Abstract
The mutation of the spatacsin gene is the single most common cause of autosomal recessive hereditary spastic paraplegia with thin corpus callosum. Common clinical, pathological and genetic features between amyotrophic lateral sclerosis and hereditary spastic paraplegia motivated us to investigate 25 families with autosomal recessive juvenile amyotrophic lateral sclerosis and long-term survival for mutations in the spatascin gene. The inclusion criterion was a diagnosis of clinically definite amyotrophic lateral sclerosis according to the revised El Escorial criteria. The exclusion criterion was a diagnosis of hereditary spastic paraplegia with thin corpus callosum in line with an established protocol. Additional pathological and genetic evaluations were also performed. Surprisingly, 12 sequence alterations in the spatacsin gene (one of which is novel, IVS30 + 1 G > A) were identified in 10 unrelated pedigrees with autosomal recessive juvenile amyotrophic lateral sclerosis and long-term survival. The countries of origin of these families were Italy, Brazil, Canada, Japan and Turkey. The variants seemed to be pathogenic since they co-segregated with the disease in all pedigrees, were absent in controls and were associated with amyotrophic lateral sclerosis neuropathology in one member of one of these families for whom central nervous system tissue was available. Our study indicates that mutations in the spatascin gene could cause a much wider spectrum of clinical features than previously recognized, including autosomal recessive juvenile amyotrophic lateral sclerosis.
Keywords: amyotrophic lateral sclerosis, hereditary spastic paraplegia, mutations, spatacsin
Introduction
Autosomal recessive hereditary spastic paraplegia with thin corpus callosum is clinically characterized by the progressive spasticity of the lower limbs, thin corpus callosum and cognitive impairment. White matter alterations may be part of the clinical phenotype. Disease onset presents itself during childhood and usually before the second decade of life (Winner et al., 2004). Mutations of the spatacsin gene on chromosome 15q15-21 (SPG11) represent the most common cause of autosomal recessive hereditary spastic paraplegia with thin corpus callosum (Stevanin et al., 2007, 2008). Autosomal recessive juvenile amyotrophic lateral sclerosis (ARJALS) is a rare disease that occurs before the age of 25 years. It is characterized by the spasticity of limb and facial muscles with distal amyotrophy of hands and feet. It follows a slowly progressive course, with cases of prolonged survival of more than three decades (Ben Hamida et al., 1990).
The overlap between hereditary spastic paraplegia and amyotrophic lateral sclerosis (ALS) (Fink, 2001; Meyer et al., 2005; Strong and Gordon, 2005) prompted us to screen unrelated families with ARJALS for mutations in SPG11.
Materials and methods
Patients
This study was performed according to a protocol reviewed and approved by the Ethics Committee of the Istituto di Ricovero e Cura a Carattere Scientifico Santa Lucia, Rome, Italy. Informed consent was obtained from all individuals. Patients were recruited by contacting neurologists in Italy, Brazil, Canada, Japan and Turkey with a special interest in motoneuron diseases and asking them to refer suitable affected subjects.
The study was based on 25 unrelated pedigrees with ARJALS. The diagnosis of clinically definite ALS was made using the revised El Escorial criteria (Brooks et al., 2000). None of the patients had autosomal recessive hereditary spastic paraplegia with thin corpus callosum in accordance with an established protocol (Boukhris et al., 2008). The autopsy confirmed the diagnosis in one patient. Intellectual quotient or Mini Mental State Examination (Folstein et al., 1975) was available for all affected subjects. Mental retardation, as defined under DSM-IV criteria (Diagnostic and Statistical Manual of Mental Disorders, fourth edition), was taken into consideration when the patient had an intellectual quotient <70 before the age of 18 years. A brain MRI was performed in all affected individuals.
Nine hundred control chromosomes were obtained from healthy volunteers of mixed ethnic origins, including 300 Caucasian, 200 Brazilian, 200 Japanese and 200 Turkish chromosomes.
Pathological studies
The post-mortem standard neuropathological evaluation consisted of histological staining of paraffin-embedded material with haematoxylin and eosin, Nïssl, Klüver-Barrera and periodic acid Schiff.
Genetic analyses
Genomic DNA from peripheral blood was extracted by using the Promega wizard genomic DNA isolation kit (http://www.promega.com).
We carried out a linkage study of all 25 families with ARJALS using three highly polymorphic genetic markers flanking the SPG11 gene locus on chromosome 15q15-21 (D15S146, D15S537 and D15S123). These are informative microsatellite markers used in previous studies (Hentati et al., 1998; Stevanin et al., 2007). The two-point logarithm of odds scores were calculated according to a genetic model based on clinical information as previously described (Orlacchio et al., 2002, 2004). No phenocopies were allowed. The primer sequences for each microsatellite marker, as well as marker allele sizes and frequencies, were found on http://www.gdb.org and http://www.cephb.fr.
The 40 coding exons of SPG11 and at least 50 base pairs of flanking intronic sequence were PCR-amplified by previously described primer pairs (Stevanin et al., 2007) and by Roche FastStart PCR Master Mix polymerase (http://www.roche-applied-science.com). All the PCR-amplified products were purified using a Qiagen PCR purification kit (http://www.qiagen.com). These purified products were sequenced with respective forward and reverse primers by a 3130 Genetic Analyser (http://www.appliedbiosystems.com). The segregation analysis and the observation of mutations in the control population were performed using a PCR-restriction fragment length polymorphism method.
The mutation analysis of ALS2/ALSIN, ALS4/SETX, ALS11/FIG4 and SPG4/SPASTIN was performed by directly sequencing all exons and their flanking introns (full details are available on request).
Results
Clinical findings of the SPG11 families
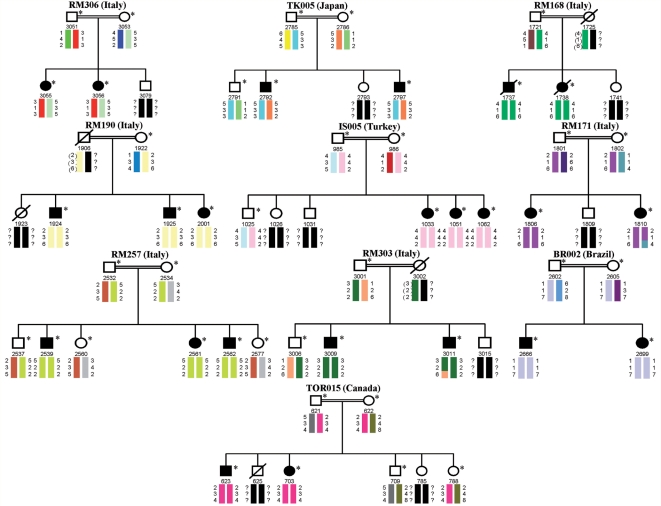
All 10 SPG11 pedigrees showed consanguinity marriage (first-degree cousins) in their parents. Six families were from Italy and one each from Brazil, Canada, Japan and Turkey (Fig. 1).
Figure 1.
Ten ARJALS families linked to the SPG11 locus. Their origin is reported in brackets. The solid symbols refer to affected individuals as well as carriers of the SPG11 mutations; circles = females; squares = males and slashes = deceased. The code numbers of all sampled individuals (asterisks) are reported below the symbols. Colour barcodes designate haplotypes in each pedigree. Haplotypes created with markers D15S146, D15S537 and D15S123 (from top to bottom) are reported under each family member investigated. The SPG11 gene is localized between markers D15S537 and D15S123. Inferred alleles are indicated by parentheses and question marks represent alleles not able to be inferred. Key recombination events are observed between markers D15S537 and D15S123 in Patient 1810 (Family RM171) as well as among markers D15S537 and D15S123 in Patient 3011 (Family RM303).
Affected subjects have a slowly progressive motor neuronopathy with upper and lower motoneuron dysfunction. There is principally distal muscle weakness and atrophy associated with pyramidal signs, including hyperreflexia, extensor plantar responses and a spastic gait. The age at onset in 23 SPG11 patients ranged from 7 to 23 with a mean of 16.3 years. The mean age at examination was 50.6 years (range 38–61) and the mean disease duration was 34.3 years (range 27–40). A wide variation of phenotypic expression was detected. Some patients were affected by mild upper and lower motoneuron symptoms, whilst there were others who might be wheelchair-bound with no functional hand use by the fifth or sixth decade of life. Pontobulbar signs, including jaw spasticity, increased facial reflexes, poor palatal elevation, weak masseter and/or pterygoids, tongue weakness, as well as muscle atrophy with fasciculations, were present in the majority of the affected individuals (Subjects 3056, Family RM306; 2792 and 2797, TK005; 1737 and 1738, RM168; 1924 and 2001, RM190; 1806, RM171; 2539 and 2562, RM257; 3009 and 3011, RM303; 2666 and 2699, BR002 and 623, TOR015). The site of disease onset was evenly split between pontobulbar (n = 12: Subjects 3056, Family RM306; 2792 and 2797, TK005; 1738, RM168; 1924 and 2001, RM190; 1806, RM171; 2539, RM257; 3009 and 3011, RM303 as well as 2666 and 2699, BR002) and limb, hand or leg (n = 11: Subjects 3055, Family RM306; 1737, RM168; 1925, RM190; 1033, 1051, and 1062, IS005; 1810, RM171; 2561 and 2562, RM257 as well as 623 and 703, TOR015). The Hoffmann sign was always present, unilaterally or bilaterally. No cognitive deficits on the Mini Mental State Examination or mental health problems (intellectual quotient >70 in all affected subjects) were observed. Brain MRI revealed the absence of thin corpus callosum and white matter alterations in all patients. Patient 1738, of pedigree RM 168, showed an intracranial arachnoid cyst in the left frontal pole. Lysosomal enzyme assay of peripheral blood leucocytes revealed that both β-hexosaminidase A and B isoenzymes were present at a normal level in one patient within each family (Subjects 3055, Family RM306; 2797, TK005; 1737, RM168; 2001, RM190; 1051, IS005; 1806, RM171; 2561, RM257; 3011, RM303; 2666, BR002 and 703, TOR015). Also, further haematological and biochemical profiles, including serum creatinine phosphokinase, were unremarkable in all subjects. Sensory symptoms were absent in all patients. No severe bladder dysfunction was found, although minor urinary dysfunctions, such as stress and urge incontinence, occurred in one individual (Subject 2792, Family TK005). The examination of the ocular fundus was normal in all affected subjects. Patients 1737 and 1738 from Family RM168 died of acute respiratory failure.
Concentric needle EMG was performed in all affected individuals and showed chronic neurogenic changes with positive sharp waves, fibrillation and fasciculation potentials. The topography of EMG signs of lower motoneuron dysfunction followed the revised El Escorial criteria (Brooks et al., 2000). The motor conduction studies revealed reduced amplitudes of compound motor action potentials, whereas the sensory conduction studies demonstrated normal amplitudes of sensory nerve-action potentials. The motor and sensory nerve conduction velocities were also normal (Table 1).
Table 1.
Clinical characteristics of 23 patients with mutations in the SPG11 gene
| Patient (sex) | Age at onset (years) | Age at examination (years) | Disease duration (years) | El Escorial category | Amyotrophy/ weakness | Pyramidal signs/ disability | Pontobulbar signs/ fasciculations | Denervation on EMG | TCC/ WMA | CD/MI | Other clinical features |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RM306-3055 (F) | 15 | 54 | 39 | Definite | Severe/moderate | Yes/3 | No/no | Yes | No/no | No/no | None |
| RM306-3056 (F) | 9 | 47 | 38 | Definite | Severe/severe | Yes/4 | Yes/yes | Yes | No/no | No/no | None |
| TK005-2792 (M) | 21 | 58 | 37 | Definite | Moderate/mild | Yes/1 | Yes/yes | Yes | No/no | No/no | UI |
| TK005-2797 (M) | 16 | 50 | 34 | Definite | Severe/moderate | Yes/3 | Yes/yes | Yes | No/no | No/no | None |
| RM168-1737 (M) | 19 | 52 | 33 | Definite | Severe/severe | Yes/5 | Yes/yes | Yes | No/no | No/no | ARF |
| RM168-1738 (F) | 7 | 38 | 31 | Definite | Severe/severe | Yes/5 | Yes/yes | Yes | No/no | No/no | ARF, IAC |
| RM190-1924 (M) | 23 | 61 | 38 | Definite | Moderate/moderate | Yes/2 | Yes/yes | Yes | No/no | No/no | None |
| RM190-1925 (M) | 20 | 59 | 39 | Definite | Moderate/moderate | Yes/2 | No/no | Yes | No/no | No/no | None |
| RM190-2001 (F) | 23 | 50 | 27 | Definite | Severe/moderate | Yes/3 | Yes/yes | Yes | No/no | No/no | None |
| IS005-1033 (F) | 20 | 55 | 35 | Definite | Moderate/moderate | Yes/2 | No/no | Yes | No/no | No/no | None |
| IS005-1051 (F) | 16 | 44 | 28 | Definite | Severe/mild | Yes/2 | No/no | Yes | No/no | No/no | None |
| IS005-1062 (F) | 10 | 42 | 32 | Definite | Severe/mild | Yes/3 | No/no | Yes | No/no | No/no | None |
| RM171-1806 (F) | 21 | 48 | 27 | Definite | Severe/severe | Yes/4 | Yes/yes | Yes | No/no | No/no | None |
| RM171-1810 (F) | 15 | 53 | 38 | Definite | Severe/severe | Yes/5 | No/no | Yes | No/no | No/no | None |
| RM257-2539 (M) | 20 | 55 | 35 | Definite | Severe/moderate | Yes/3 | Yes/yes | Yes | No/no | No/no | None |
| RM257-2561 (F) | 22 | 53 | 31 | Definite | Severe/moderate | Yes/3 | No/no | Yes | No/no | No/no | None |
| RM257-2562 (M) | 18 | 49 | 31 | Definite | Severe/severe | Yes/5 | Yes/yes | Yes | No/no | No/no | None |
| RM303-3009 (M) | 18 | 53 | 35 | Definite | Moderate/moderate | Yes/2 | Yes/yes | Yes | No/no | No/no | None |
| RM303-3011 (M) | 14 | 45 | 31 | Definite | Moderate/mild | Yes/1 | Yes/yes | Yes | No/no | No/no | None |
| BR002-2666 (M) | 12 | 52 | 40 | Definite | Moderate/mild | Yes/2 | Yes/yes | Yes | No/no | No/no | None |
| BR002-2699 (F) | 8 | 44 | 36 | Definite | Severe/severe | Yes/4 | Yes/yes | Yes | No/no | No/no | None |
| TOR015-623 (M) | 15 | 54 | 39 | Definite | Moderate/moderate | Yes/2 | Yes/yes | Yes | No/no | No/no | None |
| TOR015-703 (F) | 12 | 47 | 35 | Definite | Moderate/moderate | Yes/3 | No/no | Yes | No/no | No/no | None |
ARF = acute respiratory failure; CD = cognitive decline; F = female; IAC = intracranial arachnoid cyst; M = male; MI = mental impairment; TCC = thin corpus callosum; UI = urinary incontinence; WMA = white matter alterations. Age at onset was calculated approximately as the time when the first symptoms appeared. Disease duration was calculated by subtracting age at onset of symptoms from age at examination. Disability stages are the following: 1, no mobility problems or slight stiffness of the legs; 2, moderate gait stiffness; 3, problems in running, but ability to walk alone; 4, problems in walking; 5, wheelchair-bound.
In general, these clinical characteristics are comparable with those of previously published studies (Group 1 of the juvenile amyotrophic lateral sclerosis families described by Ben Hamida et al., 1990; ALS5 pedigrees reported by Hentati et al., 1998).
Case reports
Patient 1737 (Family RM 168)
Subject 1737 of pedigree RM168 (Fig. 1) was a 52-year-old Italian man, who first noticed muscle weakness in his left hand at the age of 19. Afterwards, he developed right hand and bilateral leg weakness, which was followed by hoarseness. One year later he started to experience dysarthria and dysphagia together with spasticity of the lower limbs, leading to the diagnosis of ALS. At the age of 27 he suffered from marked atrophy and flaccid paresis of the upper limbs, as well as unsteady gait. In addition, neurological evaluation at that time revealed a bilateral Hoffman's; sign. At the age of 34, muscle weakness and atrophy of the extremities progressed to the point where the patient became unable to sit by himself and required a walking aid. At the age of 45 he developed tongue atrophy and weakness, with visible fasciculations in the atrophic muscles. At the age of 46 he suffered from complete loss of upper limb motor function, became wheelchair-bound and his dysarthria worsened. At the age of 51 he developed breathing difficulties and the difficulty in swallowing worsened. At the age of 52 he died of sudden respiratory arrest caused by suffocation.
The total duration of the disease was 33 years. No bladder dysfunction, sensory disturbance, cognitive decline, mental impairment, ocular fundus defects, abnormal levels of β-hexosaminidase A and B isoenzymes or serum creatinine phosphokinase alterations were detected throughout the clinical course. Brain MRI performed at the age of 48 disclosed no abnormal findings. The electrodiagnostic studies showed the occurrence of a motoneuron disorder by high-amplitude polyphasic motor unit potentials, fibrillation and fasciculation potentials, incomplete interference pattern, in addition to reduced amplitudes of compound motor action potentials and normal amplitudes of sensory nerve-action potentials. The motor and sensory nerve conduction velocities were also normal. The patient fulfilled the El Escorial criteria for definite ALS (Brooks et al., 2000). The CNS at post-mortem showed neuropathological changes consistent with a diagnosis of ALS.
A family history of ALS was detected with another family member (1738) suffering from the same illness. Consanguinity of the parents was reported (Fig. 1).
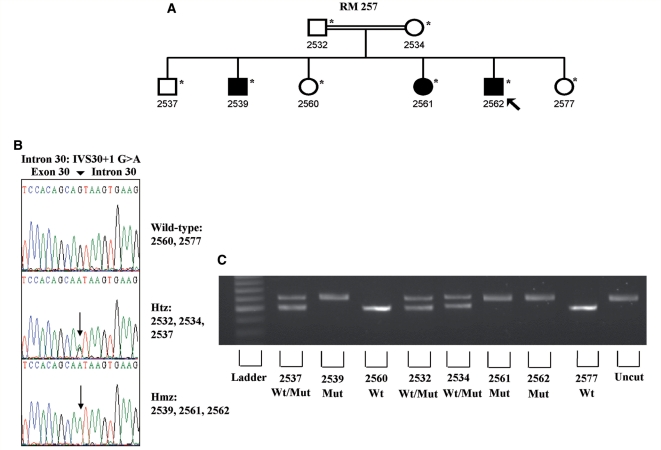
Patient 2562 (Family RM 257)
Subject 2562 of pedigree RM 257 (Figs 1 and 2) was a 49-year-old Italian man; at the age of 18, he developed a slowly progressive lower and upper motoneuron syndrome. The disease commenced with wasting and weakness of upper limbs, followed by wasting and weakness of the lower extremities. The patient had also suffered from a spastic gait since the age of 33. He became wheelchair-bound and, on neurological examination, revealed severe bilateral wasting of triceps, biceps, intrinsic hand, tibialis anterior and posterior, soleus and extensor digitorum longus muscles. Fasciculations were observed in the atrophic muscles and poor palatal elevation was also reported. Hoffman's; sign was present bilaterally. No bladder dysfunction or sensory disturbances were found. Higher mental functions, cranial nerves and ocular fundus examination were normal. Brain MRI showed normal brain images. Haematological and biochemical profiles, including serum creatinine phosphokinase, were unremarkable. The electrophysiological studies revealed normal motor and sensory nerve conduction velocities along with reduced amplitudes of compound motor action potentials and normal amplitudes of sensory nerve-action potentials. They also disclosed high-amplitude polyphasic motor unit potentials, fibrillation and fasciculation potentials, as well as incomplete interference pattern, suggesting an active muscle denervation–reinnervation process. The patient satisfied the El Escorial criteria for definite ALS (Brooks et al., 2000).
Figure 2.
(A) Family RM 257. Black symbols designate affected individuals and carriers of SPG11 mutation. White symbols symbolize unaffected subjects. Circles indicate females and squares represent males. The proband is designated with an arrow. The numbers are an internal reference for each subject. Asterisks indicate sampled individuals. (B) Electropherogram of exon/intron 30 of the SPG11 gene. The new homozygous substitution (G > A) is clearly defined in the affected individuals (Subjects 2539, 2561 and 2562) as well as the heterozygous status in the parents (Subjects 2532 and 2534) and in a sibling (Subject 2537). (C) Restriction digestion assay in the family members. Heterozygous individuals showed two bands at 600 and 500 base pairs, wild-type samples showed one band at 500 base pairs and the homozygous affected individuals remain uncleaved after digestion with EcoP15I.
Two other family members (2539 and 2561) suffered from the same illness and the consanguinity of the parents was reported (Figs 1 and 2).
Pathological assessments
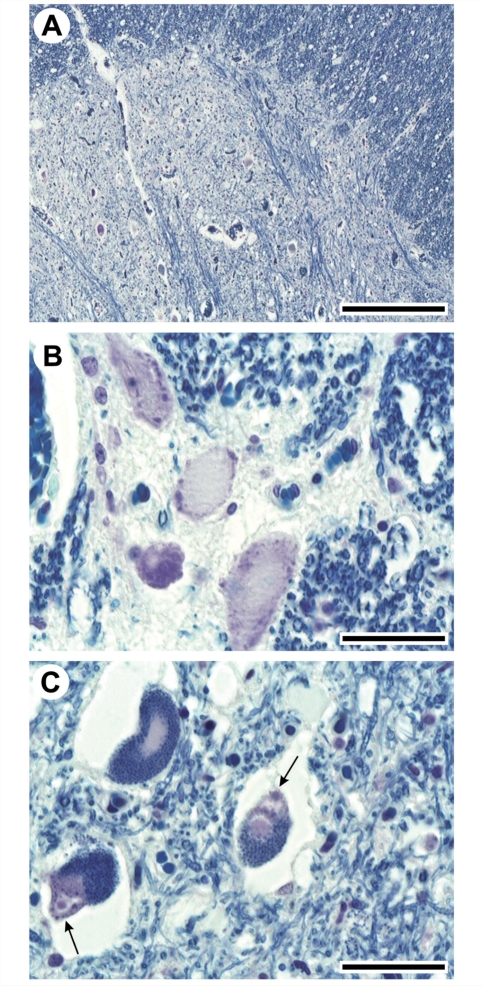
A post-mortem examination of the brain and spinal cord was performed in Patient 1737 (aged 52 years at death) from Family RM168. The brain showed no cerebral or cerebellar atrophy, and a normal corpus callosum as well as the absence of white matter alterations were found. The bilateral pyramids were small in size. The microscopic examination documented Betz cell loss in the primary cortex, whereas the cerebellum did not exhibit abnormalities. The hippocampus, parahippocampal gyrus, amygdala, nucleus basalis of Meynert, caudate nucleus, putamen, pallidum, thalamus, subthalamic nucleus appeared intact. Neuronal loss with astrocytosis was found in the glossopharyngeal and hypoglossal nuclei. The reticular formation, red nucleus, substantia nigra, locus ceruleus, oculomotor, trochlear and abducens nuclei, motor nucleus of the facial, dorsal motor nucleus of the vagus, inferior olive, in addition to other nuclei of the brainstem appeared preserved. Atrophy of the anterior roots and shrinkage of the anterior horns of spinal cord prominently in the cervical cord, as well as myelin pallor in the antero-lateral columns, were also observed. Moreover, microscopic analysis showed marked loss of anterior horn large motoneurons at all spinal cord levels, associated with loss of large myelinated fibres in the anterolateral columns (Fig. 3A). Most of the remaining spinal motoneurons displayed pathological features, such as central chromatolysis (Fig. 3B), pigmentary degeneration and round hyaline inclusions (Fig. 3C). Bunina bodies and skein-like inclusions were not observed. The posterior columns, dorsal-root ganglia, as well as sensory roots, did not show pathological changes. The main autoptic findings outside the CNS were neurogenic atrophy of the skeletal muscles and bilateral bronchopneumonia.
Figure 3.
Histopathological findings on spinal cord post-mortem samples in Patient 1737 from Family RM168. (A) Low magnification photomicrograph of the anterior horn at L1 shows a striking decrease of large motoneurons. (B) At higher magnification, several large motoneurons display central chromatolysis. (C) Most of the remaining neurons are laden with dark lipofuscin granules (pigmentary degeneration) and contain round hyaline inclusions (arrows). Klüver-Barrera staining. Bar = 800 µm (A); 100 µm (B, C).
Genetic appraisals
Eight families with ARJALS (RM168, RM190, IS005, RM171, RM257, RM303, BR002 and TOR015) showed homozygous haplotypes and produced positive logarithm of odds scores with microsatellite markers D15S146, D15S537 and D15S123 in all affected subjects, showing a cumulative two-point logarithm of odds score of 11.51 at the recombination fraction θ = 0.0 for marker D15S537 (Fig. 1). The other ARJALS families (RM306 and TK005) showed heterozygous haplotypes and produced negative or slightly significant cumulative logarithm of odds scores by the genetic program HOMOG (data not shown) (Ott, 1983).
Detailed mutations in SPG11 of 10 unrelated families with ARJALS are described in Table 2. Eight of these nucleotide changes were homozygous and two others were heterozygous compounds. The majority of mutations were truncating mutations, including five non-sense mutations, two frameshift insertions, two frameshift small deletions and two splice site mutations; furthermore, one was identified as a missense mutation. Variants segregated with the disease in all pedigrees and were absent in a panel of control chromosomes (see Materials and methods section). Eleven mutations had already been reported while IVS30 + 1 G > A, affecting the consensus splice site junction, was novel (Table 2 and Fig. 2). The sequence analysis of the ALS2, ALS4, ALS11 and SPG4 genes did not reveal any coding mutations in any of the unrelated 25 families with ARJALS.
Table 2.
Mutations identified in the SPG11 gene
| Family | Location | Mutation (cDNA) | Effect on protein | RFLP | Reference study |
|---|---|---|---|---|---|
| RM306 | Exon 1 | C118T | Gln40X | TseI (loss) | Hehr et al., 2007 |
| Exon 2 | G267A | Trp89X | — | Hehr et al., 2007 | |
| TK005 | Exon 4 | 733_734 delAT | Met245Valfs | NlaIII (loss) | Stevanin et al., 2007 |
| Exon 31 | C5974T | Arg1992X | TaqI (loss) | Stevanin et al., 2007 | |
| RM168 | Exon 11 | T2198G | Leu733X | MseI (loss) | Stevanin et al., 2007 |
| RM190 | Exon 14 | A2608G | Ile870Valfs | — | Pippucci et al., 2009 |
| IS005 | Exon 17 | 3076_3077 insA | Arg1026fs | — | Hehr et al., 2007 |
| RM171 | Exon 26 | 4461_4462 delGT | Val1468Leufs | — | Lee et al., 2008 |
| RM257 | Intron 30 | IVS30 + 1 G > A | Predicted exon 31 skipping | EcoP15I (loss) | This study |
| RM303 | Exon 31 | C5970G | Tyr1990X | BfaI (gain) | Denora et al., 2009 |
| BR002 | Exon 32 | G6157A | Val2053Met | NlaIII (gain) | Del Bo et al., 2007 |
| TOR015 | Exon 39 | 7029_7030 insT | Val2344Cysfs | — | Stevanin et al., 2007 |
del = deletion; fs = frameshift; ins = insertion; IVS = intervening sequence; X = STOP codon; RFLP = restriction fragment length polymorphism.
Discussion
SPG11 gene was analysed in patients of 25 unrelated pedigrees with ARJALS along with long-term survival. Interestingly, a high frequency of SPG11 mutations, one of which is novel, was found in 10 families (10/25 mutated pedigrees, 40%). Most of the ARJALS pedigrees carrying mutations in SPG11 originated in Italy, but mutations were also detected in families from Brazil, Canada, Japan and Turkey, showing a worldwide distribution of this clinico-genetic entity.
Clinical heterogeneity, including the presence of amyotrophy or lack of thin corpus callosum, is well-known in SPG11 (Hehr et al., 2007; Paisan-Ruiz et al., 2008; Crimella et al., 2009). Nevertheless, the phenotype of these families is different from the phenotype of hereditary spastic paraplegia with amyotrophy. This is due to the occurrence of bulbar symptoms in most patients, the presence of the typical pathological features of ALS, and the non-appearance of symptoms or signs of sensory involvement documented by nerve conduction studies. The absence of thin corpus callosum and white matter alterations, shown by the brain MRI, as well as the lack of cognitive deficits or mental health problems in any of the pedigrees, excluded the diagnosis of autosomal recessive hereditary spastic paraplegia with thin corpus callosum. Moreover, an additional distinctive trait of these families is the non-appearance of ocular abnormalities, such as macular degeneration, ptosis, skew deviation, poor upgaze, saccadic pursuit and nystagmus, which are prominent clinical features in many other SPG11 families (Paisan-Ruiz et al., 2008; Stevanin et al., 2008). Finally, the existence of symptoms of upper motoneuron involvement in all of our pedigrees rejected the diagnosis of spinal muscular atrophy.
To our knowledge, this is the first report of ARJALS occurrence in subjects with SPG11 mutations. All of our families can be classified as ALS5, since ALS5 locus for ARJALS with slow progression of symptoms maps to a 6 cM segment flanked by D15S123 and D15S146, which is the same chromosomal segment to which the SPG11 gene was mapped. This is also because the clinical phenotype of our patients is comparable to that of ALS5 (Hentati et al., 1998; Stevanin et al., 2008).
A previous study reported one patient with a non-fatal course of juvenile ALS and a long-term progression for 49 years, who presented a missense mutation in spastin (SPG4), the major gene for dominantly inherited hereditary spastic paraplegia (Meyer et al., 2005). Another study described a further missense mutation of SPG4 in a patient with a rapidly progressive spinal and bulbar upper motoneuron syndrome that progressed to ALS (Brugman et al., 2005). Similar to the SPG4 gene, the SPG11 gene is known to be involved in axonal transport (Salinas et al., 2008). Given the common finding of axonal involvement in both ALS and hereditary spastic paraplegia, SPG11 genetic variants may contribute to a common pathway in hereditary spastic paraplegia and ARJALS.
Several causative genes for juvenile ALS or ARJALS have been reported (Hadano et al., 2001; Chen et al., 2004; Meyer et al., 2005; Chow et al., 2009; Wijesekera and Leigh, 2009), although mutations are successfully identified in only a fraction of the patients. The absence of pathological mutations in ALS2, ALS4, ALS11 and SPG4 in ARJALS subjects suggests that these genes are rare causes of this clinico-genetic entity.
This study revealed pathological evidence of neurodegeneration in lower motoneurons. The absence of Bunina bodies and skein-like inclusions in the autopsied patient suggests that the pathological makeup in SPG11 might be different from that in classic ALS, similar to other variants of ALS with long survival (Honma et al., 1999). Most of the currently-known proteins involved in hereditary spastic paraplegia have a biological function in axonal transport, membrane trafficking, or biogenesis in mitochondria, and spatacsin is ubiquitously expressed in the nervous system (Salinas et al., 2008). Therefore lower motoneuron involvement in SPG11 is not a surprise. However, it is still unknown why some SPG11 mutations lead to clinical outcomes similar to ALS. Further analysis on the biological role of spatacsin is an essential requisite for the better understanding of motoneuron diseases.
In conclusion, taking into account the low frequency of ARJALS (Orlacchio et al., 2007; Wijesekera and Leigh, 2009), the pathological confirmation of the clinical diagnosis in one family, the presence of SPG11 mutations in a large number of families worldwide as well as their exclusion in control individuals, our findings indicate that the clinical spectrum caused by SPG11 mutations is much wider than previously observed, from autosomal recessive hereditary spastic paraplegia with thin corpus callosum to ARJALS with long-term survival. It will be crucial to analyse SPG11 mutations in other families suffering from motoneuron disorders with similar clinical features. The remaining question is whether genetic variants of SPG11 play any role in the common adult-onset ALS followed by long-term progression or in other forms of ALS.
Funding
The Comitato Telethon Fondazione Onlus, the Amministrazione Autonoma dei Monopoli di Stato (AAMS) and the city of Gubbio, Italy (Grant No. GGP06209 to A.O.); the Italian Ministero della Salute (Grants Nos EBRI1.O, PS05.11, PS05.21 and REG.17O to A.O.); the Italian Consiglio Nazionale delle Ricerche (CNR), International Exchange Program Year 2009 (to A.O.).
Acknowledgements
We thank the patients and their family members for taking part in this study. We also thank Michela Renna for language advice and assistance, and members of our laboratories for stimulating discussions and helpful comments on this manuscript. We are extremely grateful to the Genetic Bank of the Laboratorio di Neurogenetica, Centro Europeo del Cervello-Istituto di Ricovero e Cura a Carattere Scientifico (CERC-IRCCS) Santa Lucia, Rome, Italy (http://www.hsantalucia.it/neurogen/index_en.htm) for the service provided.
Glossary
Abbreviations
- ALS
amyotrophic lateral sclerosis
- ARJALS
autosomal recessive juvenile amyotrophic lateral sclerosis
- SPG
spastic paraplegia gene
References
- Ben Hamida M, Hentati F, Ben Hamida C. Hereditary motor system diseases (chronic juvenile amyotrophic lateral sclerosis). Conditions combining a bilateral pyramidal syndrome with limb and bulbar amyotrophy. Brain. 1990;113:347–63. doi: 10.1093/brain/113.2.347. [DOI] [PubMed] [Google Scholar]
- Boukhris A, Stevanin G, Feki I, Denis E, Elleuch N, Miladi MI, et al. Hereditary spastic paraplegia with mental impairment and thin corpus callosum in Tunisia: SPG11, SPG15, and further genetic heterogeneity. Arch Neurol. 2008;65:393–402. doi: 10.1001/archneur.65.3.393. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Brugman F, Wokke JH, Scheffer H, Versteeg MH, Sistermans EA, van den Berg LH. Spastin mutations in sporadic adult-onset upper motor neuron syndromes. Ann Neurol. 2005;58:865–9. doi: 10.1002/ana.20652. [DOI] [PubMed] [Google Scholar]
- Chen YZ, Bennett CL, Huynh HM, Blair IP, Puls I, Irobi J, et al. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4) Am J Hum Genet. 2004;74:1128–35. doi: 10.1086/421054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CY, Landers JE, Bergren SK, Sapp PC, Grant AE, Jones JM, et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am J Hum Genet. 2009;84:85–8. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimella C, Arnoldi A, Crippa F, Mostacciuolo ML, Boaretto F, Sironi M, et al. Point mutations and a large intragenic deletion in SPG11 in complicated spastic paraplegia without thin corpus callosum. J Med Genet. 2009;46:345–51. doi: 10.1136/jmg.2008.063321. [DOI] [PubMed] [Google Scholar]
- Del Bo R, Di Fonzo A, Ghezzi S, Locatelli F, Stevanin G, Costa A, et al. SPG11: a consistent clinical phenotype in a family with homozygous spatacsin truncating mutation. Neurogenetics. 2007;8:301–5. doi: 10.1007/s10048-007-0095-z. [DOI] [PubMed] [Google Scholar]
- Denora PS, Schlesinger D, Casali C, Kok F, Tessa A, Boukhris A, et al. Screening of ARHSP-TCC patients expands the spectrum of SPG11 mutations and includes a large scale gene deletion. Hum Mutat. 2009;30:E500–19. doi: 10.1002/humu.20945. [DOI] [PubMed] [Google Scholar]
- Fink JK. Progressive spastic paraparesis: hereditary spastic paraplegia and its relation to primary and amyotrophic lateral sclerosis. Semin Neurol. 2001;21:199–207. doi: 10.1055/s-2001-15265. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hadano S, Hand CK, Osuga H, Yanagisawa Y, Otomo A, Devon RS, et al. A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat Genet. 2001;29:166–73. doi: 10.1038/ng1001-166. [DOI] [PubMed] [Google Scholar]
- Hehr U, Bauer P, Winner B, Schule R, Olmez A, Koehler W, et al. Long-term course and mutational spectrum of spatacsin-linked spastic paraplegia. Ann Neurol. 2007;62:656–65. doi: 10.1002/ana.21310. [DOI] [PubMed] [Google Scholar]
- Hentati A, Ouahchi K, Pericak-Vance MA, Nijhawan D, Ahmad A, Yang Y, et al. Linkage of a commoner form of recessive amyotrophic lateral sclerosis to chromosome 15q15-q22 markers. Neurogenetics. 1998;2:55–60. doi: 10.1007/s100480050052. [DOI] [PubMed] [Google Scholar]
- Honma Y, Komori T, Kato S, Suda N, Kawata A, Oda M. An autopsy case of sporadic amyotrophic lateral sclerosis with 16-year survival without artificial ventilation. Neuropathology. 1999;19:85–92. doi: 10.1046/j.1440-1789.1999.00206.x. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Cheng TW, Hua MS, Pan MK, Wang J, Stephenson DA, et al. Mutations of the SPG11 gene in patients with autosomal recessive spastic paraparesis and thin corpus callosum. J Neurol Neurosurg Psychiatry. 2008;79:607–9. doi: 10.1136/jnnp.2007.136390. [DOI] [PubMed] [Google Scholar]
- Meyer T, Schwan A, Dullinger JS, Brocke J, Hoffmann KT, Nolte CH, et al. Early-onset ALS with long-term survival associated with spastin gene mutation. Neurology. 2005;65:141–3. doi: 10.1212/01.wnl.0000167130.31618.0a. [DOI] [PubMed] [Google Scholar]
- Orlacchio A, Bernardi G, Orlacchio A, Martino S. Genetics of amyotrophic lateral sclerosis. Recent Res Devel Neurosci. 2007;2:17–54. [Google Scholar]
- Orlacchio A, Kawarai T, Rogaeva E, Song YQ, Paterson AD, Bernardi G, et al. Clinical and genetic study of a large Italian family linked to SPG12 locus. Neurology. 2002;59:1395–401. doi: 10.1212/01.wnl.0000031423.43482.19. [DOI] [PubMed] [Google Scholar]
- Orlacchio A, Kawarai T, Totaro A, Errico A, St George-Hyslop PH, Rugarli EI, et al. Hereditary spastic paraplegia: clinical genetic study of 15 families. Arch Neurol. 2004;61:849–55. doi: 10.1001/archneur.61.6.849. [DOI] [PubMed] [Google Scholar]
- Ott J. Linkage analysis and family classification under heterogeneity. Ann Hum Genet. 1983;47:311–20. doi: 10.1111/j.1469-1809.1983.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Dogu O, Yilmaz A, Houlden H, Singleton A. SPG11 mutations are common in familial cases of complicated hereditary spastic paraplegia. Neurology. 2008;70:1384–9. doi: 10.1212/01.wnl.0000294327.66106.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippucci T, Panza E, Pompilii E, Donadio V, Borreca A, Babalini C, et al. Autosomal recessive hereditary spastic paraplegia with thin corpus callosum: a novel mutation in the SPG11 gene and further evidence for genetic heterogeneity. Eur J Neurol. 2009;16:121–6. doi: 10.1111/j.1468-1331.2008.02367.x. [DOI] [PubMed] [Google Scholar]
- Salinas S, Proukakis C, Crosby A, Warner TT. Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. Lancet Neurol. 2008;7:1127–38. doi: 10.1016/S1474-4422(08)70258-8. [DOI] [PubMed] [Google Scholar]
- Stevanin G, Azzedine H, Denora P, Boukhris A, Tazir M, Lossos A, et al. Mutations in SPG11 are frequent in autosomal recessive spastic paraplegia with thin corpus callosum, cognitive decline and lower motor neuron degeneration. Brain. 2008;131:772–84. doi: 10.1093/brain/awm293. [DOI] [PubMed] [Google Scholar]
- Stevanin G, Santorelli FM, Azzedine H, Coutinho P, Chomilier J, Denora PS, et al. Mutations in SPG11, encoding spatacsin, are a major cause of spastic paraplegia with thin corpus callosum. Nat Genet. 2007;39:366–72. doi: 10.1038/ng1980. [DOI] [PubMed] [Google Scholar]
- Strong MJ, Gordon PH. Primary lateral sclerosis, hereditary spastic paraplegia and amyotrophic lateral sclerosis: discrete entities or spectrum? Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:8–16. doi: 10.1080/14660820410021267. [DOI] [PubMed] [Google Scholar]
- Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3. doi: 10.1186/1750-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Uyanik G, Gross C, Lange M, Schulte-Mattler W, Schuierer G, et al. Clinical progression and genetic analysis in hereditary spastic paraplegia with thin corpus callosum in spastic gait gene 11 (SPG11) Arch Neurol. 2004;61:117–21. doi: 10.1001/archneur.61.1.117. [DOI] [PubMed] [Google Scholar]