Abstract
Suppression of tumorigenicity was first shown in hybrids produced by the fusion of a range of different highly malignant tumor cells with diploid fibroblasts. Cytogenetic analysis of these hybrids revealed that suppression involved a genetic region located in one specific chromosome donated to the hybrid cell by the fibroblast parent. The identity of the gene responsible for this dramatic effect has remained obscure. We now present strong evidence that the primary determinant is the gene specifying collagen XV, a proteoglycan closely associated with the basement membrane. We transfected a line of highly tumorigenic human cervical carcinoma cells with an expression vector carrying the full-length cDNA of the human collagen XV gene. We selected clones making various amounts of collagen XV, examined their growth in vitro, and tested their tumorigenicity in nude mice. High levels of collagen XV altered the growth properties of the cells in three-dimensional cultures. Moreover, we found that, in a dose-dependent manner, the production of collagen XV completely suppressed tumorigenicity in clones that synthesized this molecule at high levels. Immunohistologic studies suggest that suppression is associated with extracellular deposition of the proteoglycan at the cell periphery.
Introduction
Cytogenetic analysis of hybrids formed by fusing diploid mouse fibroblasts with a range of different malignant mouse tumor cells revealed a locus in the region between band A4 and band C3 on normal mouse chromosome 4, which had the ability to suppress the tumorigenicity of the malignant tumor cells (1). Further studies showed that the activity of this locus was dependent on gene dosage (2). The hybrid cells in which tumorigenicity was suppressed exhibited the elongated morphology of a normal mature fibrocyte and were embedded in a dense collagenous matrix; malignant segregants derived from these hybrids showed no evidence of an organized collagenous matrix (3). Scrutiny of the physical map of the mouse genome (4) revealed that the gene encoding procollagen XVa1 was located within the suppressive region identified by cytogenetic analysis. This was the only known gene in that region that had any obvious connection with a collagenous extracellular matrix. It therefore seemed reasonable to suggest that this gene might be primarily responsible for the suppression of tumorigenicity in the hybrids (5). This suggestion is consistent with what is known about the structure and biological function of collagen XV. It is a nonfibrillary proteoglycan with chondroitin, dermatan, and heparan side chains, and in many tissues it forms an integral part of the collagenous network subjacent to the basement membrane (6). The hypothesis that collagen XV might suppress the growth of malignant tumors is reinforced by the observation that invasion of the basement membrane by human ductal breast carcinoma cells is preceded by the disappearance of this molecule (7) and that this is also true for skin carcinomas and melanomas (8).
One way to test this hypothesis would be to examine the effect of inhibiting the production of this molecule in nonmalignant cells that produce it; another would be to examine the effect of expressing collagen XV in tumor cells that do not. Given that the phenotypic consequences of a null mutation of the procollagen Col15a1 gene in the mouse were not found to be severe (9), it seemed unlikely that a diploid fibroblast would be rendered malignant by inhibition of this gene alone. We therefore chose the second approach. It has been shown, however, that a syntenic region of human chromosome 9 contains a suppressor of tumorigenicity (10) and also harbors the human procollagen COL15A1 gene. We therefore directly investigated the suppressive power of the human gene. Human cervical carcinoma cells, which do not normally express collagen XV, were transfected with an expression vector containing the human procollagen COL15a1 cDNA (hcolXV). Clones of transfected cells that made high and low amounts of collagen XV were evaluated for their growth in vitro in three dimensions and for their tumorigenicity in nude mice. The expression of recombinant human collagen XV altered the growth characteristics of the cells in vitro and at high levels completely suppressed tumorigenesis.
Results and Discussion
The cell line selected for these experiments was D98. This is a hypoxanthine-guanine-phosphoribosyl transferase–negative derivative of HeLa, an established human cervical carcinoma cell line. D98 had been used in previous experiments in which tumorigenicity was shown to be suppressed in hybrid cells formed by the fusion of D98 with normal diploid epithelial cells (11, 12). D98 AP2 is a clonal derivative of D98 that has been passed serially twice through nude mice. With inocula of 106 cells, D98 AP2 cells produce tumors in 100% of recipient animals with a mean latent period of 10 days.
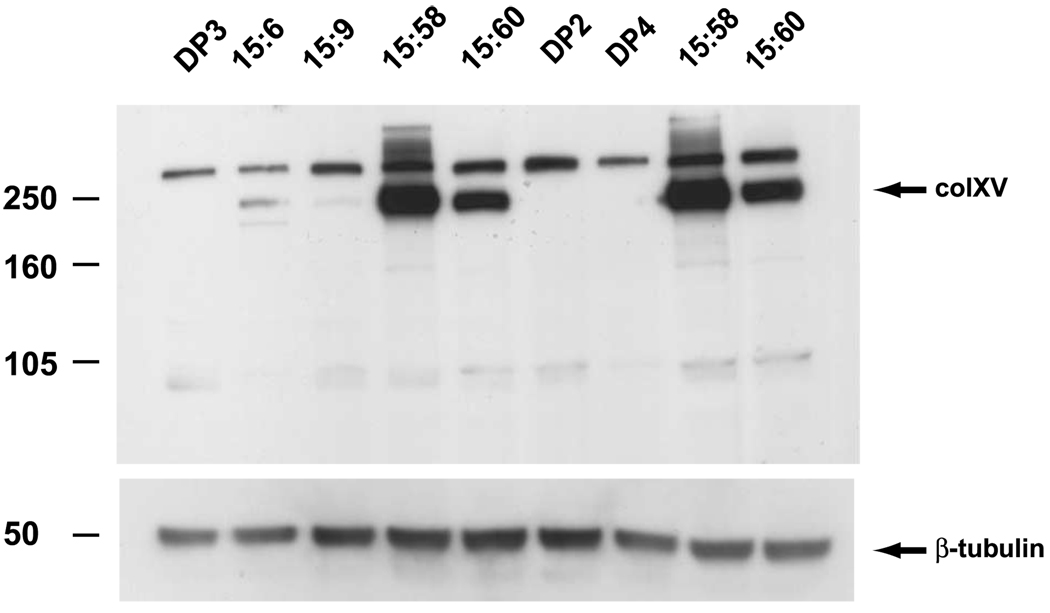
The full-length cDNA of the human collagen XV gene (hcolXV) in the mammalian expression vector pcDNA3 was transfected into D98 AP2 cells. Transfected clones were selected in zeocin. Twelve clones carrying hcolXV were evaluated for the expression of collagen XV by Western blot. Twelve clones of D98 AP2 cells transfected with the pcDNA expression vector alone served as negative controls. Four collagen-producing clones were chosen for further analysis based on their levels of collagen XV expression. Two expressed very high levels (clones 15:58 and 15:60) and two had lower levels (clones 15:6 and 15:9; Fig. 1). Three vector-only control clones were also analyzed (DP2, DP3, and DP4). The human type XV collagen protein migrates with a molecular weight of ~250 kDa (13) and the antibody to it cross-reacts with another unknown protein of higher molecular weight (which shows no correlation with collagen XV expression). This cross-reactant is also seen in the vector-only control clones and in the parental D98 AP2 line (not shown). The detection of two forms of type XV collagen in some cell types (e.g., clone 15:6) was previously reported at the time when the specificity of the antibody was established (13).
FIGURE 1.
Expression of type XV collagen in transfected cells. Western blot of whole-cell lysates from vector-only control clones DP2, DP3, and DP4 and hcolXV clones 15:6, 15:9, 15:58, and 15:60. Lysates were derived from the same passage number as that injected into mice (lanes 1–5, experiment 1; lanes 6–9, experiment 2). SDS-PAGE was carried out on 8% gels and proteins were transferred onto Immobilon P membrane and probed with a rabbit polyclonal antibody specific for the COOH terminus of human type XV collagen (top) or an antibody to β-tubulin, which served as a loading control.
Growth In vitro
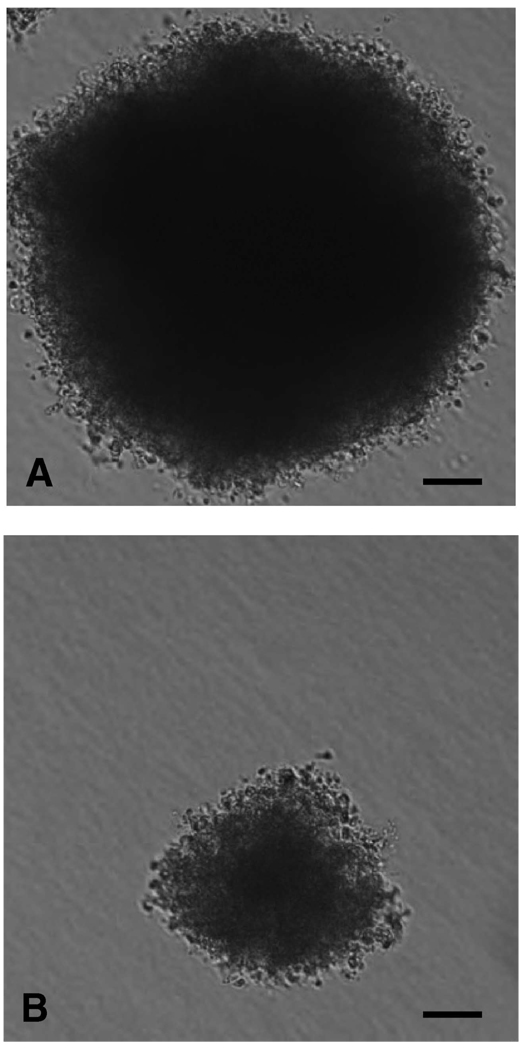
The growth in soft agar of clones expressing high levels of collagen XV and of control clones was monitored. Cells (2 × 103 and 2 × 104) were seeded into soft agar and plated in 160-mm dishes. Colony formation and morphology were evaluated after 28 days. Vector-only control clones (DP2 and DP3) formed small irregularly shaped colonies within the agar but the cells produced migrated out of the matrix onto the plastic substrate at a high frequency and then spread out to form circular monolayers. Clones 15:6 and 15:58 that produced collagen XV grew as compact, symmetrical spheres in soft agar, and few colonies released cells that migrated out onto the plastic substrate (Fig. 2). These contrasting clonal morphologies suggest that the expression of collagen XV influences the growth characteristics of the cells when grown in three dimensions, possibly by affecting the adherence of individual cells to each other or by creating an altered extracellular matrix that interacts with the tumor cells differently and thus affects their growth properties. The population doubling time of each clone was evaluated over 96 h under standard two-dimensional tissue culture conditions. No significant differences were observed between the D98 AP2 cell line, control clones carrying the vector alone, and clones expressing hcolXV (data not shown). Thus, expression of human type XV collagen does not perceptibly affect the rate of cell growth of the transfected D98 AP2 cells in two-dimensional culture.
FIGURE 2.
Growth of clones in soft agar. Cells (2 × 103 and 2 × 104) were seeded into soft agar and plated in 160-mm dishes. Representative colonies from collagen XV– producing clone 15:58 (A) and DP2 vector-only control (B). The size bar shows equivalent magnification in both images.
Growth In vivo
D98 AP2 cells grow vigorously in nude mice. In pilot experiments, we observed an incidence of tumor formation of >90%, with a mean latent period of ~10 days on injection of 106 cells. We therefore injected 1 × 106 cells subcutaneously between the scapulae of nude mice. Groups of 12 animals were used to test each clonal cell line. Mice were sacrificed once tumor diameter reached 1.5 cm, which was recorded as a terminal (death) event because we have never observed regression of tumors of this size. All the control clones containing the vector only were tumorigenic, but with an inoculum of 106 cells their take incidence was more variable than that of the D98 AP2 cells that had been repeatedly passaged through nude mice. The take incidence of these transfected control clones varied from ~50% to 100% at the time the experiment was terminated. This variability has previously been described in the unselected parental D98 line (12).
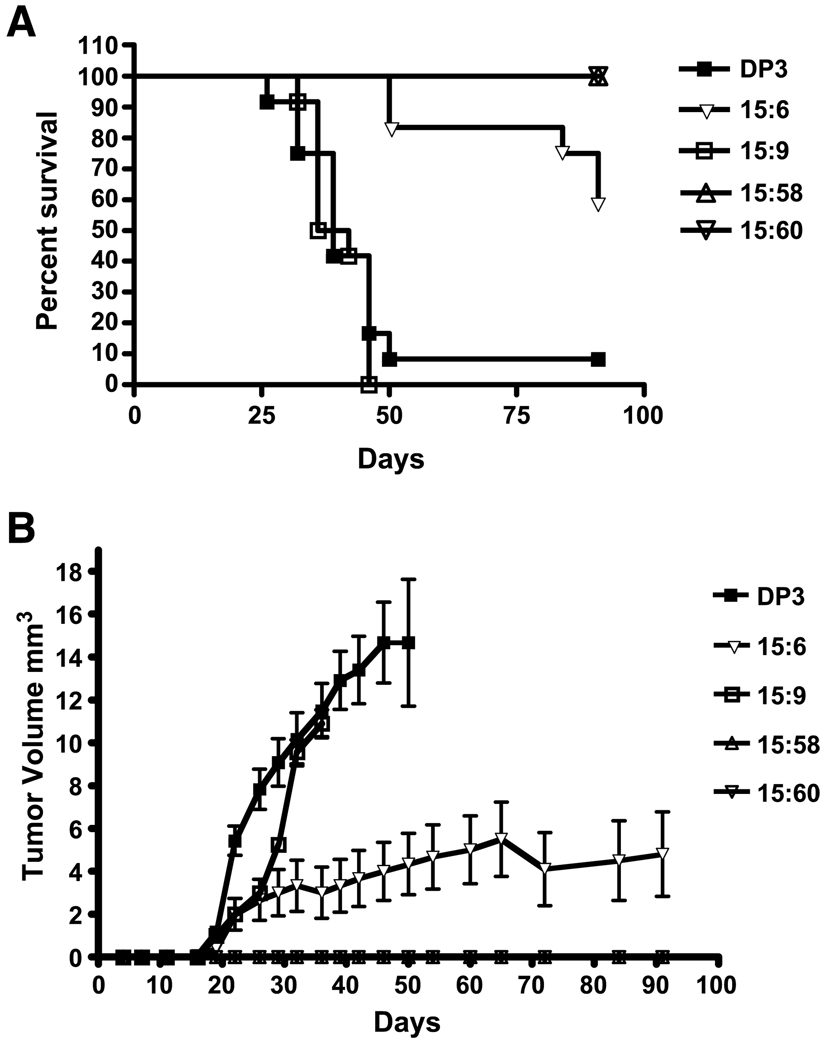
Two series of experiments were carried out and data were analyzed by the log-rank test. Kaplan-Meier plots of survival from one series of experiments are shown in Fig. 3A. Mice injected with the vector-only clone DP3 promptly produced tumors that rapidly grew to a diameter of 1.5 cm. All but one animal in the group were sacrificed by 50 days after inoculation of the cells. In the same experiment, mice injected with the 15:9 cell line that expresses only trace amounts of human type XV collagen, as estimated by Western blots, have a profile of tumor growth and survival statistically indistinguishable from the vector-only controls (log-rank test, P = 0.5704). All mice injected with this clone produced tumors of 1.5-cm diameter by 50 days and were sacrificed. Of the mice inoculated with the 15:6 clone, which has an intermediate level of type XV collagen expression, 60% survived and were tumor-free after 90 days. In contrast, no tumors appeared in the two groups of 12 mice inoculated with clones 15:58 and 15:60, which have the highest levels of type XV collagen. Note that in vitro, these two clones grow at the same rate as the other clones evaluated. The statistical differences (log-rank test) among these groups were DP3 versus 15:6, P = 0.0001; DP3 versus 15:58 (and 15:60), P < 0.0001; and 15:6 versus 15:58 (and 15:60), P = 0.0138.
FIGURE 3.
Expression of type XV collagen suppresses tumorigenicity of D98 AP2 cells in nude mice. Kaplan-Meier plots of survival of mice injected with vector-only control clones and clones expressing collagen XV (A). Inhibition of tumorigenicity correlates with levels of expression of collagen XV, with rapid tumor formation in the vector-only control clone (DP3), incomplete suppression in the clone expressing medium levels of collagen XV (15:6), and complete suppression of progressive growth in the clones expressing collagen XV at a high level (15:58 and 15:60). B. Growth curves for tumors arising in the experiment shown in A, illustrating tumor volume (mm2) at equivalent time points.
Figure 3B shows growth curves for the experimental series illustrated in Fig. 3A. The results show that clone 15:9, which expresses low levels of collagen XV, showed a slight delay in tumor growth rate initially, which increased after 25 days and approached the rate of growth of the control cells (DP3). Clone 15:6, which shows intermediate levels of expression of collagen XV, grew at a slower rate and did not show rapid progression. Clones 15:58 and 15:60 showed no tumor growth. These data support a hypothesis that expression of collagen XV in the highly tumorigenic D98 AP2 cell line inhibits tumor growth in a dose-dependent manner.
In a second experiment (data not shown), two additional vector-only control clones, DP2 and DP4, were evaluated for tumorigenicity in parallel with the 15:58 and 15:60 lines. With DP2, 11 of 12 mice produced tumors of >1.5-cm diameter in 50 days, at which point they were sacrificed. With DP4, 40% of the mice had produced tumors >1.5 cm by 110 days. Again, the two groups of 12 mice inoculated with the 15:58 and 15:60 cell lines showed 100% percent survival at 110 days. The statistical differences (log-rank test) among these groups were DP2 versus DP4, P = 0.0002; DP2 versus 15:58 (and 15:60), P < 0.0001; and DP4 versus 15:58 (and 15:60), P = 0.0136. Two of the mice in the 15:58 group developed very small nodules (<3 mm) at the site of injection after 55 days, but these did not grow progressively.
Histology of the Tumors
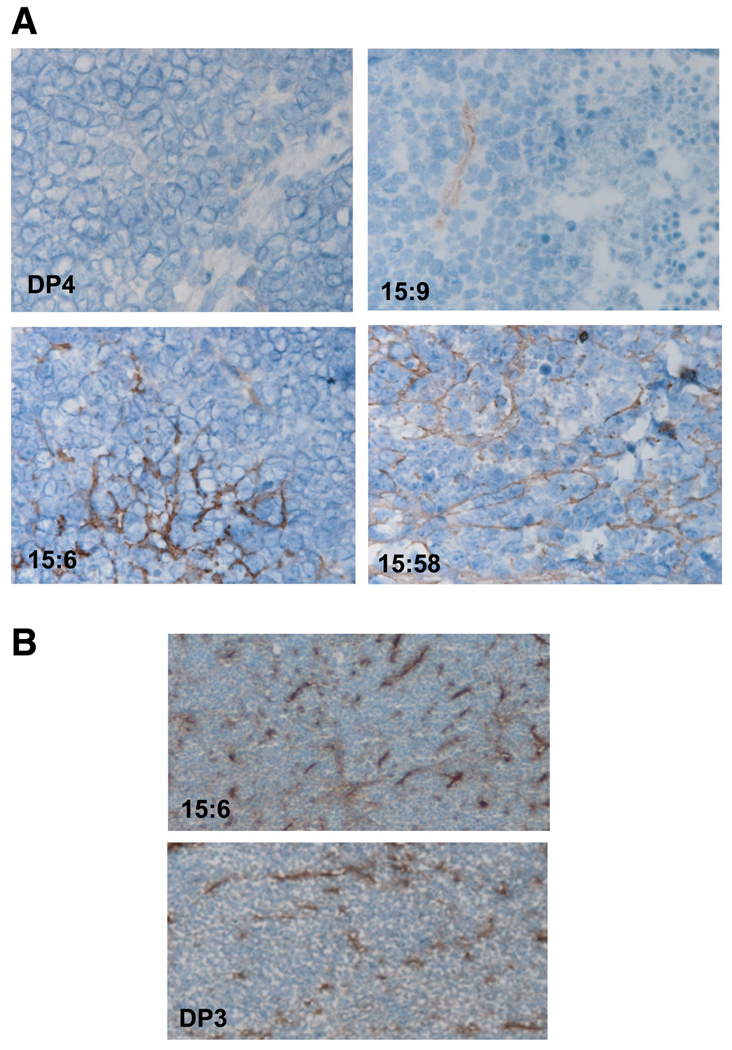
Sections of the tumors were examined for the expression of human type collagen XV by immunohistochemistry (Fig. 4A). As expected, the DP2, DP3, and DP4 lines showed no collagen XV but the tumors arising from the hcolXV-transfected clones showed two types of collagen XVexpression pattern. In the 15:9 clone expressing collagen XV at a very low level, there was a small focus of extracellular collagen XV surrounded by cells in the rest of the tumor that lacked the protein. In the 15:6 clone expressing somewhat more collagen XV, a larger focus of staining is again enclosed in a tumor mass that does not contain collagen XV. In contrast, in a nonprogressive nodule recovered from the 15:58 clone, which expressed collagen XV at a high level, an organized pattern of extracellular collagen XV is seen. Islands of tumor cells are enclosed within defined boundaries of collagen XV. This nodule failed to exceed 2mm in diameter even at 40 days after it became evident. These data are consistent with our hypothesis that collagen XV acts as a dose-dependent tumor suppressor. Thus, in the absence of extracellular collagen XV, the D98 AP2 cell line generates rapidly growing tumors in nude mice. Very low levels of collagen XV (clone 15:9) have no apparent effect on this whereas medium levels (clone 15:6) reduce the tumorigenicity of the cell line, and tumor growth in vivo is associated with loss of collagen XV expression. High levels of collagen XV (clones 15:58 and 15:60) extinguish progressive tumorigenicity, and in the minute nonprogressive nodule formed by 15.58, suppression of further growth is associated with organized extracellular deposition of secreted collagen XV.
FIGURE 4.
Distribution of type XV collagen and CD31 in tumors formed in nude mice. A. Type XV collagen. Sections of frozen tumors (20 µm) stained with the same antibody as that used in Fig. 1 for Western blots and a horseradish peroxidase – conjugated goat anti-rabbit secondary antibody. Sections were counterstained with hematoxylin. Tumors formed by vector-only control line DP4 and the 15:6, 15:9, and 15:58 hcolXV lines. The brown stain shows type XV collagen, although its intensity is not a quantitative measure of the amount of protein. B. CD31. Equivalent sections to those shown in A were stained with anti-CD31; they reveal no difference in the endothelial cell abundance or distribution in tumors generated from cell clones expressing or lacking collagen XV. The 15:6 hcolXV and DP3 control lines are shown and isotype controls (not shown) had only background levels of staining.
The mechanism whereby collagen XV inhibits tumor formation in vivo warrants further discussion because a number of properties of the molecule may be germane. That expression of collagen XV did not alter the proliferation of tumor cells in two dimensions but did affect the morphology of tumor cell growth in soft agar and suppressed tumor growth in a dose-dependent manner in vivo suggests that its effects are observed when cells are given the opportunity to grow in three dimensions. The COOH-terminal “restin” (related to endostatin) domain of collagen XV has been reported to have antiangiogenic properties that may inhibit tumor growth (14, 15). In fact, exogenous restin is not nearly as potent an inhibitor of angiogenesis as the equivalent COOH-terminal domain of collagen XVIII, endostatin. Although the two peptides show 60% identity (72% similarity), they exhibit important biological differences in vitro and in vivo. Restin and endostatin had similar inhibitory effects on cell migration that were specific to endothelial cells; however, unlike endostatin, restin did not inhibit the proliferation of endothelial cells in vitro. Furthermore, restin was not as effective as endostatin in suppressing tumor growth following i.p. injection in a renal cell carcinoma tumor xenograft model. The suppression of tumorigenicity seen in the present experiments does not seem to be caused by inhibition of angiogenesis. First, collagen XV affects the growth characteristics of the tumor cells in vitro. Second, immunohistochemical staining of endothelium with the anti-CD31 monoclonal antibody showed no difference between the densities of blood vessels in tumors that express collagen XV and those that do not (Fig. 4B). As a component of the extracellular matrix, collagen XVis thought to provide an extracellular scaffold, either alone or through interaction with other extracellular matrix components, creating novel structures that interface with the tumor cells or the surrounding stromal cells, or both. Such structures interacting directly with the tumor cells might entrain certain morphologic and functional modes of differentiation that serve to suppress tumorigenesis. These might include the formation of extracellular structural elements that permit cellular attachment and might at the same time activate morphogenetic signals that induce or influence the process of differentiation. These effects could be mediated by specific interactions between collagen XV and receptors on the surface of tumor cells. It is also possible, and not mutually exclusive, that in the animal, collagen XV interacts with surrounding stromal cells, which in turn contribute to the suppression of tumor growth.
Materials and Methods
Cell Culture and Transfection
D98 AP2 cells were routinely cultured in DMEM and 10% fetal bovine serum. Transfection was done with Lipofectin using standard protocols.
ColXV Expression Construct
The full-length human collagen XV cDNA (NM_001855) in pVL1392 (Stratagene) was kindly donated by Dr. Taina Pihlajaniemi (Department of Medical Biochemistry and Molecular Biology, University Oulu, Oulu, Finland). This clone contains the coding region, 76 bp of 5′ untranslated region, and 53 bp of 3′ untranslated region inserted into the SmaI site of the vector (16). The insert was transferred to pcDNA3neo as an XbaI-HindIII fragment at the NheI-HindIII sites, generating phcolXV.
Transfected Cell Clones
D98 AP2 clone B was used in these experiments (12). Stable clones of D98 AP2 carrying pcDNA3neo or pcolXV were generated by Lipofectin (Invitrogen) transfection followed by selection in 150 µg/mL zeocin.
Western Blots
Cells were lysed in NET buffer (pH 7.5), 1% Triton X-100 with protease inhibitor cocktail (Roche). SDS-PAGE was carried out on 8% gels and proteins transferred onto Immobilon P membrane (Millipore). Membranes were probed with a rabbit polyclonal antibody specific for the COOH terminus of human type XV collagen (13), which was kindly donated by Dr. Jeanne Myers (Department of Biochemistry and Biophysics, University of Pennsylvania School of Medicine, Philadelphia, PA). The secondary antibody was swine anti-rabbit IgG-horseradish peroxidase (DAKO) and membranes were processed by enhanced chemiluminescence (Amersham).
Subcutaneous Injections of Tumor Cells in Athymic Mice
One million cells of each indicated cell line were injected subcutaneously into congenitally athymic (nude) mice to analyze tumor growth.
Immunohistochemistry
Fresh-frozen sections of the tumors produced by the nude mouse xenografts were stained with a primary rabbit antibody raised against collagen XV or with a monoclonal antibody against the endothelial component CD31, following published techniques (17). Sections were counterstained with H&E.
Acknowledgments
We thank Timea Palmai-Pallag, Judy M. Anderson, and Thomas C. Caffrey for excellent technical assistance; Dr. Jeanne Myers for generously supplying the anti-ColXV antibody; and Dr. Taina Pihlajaniemi for the human colXV cDNA.
Grant support: National Cancer Institute grants U01CA111294 and CA36727, (M.A. Hollingsworth) and CA129258 (A. Harris).
Footnotes
Note Added in Proof
A novel fibrillary procollagen gene (colXXVII) has since been located within the suppressive region, but in the adult mouse its continued expression appears to be limited to cartilage (18) and does not, therefore, seem to be relevant to the present investigation.
References
- 1.Jonasson J, Povey S, Harris H. The analysis of malignancy by cell fusion. VII. Cytogenetic analysis of hybrids between malignant and diploid cells and of tumours derived from them. J Cell Sci. 1977;24:217–254. doi: 10.1242/jcs.24.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Evans EP, Burtenshaw MD, Brown BB, Hennion R, Harris H. The analysis of malignancy by cell fusion. IX. Re-examination and clarification of the cytogenetic problem. J Cell Sci. 1982;56:113–130. doi: 10.1242/jcs.56.1.113. [DOI] [PubMed] [Google Scholar]
- 3.Harris H. Suppression of malignancy in hybrid cells: the mechanism. J Cell Sci. 1985;79:83–94. doi: 10.1242/jcs.79.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Gregory SG, Sekhon M, Schein J, et al. A physical map of the mouse genome. Nature. 2002;418:743–750. doi: 10.1038/nature00957. [DOI] [PubMed] [Google Scholar]
- 5.Harris H. Is collagen XVa tumor suppressor? DNA Cell Biol. 2003;22:225–226. doi: 10.1089/104454903321908601. [DOI] [PubMed] [Google Scholar]
- 6.Amenta PS, Scivoletti NA, Newman MD, Sciancalepore JP, Li D, Myers JC. Proteoglycan-collagen XV in human tissues is seen linking banded collagen fibers subjacent to the basement membrane. J Histochem Cytochem. 2005;53:165–176. doi: 10.1369/jhc.4A6376.2005. [DOI] [PubMed] [Google Scholar]
- 7.Amenta PS, Hadad S, Lee MT, Barnard N, Li D, Myers JC. Loss of types XV and XIX collagen precedes basement membrane invasion in ductal carcinoma of the female breast. J Pathol. 2003;199:298–308. doi: 10.1002/path.1303. [DOI] [PubMed] [Google Scholar]
- 8.Fukushige T, Kanekura T, Ohuchi E, Shinya T, Kanzaki T. Immunohistochemical studies comparing the localization of type XV collagen in normal human skin and skin tumors with that of type IV collagen. J Dermatol. 2005;32:74–83. [PubMed] [Google Scholar]
- 9.Eklund L, Piuhola J, Komulainen J, et al. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc Natl Acad Sci U S A. 2001;98:1194–1199. doi: 10.1073/pnas.031444798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eklund LK, Islam K, Soderkvist P, Islam MQ. Regional mapping of suppressor loci for anchorage independence and tumorigenicity on human chromosome 9. Cancer Genet Cytogenet. 2001;130:118–126. doi: 10.1016/s0165-4608(01)00471-x. [DOI] [PubMed] [Google Scholar]
- 11.Peehl DM, Stanbridge EJ. Characterization of human keratinocyte X HeLa somatic cell hybrids. Int J Cancer. 1981;27:625–635. doi: 10.1002/ijc.2910270509. [DOI] [PubMed] [Google Scholar]
- 12.Harris H, Bramwell ME. The suppression of malignancy by terminal differentiation: evidence from hybrids between tumour cells and keratinocytes. J Cell Sci. 1987;87:383–388. doi: 10.1242/jcs.87.3.383. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Clark CC, Myers JC. Basement membrane zone type XV collagen is a disulfide-bonded chondroitin sulfate proteoglycan in human tissues and cultured cells. J Biol Chem. 2000;275:22339–22347. doi: 10.1074/jbc.M000519200. [DOI] [PubMed] [Google Scholar]
- 14.Ramchandran R, Dhanabal M, Volk R, et al. Antiangiogenic activity of restin, NC10 domain of human collagen XV: comparison to endostatin. Biochem Biophys Res Commun. 1999;255:735–739. doi: 10.1006/bbrc.1999.0248. [DOI] [PubMed] [Google Scholar]
- 15.John H, Radtke K, Standker L, Forssmann WG. Identification and characterization of novel endogenous proteolytic forms of the human angiogenesis inhibitors restin and endostatin. Biochim Biophys Acta. 2005;1747:161–170. doi: 10.1016/j.bbapap.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Hagg PM, Hagg PO, Peltonen S, Autio-Harmainen H, Pihlajaniemi T. Location of type XV collagen in human tissues and its accumulation in the interstitial matrix of the fibrotic kidney. Am J Pathol. 1997;150:2075–2086. [PMC free article] [PubMed] [Google Scholar]
- 17.Myers JC, Dion AS, Abraham V, Amenta PS. Type XV collagen exhibits a widespread distribution in human tissues but a distinct localization in basement membrane zones. Cell Tissue Res. 1996;286:493–505. doi: 10.1007/s004410050719. [DOI] [PubMed] [Google Scholar]
- 18.Plumb DA, Dhir V, Mironov A, et al. Collagen XXVII is developmentally regulated and forms thin fibrillar structures distinct from those of classical vertebrate fibrillar collagens. J Biol Chem. 2007;282:12791–12795. doi: 10.1074/jbc.C700021200. [DOI] [PMC free article] [PubMed] [Google Scholar]