diefenbach A. 2009. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 10:83–91 10.1038/ni.1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N., Vosshenrich C.A., Lesjean-Pottier S., Sawa S., Lochner M., Rattis F., Mention J.J., Thiam K., Cerf-Bensussan N., Mandelboim O., et al. 2008. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 29:958–970 10.1016/j.immuni.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Schmutz S., Bosco N., Chappaz S., Boyman O., Acha-Orbea H., Ceredig R., Rolink A.G., Finke D. 2009. Cutting edge: IL-7 regulates the peripheral pool of adult ROR gamma+ lymphoid tissue inducer cells. J. Immunol. 183:2217–2221 10.4049/jimmunol.0802911 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1:4 10.1186/1471-213X-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Unutmaz D., Zou Y.R., Sunshine M.J., Pierani A., Brenner-Morton S., Mebius R.E., Littman D.R. 2000. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 288:2369–2373 10.1126/science.288.5475.2369 [DOI] [PubMed] [Google Scholar]
- Takatori H., Kanno Y., Watford W.T., Tato C.M., Weiss G., Ivanov I.I., Littman D.R., O’Shea J.J. 2009. Lymphoid tissue inducer–like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206:35–41 10.1084/jem.20072713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinotte L.L., Halim T.Y., Takei F. 2008. Unique subset of natural killer cells develops from progenitors in lymph node. Blood. 111:4201–4208 10.1182/blood-2007-04-087577 [DOI] [PubMed] [Google Scholar]
- Vivier E., Spits H., Cupedo T. 2009. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat. Rev. Immunol. 9:229–234 10.1038/nri2522 [DOI] [PubMed] [Google Scholar]
- von Freeden-Jeffry U., Vieira P., Lucian L.A., McNeil T., Burdach S.E., Murray R. 1995. Lymphopenia in interleukin (IL)-7 gene–deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519–1526 10.1084/jem.181.4.1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich C.A., Ranson T., Samson S.I., Corcuff E., Colucci F., Rosmaraki E.E., Di Santo J.P. 2005. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J. Immunol. 174:1213–1221 [DOI] [PubMed] [Google Scholar]
- Vosshenrich C.A., García-Ojeda M.E., Samson-Villéger S.I., Pasqualetto V., Enault L., Richard-Le Goff O., Corcuff E., Guy-Grand D., Rocha B., Cumano A., et al. 2006. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 7:1217–1224 10.1038/ni1395 [DOI] [PubMed] [Google Scholar]
- Walzer T., Bléry M., Chaix J., Fuseri N., Chasson L., Robbins S.H., Jaeger S., André P., Gauthier L., Daniel L., et al. 2007. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl. Acad. Sci. USA. 104:3384–3389 10.1073/pnas.0609692104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y., Mansouri A., Mori S., Sugawara S., Adachi S., Nishikawa S., Gruss P. 1999. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 397:702–706 10.1038/17812 [DOI] [PubMed] [Google Scholar]
- Yoshida H., Kawamoto H., Santee S.M., Hashi H., Honda K., Nishikawa S., Ware C.F., Katsura Y., Nishikawa S.I. 2001. Expression of alpha(4)beta(7) integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J. Immunol. 167:2511–2521 [DOI] [PubMed] [Google Scholar]