Abstract
The primary biological function of the endogenous cellular prion protein has remained unclear. We investigated its biological function in the generation of cellular immune responses using cellular prion protein gene-specific small interfering ribonucleic acid in vivo and in vitro. Our results were confirmed by blocking cellular prion protein with monovalent antibodies and by using cellular prion protein-deficient and -transgenic mice. In vivo prion protein gene-small interfering ribonucleic acid treatment effects were of limited duration, restricted to secondary lymphoid organs and resulted in a 70% reduction of cellular prion protein expression in leukocytes. Disruption of cellular prion protein signalling augmented antigen-specific activation and proliferation, and enhanced T cell receptor signalling, resulting in zeta-chain-associated protein-70 phosphorylation and nuclear factor of activated T cells/activator protein 1 transcriptional activity. In vivo prion protein gene-small interfering ribonucleic acid treatment promoted T cell differentiation towards pro-inflammatory phenotypes and increased survival of antigen-specific T cells. Cellular prion protein silencing with small interfering ribonucleic acid also resulted in the worsening of actively induced and adoptively transferred experimental autoimmune encephalomyelitis. Finally, treatment of myelin basic protein1–11 T cell receptor transgenic mice with prion protein gene-small interfering ribonucleic acid resulted in spontaneous experimental autoimmune encephalomyelitis. Thus, central nervous system autoimmune disease was modulated at all stages of disease: the generation of the T cell effector response, the elicitation of T effector function and the perpetuation of cellular immune responses. Our findings indicate that cellular prion protein regulates T cell receptor-mediated T cell activation, differentiation and survival. Defects in autoimmunity are restricted to the immune system and not the central nervous system. Our data identify cellular prion protein as a regulator of cellular immunological homoeostasis and suggest cellular prion protein as a novel potential target for therapeutic immunomodulation.
Keywords: small interfering RNA, prion, immunosuppression, T cell signaling
Introduction
A physiological function for cellular prion protein (PrPc), a glycophosphatidylinositol-anchored sialoglycoprotein, is still enigmatic. So far, PrPc has mainly been studied in the realm of neurodegenerative diseases, specifically as the substrate for the replicative cycle of prions. PrPc is highly expressed in neurons (DeArmond et al., 1999). However, high expression levels of PrPc in lymphoid cells (Cashman et al., 1990; Antoine et al., 2000; Durig et al., 2000; Li et al., 2001) and myeloid cells (Burthem et al., 2001; Krebs et al., 2006) suggest a biological function of PrPc outside the CNS. The recently reported localization of PrPc in the immunological synapse, and its co-precipitation with the T cell receptor (TCR) (Mattei et al., 2004) prompted us to investigate more closely the role of PrPc in mediating T cell activation and function. The role and function of T cells in neuroinflammation can be paradigmatically examined in experimental autoimmune encephalomyelitis (EAE), an animal model of the human disorder multiple sclerosis.
Here, we silenced the PrP gene (Prnp) product with small interfering ribonucleic acid (siRNA). We observed a significant and selective decrease of PrPc expression in lymphoid tissue, but not in the CNS. Also, silencing of PrPc resulted in a significant worsening of clinical EAE. This finding was confirmed in genetic PrPc-deficient mice (Manson et al., 1994) whereas mice overexpressing PrPc (Fischer et al., 1996) were relatively resistant to EAE induction. Finally, we were able to show that PrPc silencing in T lymphocytes accounted for the observed clinical effects through enhanced T cell activation, TCR signalling and propagation of T cell differentiation towards proinflammatory T helper cell (Th)-1 and Th17 phenotypes.
Materials and methods
Peptides and antibodies
The siRNAs were purchased as purified duplexes from Dharmacon RNA Technologies:
Prnp-SiRNA 5′-GUGCACGACUCAAUAdT dT; 3′-dTdTCACGUGCUGACGCAGUUAU-5′ and nonsense siRNA 5′-CGAACGAGUACCGUACACUdTdT; 3′-dTdTGCUUGCUCAUGGCAUGUGA-5′. myelin oligodendrocyte glycoprotein (MOG)p35–55, myelin basic protein (MBP)Ac1–11, proteolipid protein (PLP)p139–151 and ovalbumine (OVA)p323–339 were synthesized by S.C. Bio. Rabbit anti-mouse PrPc monoclonal antibody ab52604 was obtained from Abcam Inc. Anti-phosphorylation clone 4G10 (Upstate), rabbit anti-mouse β-actin, goat anti-mouse Immunoglobulin G (IgG)-horseradish peroxidase and anti-rabbit IgG-horseradish peroxidase were obtained from Santa Cruz Biotechnology, Inc. Hamster anti-mouse CD3 (145-2C11) and hamster anti-mouse CD28 (37.51) monoclonal antibodies were purchased from BD Biosciences. The anti-PrP monoclonal antibody W226 was derived from PrPc-deficient mice (Bueler et al., 1992) immunized with purified PrPSc (Petsch and Stitz, manuscript in preparation). The monoclonal antibody 19C4 was derived from PrPc−/− mice immunized with recombinant mouse PrPc. The single-chain variable fragment was generated from W226 hybridoma (Muller-Schiffmann et al., 2009).
Mice
C57BL/6 and Sv129 mice (8- to 12-week-old) were bred at the animal-breeding facilities at the Friedrich-Loeffler-Institute in Tübingen, Germany. OVA323–339 TCR transgenic (OT2) mice were purchased at The Jackson Laboratories. SJL/J and B10.PL mice were purchased from the Jackson Laboratory (Bar Harbor) and bred in a barrier animal facility at the University of Texas Southwestern Medical Centre. PrP−/− mice were made available to us by Dr. Jean Manson (Manson et al., 1994). Tga20 mice (Fischer et al., 1996) were made available by Dr Adriano Aguzzi, and bred at the Friedrich Loeffler Institute at Tübingen. B10.PL mice transgenic for MBP1–11-specific TCR were a gift from Dr J. Goverman and Dr J. Lafaille. All protocols involving mice handling were approved by the respective Institutional Animal Care and Use Committee.
Experimental autoimmune encephalomyelitis
EAE was induced in 8- to 12-week-old female SJL/J mice by subcutaneous (s.c.) immunization with 100 µg of PLPp139−151, which was dissolved in complete freund's adjuvant containing 4 mg/ml of heat-killed Mycobacterium tuberculosis H37Ra (Difco Laboratories) as described (Stuve et al., 2002, 2006). Immediately thereafter and again 48 h later, mice received an intraperitoneal (i.p.) injection of 400 ng pertussis toxin (Ptx) in 0.2 ml of phosphate buffered saline (PBS). For adoptive transfer experiments, SJL/J mice were sacrificed at Day 10 after immunization with PLPp139−151. Splenocytes and lymph node cells were isolated and transfected with Prnp-siRNA or nonsense-siRNA, and then cultured in the presence of PLPp139−151 and interleukin (IL)-12 for 72 h. Viable cells were counted and 2.5 million T cell blasts were injected i.p. into naive SJL/J recipient mice. At the time of adoptive transfer, the recipient mice were treated with Prnp-siRNA or nonsense-siRNA. Mice were examined daily for clinical signs of EAE and scored as previously described (Stuve et al., 2002, 2006): 0 = no paralysis; 1 = loss of tail tone; 2 = hindlimb weakness; 3 = hindlimb paralysis; 4 = hindlimb and forelimb paralysis; 5 = moribund or dead.
Histopathology
Mice were anaesthetized and perfused transcardially with PBS and 4% paraformaldehyde. Brains and spinal cords were dissected and embedded in paraffin. Inflammation was assessed by haematoxylin and eosin staining at Day 29 after disease induction in Prp−/− (n = 6) and wild-type mice (n = 7) and expressed as the mean number of inflammatory infiltrates per spinal cord cross-section (inflammatory index). A minimum of 12 spinal cord cross-sections were examined per animal. Brains and spinal cords of siRNA-treated animals were obtained from three mice with EAE of each experimental group at Day 20 and were evaluated by an examiner, blinded to the treatment status of the animal. Quantitative studies were performed on an average of 12 anatomically matched whole cross-sections of brain and spinal cord as described earlier (Youssef et al., 2002).
siRNA transfections and treatments
For in vitro transfection of splenocytes, 2 μl TransIT-TKO transfection reagent (Mirus) was diluted in 50 μl serum-free/antibiotic-free Roswell Park Memorial Institute 1640 media per well. After 10 min incubation at room temperature, 1 μl 40 μM siRNA was added to 52 μl diluted transfection reagent. The siRNAs were incubated with the diluted transfection reagent at room temperature with gentle agitation for 30 min. The siRNAs were then added to the Vβ8.2 transgenic or B10.PL splenocyte cultures containing 5 × 106 cells in 500 μl media per well of a 24 well plate and incubated for 20 h at 37°C. On the following day, the cells were collected and washed with fresh media and then resuspended in 2 ml media and placed back in their original wells. MBPAc1–11 peptide was added at 2 μg/ml. For Vα2.3/Vβ8.2 transgenic splenocytes, 2 × 106 splenocytes were placed in each well of a 24 well plate. The transfection protocol was the same, except the cells were placed with wild-type splenocytes (6 × 106 cells/well) that had been irradiated and cultured with MBPAc1–11 after the 24 h transfection.
For in vivo experiments, 50 μg of Prnp-siRNA or nonsense-siRNA were administered intravenously (i.v.) at the time of immunization or the time of disease onset in 100 μl PBS via tail vein injection.
Immunofluorescence
Naïve mice, or mice that had been actively immunized for EAE, were injected intravenously with Cy3-labelled or DY547-labelled siRNAs. To visualize the fluorochrome-labelled siRNA in situ, tissues were prepared as described earlier (Puttaparthi et al., 2003). Briefly, the mice were sacrificed by injecting them with a lethal dose of sodium pentobarbital (250 mg/kg, i.p.) 24 or 72 h after injection of the siRNA. Anaesthetized mice were perfused transcardially with ice-cold PBS. Spinal cord, brain, liver and kidneys were removed and sectioned transversely at 250 µm intervals. Slices were transferred to Gey’s balanced salt solution at room temperature. The slices were transferred onto slides and mounted with Gel/Mount. Slides were viewed with an E800 fluorescent microscope (Nikon Instruments Inc.) using Metamorph software.
Tracking of siRNA-labelled CD4 + T cells in vivo
Single cell suspensions of splenocytes obtained from Vα2.3Vß8.2 TCR transgenic mice were transfected with DY547-labelled Prnp-siRNA or nonsense-siRNA as described in the previous section. After 72 h of in vitro stimulation with MBPAc1–11 (6 µg/ml) and IL-12 (0.5 ng/ml) the cells were collected, washed and resuspended at 5 × 106/200 µl. Each mouse (n = 4/siRNA) received 200 µl of siRNA transfected cells via i.p. injection. Seventy-two hours post injection, mice were euthanized and perfused with cold PBS. Numerous tissues, including the lymph nodes, spleen, lung, liver, brain and spinal cord, were collected, processed and examined for expression of DY547-labelled siRNA by flow cytometry (as described below) and immunofluorescence microscopy (described above).
CD4 T cell purification
Mouse CD4+ T cells were purified from a bulk spleen population using a mouse CD4 T lymphocyte enrichment set (BD IMag™). The purity of CD4+ T cells was assessed by flow cytometry and exceeded 95%.
Proliferation assays
For primary proliferation assays, splenocytes or lymph node cells were isolated from SJL/J mice that had been immunized with PLPp139–151 seven days before, and that had been concomitantly treated with Prnp-siRNA or nonsense-siRNA. Cells were cultured with antigen in 96 well microtitre plates at a concentration of 5 × 106 cells/ml. Culture medium consisted of Roswell Park Memorial Institute medium 1640 (Invitrogen) supplemented with l-glutamine (2 mM), sodium pyruvate (1 mM), non-essential amino acids (0.1 mM), penicillin (100 U/ml), streptomycin (0.1 mg/ml), 2-mercaptoethanol (5 × 10−5 M) and 1% (v/v) autologous normal mouse serum. Splenocytes were incubated for 72 h. Cultures were then pulsed for 18 h with 1 µCi per well of [3H]thymidine before harvesting. Results are shown as mean of triplicates ± SEM.
For proliferation assays in which irradiated B10.PL splenocytes served as the antigen presenting cell, and MBPAc1–11 TCR transgenic CD4+-enriched T cells served as effector cells, splenocytes from B10.PL and MBPAc1–11 TCR transgenic mice were brought into single cell suspension, and transfected with Prnp-siRNA or nonsense-siRNA. In other experiments, cells were treated for 24 h with the anti-mouse PrPc monoclonal antibodies 19C4 or W226, or an anti-PrPc single-chain variable fragment and the appropriate isotype control mouse IgG1. Cells were then washed, irradiated and pulsed with MBPAc1–11. Cells were harvested on glass filters using a Tomtec harvester (Tomtec), and incorporated [methyl-3H]thymidine was measured with a Betaplate counter (PerkinElmer).
Cytokine analysis
Supernatants from splenocytes cultured in parallel with those cells used in proliferation assays were used for cytokine analysis. Supernatants were collected for interleukin (IL)-2 (48 h), interferon gamma (IFNγ) and IL-17 (72 h), IL-4, IL-5 and IL-10 (120 h). Quantitative enzyme-linked immunosorbent assay (ELISA) was performed using paired monoclonal antibodies specific for corresponding cytokines as per manufacturer’s recommendations (BD PharMingen). The results of ELISA experiments are expressed as an average of triplicate wells ± SD. A SOFTmax ELISA plate reader and software was used for data analysis (Molecular Devices).
Western blotting
Splenocytes were transfected as described above. For preparation of total cell lysates, cells were collected, spun down and resuspended in sodium dodecyl sulfate-lysis buffer. Cells were lysed on ice for 30 min and spun down to remove cell debris. Total cell lysates were also made from the brains of mice using a tissue homogenizer and lysing with sodium dodecyl sulfate-lysis buffer. Protease inhibitors (aprotinin, leupeptin and pepstatin) were added to all lysates at the time of preparation. Prior to the western blot, the protein concentration of these extracts was determined by using the BioRad Protein Assay. The extracts were diluted in sodium dodecyl sulfate loading buffer, boiled for 3 min and were electrophorectically separated on 4–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels. After transfer of the gels to nitrocellulose membranes, the membranes were blocked with 5% milk in tris-buffered saline. The primary antibody was diluted 1:1000 in blocking buffer and was added to the membrane for 2 h. The membrane was washed three times in tris-buffered saline/Tween. The secondary antibody was diluted 1:1000 and was added to the membrane for 1 h. The membrane was washed three times and a chemiluminescent substrate (Bio-rad Inc.) was added for 1 min; the blot was then exposed to film for various times (0.05–10 min). The density of the bands was determined by using ImageQuant 5.2 program. Data were normalized by dividing the density of the PrPc band by the density of the β-actin band. The data are representative of at least five independent experiments. It is difficult to normalize between experiments, therefore, no error bars are shown.
Flow cytometry
The following antibodies were used: mouse anti-mouse PrP antibody (W226), fluorescein isothiocyanate-conjugated mouse IgG1 (BD Biosciences) and antigen presenting cell-conjugated anti-CD3 (eBioscience). Mouse spleens were pressed through a 70 µm nylon mesh cell strainer. Then, splenocytes were treated with RBC lysing buffer (Sigma-Aldrich). For flow cytometry, cells were counted, washed and resuspended in staining buffer (4% foetal calf serum and 0.1% sodium azide in PBS). Fc receptors were blocked with anti-CD16/32 (BD Biosciences) and the cells were incubated with 1 µg W226 monoclonal antibody for 45 min at 4°C. W226 staining was revealed with fluorescein isothiocyanate conjugated rat anti-mouse IgG1 followed by a 30 min blocking step with purified mouse immunoglobulins. After washing twice with staining buffer, cells were stained with directly labelled anti-CD3 for 30 min on ice, washed and then fixed with 1% paraformaldehyde. Twenty thousand gated events were acquired on a FACSCalibur (BD Biosciences) and analysed using FlowJo software (Tree Star).
Phosphorylation assay
CD4+ T cells were activated by suspending cells in media with biotinylated anti-CD3 antibody (5 µg/ml) for 20 min, goat anti-hamster IgG (10 µg/ml Jackson Immunoresearch) for 1 min and then treated with anti-CD28 for 1 and 5 min, respectively. Sodium orthovanadate was then added to stop the phosphorylation and western blot analysis was performed.
T cells activation assays
A fast-growing variant of the D10 T cell clone (Kaye et al., 1983) was obtained from M. Krummel (University of California, San Francisco) and cultured as described (Kane et al., 2004). D10 cells (15 million) were transfected by electroporation as described (de Souza et al., 2005). For luciferase assays, cells were resuspended 18–20 h later at 2 million/ml in fresh media and stimulated for 6 h with biotinylated anti-CD3 and anti-CD28 antibodies (1 μg/ml each; BD Biosciences) plus streptavidin (5 μg/ml; Zymed) or phorbol 12-myristate 13-acetate (PMA) (25 ng/ml; Calbiochem) plus ionomycin (1 μm; Sigma). Luciferase activity was then measured as described (Kane et al., 2001). For determination of activation markers, cells were re-suspended the day after transfection at 1 million/ml and stimulated for 18 h with either media alone or anti-CD3/CD28 antibodies at 1 μg/ml each. Cells were then stained with phycoerythrin-conjugated antibodies to murine CD25 or CD69 (Caltag) and analysed on a BD LSR II flow cytometer.
Statistical analysis
Correlations between continuous and categorical variables were assessed using the Mann–Whitney U-test. The means of two normally distributed samples were compared by Student's t-test. All other statistical comparisons between groups were examined using one-way multiple range ANOVA test for multiple comparison. P-values <0.05 were considered significant.
Results
Peripheral silencing of PrPC with siRNA does not affect central nervous system PrPC expression
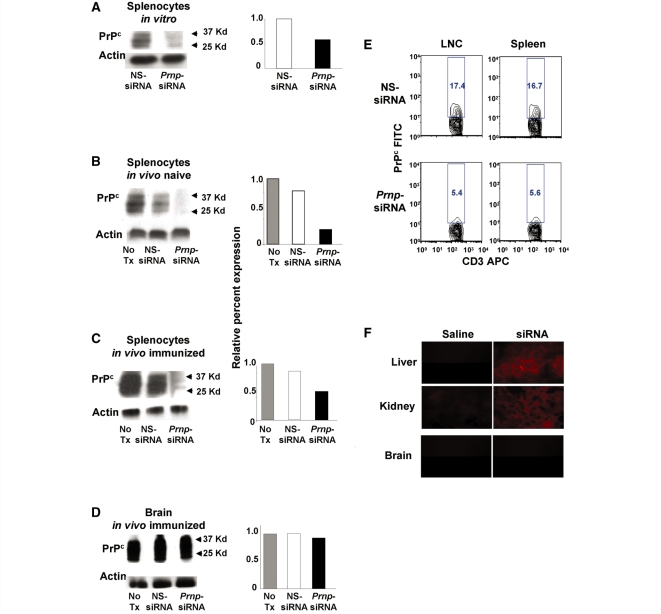
Prnp-specific siRNA and control NS-siRNA were generated to investigate the tissue specific role of PrPc in activating and perpetuating cellular immune responses. By western blot analyses, we demonstrated that in vitro transfection of murine splenocytes with Prnp-siRNA substantially decreased PrPc expression (Fig. 1A). On Day 3 after in vivo intravenous treatment of naïve SJL/J mice (Fig. 1B), and SJL/J mice immunized for EAE with 50 μg of Prnp-siRNA (Fig. 1C), PrPc expression was decreased in splenocytes, but not in the brain (Fig. 1D). When evaluated by flow cytometry, PrPc expression was found to be decreased by ∼70% in CD3+ T cells from Prnp-siRNA-treated mouse lymph node cells and splenocytes compared with nonsense-siRNA-treated samples (Fig. 1E). To investigate the bioavailability of Prnp-siRNA further, Cy3-labelled siRNA or DY547-labelled siRNA was injected intravenously into naïve experimental animals, or into mice in which EAE had been induced by active immunization. Fluorescence was detectable in the tissue sections of liver and kidney 72 h after injection (Fig. 1F), but not in the brain and spinal cord of naïve mice (Fig. 1F) or mice with active EAE (data not shown). Thus, Prnp-siRNA treatment created chimeric mice that expressed normal levels of PrPc in the CNS, but substantially decreased levels of PrPc in peripheral organs.
Figure 1.
Decreased PrPc expression levels on peripheral leucocytes but not in the CNS in mice treated with Prnp-siRNA. (A) Transfection with Prnp-siRNA decreased PrPc expression in murine splenocytes, compared with nonsense (NS)-siRNA treatment. On Day 3 after in vivo treatment of (B) unimmunized SJL/J mice and (C) SJL/J mice immunized with PLPp139−151 with 50 μg of Prnp-siRNA, PrPc expression was decreased in splenocytes, but not in (D) brain homogenate of immunized mice. (A–D) Densitometry was performed, and relative PrPc expression was determined by normalizing the PrPc to β-actin levels. (E) Using flow cytometry three days after immunization and siRNA treatment, lymph node cells (LNC) and splenocytes were gated on CD3 and PrPc. The top two panels show the percentage of PrPc+ cells within the CD3+ cell population from nonsense-siRNA-treated murine cells. The lower two panels show the percentage of PrPc+ cells within the CD3+ cell population from Prnp-siRNA-treated murine cells. PrPc expression was decreased by ∼70% following Prnp-siRNA treatment. (F) After injecting Cy3-labelled siRNA or DY547-labelled siRNA intravenously, fluorescence was detectable 72 h later in liver and kidney tissue but not in the brain of naïve mice or mice in which EAE had been induced by active immunization (data not shown).
Silencing PrPc with siRNA worsens clinical and neuropathological central nervous system autoimmune disease
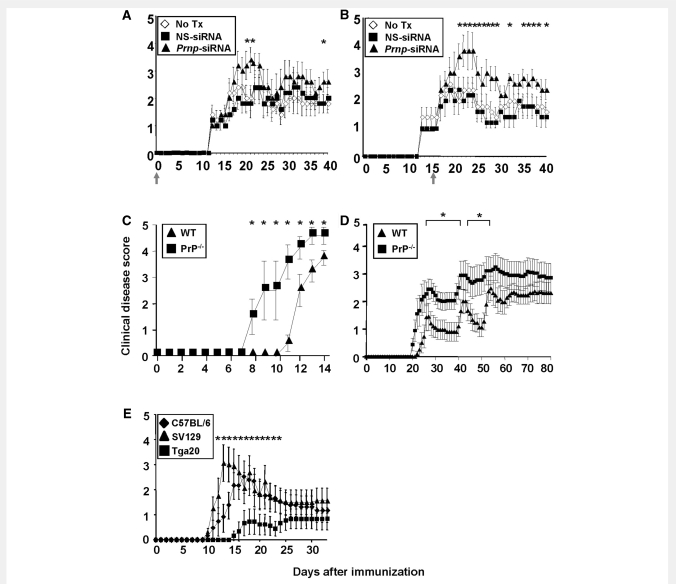
To test the role of PrPc in CNS autoimmune disease, EAE was induced by active immunization of SJL/J mice with PLPp139−151. A single dose of 50 μg Prnp-siRNA or nonsense-siRNA was injected i.v. via the tail vein in 100 μl PBS according to published methods (Lovett-Racke et al., 2004). Experimental animals were monitored for 40 days. In a set of ‘prevention’ experiments, treatment with Prnp-siRNA resulted in significantly worse clinical EAE within the first 10 days after disease onset than treatment with nonsense-siRNA or no treatment (Fig. 2A, Supplementary Table S1A). In a second set of ‘treatment’ experiments, mice treated with Prnp-siRNA had a significantly worse initial clinical exacerbation than control mice and continued to do significantly worse throughout the observation period (Fig. 2B, Supplementary Table S1B)
Figure 2.
Prnp-siRNA treated and PrP-deficient (PrP−/−) mice develop more severe EAE whereas mice overexpressing PrPC are protected. (A and B) In vivo silencing of PrPc worsens the clinical course of actively induced EAE in SJL/J mice. (A) In an EAE ‘prevention’ experiment, SJL/L mice were injected intravenously with a single dose of Prnp-siRNA or nonsense (NS)-siRNA at the time of immunization with PLPp139−151, as indicated by a grey arrow. Prnp-siRNA treatment resulted in clinically more severe disease. (B) In an EAE ‘treatment’ experiment, Prnp-siRNA or nonsense-siRNA was injected at the time of clinical onset of EAE (grey arrow). Mice treated with Prnp-siRNA had a significantly worse initial clinical exacerbation. (C) Male and (D) female PrP−/− mice had earlier disease onset and significantly higher disease scores than male wild-type (WT) mice. (E) Tga20 mice had a delayed disease onset, and developed only mild EAE compared to C57BL/6 and Sv129 wild-type mice.
Histological evaluation of CNS tissue with haematoxylin and eosin (data not shown) revealed a significantly increased number of inflammatory foci in the brain (P = 0.046; data not shown) and spinal cord parenchyma (P = 0.04; Supplementary Fig. S1D) in EAE mice treated with Prnp-siRNA. In the brain, the difference in inflammatory infiltrates was also significant within the meninges (P = 0.008; data not shown).
Next, we performed active immunization to induce EAE in PrP−/− mice (Manson et al., 1994) on the Sv129/OLA background (H-2b), and in Sv129/OLA wild-type mice. Male PrP−/− mice had earlier disease onset and significantly higher disease scores than male wild-type mice (Fig. 2C, Supplementary Table S1C). Similarly, female PrP−/− mice had earlier disease onset and worse clinical disease than female wild-type mice over a longer period of 80 days (Fig. 2D, Supplementary Table S1D). Histopathology obtained at Day 29 after disease induction revealed significantly higher numbers of inflammatory infiltrates in PrP−/− mice compared with wild-type controls (Supplementary Fig. S1A–C).
To determine whether the increased number of inflammatory cells in the CNS of mice treated with Prnp-siRNA are antigen-specific T cells in which Prnp was silenced, we tracked Vα2.3Vß8.2 TCR transgenic CD4+ T cells transfected with DY547-labelled Prnp-siRNA or nonsense-siRNA in vivo. Numerous tissues, including the lymph nodes, spleen, lung, liver, brain and spinal cord were collected, processed and examined for expression of DY547-labelled siRNA by flow cytometry and immunofluorescence microscopy. With regard to absolute cell numbers and percent of fluorochrome-labelled CD4+ T cells there was no difference in any of the organs between cells in which Prnp had been silenced, and in those in which it had not been silenced by siRNA (data not shown).
Mice overexpressing PrPc are protected from severe experimental autoimmune encephalomyelitis
Tga20 mice that ubiquitously overexpress PrPc (Fischer et al., 1996) had a later disease onset and developed milder disease than C57BL/6 wild-type mice and Sv129 wild-type mice that were used as controls (Fig. 2E, Supplementary Table S1E). Consistent with published data (Zabel et al., 2009), we observed a significantly lower absolute number of CD4+ and CD8+ T cells in the spleen of Tga20 mice compared with wild-type controls (data not shown). Other investigators had previously demonstrated that mRNA transcripts from pre-TCR-α, a T-cell development gene located on mouse chromosome 17, are substantially reduced in Tga20 mice (Zabel et al., 2009). Interestingly, the percentage of FoxP3+ T regulatory T cells was increased in these mice compared with wild-type controls (data not shown).
Silencing PrPc on antigen-specific T cells promotes the differentiation into Th1 and Th17 phenotypes
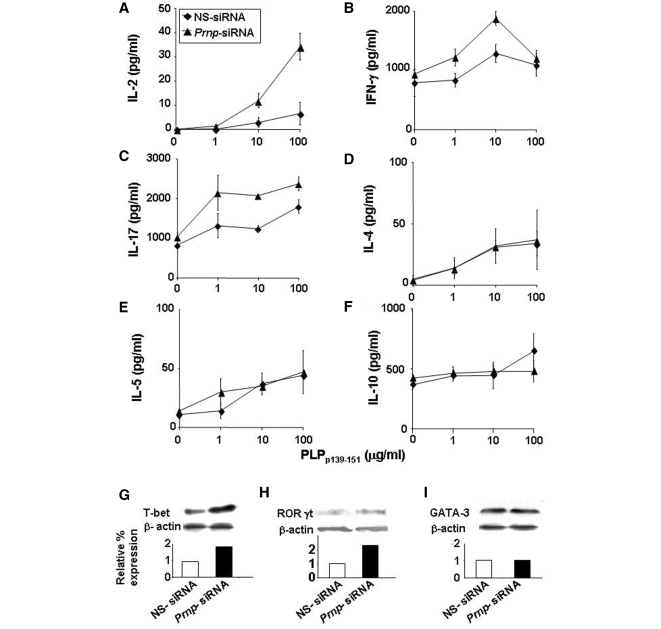
Splenocytes from SJL/J mice immunized with PLPp139–151 and treated intravenously with 50 µg of Prnp-siRNA or nonsense-siRNA were brought into single cell suspension 3 days after immunization and were pulsed with PLPp139–151. IL-2, IFNγ, IL-17, IL-4, IL-5 and IL-10 cytokine expression in culture supernatants was measured by ELISA. Treatment of mice with Prnp-siRNA significantly increased the expression of IL-2 (Fig. 3A), interferon-γ (Fig. 3B) and IL-17 (Fig. 3C). In contrast, there was no effect of silencing PrPc with siRNA on the expression of IL-4 (Fig. 3D), IL-5 (Fig. 3E) and IL-10 (Fig. 3F). Protein expression of T box expressed in T cells, a transcription factor that regulates IFNγ and IL-17 expression, and (H) retinoic acid receptor-related orphan receptor (ROR)γt, a regulator of IL-17 was increased in splenocytes of mice treated with Prnp-siRNA compared with nonsense-siRNA-treated cells (Fig. 3G and H). The expression of the Th2 transcription factor GATA binding protein (GATA)-3 was not altered (Fig. 3I).
Figure 3.
Silencing PrPc in antigen specific splenocytes increases the expression of Th1 cytokines by upregulating T box expressed in T cells (T-bet). Splenocytes from SJL/J mice immunized with PLPp139–151 and treated concomitantly intravenously with 50 µg of Prnp-siRNA or nonsense (NS)-siRNA, were brought into single cell suspension 3 days after immunization and were pulsed with PLPp139–151. Interleukin (IL)-2, interferon (IFN)-γ, IL-17, IL-4, IL-5 and IL-10 cytokine expression was measured by ELISA. Silencing of PrPc significantly increased the expression of (A) IL-2, (B) IFN-γ and (C) IL-17. In contrast, silencing of PrPc had no effect on the expression of (D) IL-4, (E) IL-5 and (F) IL-10. (G) Protein expression of T box expressed in T cells and (H) retinoic acid receptor related orphan receptor (ROR)γt in splenocytes of mice treated with Prnp-siRNA was increased compared with nonsense-siRNA-treated cells. (I) In contrast, the expression of GATA binding protein (GATA)-3 was not altered. The data are shown as mean clinical score ± standard deviation.
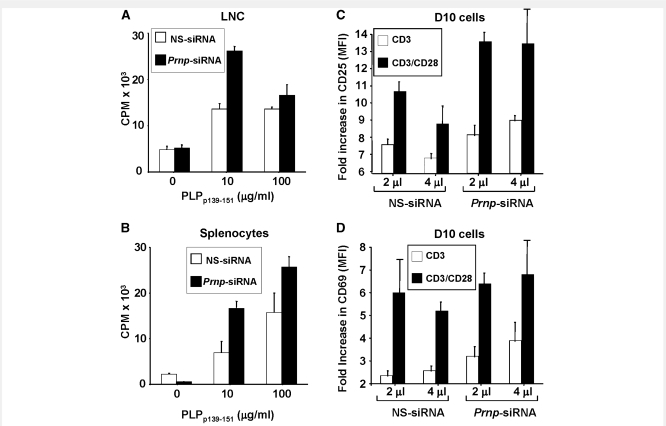
Silencing of PrPc augments antigen-specific T cell proliferation and activation
Lmph node cells (Fig. 4A) and splenocytes (Fig. 4B) from Prnp-siRNA-treated mice showed significantly increased antigen-specific proliferation compared with cells from nonsense-siRNA-treated control mice. In an effort to understand the function of PrPc in T cells further, we tested the activation status of the Prnp-siRNA-treated T cells. As shown in Fig. 4C, silencing of PrPc augmented the expression of the activation marker CD25 in T cells stimulated with anti-CD3, with or without addition of anti-CD28 monoclonal antibody. The effect on CD25 appeared to be most pronounced in the presence of both anti-CD3 and anti-CD28. There was less of an effect of Prnp-siRNA treatment on the expression of CD69, an early T cell activation marker (Fig. 4D) (Testi et al., 1989), although some increase in CD69 expression was observed in conjunction with anti-CD3 stimulation alone.
Figure 4.
Silencing PrPc in antigen-specific T cells increases T cell proliferation and activation markers. (A) Lymph node cells (LNC) and (B) splenocytes from SJL/J mice immunized with PLPp139–151 and treated with Prnp-siRNA or nonsense (NS)-RNA at the time of immunization were brought into single cell suspension on day 10 after immunization. Both (A) Lymph node cells and (B) splenocytes from Prnp-siRNA-treated mice showed increased proliferation. (C) Prnp-siRNA treatment also significantly increased the mean fluorescence intensity (MFI) of the activation marker CD25 in D10 T cells activated by anti-CD3, with or without anti-CD28 monoclonal antibody. (D) There was less of an effect of Prnp-siRNA treatment on the expression of CD69, an early T cell activation marker. Proliferation was measured as counts per minute (CPM).
T cells, not antigen presenting cells, mediate the proinflammatory effects of PrPC silencing
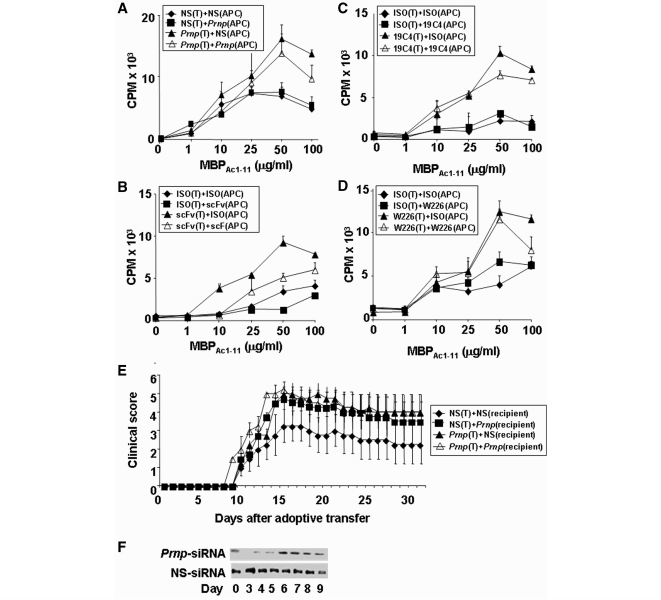
PrPc is constitutively expressed in T cells (Cashman et al., 1990; Durig et al., 2000; Li et al., 2001) and cells that can serve as antigen-presenting cells, including B cells and dendritic cells. Thus, we investigated whether the effect of PrPc on T cell proliferation was predominantly mediated through its effects on T cells, or antigen-presenting cells or both. Specifically, sublethally irradiated splenocytes from B10.PL mice were used as antigen-presenting cells and presented MBP peptide Ac1–11 to CD4+-enriched MBP1–11 TCR transgenic T cells (Goverman et al., 1993). Either antigen-presenting cells or T cells were treated with Prnp-siRNA (Fig. 5A), a recombinant anti-PrPc single chain variable fragment (Fig. 5B), or two distinct anti-PrPc monoclonal antibodies (Fig. 5C and D) at a dose of 1 µg/ml for 24 h prior to the proliferation assay (for details, see Experimental Procedures section). Silencing of PrPc in antigen-specific T cells resulted in increased proliferation of these cells (Fig. 5A–D). In contrast, silencing of PrPc in antigen-presenting cells had no apparent effect on antigen-specific T cell proliferation (Fig. 5A–D).
Figure 5.
Antigen-specific T cells are the main target of Prnp-siRNA. (A) Irradiated splenocytes from B10PL mice that served as antigen presenting cells (APC), or MBP1–11 TCR transgenic CD4+ T cells were treated with Prnp-siRNA and/or nonsense-siRNA, and incubated with antigen. Silencing of PrPc in T cells results in increased proliferation, whereas silencing of PrPc in antigen presenting cells had no apparent effect on T cell proliferation. Targeting PrPc in T cells with a (B) single chain variable fragment (scFv), or two monoclonal mouse anti-mouse PrP IgG1 monoclonal antibodies (C) 19C4, (D) W226 also resulted in the augmented proliferation of antigen-specific T cells. (E) Mice treated with Prnp-siRNA as recipients of Prnp-siRNA-treated adoptively transferred T cells, as recipients of direct Prnp-siRNA treatment, or both, developed significantly worse EAE than controls. (F) PrPc expression after Prnp-siRNA treatment is suppressed for 5 days.
To test the in vivo relevance of this observation, reciprocal adoptive transfer EAE experiments were conducted. PLPp139–151-specific T cells that were treated with Prnp-siRNA or nonsense-siRNA were adoptively transferred into recipient mice treated with either Prnp-siRNA or nonsense-siRNA. Significant worsening of clinical EAE was observed in (i) recipient mice treated with Prnp-siRNA that received untreated donor cells; (ii) recipient mice treated with Prnp-siRNA that received Prnp-siRNA-treated donor cells and (iii) recipient mice treated with nonsense-siRNA that received Prnp-siRNA-treated donor cells (Fig. 5E, Supplementary Table S1F). PrPc expression in draining lymph nodes was diminished in lymph node cells until Day 6 post siRNA administration (Fig. 5F).
Silencing of PrPc in anti-CD3/CD28 activated T cells increases zeta-chain-associated protein-70 phosphorylation and nuclear factor of activated T cells/activator protein 1 reporter activity
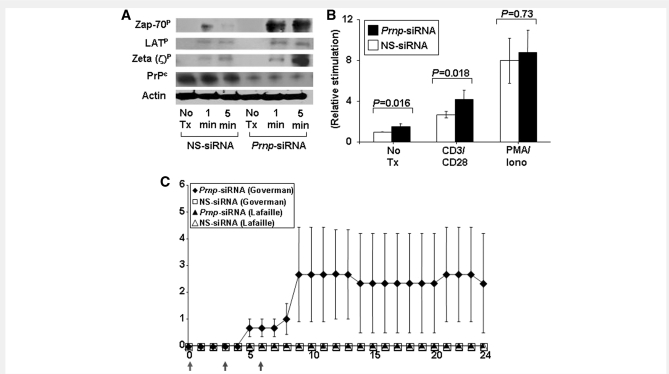
Our earlier results on the effects of PrPc on antigen-specific T cell activation, proliferation and differentiation suggested that PrPc may serve as an immunomodulatory molecule and specifically that the absence of PrPc amplified TCR signalling. Zeta-chain-associated protein (ZAP)-70, a member of the protein tyrosine kinase family, is constitutively expressed in T lymphocytes. The phosphorylation of ZAP-70 and its association with the zeta chain are upstream events in the TCR signalling pathway. Prnp-siRNA, but not nonsense-siRNA, treatment was associated with increased phosphorylation of ZAP-70 and the zeta chain of the TCR/CD3 complex (Fig. 6A). There was a less pronounced but detectable increase in tyrosine phosphorylation of the ZAP-70 substrate linker of activated T cells (Fig. 6A).
Figure 6.
Silencing PrPc on T cells increases ZAP-70 phosphorylation and NFAT/AP-1 reporter activity. (A) Phosphorylation of ZAP-70 and the zeta chain of the TCR/CD3 complex was increased in activated CD4+ T cells treated with Prnp-siRNA. (B) Transcription of an NFAT/AP-1 luciferase reporter was also significantly increased in activated and unstimulated T cells treated with Prnp-siRNA. In contrast, NFAT/AP-1 reporter activity was not altered in PMA/ionomycin-stimulated T cells. (C) MBP peptide AC1–11 TCR transgenic mice on a B10.PL background (I-Au) generated by Joan Goverman and coworkers developed EAE after in vivo intravenous treatment with Prnp-siRNA on Days 0, 3 and 6 of the observation period (indicated by grey arrows), but not after treatment with nonsense-siRNA. In contrast, MBP1–11 TCR transgenic mice generated by Lafaille and coworkers did not develop disease.
After activation with anti-CD3 and anti-CD28 monoclonal antibodies, transcription of an nuclear factor of activated T cells (NFAT)/activator protein 1 (AP-1) luciferase reporter was significantly increased in T cells treated with Prnp-siRNA compared with nonsense-siRNA (Fig. 6B). There was also a significant increase in unstimulated T cells. In contrast, NFAT/AP-1 reporter activity was not altered in phorbol 12-myristate 13-acetate and ionomycin stimulated T cells (Fig. 6B), consistent with the notion that the effects of PrPc silencing were primarily at the level of TCR-proximal signalling.
Silencing PrPc induces spontaneous experimental autoimmune encephalomyelitis in MBP1–11 T cell receptor transgenic mice
We treated two distinct MBP1–11 TCR transgenic mice generated by Goverman et al. (1993) and Lafaille et al. (1994) with Prnp-siRNA. MBP1–11 TCR transgenic mice on a B10.PL background (I-Au) generated by Goverman and coworkers (1993) were reported to have a higher incidence of spontaneous EAE in non-pathogen-free conditions than the mice created by Lafaille et al. (1994). Without the addition of antigen, only mice generated by Goverman et al. rapidly developed spontaneous EAE after treatment with Prnp-siRNA on Days 0, 3 and 6 of the observation period (indicated by red arrows), but not after treatment with nonsense-siRNA (Fig. 6C).
Silencing PrPc promotes T cell survival
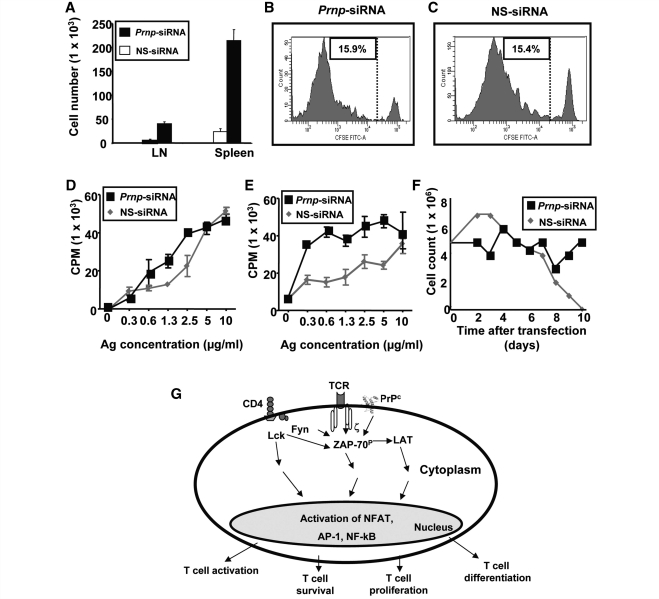
To investigate whether an effect of PrPc on the survival of activated antigen-specific T cells may account for some of its effects on CNS autoimmune disease, Prnp-siRNA or nonsense-siRNA-transfected purified OVA323–339 TCR transgenic mice CD45.2+ CD4+ T cells were transferred intravenously into C57BL/6 CD45.1+ wild-type mice that were immunized with OVA323–339 in complete freund's adjuvant 24 h after cell transfer. We were able to recover a significantly higher number of Prnp-siRNA-transfected CD45.2+ donor OVA323–339 TCR transgenic CD4+ T cells from lymph nodes and spleens than nonsense-siRNA-transfected cells (Fig. 7A). At Day 5 post transfer, there was no difference with regard to in vivo antigen-specific proliferation between OVA323–339 TCR transgenic CD4+ T cells transfected with Prnp-siRNA (Fig. 7B), or with nonsense-siRNA (Fig. 7C) as shown by flow cytometric proliferation analysis of 5- (and 6-) carboxy fluorescein diacetate succinimidyl ester-labelled OVA323–339 TCR transgenic CD45.2+ CD4+ T cells. When lymph nodes (Fig. 7D) and splenocytes (Fig. 7E) of recipient mice were resected on Day 3 after immunization, and recall assays were performed with OVA323–339, we observed a significant increase in cell proliferation in both compartments. Thus, while there was no change in proliferative responses in antigen-specific T cells in both secondary lymphoid organs, there appeared to be bystander-activation of other cells.
Figure 7.
Silencing of PrPc improves T cell survival. Prnp-siRNA or nonsense-siRNA-transfected purified OVA323–339 TCR transgenic CD45.2+ CD4+ T cells were transferred intravenously into C57BL/6 CD45.1+ wild-type mice that were immunized with OVA323–339 in complete freund's adjuvant 24 h after cell transfer. (A) A significantly higher number of Prnp-siRNA-transfected CD45.2+ donor OVA323–339 TCR transgenic CD4+ T cells were recovered from lymph nodes and spleens than nonsense-siRNA-transfected cells. At Day 5 post transfer, there was no difference with regard to in vivo antigen-specific proliferation between OVA323–339 TCR transgenic CD4+ T cells transfected with (B) Prnp-siRNA, or with (C) nonsense-siRNA. When (D) lymph nodes and (E) splenocytes of recipient mice were resected on Day 3 after immunization, and recall assays were performed with OVA323–339, a significant increase in cell proliferation in both compartments was observed. (F) The number of purified antigen-activated CD4+ MBP1–11 TCR transgenic T cells transfected with nonsense-siRNA started to decline in vitro on Day 3, and none of the cells were viable on Day 10. Cell numbers remained relatively stable in the CD4+ MBP1–11 TCR transgenic T cells treated with Prnp-siRNA. (G) Schematic of proposed mechanisms of action of PrPc in antigen-specific T cells.
Next, purified CD4+ MBP1–11 TCR transgenic T cells from Lafaille et al. (1994) MBP1–11 TCR transgenic mice were activated in vitro with 2 µg/ml MBP1–11 for 72 h. The number of nonsense-siRNA-transfected T cells started to decline on Day 3 after putting the cells into culture, and none of the cells in this experimental group were viable on Day 10 (Fig. 7F). In contrast, cell numbers remained relatively stable in the CD4+ MBP1–11 TCR transgenic T cells transfected with Prnp-siRNA (Fig. 7F).
Discussion
Although it is well-known as a substrate for PrPSc in prion diseases, the normal functions of PrPc in cellular processes and non-prion CNS diseases remain enigmatic. In the EAE model of CNS autoimmunity, we describe that PrPc is an important inhibitor of peripheral immune function. Deletion, knockdown or blockade of PrPc resulted in accelerated and more severe autoimmunity while PrPc overexpression (on a PrP knockout background) resulted in diminished autoimmunity.
Consistent with recently published findings (Tsutsui et al., 2008; Ingram et al., 2009), we have found that EAE severity was significantly increased in PrP knockout mice. Based purely on the knockout results, however, it remained unclear whether the effects of PrPc on CNS autoimmunity were due to alterations in the susceptibility of the CNS to inflammation (i.e. neuronal survival) or due to alterations of the inflammatory response. Additionally, because the knockout animals lack all PrPc expression, it was not apparent whether alterations in immune function were due to defects in development or thymic negative selection. Pharmacological in vivo silencing of PrPc using Prnp-siRNA allowed us to define the role of this molecule more precisely in different disease stages. PrPc expression was effectively silenced solely in cells of the peripheral immune system but not brain tissue after treatment with Prnp-siRNA. In addition, Cy3-labelled siRNA was undetectable in CNS tissue indicating that the major location of siRNA knockdown is the periphery. Consequently, we conclude that the major effects of PrPc in EAE pathogenesis are based on the decrease in PrPc expression in lymphatic tissues. Furthermore, Prnp-siRNA administration in adult mice resulted in rapidly enhanced immune responses, thereby bypassing the potential for thymic defects to account for the observed exacerbated autoimmunity. Although we cannot discount the function of PrPc in the thymic maturation processes in the knockout mouse, it appears that PrPc disruption in the peripheral immune system is sufficient to enhance immune responses.
The finding that PrPc-silencing could exacerbate disease when delivered at the time of immunization, onset of disease, or at later stages of autoimmunity strongly suggests that PrPc is an important negative regulator of T cell responses.
Our data on in vivo T cell tracking of cells treated with fluorescent Prnp-siRNA or nonsense-siRNA strongly suggest that some of the effects we observed in mice treated with Prnp-siRNA, including the increased number of inflammatory infiltrates in the CNS, may be an indirect effect of Prnp-silencing on other lymphocyte populations, resulting in an amplification of the initial immune response. These data are complementary to observations we made in our adoptive transfer experiments (Fig. 5E, Supplementary Table S1F) and in some of the survival experiments. Specifically, Prnp-siRNA or nonsense-siRNA-transfected purified OVA323–339 TCR transgenic CD45.2+ CD4+ T cells were transferred intravenously into C57BL/6 CD45.1+ wild-type mice that were immunized with OVA323–339 in complete freund's adjuvant 24 h after cell transfer to investigate whether an effect of PrPc on the survival of activated antigen-specific T cells may account for some of its effects on CNS autoimmune disease. At Day 5 post transfer, there was no difference with regard to in vivo antigen-specific proliferation between OVA323–339 TCR transgenic CD4+ T cells transfected with Prnp-siRNA (Fig. 7B), or with nonsense-siRNA (Fig. 7C). In contrast, silencing of Prnp in vitro resulted in increased proliferation of bulk T cell populations obtained from splenocytes (Fig. 5A–D).
To study the role of PrPc as a negative regulator of T lymphocyte responses in more detail, we examined the effects of PrPc knockdown on T cell activation and survival.
PrPc is a negative regulator of TCR signalling
We consistently observed an increased antigen-specific proliferation when PrPc was silenced only on T cells, rather than on T cells and antigen-presenting cells. At this point, we are investigating the differential effect of PrPc silencing and overexpression on different cell types. From preliminary data it appears that silencing of PrPc in dendritic cells may be pro-apoptotic, while it is anti-apoptotic in T cells.
TCR signalling is critical to the development of EAE at the immunization and elicitation phases of the disease. Among the earliest events of TCR engagement is recruitment of leukocyte-specific protein tyrosine kinase and subsequent phosphorylation of the TCR zeta chain. Phosphorylated zeta serves as a binding site for ZAP-70 (Chan et al., 1992), which then phosphorylates numerous substrates including linker of activated T cells. Although it is so far unclear how PrPc interacts with or influences ZAP-70, PrPc immunoprecipitated with ZAP-70 in activated T cells in a previous study (Mattei et al., 2004).
We found an increased phosphorylation of ZAP-70 in activated T cells after PrPc silencing. Suppression of the ZAP-70 dependent pathway by PrPc was confirmed by demonstrating that basal and CD3/CD28-stimulated transcription from NFAT/AP-1, two transcription factors crucial in T cell activation and differentiation, were also enhanced upon silencing PrPc in T cells (Fig. 6B). Interestingly, NFAT transcription was not altered in the PMA/ionomycin-stimulated T cells. Due to the fact that PMA/ionomycin induces NFAT/AP-1-dependent transcription by activation of protein kinase C/Ras/mitogen-activated protein kinase and calcium mobilization, respectively (Chatila et al., 1989; Liu and Heckman 1998), our results support the notion that PrPc functions as a regulator of TCR-proximal signalling. The in vivo importance of PrPc as a negative regulator of TCR signals was demonstrated by the increased incidence of spontaneous EAE in the autoimmune-prone MBP1–11 TCR-transgenic mice after treatment with Prnp-siRNA (Fig. 6C).
We also demonstrate that PrPc may exert an indirect immunosuppressive function by promoting T cell death. Increased numbers of antigen-specific T cells in vivo after silencing of Prnp allow several explanations: (i) a decreased sequestration of these cells from secondary lymphoid organs into other compartments; (ii) a decrease of Fas-mediated apoptotic cell death; (iii) a diminished growth factor withdrawal due to decreased antigen-specific proliferation or (iv) a push of these cells into cell cycle. The latter scenario was basically ruled out by showing that there was no difference in proliferation of antigen-specific T cells transfected with Prnp-siRNA or nonsense-siRNA. Furthermore our results on increased T cell survival in vitro after silencing of PrPc strongly argue against the hypothesis of diminished growth factor withdrawal from the Prnp-siRNA-treated cells, as more cells were competing for growth factors in a closed system for a 10 day period. These results also rule out differences in tissue sequestration between the two treatment groups. Thus, PrPc may promote apoptosis in activated antigen-specific T cells, possibly mediated through a Fas pathway, or other tumor necrosis factor receptor superfamily member pathways.
Summary and proposed model for PrPc
In summary, we reveal PrPc as a molecule critically influencing T cell functions, by modulating TCR signals and limiting survival. We can thereby clearly assign an essential function to PrPc beyond being the replicative substrate or transport molecule for prions. Whether the conversion from PrPc to the infectious PrPSc conformer also involves changes in T cell function, and whether these have anything to do with prion disease mechanisms is currently unclear. It is possible that the effect for PrPc on regulating TCR signals may suggest a similar function in other cell types. Other cellular receptors, including the B cell receptor, Fc receptor, epidermal growth factor receptor and insulin receptor possess a similar signalling architecture as the TCR. Our observations put PrPc at the intersection of neuroinflammation and neurodegeneration.
Funding
Funding for this project was provided through a Merit Award from the Department of Veterans Affairs. Start-up Grant from the Dallas VA Research Corporation; New Investigator Award from VISN 17 from the Department of Veterans Affairs; Clinical Scientist Development Award from the Doris Duke Charitable Foundation; Merit Award from the Department of Veterans Affairs; grant from the Viragh Foundation (to Dr O.S.); grant from the VW foundation; grants from the FP6, EU Research Commission Anteprion (LSHB-CT-2006-019090) and StrainBarrier (FP6 2004 Food 3B 023183) (to C.K.); Fritz Thyssen foundation (AZ 10.08.1.178 to S.N.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We thank Ryan Jordanhazy and Dr Paul Carson for excellent technical assistance, and Katherine Deming for critical review of the manuscript. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Glossary
Abbreviations
- AP-1
activator protein 1
- EAE
experimental autoimmune encephalomyelitis
- ELISA
enzyme-linked immunosorbent assay
- IgG
immunoglobulin G
- IL
interleukin
- MBP
myelin basic protein
- NFAT
nuclear factor of activated T cells
- PBS
phosphate buffered saline
- PMA
phorbol 12-myristate 13-acetate
- PrP
prion protein
- Prnp
prion protein gene
- siRNA
small interfering ribonucleic acid
- TCR
T cell receptor
- Th
T helper cell
- Zap70
zeta-chain-associated protein-70
References
- Antoine N, Cesbron JY, Coumans B, Jolois O, Zorzi W, Heinen E. Differential expression of cellular prion protein on human blood and tonsil lymphocytes. Haematologica. 2000;85:475–80. [PubMed] [Google Scholar]
- Bueler H, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, et al. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–82. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- Burthem J, Urban B, Pain A, Roberts DJ. The normal cellular prion protein is strongly expressed by myeloid dendritic cells. Blood. 2001;98:3733–8. doi: 10.1182/blood.v98.13.3733. [DOI] [PubMed] [Google Scholar]
- Cashman NR, Loertscher R, Nalbantoglu J, Shaw I, Kascsak RJ, Bolton DC, et al. Cellular isoform of the scrapie agent protein participates in lymphocyte activation. Cell. 1990;61:185–92. doi: 10.1016/0092-8674(90)90225-4. [DOI] [PubMed] [Google Scholar]
- Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–62. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Chatila T, Silverman L, Miller R, Geha R. Mechanisms of T cell activation by the calcium ionophore ionomycin. J Immunol. 1989;143:1283–9. [PubMed] [Google Scholar]
- de Souza AJ, Oriss TB, O’malley KJ, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc Natl Acad Sci USA. 2005;102:17113–8. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeArmond SJ, Qiu Y, Sanchez H, Spilman PR, Ninchak-Casey A, Alonso D, et al. PrPc glycoform heterogeneity as a function of brain region: implications for selective targeting of neurons by prion strains. J Neuropathol Exp Neurol. 1999;58:1000–9. doi: 10.1097/00005072-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Durig J, Giese A, Schulz-Schaeffer W, Rosenthal C, Schmucker U, Bieschke J, et al. Differential constitutive and activation-dependent expression of prion protein in human peripheral blood leucocytes. Br J Haematol. 2000;108:488–95. doi: 10.1046/j.1365-2141.2000.01881.x. [DOI] [PubMed] [Google Scholar]
- Fischer M, Rulicke T, Raeber A, Sailer A, Moser M, Oesch B, et al. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996;15:1255–64. [PMC free article] [PubMed] [Google Scholar]
- Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–60. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- Ingram RJ, Isaacs JD, Kaur G, Lowther DE, Reynolds CJ, Boyton RJ, et al. A role of cellular prion protein in programming T-cell cytokine responses in disease. FASEB J. 2009;23:1672–84. doi: 10.1096/fj.08-116087. [DOI] [PubMed] [Google Scholar]
- Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- Kane LP, Mollenauer MN, Weiss A. A proline-rich motif in the C terminus of Akt contributes to its localization in the immunological synapse. J Immunol. 2004;172:5441–9. doi: 10.4049/jimmunol.172.9.5441. [DOI] [PubMed] [Google Scholar]
- Kaye J, Porcelli S, Tite J, Jones B, Janeway CA., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983;158:836–56. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs B, Dorner-Ciossek C, Schmalzbauer R, Vassallo N, Herms J, Kretzschmar HA. Prion protein induced signaling cascades in monocytes. Biochem Biophys Res Commun. 2006;340:13–22. doi: 10.1016/j.bbrc.2005.11.158. [DOI] [PubMed] [Google Scholar]
- Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- Li R, Liu D, Zanusso G, Liu T, Fayen JD, Huang JH, et al. The expression and potential function of cellular prion protein in human lymphocytes. Cell Immunol. 2001;207:49–58. doi: 10.1006/cimm.2000.1751. [DOI] [PubMed] [Google Scholar]
- Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–42. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, Hussain RZ, Ratts RB, et al. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity. 2004;21:719–31. doi: 10.1016/j.immuni.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Manson JC, Clarke AR, Hooper ML, Aitchison L, McConnell I, Hope J. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol Neurobiol. 1994;8:121–7. doi: 10.1007/BF02780662. [DOI] [PubMed] [Google Scholar]
- Mattei V, Garofalo T, Misasi R, Circella A, Manganelli V, Lucania G, et al. Prion protein is a component of the multimolecular signaling complex involved in T cell activation. FEBS Lett. 2004;560:14–18. doi: 10.1016/S0014-5793(04)00029-8. [DOI] [PubMed] [Google Scholar]
- Muller-Schiffmann A, Petsch B, Leliveld SR, Muyrers J, Salwierz A, Mangels C, et al. Complementarity determining regions of an anti-prion protein scFv fragment orchestrate conformation specificity and antiprion activity. Mol Immunol. 2009;46:532–40. doi: 10.1016/j.molimm.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Puttaparthi K, Wojcik C, Rajendran B, DeMartino GN, Elliott JL. Aggregate formation in the spinal cord of mutant SOD1 transgenic mice is reversible and mediated by proteasomes. J Neurochem. 2003;87:851–60. doi: 10.1046/j.1471-4159.2003.02028.x. [DOI] [PubMed] [Google Scholar]
- Stuve O, Youssef S, Slavin AJ, King CL, Patarroyo JC, Hirschberg DL, et al. The role of the MHC class II transactivator in class II expression and antigen presentation by astrocytes and in susceptibility to central nervous system autoimmune disease. J Immunol. 2002;169:6720–32. doi: 10.4049/jimmunol.169.12.6720. [DOI] [PubMed] [Google Scholar]
- Stuve O, Youssef S, Weber MS, Nessler S, von Budingen HC, Hemmer B, et al. Immunomodulatory synergy by combination of atorvastatin and glatiramer acetate in treatment of CNS autoimmunity. J Clin Invest. 2006;116:1037–44. doi: 10.1172/JCI25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testi R, Phillips JH, Lanier LL. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989;142:1854–60. [PubMed] [Google Scholar]
- Tsutsui S, Hahn JN, Johnson TA, Ali Z, Jirik FR. Absence of the cellular prion protein exacerbates and prolongs neuroinflammation in experimental autoimmune encephalomyelitis. Am J Pathol. 2008;173:1029–41. doi: 10.2353/ajpath.2008.071062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- Zabel M, Greenwood C, Thackray AM, Pulford B, Rens W, Bujdoso R. Perturbation of T-cell development by insertional mutation of a PrP transgene. Immunology. 2009;127:226–36. doi: 10.1111/j.1365-2567.2008.02944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]